Key Points
Only high HHV6 viremia (>105 copies/mL) affects late but not early T-cell reconstitution after HCT.
Antivirals improve T-cell reconstitution probability in the context of HHV6 viremia after HCT.
Abstract
Human herpesvirus 6 (HHV6) viremia is a common cause of morbidity following allogeneic hematopoietic cell transplantation (HCT). We previously associated T-cell reconstitution with HHV6 viremia. Here, we investigated whether HHV6 viremia affects T-cell reconstitution after HCT in a time-dependent retrospective analysis. We included 273 pediatric patients (0.1-22.7 years; median follow-up, 58 months) receiving a first HCT between 2004 and 2014. HHV6 was screened weekly in plasma via polymerase chain reaction and occurred in 79 patients (29%) at a median time of 19 days after transplant. Main outcome of interest was immune reconstitution (IR) (CD3/CD4/CD8 T cells), measured biweekly until 12 weeks and monthly thereafter. Cox proportional-hazard models were used with IR and HHV6 as time-dependent variables in multivariate analysis with serotherapy in conditioning, graft source, graft-versus-host disease, age, and other viruses (Epstein-Barr virus, cytomegalovirus, and adenovirus) as covariates. Only patients with very high HHV6 viremia (>105 copies/mL) showed hampered CD4+ (hazard ratio [HR], 0.913; 95% confidence interval [CI], 0.892-0.934; P < .001) and CD8+ (HR, 0.912; 95% CI, 0.891-0.933; P < .001) reconstitution in comparison with patients without HHV6, from ∼6 months after HCT. Especially naïve CD4+ IR was affected (P = .028) but not effector memory CD4+ IR (P = .33). Interestingly, T-cell reconstitution was improved in patients treated with antivirals (HR, 1.572; 95% CI, 1.463-1.690; P < .001). These findings suggest that HHV6 viremia affects late but not early T-cell reconstitution.
Visual Abstract
Introduction
Viral reactivations severely hamper the success of allogeneic hematopoietic stem cell transplantation (HCT).1-8 We recently showed that reactivation of human herpesvirus 6 (HHV6) increases the risk of developing acute graft-versus-host disease (aGVHD) but only in patients without successful CD4+ T-cell immune reconstitution (CD4+ immune reconstitution [IR]; defined as having 2 consecutive measurements of ≥50 × 106 CD4+ cells/L of blood, within 100 days after HCT).8 Moreover, HHV6 counts were lower in patients with adequate CD4+ IR. This was also found for adenovirus (AdV) and Epstein-Bar virus (EBV). Nevertheless, in vitro and in vivo models have shown that HHV6 predominantly infects and kills CD4+ T cells.9-13 Therefore, the question arises whether CD4 T-cell reconstitution can be hampered by HHV6 viremia so that a high HHV6 load would result in lower CD4+ T-cell counts, and vice versa. We here investigate the causality of the relation between CD4+ T-cell recovery after HCT and HHV6 load. We applied multivariate Cox proportional-hazard modeling to evaluate the effect of HHV6 viremia on T-cell reconstitution after HCT, which enables time-dependent analysis.
Patients and methods
We included pediatric patients receiving their first allogeneic HCT between 2004 and 2014 at the University Medical Centre in Utrecht, The Netherlands. Patients were treated as has been previously described.8 Blood samples of peripheral blood mononuclear cells and outcome data were collected and registered prospectively. After reaching a leukocyte count of at least 0.3 × 109/L, absolute numbers of lymphocytes were measured by flow cytometry at least every other week up to 12 weeks post-HCT and monthly thereafter, up to 6 months post-HCT in EDTA-treated whole blood using TruCOUNT tubes (BD Biosciences).
HHV6, AdV, EBV, and cytomegalovirus (CMV) were screened weekly via polymerase chain reaction following HCT. For HHV6 viremia, no distinction was made between reactivation or primo infection. Patients with HHV6 reactivation were treated with foscarnet only in high clinical suspicion of HHV6-associated disease (eg, encephalitis, bone marrow suppression). Treatment of viral reactivations was stopped when viral load was undetectable in 2 subsequent samples or in case of severe toxicity after acquiring low viral loads (<1000 copies/mL). Patients were enrolled, and data were collected after written informed consent in accordance with the Helsinki Declaration. The study was approved by the local ethical committee (trial numbers 05-143 and 11-063k).
Statistical analyses were performed using R 3.0.1 using the packages survival and cmprsk. Cox proportional-hazard models were used to analyze the relation between HHV6 viremia and the probability of CD3 (twice ≥100 × 106 CD3+ cells/L of blood), CD4 (twice ≥50 × 106 CD3+CD4+ cells/L of blood), and CD8 (twice ≥50 × 106 CD3+CD8+ cells/L of blood) reconstitution at any time after HCT. Conditioning with serotherapy (eg, antithymocyte globulin, alemtuzumab), graft source, grade II to IV aGVHD (treated with steroids), age, and other viruses were evaluated as covariates to exclude additional T-cell depleting effects. The effect of antiviral therapy was evaluated with patients receiving cidofovir (for adenovirus reactivation of >1000 copies/mL), ganciclovir, or foscarnet (for CMV reactivation of >1000 copies/mL). Linear regression curves were used to analyze the effect of HHV6 viremia on naïve (CD45RA+CCR7+) and effector memory (CD45RO+CCR7+/−) CD4+ T-cell reconstitution. Locally weighted smoothing (LOESS) regression curves were fitted to the data for visualization of differences between groups.
Results
We evaluated whether HHV6, as well as AdV, CMV, and EBV, independently affects T-cell reconstitution after HCT in 273 pediatric patients (Table 1). HHV6 viremia occurred in 79 patients (29%), AdV in 41 patients (15%), CMV in 50 patients (18%), and EBV in 29 patients (11%). Of these patients, 31 (11%) were single-positive for HHV6 viremia, 14 (5%) for AdV, 25 (9%) for CMV, and 9 (3%) for EBV viremia. In multivariate analysis, evaluating viremia as time-varying variables, HHV6 viremia decreased both CD4+ (hazard ratio [HR], 0.913; 95% CI, 0.892-0.934; P < .001) and CD8+ (HR, 0.912; 95% CI, 0.891-0.933; P < .001) IR. This translates to the chance of having CD4+ IR being reduced 8.7% with every increase of 105 HHV6 copies/mL of plasma. Median time to CD4+ IR was 78 days in patients without HHV6 viremia and 84 days in patients with HHV6 viremia, independent of aGVHD. EBV had less effect on CD8+ (HR, 0.962; 95% CI, 0.934-0.989; P = .01) and CD4+ (HR, 0.964; 95% CI, 0.937-0.993; P = .01) IR, indicating a 3.6% reduced chance of having CD4+ IR with every increase of 105 EBV copies/mL of plasma. CMV and AdV did not affect T-cell recovery in multivariate analysis. Nevertheless, the actual effect is limited, and only a few patients have >105 HHV6 copies/mL of viremia, indicating that this effect of HHV6 on T-cell reconstitution after HCT is not clinically relevant.
Table 1.
Uni- and multivariate analyses of the impact of viremia on T-cell reconstitution after HCT
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Outcome | P | HR | 95% CI | P |
| HHV6 | ||||
| CD3+ IR | <.001*** | 0.922 | 0.902-0.942 | <.001*** |
| CD4+ IR | <.001*** | 0.913 | 0.892-0.934 | <.001*** |
| CD8+ IR | <.001*** | 0.912 | 0.891-0.933 | <.001*** |
| EBV | ||||
| CD3+ IR | .006** | 0.963 | 0.937-0.991 | .01* |
| CD4+ IR | .006** | 0.964 | 0.937-0.993 | .01* |
| CD8+ IR | .015* | 0.962 | 0.934-0.989 | .01* |
| CMV | ||||
| CD3+ IR | .034* | 0.971 | 0.882-1.068 | .54 |
| CD4+ IR | .033* | 0.972 | 0.877-1.077 | .58 |
| CD8+ IR | .081 | 1.015 | 0.936-1.101 | .71 |
| AdV | ||||
| CD3+ IR | .58 | 0.999 | 0.999-1.000 | .55 |
| CD4+ IR | .57 | 0.999 | 0.999-1.000 | .58 |
| CD8+ IR | .63 | 0.999 | 0.999-1.000 | .64 |
| Antiviral treatment | ||||
| CD3+ IR | <.001*** | 1.566 | 1.459-1.681 | <.001*** |
| CD4+ IR | <.001*** | 1.572 | 1.463-1.690 | <.001*** |
| CD8+ IR | <.001*** | 1.707 | 1.586-1.837 | <.001*** |
Virus and cell recovery were considered time-varying variables. Analyses were corrected for variables other than viruses that are known to affect immune reconstitution (serotherapy, graft source, steroid-treated aGVHD, age). Cox proportional hazard models were used. Hazard ratio (HR) on T-cell reconstitution probability for every 105 viral copies/mL plasma.
CI, confidence interval.
P < .05; **P < .01; ***P < .001.
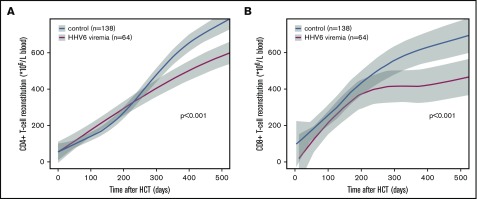
Figure 1A shows that HHV6 especially affects long-term CD4+ T-cell recovery, but also CD8+ T-cell recovery (Figure 1B), after about 250 days, whereas no effects were observed in the first 100 days after HCT (P = .83 in comparison with patients without HHV6 viremia, not shown). In addition, CD4+ T-cell counts in patients with or without HHV6 viremia were comparable before the onset of the reactivations (P = .11, not shown), which mostly occur early after HCT (median time, 19 days). These findings suggest that the CD4+ T-cell depleting effect of HHV6 found in vitro and in animal models (in a nonlymphodepleted setting)9-13 does not directly influence CD4+ T-cell recovery after HCT in vivo in humans. In addition, we observed both an effect on long-term CD4+ and CD8+ T-cell reconstitution, whereas in vitro, HHV6 showed only low apoptosis in CD8+ T cells.9-13 T cells recover early after transplantation by homeostatic peripheral expansion (HPE) when T cells from the graft proliferate, driven by cytokines and a lymphopenic environment.14 Therefore, it might be that the T cells that were depleted by HHV6 are efficiently replaced through HPE of the remaining T cells.
Figure 1.
The impact of HHV6 viremia on CD4+and CD8+T-cell reconstitution. LOESS curves of CD4+ (A) and CD8+ (B) T-cell counts in blood over time after HCT are depicted in patients with (n = 64; red lines) and without (n = 138; blue lines) HHV6 viremia. Patients with steroid-treated severe aGVHD were excluded from these graphs. Gray areas depict 95% CIs of LOESS fit.
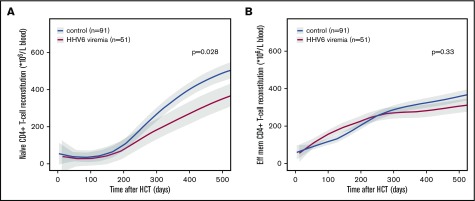
Although HPE is responsible for early T-cell recovery, late T-cell reconstitution is dependent on thymopoiesis,14 which takes at least 6 months to occur after HCT. The observation that HHV6 viremia affects T-cell reconstitution at least 6 months after transplantation, and has no effect on recovery of other immune cells (data not shown), suggests that HHV6 might affect thymopoiesis. In addition, further analysis shows that HHV6 affects naïve CD4+ T-cell reconstitution after 300 days (P = .028; Figure 2A) but not effector memory CD4+ IR (P = .33; Figure 2B). Indeed, an effect of HHV6 on thymus cells has been recently shown in an in vivo mouse model.12 Future studies should investigate whether HHV6 infects the human thymus, causing delayed T-cell reconstitution after HCT. In addition, it would be interesting to evaluate whether pre-emptive or prophylactic antiviral therapy could neutralize this possible effect on thymopoiesis in a prospective study.
Figure 2.
Naïve and effector memory CD4+T-cell reconstitution in context HHV6 viremia. LOESS curves of naïve (A) and effector memory (B) CD4+ T-cell counts in blood over time after HCT are depicted in patients with (n = 51; red lines) and without HHV6 viremia (n = 91; blue lines). Patients with steroid-treated severe aGVHD were excluded from these graphs. Gray areas depict 95% CIs of LOESS fit.
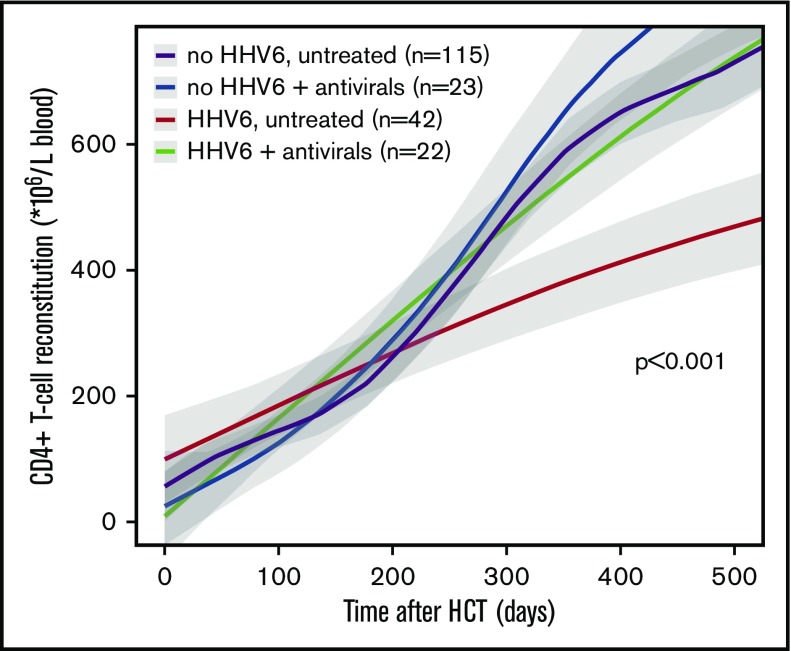
In our cohort, HHV6 reactivation was treated in only 11 patients (only in case of encephalitis or bone marrow suppression suspected to be associated with HHV6 reactivation), whereas antivirals were given to the majority of patients with a CMV or AdV reactivation (who had a HHV6 reactivation at the same time). T-cell reconstitution was not delayed in patients with HHV6 viremia who received antivirals (for any virus) with effectivity against HHV6. Figure 3 illustrates CD4+ T-cell recovery after HCT in patients with and without HHV6 viremia, who did or did not receive antiviral treatment. In multivariate analysis, antiviral treatment increased the chance of CD4+ IR by 42.8%, despite viremia (HR, 1.572; 95% CI, 1.463-1.690; P < .001).
Figure 3.
The impact of HHV6 viremia on CD4+T-cell reconstitution in context of antiviral therapy. LOESS curves of CD4+ T-cell counts in patients, grouped as having no HHV6 and no antiviral treatment (n = 115; purple line); having no HHV6 with antiviral treatment (n = 23; blue line); having HHV6 viremia and no antiviral treatment (n = 42; red line); or having HHV6 viremia with antiviral treatment (n = 22; green line). Patients with steroid-treated severe aGVHD were excluded from this graph. Gray areas depict 95% CIs of LOESS fit.
Discussion
Our findings indicate that late T-cell reconstitution after HCT is affected only by the presence of high HHV6 viremia. This indicates that the association between early CD4+ IR and lower HHV6 viremia, and subsequent aGVHD risk,8 which we previously reported, does not merely result from CD4 T-cell depletion due to HHV6 viremia after HCT. Antiviral treatment might be able to neutralize the delay in T-cell reconstitution caused by HHV6, which may, however, cause toxicity.15 Currently, alternative antiviral therapies based on virus-specific T-cell immunotherapy show successful control of viruses, including HHV6 viremia.16-19 Because HHV6 is associated with increased aGVHD risk in patients with delayed CD4+ IR,8 intensive monitoring, with additional antiviral pre-emptive or prophylactic treatment, may be considered to improve the outcomes of HCT recipients with a high HHV6 load.
Acknowledgments
The authors would like to thank Kristin Loomis and Michael Boeckh for excellent expert feedback during the data evaluation and preparation of this manuscript.
This work was supported by Foundation Children Cancerfree (KiKa) (project 142).
Authorship
Contribution: J.J.B., S.N., and C.d.K. designed the study; C.d.K. wrote the manuscript; C.d.K. and R.A. analyzed the data; J.J.B. and S.N. provided critical comments; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaap Jan Boelens, Pediatric Blood and Marrow Transplantation Program, Lundlaan 6: KC 0.30.063.0, 3584 CX Utrecht, The Netherlands; e-mail: j.j.boelens@umcutrecht.nl.
References
- 1.Pourgheysari B, Piper KP, McLarnon A, et al. . Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853-861. [DOI] [PubMed] [Google Scholar]
- 2.Broers AE, van Der Holt R, van Esser JW, et al. . Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95(7):2240-2245. [PubMed] [Google Scholar]
- 3.Hiwarkar P, Gaspar HB, Gilmour K, et al. . Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48(6):803-808. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Leisenring W, Riddell SR, et al. . Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407-414. [DOI] [PubMed] [Google Scholar]
- 5.Teira P, Battiwalla M, Ramanathan M, et al. . Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341-352. [DOI] [PubMed] [Google Scholar]
- 7.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40(7):932-940. [DOI] [PubMed] [Google Scholar]
- 8.Admiraal R, de Koning C, Bierings MB, et al. . Viral reactivations and associated outcomes in context of immune reconstitution after pediatric hematopoieitc cell transplantation. J Allergy Clin Immunol. 2017;140(6):1643-1650. [DOI] [PubMed] [Google Scholar]
- 9.Yasukawa M, Inoue Y, Ohminami H, Terada K, Fujita S. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J Gen Virol. 1998;79(Pt 1):143-147. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Yasukawa M, Fujita S. Induction of T-cell apoptosis by human herpesvirus 6. J Virol. 1997;71(5):3751-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K, Sonoda S, Higashi K, et al. . Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989;63(7):3161-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SJ, Zhao G, Penna VR, et al. . A murine herpesvirus closely related to ubiquitous human herpesviruses causes T-cell depletion. J Virol. 2017;91(9):e02463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichimi R, Jin-no T, Ito M. Induction of apoptosis in cord blood lymphocytes by HHV-6. J Med Virol. 1999;58(1):63-68. [DOI] [PubMed] [Google Scholar]
- 14.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71(2-3):154-163. [DOI] [PubMed] [Google Scholar]
- 16.Leen A, Gee AP, Leung K, et al. . Multi-virus-specific T-cell therapy for patients after hematopoietic stem cell and cord blood transplantation. Blood. 2013;122(21):140. [Google Scholar]
- 17.Heslop HE, Leen AM. T-cell therapy for viral infections. Hematology Am Soc Hematol Educ Program. 2013;2013:342-347. [DOI] [PubMed] [Google Scholar]
- 18.Hanley PJ, Lam S, Shpall EJ, Bollard CM. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. J Vis Exp. 2012;7(63):e3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou A, Gerdemann U, Katari UL, et al. . Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6(242):242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]