Key Points
Less immunogenic FVIII muteins were designed by defining and replacing MHCII anchor residues with amino acids that reduced MHCII binding.
Patient-derived T-cell clones show lower proliferation in response to FVIII-F2196K, which had normal FVIII activity and expression level.
Abstract
Factor VIII (FVIII)–neutralizing antibodies (inhibitors) are a serious complication in hemophilia A (HA). The peptide FVIII2194-2213 contains an immunodominant HLA-DRA*01-DRB1*01:01 (DRB1*01:01)-restricted epitope recognized by CD4+ T-effector cells from HA subjects. The aim of this study was to identify amino acid substitutions to deimmunize this epitope while retaining procoagulant function and expression levels comparable to those of wild-type (WT) FVIII proteins. The shortest DRB1*01:01-binding peptide was FVIII2194-2205, and residues important for affinity were identified as F2196, M2199, A2201, and S2204. T-cell proliferation experiments with Ala-substituted FVIII2194-2205 peptides identified F2196A as a substitution that abrogated proliferation of clones specific for the WT sequence. T-cell clones that were stimulated by recombinant WT-FVIII-C2 (rWT-FVIII-C2) protein did not proliferate when cultured with rFVIII-C2-F2196A, indicating the immunogenic peptide includes a naturally processed T-cell epitope. Additional amino acid substitutions at F2196 and M2199 were evaluated by peptide-MHC class II (MHCII)–binding assays, T-cell proliferation assays, epitope prediction algorithms, and sequence homologies. Six B-domain–deleted (BDD)-FVIII proteins with substitutions F2196A, F2196L, F2196K, M2199A, M2199W, or M2199R were produced. Proliferation of T-cell clones and polyclonal lines in response to rBDD-FVIII-F2196K and rBDD-FVIII-M2199A was reduced compared with responses to WT-BDD-FVIII. The BDD-FVIII-F2196K sequence modification appears to be the most promising sequence variant tested here, due to its effectiveness at eliminating DRB1*01:01-restricted immunogenicity, low potential immunogenicity in the context of other MHCII alleles, expression level comparable to WT-BDD-FVIII, and retained procoagulant activity. These results provide proof of principle for the design of less immunogenic FVIII proteins targeted to specific subsets of HA patients.
Visual Abstract
Introduction
Hemophilia A (HA) is a bleeding disorder caused by factor VIII (FVIII) deficiency. The reduction of FVIII procoagulant cofactor activity compared with normal (100%) activity determines the severity of HA, which is categorized as mild (>5%-40%), moderate (1%-5%), or severe (<1%).1,2 Bleeding is best managed with FVIII replacement therapy, however, FVIII-neutralizing antibodies (“inhibitors”) develop in up to 40% of severe3 and ∼13% of mild/moderately severe4 HA patients, often resulting in significant morbidity and an ongoing risk of uncontrolled bleeds.2,5
Antibody development is initiated when FVIII is internalized and processed by professional antigen-presenting cells and FVIII peptides are presented by major histocompatibility complex class II (MHCII) proteins to T-cell receptors (TCRs) on CD4+ T-effector cells.6-10 Binding of antigenic peptides to a particular MHCII is dictated by polymorphisms in 4 or 5 major pockets within its peptide-binding groove. Peptide side chains at relative “anchor” positions 1, 4, 6, 7, and 9 bind to these pockets, with size, shape, and charge or hydrophobic complementarity determining their affinity.11-13 Recognition of MHCII-peptide complexes by specific TCR, in the context of costimulatory engagement, results in T-cell activation and proliferation. Activated T-effectors traffic to B-cell follicles where they engage with B cells via MHCII-peptide-TCR interactions and induce germinal center formation, wherein activated B cells proliferate and terminally differentiate into anti-FVIII antibody-secreting plasma cells.
An immunodominant HLA-DRA*01-DRB1*01:01 (abbreviated DRB1*01:01)-restricted epitope within overlapping peptides FVIII2186-2205 and FVIII2194-2213 (legacy FVIII numbering,14 sequence: SYFTNMFATWSPSKARLHLQ) was identified in 3 HA subjects.15-17 This allele is found in ∼8% to 9% of whites and ∼1% to 7% of other racial/ethnic groups in the United States.18 The epitope was first identified by MHCII tetramer-guided epitope mapping using peripheral blood mononuclear cells (PBMCs) from a mild HA subject with an A2201P missense substitution who developed a high-titer inhibitor.15 His brother, who had a subclinical inhibitor, also had CD4+ T cells that responded to these 2 peptides but differed in their T-helper phenotype.16,19 Interestingly, tetramer-guided epitope mapping carried out using tetramers loaded with peptides spanning the FVIII-A2, C1, and C2-domains for a severe HA subject with a large F8 gene deletion and a persistent inhibitor identified only this 1 high-affinity epitope.17 Thirty T-cell clones and 5 polyclonal lines specific for this epitope were isolated from these 3 subjects and their TCRB genes sequenced, revealing that the FVIII-specific cells that bound DRB1*01:01-FVIII2194-2213 tetramers with high avidity had a highly oligoclonal TCR repertoire.17
Removal of T-cell epitopes in FVIII through strategic sequence modifications, sometimes termed “deimmunization,” may be an effective strategy to reduce the incidence of FVIII-neutralizing antibodies.20 Jones et al initiated this approach by modifying a promiscuous epitope, FVIII2098-2112, recognized by highly expanded CD4+ T cells from 1 HA and 1 healthy control donor.21 Epitope modification has been explored for other biotherapeutics including erythropoietin,22 interferon-β (IFN-β),23 staphylokinase,24 β-lactamase,25 antibodies,26 and anti-cancer immunotoxins.27-31 Herein, experiments to nullify an immunodominant DRB1*01:01-restricted T-cell epitope in FVIII are described.
Materials and methods
Human subjects and T-cell clones
Subjects were enrolled in “Genetic Studies in Hemophilia and von Willebrand Disease” (GS1) and provided informed consent according to the principles of Helsinki. Protocols were approved by the Seattle Children’s Hospital, University of Washington, and Uniformed Services University of the Health Sciences Institutional Review Boards. HA subjects GS1-17A, GS1-32A, and GS1-56A were described previously.15-17,19 All T-cell clones and polyclonal lines from these subjects were DRB1*01:01-restricted and were specific for an epitope within overlapping peptides FVIII2186-2205 and FVIII2194-2213. Clones and polyclonal lines were expanded by stimulation with irradiated PBMCs from an HLA-mismatched individual plus phytohemagglutinin (Thermo Scientific Remel) in the presence of human interleukin-2 (IL-2; Hemagen Diagnostics, Columbia, MD) in T-cell medium (RPMI 1640 with 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 15% human serum [MP Biomedicals, Solon, OH], 2 mM L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin) as described.17 Blood samples were also obtained from DRB1*01:01-mismatched and DRB1*01:01-matched healthy controls. PBMCs were obtained by Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Marlborough, MA) underlay and either frozen in 7% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) in fetal bovine serum (FBS; GE Healthcare Bio-Sciences HyClone) or used immediately.
FVIII peptides and MHCII-peptide binding
FVIII2194-2213 and FVIII peptides truncated and/or with sequence modifications, as well as reference peptides, were obtained from commercial vendors (Mimotopes, Clayton, VIC, Australia; Global Peptide Inc, Ft. Collins, CO; Synpep, Dublin, CA; Anaspec, Fremont, CA). Molecular weights were confirmed by mass spectrometry. Ten recombinant HLA-DR proteins and reference non-FVIII peptides with known MHCII-binding affinities are listed in supplemental Table 1. Affinities of FVIII peptides for the HLA-DR proteins were determined by competition assays using these reference peptides as described.15,32-34 MHCII-peptide–binding FVIII sequences were also predicted using ProPred35 and Immune Epitope Database (IEDB)36,37 algorithms. MHCII-binding motifs within these sequences were predicted using the SYFPEITHI database.38
Bioinformatics
FVIII and FV protein sequences were obtained from the National Center for Biotechnology Information (NCBI) reference sequence database (www.ncbi.nlm.nih.gov/refseq/) and were aligned using Clustal Omega.39
Recombinant FVIII-C2 and recombinant BDD-FVIII proteins
Amino acid substitution F2196A was introduced into a pET16b/wild-type (WT) FVIII-C2 plasmid containing a His tag using QuikChange II site-directed mutagenesis (Agilent, Santa Clara, CA), and proteins were expressed in Origami B(DE3)pLysS cells (EMD Millipore, Billerica, MA) and purified using a Ni-charged column (EMD Millipore) as described.40 The purification procedure included a wash step with buffer containing 0.1% Triton X-114 to remove endotoxins.41 Purified proteins were dialyzed into sterile Ca++/Mg++-free Dulbecco's phosphate-buffered saline (D-PBS) containing 5% glycerol. Endotoxin levels were tested with the ToxinSensor Chromogenic LAL endotoxin assay kit (GenScript Corporation, Piscataway, NJ).
B-domain–deleted (BDD)-FVIII muteins with single amino acid substitutions F2196A, F2196L, F2196K, M2199A, M2199W, or M2199R were stably expressed in BHK-M cells42 as described in supplemental Methods. Briefly, mutations were introduced with splicing by overlap extension43 using the primers in Table 1 and products cloned into HSQ/AvrII/ReNeo.44-46 Cells were expanded with Dulbecco's modified Eagle medium/F12 medium with L-glutamine and HEPES supplemented with 10% fetal bovine serum, 50 U/mL penicillin, 50 μg/mL streptomycin, and 100 μg/mL geneticin. Upon reaching ≥70% confluency, cells were washed with Ca++/Mg++-free D-PBS and transferred to AIM-V serum-free medium (Thermo Fisher Scientific) to collect supernatants. Proteins were purified by ion exchange and assayed for FVIII activity.45
Table 1.
Primers to introduce mutations into BDD-F8 by splicing overlap extension
| Location | Mutation* | Primer name | Sequence |
|---|---|---|---|
| External | F8-4162f-P1† | GCTGGGATGAGCACACTTTT | |
| External | F8-5302r-P4† | CCACCAAAGAAATGCAGGAC | |
| Internal | 2196A | F8-4709f-2196A-P2‡ | ATTACTGCTTCATCCTACGCTACCAATATGTTTGCCACC |
| Internal | 2196A | F8-4748r-2196A-P3‡ | GGTGGCAAACATATTGGTAGCGTAGGATGAAGCAGTAAT |
| Internal | 2196L | F8-4709f-2196L-P2 | ATTACTGCTTCATCCTACCTTACCAATATGTTTGCCACC |
| Internal | 2196L | F8-4748r-2196L-P3 | GGTGGCAAACATATTGGTAAGGTAGGATGAAGCAGTAAT |
| Internal | 2196K | F8-4709f-2196K-P2 | ATTACTGCTTCATCCTACAAAACCAATATGTTTGCCACC |
| Internal | 2196K | F8-4748r-2196K-P3 | GGTGGCAAACATATTGGTTTTGTAGGATGAAGCAGTAAT |
| Internal | 2199A | F8-4718f-2199A-P2 | TCATCCTACTTTACCAATGCTTTTGCCACCTGGTCTCCT |
| Internal | 2199A | F8-4757r-2199A-P3 | AGGAGACCAGGTGGCAAAAGCATTGGTAAAGTAGGATGA |
| Internal | 2199W | F8-4718f-2199W-P2 | TCATCCTACTTTACCAATTGGTTTGCCACCTGGTCTCCT |
| Internal | 2199W | F8-4757r-2199W-P3 | AGGAGACCAGGTGGCAAACCAATTGGTAAAGTAGGATGA |
| Internal | 2199R | F8-4718f-2199R-P2 | TCATCCTACTTTACCAATCGTTTTGCCACCTGGTCTCCT |
| Internal | 2199R | F8-4757r-2199R-P3 | AGGAGACCAGGTGGCAAAACGATTGGTAAAGTAGGATGA |
Production of BDD-FVIII muteins F2196A, F2196K, and M2199A was reported previously.53
F8-4162f-P1 and F8-5302r-P4 primers hybridize at the ends of the target sequence and amplify an ApaI-NotI fragment.
The P2 and P3 primers are internal primers that contain the mutation, which are underlined within the primer sequences.
Antigen-presentation assays
FVIII peptides (0.01-100 μM) and FVIII-C2 domain proteins (1-1000 nM) were added to 100 000 irradiated PBMCs from a donor with the DRB1*01:01 allele. After a 4-hour incubation at 37°C, T-cell clones (10 000) were added to each well. At 48 hours, 50 μL of supernatant was removed for cytokine analysis and replaced with [3H]thymidine (1 μCi per well) in T-cell medium. Cells were harvested after 18 hours of further incubation and [3H]thymidine incorporation was measured as described.16 IFN-γ and IL-4 in cell supernatants were measured by sandwich enzyme-linked immunosorbent assays (ELISAs) as described.32 Fifty percent effective concentration (EC50) values (concentration at which half-maximal levels occur) were determined with Systat (Systat Software, San Jose, CA) using a 3-parameter sigmoid model. Stimulation indices were calculated by dividing the counts per minute (cpm) of [3H]thymidine incorporated in antigen-stimulated cells by the cpm of unstimulated cells.
Immature monocyte-derived dendritic cells (iMo-DCs) from a DRB1*01:01 donor were generated in vitro from CD14+ cells as an alternative source of antigen-presenting cells (supplemental Methods). BDD-FVIII proteins (1.6-50 nM) were added to 5000 irradiated iMo-DCs. After a 4-hour incubation at 37°C with antigen, iMo-DCs were washed twice and resuspended in T-cell medium. T-cell clones (50 000) and antigen-pulsed iMo-DCs were incubated together. At 48 hours, 50 μL of supernatant was removed from each well and replaced with [3H]thymidine (1 μCi per well) in T-cell medium. Cells were harvested after 18 hours of further incubation, [3H]thymidine incorporation was measured, and stimulation indices calculated.
FVIII activity and ELISAs
To measure BDD-FVIII activity in cell supernatants, BHK-M cells were first washed with Ca++/Mg++-free D-PBS to remove serum-containing medium and transferred to AIM-V serum-free medium when ≥70% confluent. Cell supernatants were collected 1 day later, centrifuged to pellet and remove the remaining cells, and stored as aliquots at −80°C. FVIII activity was measured with 1- and 2-stage coagulation assays47 (supplemental Methods) and with a chromogenic assay using the Chromogenix COAMATIC Factor VIII kit (DiaPharma) following the microplate method. BDD-FVIII expression levels were measured using a sandwich ELISA with anti-human FVIII ESH-4 (Sekisui Diagnostics, Lexington, MA) and biotinylated anti-human FVIII ESH-8 (Sekisui Diagnostics) as the coating and detection antibodies, respectively (supplemental Methods).
Results
Mapping the minimal DRB1*01:01-restricted binding anchors and TCR contact sites
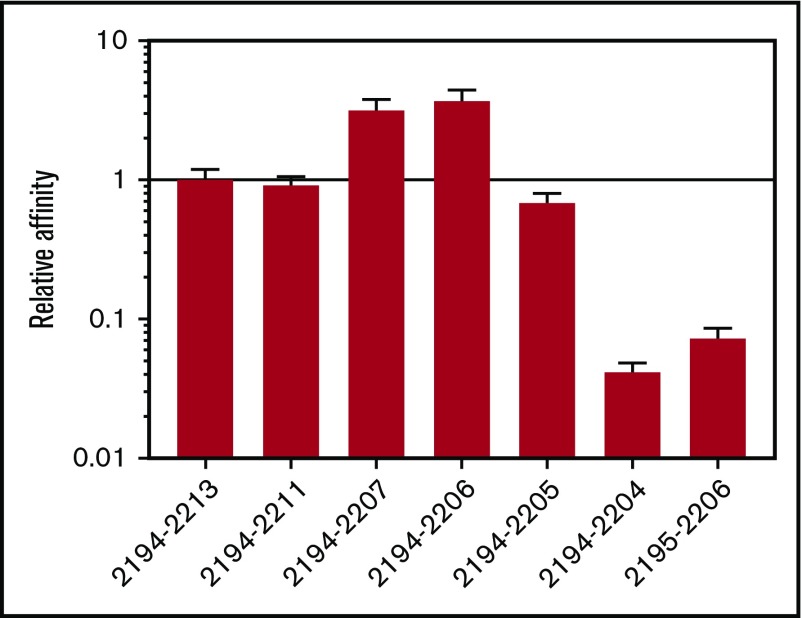
The ProPred and IEDB algorithms predicted the same minimal 9-residue DRB1*01:01-restricted binding sequence at FVIII2196-2204.17 To test this prediction, serial N-terminal and C-terminal truncations of FVIII2194-2213 were tested for DRB1*01:01 affinity by competition assays. FVIII2194-2204 affinity was reduced >10-fold compared with FVIII2194-2205, and that of FVIII2195-2206 was >10-fold reduced compared with FVIII2194-2206, indicating that the minimal peptide for high-affinity binding to recombinant DRB1*01:01 (rDRB1*01:01) is the 12-residue sequence FVIII2194-2205 (Figure 1).
Figure 1.
FVIII2194-2205constitutes the minimal binding peptide to rDRB1*0101. FVIII2194-2213 and peptides truncated from the C and N termini were tested for binding to rDRB1*01:01 using a competition assay measuring displacement of the high-affinity reference peptide HA306-318. The DRB1*01:01 protein was incubated with 0.05, 0.1, 0.5, 1, 5, 10, and 50 μM FVIII peptides plus biotinylated HA306-318 and immobilized in wells coated with anti-DR antibody L243. After washing, biotinylated peptide bound to HLA-DR was labeled using europium-conjugated streptavidin and quantified using a time-resolved fluorometer. Sigmoidal binding curves were simulated and IC50 values calculated. Results are expressed as relative affinities (IC50 of FVIII2194-2213/IC50 of the indicated truncated peptide) ± standard error (SE). The IC50 of FVIII2194-2213 was 0.22 ± 0.03 μM.
The SYFPEITHI database indicated that the binding anchor residues to DRB1*01:01 pockets 1, 4, 6, and 9 were F2196, M2199, A2201, and S2204. This prediction was tested systematically using FVIII2194-2205 peptides substituted with Arg (Figure 2A) or Ala (Figure 2B) at each possible position. More than 10-fold reduced binding to rDRB1*01:01 was seen for peptides with the following substitutions: F2196R, M2199R, A2201R, S2204R, F2196A, and M2199A, thereby confirming the predicted DRB1*01:01-restricted anchor residues.
Figure 2.
FVIII residues 2196, 2199, 2201, and 2204 contribute to DRB1*01:01 binding. The anchor residues contributing peptide-DRB1*01:01 affinity were determined by competition binding assays with reference peptide HA306-318 using FVIII2194-2205 peptides having single Arg (A) or Ala (B) substitutions at every possible position. The Arg substitution was chosen to map the anchor residues as this bulky, charged side chain is rarely accommodated by pockets within the MHCII peptide-binding groove. Ala substitutions are also informative, as they can perturb binding by removing interactions contributing to peptide-MHCII affinity. Results are expressed as relative affinities (IC50 of FVIII2194-2205/IC50 of the substituted peptide) ± SE. The IC50 of FVIII2194-2205 was 0.26 ± 0.02 μM.
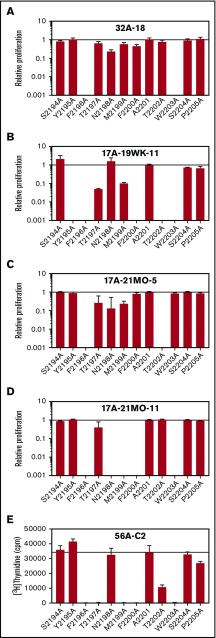
FVIII2194-2205 peptides with alanine substitutions at each position were next tested for immunogenicity by measuring T-cell proliferation and cytokine secretion of patient-derived T-cell clones having distinct TCRBV sequences17 (Table 2; Figure 3). Cytokine secretion results generally mirrored proliferation (supplemental Figure 1), identifying Y2195, T2197, N2198, F2200, T2202, and W2203 (relative peptide [p] positions p-1, p2, p3, p5, p7, and p8) as TCR contact residues. Only the substitution F2196A (MHCII anchor residue 1) abrogated immune responses of all clones.
Table 2.
Representative DRB1*01:01-restricted T-cell clones recognizing FVIII2194-2213
| T-cell clone | TCRBV* | TCRBD* | TCRBJ* | No. of clones† |
|---|---|---|---|---|
| 17A-19WK-11 | 20-1‡ | 2*01 or *02 | 2-7*01 | 3 |
| 17A-21MO-5 | 6-6*01 or *03 | 1*01 | 2-2*01 | 5 |
| 17A-21MO-11 | 5-1*01 | 1*01 | 1-2*01 | 3 |
| 32A-18 | 6-1*01 | 1*01 | 2-7*01 | 6 |
| 56A-C2 | 27*01 | Not identified | 1-1*01 | 10 |
Five CD4+ T-cell clones having unique TCRB sequences were chosen from 27 clones isolated from subjects GS1-17A, GS1-32A, and GS1-56A.17 These clones all bound with medium or high avidity to a DRB1*01:01 tetramer loaded with FVIII2194-2213, expanded well in culture, and proliferated in response to FVIII2194-2213. One clone for each unique TCRB sequence was selected to evaluate immune responses to FVIII peptides and proteins.
TCR nomenclature is in accordance with the international reference IMGT/GENE-DB.70
The total number of clones isolated with this TCRB gene.
Sequence was identical to 20-1*01, *02, *04, *05, and *06 alleles.
Figure 3.
Effect of systematic alanine substitutions in FVIII2194-2205on T-cell proliferation of clones. WT and Ala-substituted FVIII2194-2205 peptides were added to 5 T-cell clones isolated from 3 HA subjects, incubated with irradiated PBMCs from a donor with an DRB1*01:01 allele as antigen-presenting cells. For clones 32A-18 (A), 17A-19WK-11 (B), 17A-21MO-5 (C), and 17A-21MO-11 (D) each peptide was added at final concentrations of 0.01, 0.1, 1, 5, 10, 50, and 100 μM, cpm of [3H] thymidine incorporated was measured, and EC50 values were calculated. Clone 56A-C2 (E) was stimulated with peptides at 0.05, 0.1, 0.5, 1, and 10 μM final concentrations. The curves that resulted from these data were not sufficient to calculate accurate EC50 values, therefore, cpm in response to 10 μM peptide was plotted. Results are expressed as relative T-cell proliferation (EC50 or cpm of FVIII2194-2205/EC50 or cpm of the indicated Ala-substituted peptide) ± SE. The EC50 values for clonal responses to FVIII2194-2205 were 5.7 ± 0.9 μM (A), 48.2 ± 2.1 μM (B), 10.5 ± 0.9 μM (C), and 52.5 ± 3.1 μM (D). Note that the WT peptide already contains an Ala at position 2201. These 5 clones had distinct TCR sequences and were previously shown to bind tetramers with high avidity and to proliferate in response to FVIII2194-2213 (Table 2). Although the binding affinities of FVIII2194-2213 and FVIII2194-2205 to rDRB1*01:01 protein were almost identical, the T-cell clones proliferated more robustly in response to FVIII2194-2213 compared with FVIII2194-2205 (supplemental Figure 8). Cytokine analysis of supernatants from stimulated clones is in supplemental Figure 1.
Strategy for designing less immunogenic rFVIII-C2 and BDD-FVIII proteins
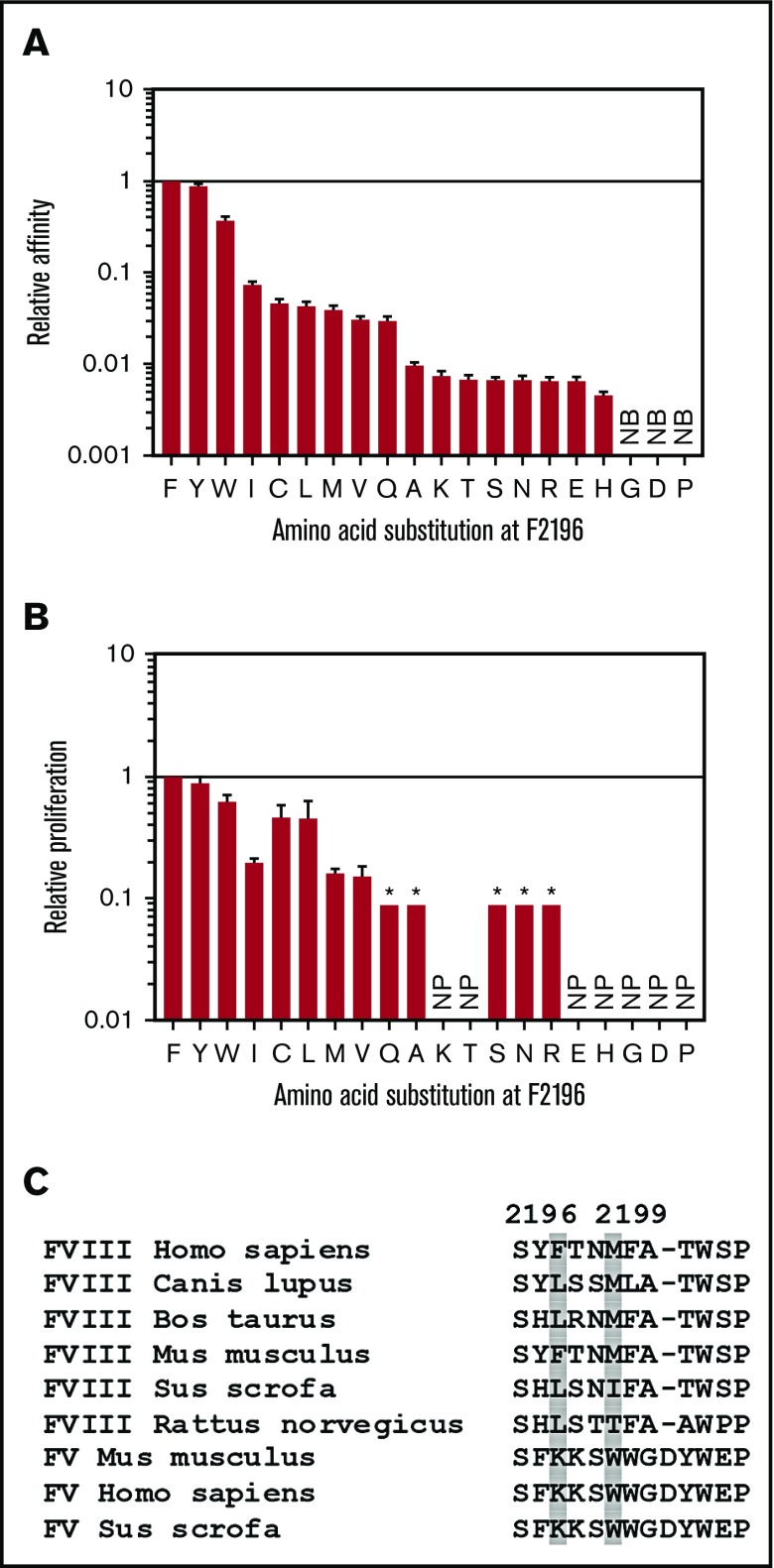
Our primary goal was to design FVIII muteins with amino acid substitutions that would block MHCII binding without significantly reducing procoagulant activity. The rFVIII-C2 crystal structure48 revealed that the FVIII-F2196 side chain interacts with T2202, stabilizing a β turn at a FVIII membrane-binding region, suggesting that substitution F2196A might not be optimum for preserving function. Therefore, 20 FVIII2194-2205 peptides, containing each possible amino acid at position 2196, were evaluated for DRB1*01:01-restricted immunogenicity by measuring affinity for rDRB1*01:01 and proliferation of T-cell clone 32A-18. Binding and proliferation assay results were broadly consistent (Figure 4A-B). Eleven of the substitutions reduced the affinity ≥100-fold relative to that of the WT peptide, and 7 abrogated T-cell proliferation.
Figure 4.
Effects of all possible amino acid substitutions at FVIII-F2196 on DRB1*01:01 binding and CD4+T-cell proliferation, and homologous/orthologous residues to human FVIII anchor residues F2196 and M2199. (A) All 19 non-Phe amino acids were substituted into FVIII2194-2205 at position F2196. Peptides were tested for binding to rDRB1*01:01 by a competition assay measuring displacement of the DRB1*01:01 reference peptide HA306-318. Binding of the F2196C-substituted peptide was tested with 10 mM β-mercaptoethanol in the binding buffer as described.72 Results are expressed as relative binding affinities (IC50 of FVIII2194-2205/IC50 of the indicated substituted peptide) ± SE. The IC50 of FVIII2194-2205 was 0.34 ± 0.02 μM. (B) Peptides were also tested for presentation on irradiated DRB1*01:01 PBMCs to the T-cell clone 32A-18 by measuring [3H]thymidine incorporation. Results are expressed as relative T-cell proliferation (EC50 of FVIII2194-2205/EC50 of the indicated substituted peptide) ± SE. The EC50 of FVIII2194-2205 in the proliferation assay was 8.9 ± 0.7 μM. The asterisk (*) indicates that, for those peptides, the relative proliferation was <0.09. (C) FVIII and FV protein sequences aligning with human FVIII residues 2194-2205 from different mammalian species were collected from the NCBI reference sequence database including FVIII Homo sapiens, NP_000123.1; FVIII Canis lupus familiaris, NP_001003212.1; FVIII Bos taurus, NP_001138980.1; FVIII Mus musculus, NP_032003.2; FVIII Sus scrofa, NP_999332.1; FVIII Rattus norvegicus, NP_899160.1; FV M musculus, NP_032002.1; FV H sapiens, NP_000121.2; and FV S scrofa, NP_999285.1. Amino acids homologous/orthologous to anchor residues FVIII-F2196 and M2199 are highlighted with gray shading. NB, no detectable binding at 50 μM peptide; NP, no detectable proliferation even at the highest peptide concentration, 100 μM.
The predicted effects of amino acid substitutions at anchor residue 4, M2199, on MHCII affinities were evaluated using the ProPred and IEDB algorithms, identifying candidates for substitutions to decrease affinities for DRB1*01:01 (Table 3). In addition, the human FVIII sequence was aligned with FVIII sequences from other species, and with the homologous human factor V sequence,49 to determine the conservation of amino acids F2196 and M2199 (Figure 4C). Finally, a literature search identified 3 FVIII proteins with substitutions at amino acids F2196 and M2199 that retained FVIII procoagulant function: BDD-FVIII-M2199I,50 BDD-FVIII-M2199A,51 and BDD-FVIII-F2196L.52 On the basis of all of these results, amino acid substitutions F2196A, F2196L, F2196K, M2199A, M2199W, and M2199R were selected as potential sequence modifications and the corresponding peptides were screened for their binding to a series of recombinant HLA proteins as an indicator of their potential immunogenicity.
Table 3.
Predicted binding of FVIII peptides with substitutions at M2199 to DRB1*01:01
| Substitution* | ProPred score† | IEDB consensus percentile rank‡ |
|---|---|---|
| None (WT peptide) | 1.09 | 6.87 |
| M2199L | 1.19 | 6.53 |
| M2199I | 0.79 | 14.35 |
| M2199Q | 0.39 | 13.37 |
| M2199F | 0.37 | 3.45 |
| M2199N | 0.33 | 13.67 |
| M2199A | 0.29 | 11.97 |
| M2199C | 0.29 | 15.04 |
| M2199V | 0.24 | 9.07 |
| M2199Y | <0 | 4.14 |
| M2199S | <0 | 18.03 |
| M2199T | <0 | 18.03 |
| M2199G | <0 | 12.73 |
| M2199H | <0 | 12.73 |
| M2199E | <0 | 18.03 |
| M2199W | <0 | 10.95 |
| M2199K | <0 | 18.03 |
| M2199R | <0 | 11.55 |
| M2199P | <0 | 18.03 |
| M2199D | <0 | 18.03 |
The input sequence for the ProPred and IEBD algorithms was FVIII2185-2213, which spans all possible 15-mer sequences that include the substitution site at M2199.
Predicted affinities from the ProPred algorithm.35 Possible scores range from <0 (no binding) to 6 (highest possible affinity) for DRB1*01:01. All 9 sequences with scores >0 had the same predicted binding motif, with F2196 in MHCII anchor position 1. None of the substitutions resulted in additional predicted binding modes (score >0) that would indicate creation of a potential DRB1*01:01-restricted neoepitope. Scores ≥0.14 (bolded) correspond to predicted peptide-DRB1*01:01 affinities in the top 3% of possible values. ProPred recommends a threshold of 3% for selecting potential physiologically relevant interactions.
Predicted affinities from the IEDB consensus method, which combines scores generated by 3 prediction algorithms: combinatorial library, NN-align (netMHCII-2.2), and SMM-align (netMHCII-1.1).36,37 The final scores reflect the predicted peptide affinities for DRB1*01:01. The highest predicted consensus affinity score, expressed as a percentage ranking, for each input sequence is listed. IEDB recommends a threshold of 10% for selecting potential physiologically relevant interactions (bolded). All 3 algorithms in the consensus method predicted a 9-mer MHCII-binding core for the WT sequence with F2196 in anchor position 1. The 4 modified FVIII sequences that ranked in the top 10% all had alternative predicted MHCII core sequences with either F2196 or F2200 in anchor position 1.
Binding promiscuity of FVIII2194-2205 and modified peptides to MHCII
MHCII affinities of WT and sequence-modified peptides within FVIII2182-2213 were predicted using ProPred and IEDB algorithms. This FVIII region spans all possible 15-mer sequences that include the proposed amino acid substitution sites at F2196 or M2199 (MHCII anchor residues 1 and 4). The ProPred algorithm predicted that, with an affinity threshold of 3% (the recommended cutoff for physiologically relevant affinities), WT, F2196L-, and M2199W-substituted peptides would bind to 12, 8, and 5 HLA-DR, respectively, of 51 HLA-DR proteins for which quantitative matrices are available; that F2196A and F2196K substitutions would prevent binding to all 51 HLA-DR; and that M2199A- and M2199R-substituted peptides would bind to 2 HLA-DR each (Table 4). Therefore, all 6 substitutions were predicted to reduce MHCII-binding promiscuity relative to the WT sequence. Substitutions F2196L, M2199W, and M2199R were predicted to increase affinity for 1 to 4 of the 51 MHCII alleles in the database. The IEDB predictions were run using the HLA class II reference set, which contains representative alleles for ∼99% of the human population. Results were in good agreement with ProPred in predicting high-affinity binding to DRB1*01:01, DRB1*0401, DRB1*04:05, and DRB5*01:01, which were included in reference databases for both programs (Table 5). The IEDB method predicted high-affinity binding of the WT peptides to an additional 11 MHCII alleles, and the proposed sequence modifications were predicted to eliminate high-affinity binding to 3 to 6 of these MHCII alleles. Increased affinities for 0 to 2 MHCII alleles per sequence modification were also predicted.
Table 4.
ProPred-predicted binding of FVIII peptides to 51 HLA-DR alleles
| FVIII peptide | 1% Threshold | 3% Threshold | 10% Threshold |
|---|---|---|---|
| WT | 04:05,* 04:08 | 01:01, 01:02, 04:01, 04:04, 04:05, 04:08, 04:21, 04:23, 04:26, 08:13, DRB5*01:01, DRB5*01:05 | 30 HLA-DR |
| F2196A | None | None | 15 HLA-DR |
| F2196K | None | None | 15 HLA-DR |
| F2196L | 04:04,† 04:08, 04:23 | 01:02, 04:02, 04:04, 04:05, 04:08, 04:10, 04:23, 08:13 | 42 HLA-DR |
| M2199A | None | 01:01, 04:08 | 26 HLA-DR |
| M2199W | None | 04:01, 04:02, 04:08, DRB5*01:01, DRB5*01:05 | 22 HLA-DR |
| M2199R | 08:13 | 08:02, 08:13 | 20 HLA-DR |
The input sequences for the ProPred algorithm were WT and sequence-modified FVIII2182-2213, which spans all possible 15-mer sequences that include the substitution sites at F2196 or M2199. ProPred predicts binding to 49 different HLA-DRB1 alleles and 2 HLA-DRB5 alleles.35 Interactions with predicted affinities in the top 1%, 3%, and 10% for each HLA-DR are listed. The predicted highest-affinity interactions are in the 1% threshold column. A threshold stringency of 3% is recommended to predict potential physiologically relevant binding affinities. “None” indicates that no HLA-DR were predicted to bind this FVIII peptide at or above the indicated affinity threshold. At the least-stringent affinity threshold (top 10%), the number of HLA-DRs predicted to bind 1 or more motifs within FVIII2182-2213 is listed for the WT sequence and for the 6 modified sequences.
Four-digit numbers correspond to HLA-DRB1* alleles, unless an HLA-DRB5* allele is specified.
Bolded allele numbers indicate that the amino acid substitution is predicted to result in binding (at this threshold level) that was not predicted for the corresponding WT FVIII sequence. Such high-affinity MHCII binding could create neoepitopes restricted to these alleles.
Table 5.
IEDB-predicted binding of FVIII peptides to an HLA class II allele reference set
| IEDB percentile rank, % | |||||||
|---|---|---|---|---|---|---|---|
| HLA allele | WT | F2196A | F2196K | F2196L | M2199A | M2199W | M2199R |
| DRB1*01:01 | 6.87 | 13.70* | 15.40* | 13.04* | 11.97* | 10.95* | 11.55* |
| DRB1*03:01 | 16.60 | 18.81 | 18.81 | 9.61† | 16.6 | 19.22 | 19.22 |
| DRB1*04:01 | 2.32 | 6.65 | 6.65 | 4.78 | 4.20 | 5.34 | 7.80 |
| DRB1*04:05 | 4.58 | 22.26* | 15.75* | 6.09 | 8.21 | 18.55* | 15.58* |
| DRB1*07:01 | 4.21 | 4.21 | 4.21 | 2.39 | 4.10 | 4.14 | 4.03 |
| DRB1*08:02 | 6.00 | 5.81 | 5.95 | 5.05 | 8.78 | 8.78 | 5.15 |
| DRB1*09:01 | 0.94 | 2.18 | 2.18 | 1.97 | 1.21 | 1.61 | 1.88 |
| DRB1*11:01 | 5.04 | 11.79 | 11.79 | 9.49 | 9.49 | 10.04 | 8.25 |
| DRB1*12:01 | 19.66 | 26.08 | 26.08 | 13.79 | 18.40 | 26.12 | 21.62 |
| DRB1*13:02 | 38.79 | 34.21 | 41.41 | 25.8 | 39.01 | 39.11 | 38.97 |
| DRB1*15:01 | 18.10 | 22.85 | 22.85 | 10.98 | 18.64 | 12.23 | 18.64 |
| DRB3*01:01 | 8.08 | 11.50* | 9.71 | 14.09* | 4.92 | 12.11* | 10.21* |
| DRB3*02:02 | 8.64 | 8.64 | 8.64 | 8.64 | 12.31* | 8.54 | 10.02* |
| DRB4*01:01 | 21.82 | 21.82 | 21.82 | 21.82 | 21.82 | 21.82 | 21.82 |
| DRB5*01:01 | 2.34 | 4.69 | 4.69 | 3.86 | 4.69 | 2.24 | 4.69 |
| DQA1*05:01/DQB1*02:01 | 18.27 | 26.97 | 47.89 | 24.05 | 21.10 | 27.65 | 30.94 |
| DQA1*05:01/DQB1*03:01 | 16.93 | 15.87 | 16.85 | 14.85 | 16.93 | 16.93 | 16.93 |
| DQA1*03:01/DQB1*03:02 | 18.96 | 15.01 | 22.61 | 18.98 | 7.86† | 19.21 | 21.68 |
| DQA1*04:01/DQB1*04:02 | 10.25 | 16.25 | 20.51 | 13.13 | 10.25 | 10.25 | 10.25 |
| DQA1*01:01/DQB1*05:01 | 5.64 | 16.23* | 43.15* | 13.43* | 6.89 | 0.66 | 10.95* |
| DQA1*01:02/DQB1*06:02 | 11.91 | 4.87† | 12.38 | 9.18† | 11.91 | 11.91 | 11.91 |
| DPA1*02:01/DPB1*01:01 | 7.77 | 9.05 | 27.15* | 12.90* | 7.77 | 7.77 | 7.77 |
| DPA1*01:03/DPB1*02:01 | 0.96 | 1.68 | 3.90 | 1.43 | 2.13 | 1.71 | 0.65 |
| DPA1*01/DPB1*04:01 | 0.66 | 0.79 | 1.02 | 0.81 | 2.14 | 0.02 | 0.30 |
| DPA1*03:01/DPB1*04:02 | 13.93 | 20.86 | 27.97 | 12.32 | 29.03 | 14.60 | 20.61 |
| DPA1*02:01/DPB1*05:01 | 8.18 | 14.02* | 12.45* | 19.14* | 14.44* | 19.94* | 13.74* |
| DPA1*02:01/DPB1*14:01 | 10.89 | 23.13 | 21.84 | 13.05 | 11.25 | 16.11 | 9.36† |
Predicted binding of WT and sequence-modified 15-mer peptides spanning FVIII2182-2213 to the IEDB HLA class II reference set, which consists of the most frequent MHCII (HLA class II) loci worldwide representing 46% of DRB1, 77% of DRB3/4/5, 57% of DQA1/DQB1, and 76% of DPA1/DPB1 alleles.63 The highest predicted peptide affinity (lowest percentile rank) score for each MHCII is listed. Predictions were generated using the “recommended IEDB method,” which defaults to the IEDB consensus method when combined consensus scores are possible.36,37 The consensus method was not applicable to the DRB1*12:01, DRB3*02:02, and DPA1*02:01/DPB1*14:01 alleles, thus these predictions were generated by the SMM-align, NetMHCIIpan, and NetMHCIIpan algorithms, respectively. Interactions predicted to be in the top 10% of possible peptide affinities for the indicated MHCII are bolded. A threshold of 10% is recommended by IEDB for predicting potential physiologically relevant binding interactions. Percentile ranks (predicted affinities) of the sequence-modified FVIII peptides (columns 3-8) were compared with those of the WT FVIII peptides (column 2).
Substitutions predicted to decrease the peptide affinity for a particular MHCII to below the top 10%, and in which the affinity of the WT peptide ranks in the top 10%, indicating that this substitution may decrease immunogenicity restricted to this allele.
Substitutions predicted to increase the peptide affinity for a particular MHCII to within the top 10%, and in which the affinity of the WT peptide ranks below the top 10%, indicating that this substitution may create a neoepitope restricted to this allele.
Affinities of the minimal epitope FVIII2194-2205 for 10 recombinant HLA-DR proteins were determined experimentally using a competitive binding assay. FVIII2194-2205 bound strongly to DRB1*01:01, with moderate affinity to DRB1*15:01 and DRB1*10:01, and weakly to DRB1*04:01, DRB1*04:04, and DRB1*09:01. The F2196A, F2196K, and M2199R-substituted peptides had 50% inhibitory concentration (IC50) values >50 μM for each HLA-DR protein tested, whereas the M2199A-substituted peptide bound with moderate affinity only to DRB1*09:01, weakly to DRB1*01:01 and DRB1*03:01, and with IC50 values >50 μM for all other tested HLA-DR proteins (Table 6). The binding results suggested that all 6 substitutions would reduce MHCII-binding promiscuity, as increased affinity was observed only for DRB1*09:01 binding to the M2199A-substituted peptide. Therefore, rBDD-FVIII proteins with these 6 sequence modifications were designed and produced.
Table 6.
Experimental affinities of FVIII2194-2205 peptides for 10 HLA-DR proteins
| Reference peptide* | FVIII2194-2205 | F2196A | F2196K | M2199A | M2199R | |
|---|---|---|---|---|---|---|
| DRB1*01:01 | 0.3 ± 0.0 | 0.3 ± 0.0 | >50 | >50 | 39.8 ± 3.2 | >50 |
| DRB1*03:01 | 3.9 ± 0.7 | >50 | >50 | >50 | 28.5 ± 2.9 | >50 |
| DRB1*04:01 | 1.1 ± 0.2 | 36.3 ± 4.8 | >50 | >50 | >50 | >50 |
| DRB1*04:04 | 0.02 ± 0.00 | 39.1 ± 5.0 | >50 | >50 | >50 | >50 |
| DRB1*07:01 | 0.3 ± 0.1 | >50 | >50 | >50 | >50 | >50 |
| DRB1*09:01 | 0.1 ± 0.0 | 42.0 ± 5.2 | >50 | >50 | 3.5 ± 0.3 | >50 |
| DRB1*10:01 | 2.9 ± 0.4 | 7.3 ± 0.7 | >50 | >50 | >50 | >50 |
| DRB1*11:01 | 4.4 ± 0.7 | >50 | >50 | >50 | >50 | >50 |
| DRB1*11:04 | 3.8 ± 0.5 | >50 | >50 | >50 | >50 | >50 |
| DRB1*15:01 | 0.06 ± 0.01 | 5.8 ± 0.5 | >50 | >50 | >50 | >50 |
Affinities were determined using a competitive binding assay. HLA-DR proteins were incubated with 0.05, 0.1, 0.5, 1, 5, 10, and 50 μM FVIII peptides plus the relevant biotinylated reference peptides as competitors for each HLA-DR (supplemental Table 1) and immobilized in wells coated with the anti-DR antibody L243. After washing, the remaining bound biotinylated peptide was labeled using europium-conjugated streptavidin and quantified using a time-resolved fluorometer.
Immunogenicity of WT vs sequence-modified rFVIII-C2
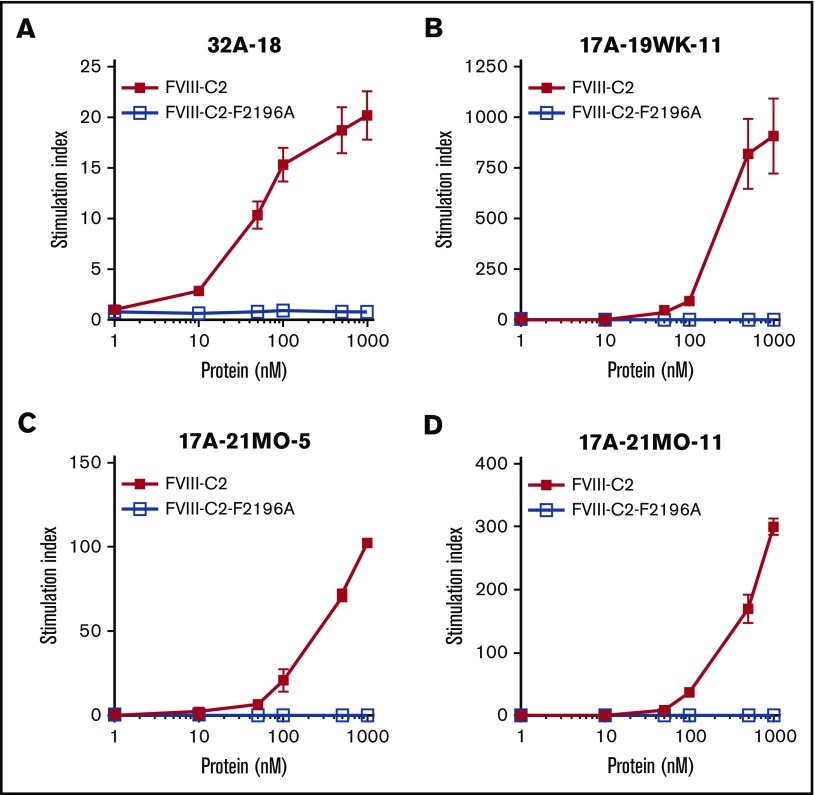
As proof of principle, and to test whether the DRB1*01:01-restricted epitope identified using peptide assays was also a naturally processed epitope, rFVIII-C2 and rFVIII-C2-F2196A were generated, purified (supplemental Figure 2), and tested for immunogenicity. The endotoxin level in the purified samples was 0.20 endotoxin units (EU)/mL, comparable to the low level in the human serum used in the T-cell medium. Immunogenicity was evaluated by culturing the proteins with 4 patient-derived T-cell clones (Figure 5). All 4 clones showed dose-responsive proliferation in response to rFVIII-C2 but not rFVIII-C2-F2196A.
Figure 5.
CD4+T-cell clones did not proliferate when stimulated with rFVIII-C2-F2196A. T-cell clones 32A-18 (A), 17A-19WK-11 (B), 17A-21MO-5 (C), and 17A-21MO-11 (D) were stimulated with purified recombinant rWT-FVIII-C2 and rFVIII-C2-F2196A presented by irradiated PBMCs from an DRB1*01:01 donor. Proteins were added at final concentrations of 1, 10, 50, 100, 500, and 1000 nM. T-cell proliferation was measured by [3H]thymidine incorporation. Results are averages of triplicate determinations ± standard deviation (SD). Stimulation indices were calculated by dividing the mean cpm of [3H]thymidine incorporated into protein-stimulated cells by the mean cpm of [3H]thymidine incorporated into unstimulated cells. Results are also plotted as proliferation (cpm) in supplemental Figure 9.
Expression, function, and immunogenicity of BDD-FVIII muteins
BDD-FVIII muteins F2196A, F2196L, F2196K, M2199A, M2199W, and M2199R were generated and the activities of the 3 highest-expressing cell lines per protein determined (supplemental Figures 3 and 4A). Specific activities are in Table 7 and supplemental Figure 4. Median specific activities were similar to WT-BDD-FVIII–specific activity for all muteins except F2196A.
Table 7.
Activities and AQs of BDD-FVIII muteins relative to WT-BDD-FVIII in cell supernatants and after purification
| BDD-FVIII | Specific activity, IU/mg or AQ ± SD cell supernatants* | % WT ± SD cell supernatants* | Specific activity, IU/mg or AQ ± SD purified proteins† | % WT ± SD purified proteins† |
|---|---|---|---|---|
| 1-Stage coagulation assay | ||||
| WT* | 4000 ± 590 | 12 000 ± 1400 | ||
| F2196A | 2600 ± 200 | 65 ± 5 | ||
| F2196L | 4000 ± 120 | 100 ± 30 | ||
| F2196K | 4300 ± 1700 | 110 ± 42 | 9 500 ± 1700 | 81 ± 18 |
| M2199A | 4000 ± 710 | 100 ± 18 | 9 400 ± 1800 | 81 ± 18 |
| M2199W | 4400 ± 2100 | 110 ± 53 | ||
| M2199R | 3700 ± 1500 | 92 ± 37 | ||
| Kogenate† | 3 100 ± 190 | |||
| AQ‡ | ||||
| WT* | 27 ± 9 | 20 ± 4 | ||
| F2196A | 25 ± 6 | 92 ± 21 | ||
| F2196L | 28 ± 3 | 100 ± 12 | ||
| F2196K | 26 ± 7 | 98 ± 26 | 20 ± 2 | 97 ± 24 |
| M2199A | 26 ± 4 | 95 ± 14 | 43 ± 14 | 210 ± 83 |
| M2199W | 19 ± 7 | 72 ± 26 | ||
| M2199R | 24 ± 4 | 88 ± 14 | ||
| Kogenate† | 23 ± 3 | |||
| Chromogenic assay | ||||
| WT* | 3600 ± 33 | 8 500 ± 220 | ||
| F2196A | 1900 ± 300 | 52 ± 8 | ||
| F2196L | 2700 ± 590 | 74 ± 17 | ||
| F2196K | 2200 ± 610 | 61 ± 17 | 9 600 ± 500 | 110 ± 6 |
| M2199A | 2700 ± 130 | 77 ± 4 | 13 000 ± 310 | 150 ± 5 |
| M2199W | 3300 ± 780 | 93 ± 22 | ||
| M2199R | 2700 ± 660 | 76 ± 18 | ||
| Kogenate† | 2 800 ± 110 |
AQ, activation quotient.
FVIII activities (IU/mL) in BHK-M cell supernatants were measured in triplicate using a 1-stage coagulation assay, 1- and 2-stage coagulation assays to calculate AQs, and chromogenic assays for 3 cell lines per FVIII protein. Specific activities (IU/mg) were calculated using the concentrations of FVIII in cell supernatants as measured in an ELISA. The average specific activity for each protein was calculated from the specific activities of the individual cell lines ± standard deviations (SDs) as shown in “1-Stage coagulation assay” and “Chromogenic assay.” Percentage WT (% WT) activity for each BDD-FVIII mutein = (average specific activity or AQ of the cell lines expressing the mutein/average specific activity or AQ of the cell lines expressing WT-BDD-FVIII) × 100. Details regarding individual cell lines are provided in supplemental Figure 4. The WT-BDD-FVIII cell line is HSQ/AvrII/RENEO–expressing BHK-M cells, kindly provided by Pete Lollar’s laboratory (Emory University, Atlanta, GA).
The FVIII activities (IU/mL) of the purified proteins were measured using 1-stage coagulation (n = 4), 1- and 2-stage coagulation to calculate AQs (n = 4), and chromogenic assays (n = 6). Specific activities (IU/mg) were calculated using the concentrations of FVIII determined by measuring A280 of the purified protein solutions and using an extinction coefficient of 256 300 M−1cm−1. Research-grade Kogenate, provided by Bayer at a specific activity of 3600 IU/mg, was used as a control for the assay.
AQs are calculated as ratios of 2-stage FVIII activity divided by 1-stage FVIII activity. The expected AQ for human FVIII that has not been significantly preactivated is 15-25.47
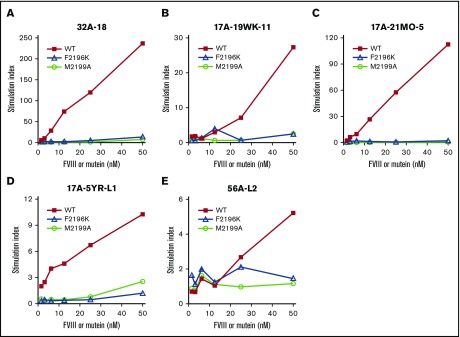
WT-BDD-FVIII and BDD-FVIII-F2196K and -M2199A were highly purified (supplemental Figure 5) and had specific activities of ∼10 000 IU/mg53 (Table 7). These proteins, and T-cell clones and polyclonal lines from 3 inhibitor subjects, were used to test whether the DRB1*01:01-restricted immunogenicity of BDD-FVIII was decreased by sequence modifications F2196K and M2199A. The polyclonal T-cell lines 17A-5YR-L1 and 56A-L217 and T-cell clones 32A-18, 17A-19WK-11, and 17A-21MO-5, were cultured with these proteins with DRB1*01:01-matched antigen-presenting cells (supplemental Figure 6). All clones and lines proliferated in a dose-dependent manner to WT-BDD-FVIII, as expected, whereas the responses to BDD-FVIII-F2196K and BDD-FVIII-M2199A were greatly reduced (Figure 6).
Figure 6.
Markedly reduced proliferation of CD4+T-cell clones and polyclonal lines to BDD-FVIII-F2196K and BDD-FVIII-M2199A. FVIII2194-2213-specific T-cell clones 32A-18 (A), 17A-19WK-11 (B), 17A-21MO-5 (C), and polyclonal lines 17A-5YR-L1 (D) and 56A-L2 (E) were stimulated with purified recombinant WT-BDD-FVIII, BDD-FVIII-F2196K, and BDD-FVIII-M2199A presented by iMo-DCs. The iMo-DCs were differentiated from CD14+ cells isolated from an DRB1*01:01 donor and pulsed with FVIII proteins at final concentrations of 1.6, 3.1, 6.2, 12.5, 25, and 50 nM. CD4+ T-cell proliferation was measured by [3H]thymidine incorporation. Stimulation indices were calculated by dividing the cpm of [3H]thymidine incorporated into protein-stimulated cells by the cpm of [3H]thymidine incorporated into unstimulated cells. Results are also plotted as proliferation (cpm) in supplemental Figure 10.
Discussion
Development of inhibitors in response to FVIII replacement therapy is the most serious complication in the treatment of HA. Whether alloimmunization occurs is dependent on the degree of structural difference between therapeutic FVIII and the patient’s endogenous, dysfunctional FVIII protein (if any protein is expressed), on additional genetic variations, and on environmental factors including intensity of FVIII treatment and inflammatory status of the patient. CD4+ T cells are critical to the production and maintenance of inhibitors, as demonstrated by the observation that HIV+ HA patients experienced declines in inhibitor titers that were, unfortunately, reversed when T-cell levels rebounded with effective antiretroviral therapy.54 Attenuation of CD4+ T-effector help, therefore, is a promising strategy to prevent or reverse anti-FVIII antibody responses. This may be accomplished by manipulating the cellular response, for example, by expanding natural or engineered regulatory T cells55 or, alternatively, by modifying the immunogenicity of the antigen itself. This study explores the potential use of the latter approach.
An important consideration in assessing the feasibility of deimmunization strategies through modification of T-cell epitopes is the number of immunodominant epitopes that drive initial anti-FVIII immune responses and that maintain persistent inhibitor titers. Epitope mapping of T-cell epitopes recognized by humanized (DRB1*15:01) FVIII-KO mice identified 8 immunodominant epitopes in multiple FVIII domains.56 In a recent study of a severe HA subject with a persistent high-titer inhibitor, MHCII tetramer-based epitope mapping to evaluate the immunogenicity of peptides spanning the FVIII A2, C1, and C2 domains revealed a highly oligoclonal CD4+ T-cell response to only 1 epitope (there was insufficient blood available to test responses to peptides spanning the entire FVIII sequence). This subject had a multiexon F8 gene deletion and had failed immune tolerance induction (ITI) therapy.17 There is still no adequate murine model of human ITI protocols; however, these earlier results suggest that functional peripheral tolerance to FVIII is achieved, in part, through deletion and anergy of initially responsive CD4+ T-cell clones. This in turn suggests that identification and modification of only a small set of immunodominant T-cell epitopes in FVIII may be a feasible strategy to avoid stimulating T-effector cells that can initiate or maintain inhibitor responses.20,57 Each sequence modification has the potential to compromise FVIII activity and/or generate a neoepitope that could have the opposite of the desired effect on FVIII immunogenicity. Porcine FVIII has proven an effective “bypass” therapy to stabilize hemostasis in some inhibitor patients, however, subsequent immune responses to porcine FVIII may develop, thereby negating its clinical benefits.58,59 Strategic amino acid substitutions in immunodominant T-cell epitopes with known HLA restriction would, in principle, be less likely to provoke additional neutralizing antibodies. In other words, the principles of precision medicine could be applied to design less immunogenic FVIII proteins targeted to HA patients with specific HLA alleles.
Toward the goal of generating less immunogenic FVIII proteins, the present study identifies the precise binding mode of an immunodominant T-cell epitope15-17,19 in FVIII to DRB1*01:01 and tests the effects of sequence modifications to disrupt MHCII binding while preserving FVIII procoagulant function. To systematically test the effects of amino acid sequence modifications within the minimal epitope, FVIII2194-2205, peptides with Ala or Arg at every possible position, or with 20 different amino acids substituted for anchor residue 1 (F2196), were synthesized. IC50 values for the substituted peptides clearly established the DRB1*01:01 anchor residues (F2196, M2199, A2201, and S2204) and identified additional substitutions of F2196 that would prevent or reduce the affinity for DRB1*01:01. Prediction algorithms further suggested substitutions of M2199 that could interfere with productive MHCII binding.
The MHCII anchor residues identified were consistent with the known properties of the DRB1*01:01 peptide-binding groove that were determined by crystallization with the high-affinity peptide HA306-318.11 The anchor residue at position 1 plays a major role in contributing affinities of peptides for most HLA-DR alleles. The specificity of this pocket is dictated by the Gly/Val dimorphism at β86.11-13,60-62 HLA-DR alleles with β86Gly, including DRB1*01:01, allow primarily large hydrophobic aromatic residues at position 1 and large aliphatic residues secondarily, whereas HLA-DR alleles with β86Val have the reverse specificities. Accordingly, F2196 was found to be critical for DRB1*01:01 affinity and for the CD4+ T-cell response. Binding of F2196-substituted peptides to DRB1*01:01 showed the expected preference for large hydrophobic/aromatic amino acids.
To test effects of amino acid substitutions on immunogenicity, 5 patient-derived T-cell clones with distinct TCRs were cultured with antigen-presenting cells from a donor with a DRB1*01:01 allele and FVIII2194-2205 peptides having an Ala substitution at each position. Only the F2196A substitution abrogated proliferation of all 5 clones, whereas the M2199A substitution reduced or abrogated proliferation of the same clones. Ala substitutions of non-MHCII-anchor residues had variable effects on proliferation, consistent with the diverse TCR sequences.17 The substitution F2196A in a recombinant FVIII-C2 protein abrogated proliferation of T-cell clones, clearly demonstrating that the epitope identified by MHCII tetramer mapping and peptide-DRB1*01:01–binding assays is indeed a physiologically relevant, naturally processed epitope.
Six FVIII sequence modifications at DRB1*01:01 anchor residues 1 and 4, each having a lower predicted DRB1*01:01-restricted immunogenicity than the WT sequence, were chosen based on binding and T-cell assay results and predictions and homologies with other FVIII proteins. Binding of WT and sequence-modified FVIII peptides to additional MHCII was tested directly using 10 recombinant HLA-DRB1 proteins and was evaluated for additional HLA class II alleles using the IEDB36,37 and ProPred35 prediction algorithms. The IEDB HLA class II reference set corresponds to a panel of the most frequent MHCII loci worldwide.18,63 Most substitutions evaluated were predicted to lower MHCII-binding promiscuity. However, several substitutions that could increase the affinities for specific MHCII were identified, which could conceivably result in presentation of neoepitopes. The IEDB and ProPred algorithms performed well in predicting which substitutions would disrupt binding to DRB1*01:01 (supplemental Table 2), however, the correlations between IC50 values and predicted affinities were poor (supplemental Figure 7).
The 6 BDD-FVIII muteins described here each retained at least half of the specific activity of WT-FVIII. Purified BDD-FVIII-F2196K and BDD-FVIII-M2199A bound effectively to von Willebrand factor53 and had specific activities similar to those reported for rWT-FVIII preparations.46,64-66 These 2 amino acid substitutions profoundly reduced the proliferation of patient-derived CD4+ T-cell clones and polyclonal lines that were specific for the WT FVIII2194-2205 epitope. The BDD-FVIII-F2196K sequence modification appears to be the most promising sequence modification tested here, due to its effectiveness at eliminating DRB1*01:01-restricted immunogenicity, its low potential immunogenicity restricted to additional representative MHCII alleles, an expression level comparable to WT-BDD-FVIII, and its retained procoagulant activity. Furthermore, the F2196K substitution prevented neutralization of FVIII activity by the inhibitory antibodies BO2C11 and 1B5,53 suggesting that it may also avoid stimulation of relevant naive and/or memory B cells.
The present study demonstrates effective removal of an immunodominant DRB1*01:01-restricted T-cell epitope in FVIII. Future experiments will test for reduced immunogenicity and antigenicity of sequence-modified FVIII proteins using human PBMCs and animal models. Some recently introduced therapeutic FVIII proteins have extended half-lives achieved through conjugation to Fc67 or polyethylene glycol,67,68 or sequence modifications to generate a single-chain FVIII,69 etc. The effects of these modifications on immunogenicity are subjects of ongoing research. As epitope-mapping efforts are applied to additional HA subjects, FVIII sequence modifications to remove immunodominant epitopes may be incorporated into precision medicine strategies to match individual patients with optimal therapeutic FVIII proteins.
Potential limitations of our FVIII deimmunization approach include the possibility that subdominant epitopes could drive new inhibitor responses following infusions with a rationally sequence-modified FVIII. Animal model studies are required to test this possibility in the context of FVIII immunogenicity. An earlier study of sequence-modified IFN-β did not identify emerging subdominant epitopes following removal of 1 immunodominant epitope,23 which is encouraging. Some epitopes may consist of residues that cannot be modified without compromising FVIII procoagulant function, and generating multiple sequence-modified FVIII proteins would be costly. Memory B cells are critical to maintenance of inhibitors, and it remains to be seen whether decreasing the T-effector response to FVIII will also significantly reduce inhibitor titers. Mapping and modifying B-cell epitopes also remains a priority, especially in designing less antigenic proteins as potential therapeutics for patients with an existing inhibitor.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Charles Cooper, Mark Bray, and Colette Norby-Slycord for enrolling subjects; Shelley Fletcher for sequencing; the Benaroya Research Institute Tetramer Core for producing HLA-DR proteins; Pete Lollar, John Healey, and Ernest Parker for the FVIII expression system and technical support; Douglas Bolgiano for statistical assistance; and David W. Scott and Ai-Hong Zhang for critical reading of the manuscript. The authors thank all subjects for their generous blood donations.
This work was supported by an Investigator-Initiated Research award from Pfizer (study identification no. WS1907291) (K.P.P.). Additional funding was from National Institutes of Health, National Heart, Lung, and Blood Institute grants 1RC2-HL101851 and 1R01-HL-130448 and Uniformed Services University of the Health Sciences startup funding (K.P.P.), and from National Heart, Lung, and Blood Institute grant R01-07109 (A.R.T.). J.A.L. was supported by an American Society of Hematology trainee award; all of J.A.L.’s work on this project was carried out while employed or volunteering at the Puget Sound Blood Center (Seattle, WA).
Pfizer had no role in the study design, data collection, statistical analysis, interpretation, or writing of the manuscript. Shire had no role in the work, or in the writing or publication of this project.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Authorship
Contribution: K.P.P. is responsible for the content of the manuscript and the decision to submit for publication; R.A.E., E.A.J., A.R.T., and K.P.P. conceived and designed experiments; R.A.E., J.A.L., D.G., and K.P. performed experiments; A.R.T. and K.P.P. enrolled subjects; E.A.J. contributed reagents; R.A.E., J.A.L., D.G., K.P., and K.P.P. analyzed data; R.A.E. and K.P.P. wrote the paper; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: K.P.P., R.A.E., and E.A.J. are inventors on FVIII patents. J.A.L. is currently an employee of Shire. The remaining authors declare no competing financial interests.
Correspondence: Kathleen P. Pratt, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD 20814; e-mail: kathleen.pratt@usuhs.edu.
References
- 1.Konkle BA, Huston H, Nakaya Fletcher S. Hemophilia A. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. Seattle, WA: University of Washington; 2000. [Google Scholar]
- 2.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. [DOI] [PubMed] [Google Scholar]
- 3.Gouw SC, van den Berg HM, Oldenburg J, et al. . F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119(12):2922-2934. [DOI] [PubMed] [Google Scholar]
- 4.Castaman G, Fijnvandraat K. Molecular and clinical predictors of inhibitor risk and its prevention and treatment in mild hemophilia A. Blood. 2014;124(15):2333-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124(23):3365-3372. [DOI] [PubMed] [Google Scholar]
- 6.André S, Meslier Y, Dimitrov JD, et al. . A cellular viewpoint of anti-FVIII immune response in hemophilia A. Clin Rev Allergy Immunol. 2009;37(2):105-113. [DOI] [PubMed] [Google Scholar]
- 7.Wroblewska A, Reipert BM, Pratt KP, Voorberg J. Dangerous liaisons: how the immune system deals with factor VIII. J Thromb Haemost. 2013;11(1):47-55. [DOI] [PubMed] [Google Scholar]
- 8.Scott DW, Pratt KP, Miao CH. Progress toward inducing immunologic tolerance to factor VIII. Blood. 2013;121(22):4449-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacquemin M, Saint-Remy JM. T cell response to FVIII. Cell Immunol. 2016;301:8-11. [DOI] [PubMed] [Google Scholar]
- 10.Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: preclinical assessment and mitigation. Clin Immunol. 2013;149(3):534-555. [DOI] [PubMed] [Google Scholar]
- 11.Stern LJ, Brown JH, Jardetzky TS, et al. . Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368(6468):215-221. [DOI] [PubMed] [Google Scholar]
- 12.Moustakas AK, Papadopoulos GK. Use of MHC II structural features in the design of vaccines for organ-specific autoimmune diseases. Curr Pharm Des. 2009;15(28):3262-3273. [DOI] [PubMed] [Google Scholar]
- 13.Stern LJ, Calvo-Calle JM. HLA-DR: molecular insights and vaccine design. Curr Pharm Des. 2009;15(28):3249-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodeve AC, Reitsma PH, McVey JH; Working Group on Nomenclature of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Nomenclature of genetic variants in hemostasis. J Thromb Haemost. 2011;9(4):852-855. [DOI] [PubMed] [Google Scholar]
- 15.James EA, Kwok WW, Ettinger RA, Thompson AR, Pratt KP. T-cell responses over time in a mild hemophilia A inhibitor subject: epitope identification and transient immunogenicity of the corresponding self-peptide. J Thromb Haemost. 2007;5(12):2399-2407. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. HLA-DR-restricted T-cell responses to factor VIII epitopes in a mild haemophilia A family with missense substitution A2201P. Haemophilia. 2010;16(102):44-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ettinger RA, Paz P, James EA, et al. . T cells from hemophilia A subjects recognize the same HLA-restricted FVIII epitope with a narrow TCR repertoire. Blood. 2016;128(16):2043-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Galarza FF, Takeshita LY, Santos EJ, et al. . Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43(database issue):D784-D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114(7):1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt KP. Engineering less immunogenic and antigenic FVIII proteins. Cell Immunol. 2016;301:12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TD, Phillips WJ, Smith BJ, et al. . Identification and removal of a promiscuous CD4+ T cell epitope from the C1 domain of factor VIII. J Thromb Haemost. 2005;3(5):991-1000. [DOI] [PubMed] [Google Scholar]
- 22.Tangri S, Mothé BR, Eisenbraun J, et al. . Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol. 2005;174(6):3187-3196. [DOI] [PubMed] [Google Scholar]
- 23.Yeung VP, Chang J, Miller J, Barnett C, Stickler M, Harding FA. Elimination of an immunodominant CD4+ T cell epitope in human IFN-beta does not result in an in vivo response directed at the subdominant epitope. J Immunol. 2004;172(11):6658-6665. [DOI] [PubMed] [Google Scholar]
- 24.Warmerdam PA, Plaisance S, Vanderlick K, et al. . Elimination of a human T-cell region in staphylokinase by T-cell screening and computer modeling. Thromb Haemost. 2002;87(4):666-673. [PubMed] [Google Scholar]
- 25.Harding FA, Liu AD, Stickler M, et al. . A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol Cancer Ther. 2005;4(11):1791-1800. [DOI] [PubMed] [Google Scholar]
- 26.Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2(3):256-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazor R, Eberle JA, Hu X, et al. . Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc Natl Acad Sci USA. 2014;111(23):8571-8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cizeau J, Grenkow DM, Brown JG, Entwistle J, MacDonald GC. Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope-depleted variant of the plant toxin bouganin. J Immunother. 2009;32(6):574-584. [DOI] [PubMed] [Google Scholar]
- 29.Griswold KE, Bailey-Kellogg C. Design and engineering of deimmunized biotherapeutics. Curr Opin Struct Biol. 2016;39:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazor R, Zhang J, Xiang L, et al. . Recombinant immunotoxin with T-cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin-expressing tumors. Mol Cancer Ther. 2015;14(12):2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazor R, Onda M, Park D, et al. . Dual B- and T-cell de-immunization of recombinant immunotoxin targeting mesothelin with high cytotoxic activity. Oncotarget. 2016;7(21):29916-29926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James EA, van Haren SD, Ettinger RA, et al. . T-cell responses in two unrelated hemophilia A inhibitor subjects include an epitope at the factor VIII R593C missense site. J Thromb Haemost. 2011;9(4):689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James EA, Moustakas AK, Bui J, Nouv R, Papadopoulos GK, Kwok WW. The binding of antigenic peptides to HLA-DR is influenced by interactions between pocket 6 and pocket 9. J Immunol. 2009;183(5):3249-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James EA, Moustakas AK, Bui J, et al. . HLA-DR1001 presents “altered-self” peptides derived from joint-associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. 2010;62(10):2909-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17(12):1236-1237. [DOI] [PubMed] [Google Scholar]
- 36.Wang P, Sidney J, Dow C, Mothé B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLOS Comput Biol. 2008;4(4):e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, Sidney J, Kim Y, et al. . Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010;11(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50(3-4):213-219. [DOI] [PubMed] [Google Scholar]
- 39.Sievers F, Wilm A, Dineen D, et al. . Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7(1):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen PC, Lewis KB, Ettinger RA, et al. . High-resolution mapping of epitopes on the C2 domain of factor VIII by analysis of point mutants using surface plasmon resonance. Blood. 2014;123(17):2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichelt P, Schwarz C, Donzeau M. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr Purif. 2006;46(2):483-488. [DOI] [PubMed] [Google Scholar]
- 42.Lubin IM, Healey JF, Scandella D, Runge MS, Lollar P. Elimination of a major inhibitor epitope in factor VIII. J Biol Chem. 1994;269(12):8639-8641. [PubMed] [Google Scholar]
- 43.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51-59. [DOI] [PubMed] [Google Scholar]
- 44.Lind P, Larsson K, Spira J, et al. . Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur J Biochem. 1995;232(1):19-27. [DOI] [PubMed] [Google Scholar]
- 45.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High level expression of recombinant porcine coagulation factor VIII. J Biol Chem. 2002;277(41):38345-38349. [DOI] [PubMed] [Google Scholar]
- 46.Parker ET, Healey JF, Barrow RT, Craddock HN, Lollar P. Reduction of the inhibitory antibody response to human factor VIII in hemophilia A mice by mutagenesis of the A2 domain B-cell epitope. Blood. 2004;104(3):704-710. [DOI] [PubMed] [Google Scholar]
- 47.Lollar P, Fay PJ, Fass DN. Factor VIII and factor VIIIa. Methods Enzymol. 1993;222:128-143. [DOI] [PubMed] [Google Scholar]
- 48.Pratt KP, Shen BW, Takeshima K, Davie EW, Fujikawa K, Stoddard BL. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999;402(6760):439-442. [DOI] [PubMed] [Google Scholar]
- 49.Fuentes-Prior P, Fujikawa K, Pratt KP. New insights into binding interfaces of coagulation factors V and VIII and their homologues—lessons from high resolution crystal structures. Curr Protein Pept Sci. 2002;3(3):313-339. [DOI] [PubMed] [Google Scholar]
- 50.Barrow RT, Healey JF, Jacquemin MG, Saint-Remy JM, Lollar P. Antigenicity of putative phospholipid membrane-binding residues in factor VIII. Blood. 2001;97(1):169-174. [DOI] [PubMed] [Google Scholar]
- 51.Lewis DA, Pound ML, Ortel TL. Contributions of Asn2198, Met2199, and Phe2200 in the factor VIII C2 domain to cofactor activity, phospholipid-binding, and von Willebrand factor-binding. Thromb Haemost. 2003;89(5):795-802. [PubMed] [Google Scholar]
- 52.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007;110(13):4234-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin JC, Ettinger RA, Schuman JT, et al. . Six amino acid residues in a 1200 Å2 interface mediate binding of factor VIII to an IgG4κ inhibitory antibody. PLoS One. 2015;10(1):e0116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bray GL, Kroner BL, Arkin S, et al. . Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am J Hematol. 1993;42(4):375-379. [DOI] [PubMed] [Google Scholar]
- 55.Parvathaneni K, Abdeladhim M, Pratt KP, Scott DW. Hemophilia A inhibitor treatment: the promise of engineered T-cell therapy. Transl Res. 2017;187:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinitz KN, van Helden PM, Binder B, et al. . CD4+ T-cell epitopes associated with antibody responses after intravenously and subcutaneously applied human FVIII in humanized hemophilic E17 HLA-DRB1*1501 mice. Blood. 2012;119(17):4073-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pipe SW. Hemophilia: new protein therapeutics. Hematology Am Soc Hematol Educ Program. 2010;2010:203-209. [DOI] [PubMed] [Google Scholar]
- 58.Hay CR, Lozier JN, Lee CA, et al. . Safety profile of porcine factor VIII and its use as hospital and home-therapy for patients with haemophilia-A and inhibitors: the results of an international survey. Thromb Haemost. 1996;75(1):25-29. [PubMed] [Google Scholar]
- 59.Hay CR, Lozier JN, Lee CA, et al. . Porcine factor VIII therapy in patients with congenital hemophilia and inhibitors: efficacy, patient selection, and side effects. Semin Hematol. 1994;31(2 suppl 4):20-25. [PubMed] [Google Scholar]
- 60.Busch R, Hill CM, Hayball JD, Lamb JR, Rothbard JB. Effect of natural polymorphism at residue 86 of the HLA-DR beta chain on peptide binding. J Immunol. 1991;147(4):1292-1298. [PubMed] [Google Scholar]
- 61.Ong B, Willcox N, Wordsworth P, et al. . Critical role for the Val/Gly86 HLA-DR beta dimorphism in autoantigen presentation to human T cells. Proc Natl Acad Sci USA. 1991;88(16):7343-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davenport MP, Quinn CL, Chicz RM, et al. . Naturally processed peptides from two disease-resistance-associated HLA-DR13 alleles show related sequence motifs and the effects of the dimorphism at position 86 of the HLA-DR beta chain. Proc Natl Acad Sci USA. 1995;92(14):6567-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63(6):325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandberg H, Kannicht C, Stenlund P, et al. . Functional characteristics of the novel, human-derived recombinant FVIII protein product, human-cl rhFVIII. Thromb Res. 2012;130(5):808-817.23010293 [Google Scholar]
- 65.Orlova NA, Kovnir SV, Gabibov AG, Vorobiev II. Stable high-level expression of factor VIII in Chinese hamster ovary cells in improved elongation factor-1 alpha-based system. BMC Biotechnol. 2017;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shestopal SA, Hao JJ, Karnaukhova E, et al. . Expression and characterization of a codon-optimized blood coagulation factor VIII. J Thromb Haemost. 2017;15(4):709-720. [DOI] [PubMed] [Google Scholar]
- 67.Young G, Mahlangu J, Kulkarni R, et al. . Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967-977. [DOI] [PubMed] [Google Scholar]
- 68.Shapiro AD. Long-lasting recombinant factor VIII proteins for hemophilia A. Hematology Am Soc Hematol Educ Program. 2013;2013:37-43. [DOI] [PubMed] [Google Scholar]
- 69.Schulte S. Innovative coagulation factors: albumin fusion technology and recombinant single-chain factor VIII. Thromb Res. 2013;131(suppl 2):S2-S6. [DOI] [PubMed] [Google Scholar]
- 70.Giudicelli V, Chaume D, Lefranc MP. IMGT/GENE-DB: a comprehensive database for human and mouse immunoglobulin and T cell receptor genes. Nucleic Acids Res. 2005;33(database issue):D256-D261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunasekera D, Ettinger RA, Nakaya Fletcher S, et al. ; Personalized Approaches to Therapies for Hemophilia (PATH) Study Investigators. Factor VIII gene variants and inhibitor risk in African American hemophilia A patients. Blood. 2015;126(7):895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ettinger RA, Kwok WW. A peptide binding motif for HLA-DQA1*0102/DQB1*0602, the class II MHC molecule associated with dominant protection in insulin-dependent diabetes mellitus. J Immunol. 1998;160(5):2365-2373. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.