Abstract
Purpose
Metabolic syndrome is associated with an increased risk of cardiovascular disease, type 2 diabetes, and breast cancer recurrence in survivors of breast cancer. This randomized controlled trial assessed the effects of a 16-week combined aerobic and resistance exercise intervention on metabolic syndrome, sarcopenic obesity, and serum biomarkers among ethnically diverse, sedentary, overweight, or obese survivors of breast cancer.
Methods
Eligible survivors of breast cancer (N = 100) were randomly assigned to exercise (n = 50) or usual care (n = 50). The exercise group participated in supervised moderate-to-vigorous—65% to 85% of heart rate maximum—aerobic and resistance exercise three times per week for 16 weeks. Metabolic syndrome z-score (primary outcome), sarcopenic obesity, and serum biomarkers were measured at baseline, postintervention (4 months), and 3-month follow-up (exercise only).
Results
Participants were age 53 ± 10.4 years, 46% were obese, and 74% were ethnic minorities. Adherence to the intervention was 95%, and postintervention assessments were available in 91% of participants. Postintervention metabolic syndrome z-score was significantly improved in exercise versus usual care (between-group difference, −4.4; 95% CI, −5.9 to −2.7; P < .001). Sarcopenic obesity (appendicular skeletal mass index, P = .001; body mass index, P = .001) and circulating biomarkers, including insulin (P = .002), IGF-1 (P = .001), leptin (P = .001), and adiponectin (P = .001), were significantly improved postintervention compared with usual care. At 3-month follow-up, all metabolic syndrome variables remained significantly improved compared with baseline in the exercise group (P < .01).
Conclusion
Combined resistance and aerobic exercise effectively attenuated metabolic syndrome, sarcopenic obesity, and relevant biomarkers in an ethnically diverse sample of sedentary, overweight, or obese survivors of breast cancer. Our findings suggest a targeted exercise prescription for improving metabolic syndrome in survivors of breast cancer and support the incorporation of supervised clinical exercise programs into breast cancer treatment and survivorship care plans.
INTRODUCTION
Metabolic syndrome includes visceral adiposity, hyperglycemia, low-serum, high-density lipoprotein cholesterol, hypertriglyceridemia, and hypertension,1 and is associated with a 17% increase in the risk of breast cancer,2-4 a three-fold increase in breast cancer recurrence, and an approximate two-fold increase in breast cancer–specific mortality.5,6 Metabolic syndrome is exacerbated by obesity, sedentary lifestyle, and the receipt of chemotherapy in patients with breast cancer. Interventions to improve metabolic syndrome among survivors of breast cancer are needed to reduce the risk of comorbidities and improve breast cancer outcomes.
Exercise improves individual components of metabolic syndrome in survivors of breast cancer7-9; however, consensus is lacking that it improves metabolic syndrome as a collective entity, particularly in high-risk survivors of breast cancer—racial and/or ethnic minorities with obesity.5 The purpose of this trial was to examine the effects of a 16-week supervised aerobic and resistance exercise intervention on comprehensive metabolic syndrome parameters among ethnically diverse, sedentary, overweight, or obese survivors of breast cancer. Secondary outcomes included sarcopenic obesity and circulating biomarkers. We also examined outcomes in the exercise group 3-months after the completion of the intervention.
METHODS
This two-arm randomized controlled trial compared a progressive combined—aerobic and resistance—exercise intervention with usual care on baseline to 4-month changes in metabolic syndrome, body composition, and circulating biomarkers. The protocol and informed consent were approved by the institutional review board (HS-12-00141) and registered with ClinicalTrials.gov. Detailed methods have been published.10 End points were assessed at baseline, postintervention (month 4), and at 3-month follow-up (exercise group only).
Participants, Recruitment, and Random Assignment
Eligible participants were < 6 months post-treatment for stage 0 to 3 breast cancer and were nonsmokers, sedentary (< 60 minutes of structured exercise per week), with body mass index (BMI) ≥ 25.0 kg/m2 (or body fat > 30%) and waist circumference > 88 cm. Women were eligible regardless of baseline metabolic syndrome status and were prescreened on the basis of waist circumference given its strong association with insulin resistance.11,12
Recruitment occurred between August 1, 2012, and December 31, 2016, from the University of Southern California Norris Comprehensive Cancer Center and Los Angeles County Hospital. Participants were stratified by menopausal status and randomly assigned (block size = 10 patients) to exercise or usual care after baseline testing using concealed randomization lists.
Outcome Measures
Primary outcome was metabolic syndrome z-score that was calculated from modified z-scores of the following variables13-16: waist circumference; systolic blood pressure; diastolic blood pressure (DBP); HDL cholesterol (HDL-C); triglycerides (TGs); and glucose using individual participant data, US National Cholesterol Education Program Adult Treatment Panel III (ATP III) criteria, and standard deviations (SDs; denominator of each factor in the formulas) using baseline data of the entire study cohort (N = 100)13,16:
Metabolic syndrome criteria.
On the basis of the ATP III definition,17 metabolic syndrome constituted three or more of the following risk factors: waist circumference ≥ 88 cm, SBP ≥ 130 mm Hg or Hg DBP ≥ 85 mm or taking blood pressure medication, fasting levels of HDL-C < 50 mg/dL, TGs ≥ 150 mg/dL, and glucose ≥ 100 mg/dL or taking diabetes medication. The ATP III score of each participant was calculated by summing ATP III criteria met at each timepoint.
Serum biomarkers.
Fasting (≥ 12 hours) blood (∼ 30 cc) was obtained from the antecubital vein by trained phlebotomists. Serum was stored at −80° celsius until batch analysis at study completion. Glucose, HDL-C, LDL cholesterol, total cholesterol, TGs, and glycosylated hemoglobin (HbA1c) were measured on a Vitros 4600 Analyzer (Ortho Clinical Diagnostics, Rochester, NY). High-sensitivity C-reactive protein was measured by immunoturbidmetric assay. Enzyme-linked immunosorbent assays were used to measure insulin, IL-6, IL-8, TNF-α, leptin, and adiponectin. Homeostasis model assessment (HOMA-IR) was used to estimate insulin resistance using the validated equation: Fasting Plasma Insulin × Fasting Plasma Glucose (mmol/L)/22.5.18
Estradiol and testosterone were determined by liquid chromatography–mass spectrometry. Sex hormone binding globulin was measured by double-antibody radioimmunoassay (Roche Cobas: SHBG:03052001; Indianapolis, IN). Duplicate testing was performed with coefficients of variation for all samples < 10%.
Anthropometrics/dual-energy X-ray absorptiometry.
Weight was measured to the nearest 0.1 kg on an electronic scale with the patient wearing a hospital gown and no shoes. Height was measured to the nearest 0.5 cm with a fixed stadiometer. Waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the iliac crest. Hip circumference was measured around the widest portion of the buttocks. Whole-body dual-energy X-ray absorptiometry scans were performed to assess percent body fat, fat mass, appendicular skeletal mass, and lean mass (Lunar GE iDXA; Fairfield, CT). Sarcopenic obesity was defined as appendicular skeletal mass index < 5.45 kg/m2 and BMI ≥ 30.0 kg/m2.19
Blood pressure.
After 5 minutes of quiet sitting, blood pressure was measured by using the arm contralateral to the affected breast with an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY).
Covariate Measures
Physical activity/dietary assessments.
Physical activity history was assessed at baseline by using an interviewer-administered, validated questionnaire.20 Three-day dietary records—2 weekdays and 1 weekend day—were completed at baseline, postintervention, and 3-month follow-up (exercise group only) within 1 week of each assessment and were analyzed using Nutritionist Pro (Axxya Systems, Woodinville, WA). Participants were asked to maintain dietary behaviors throughout the trial.
Medical history.
Participants completed the Charlson comorbidity questionnaire.21 Cancer-related information—disease stage, hormone receptor status, histologic grade, endocrine therapy, type and duration of chemotherapy, duration of radiation therapy, and surgery—was abstracted from medical records.
Physical Fitness Measures
A single-stage submaximal treadmill test was used to estimate maximal oxygen uptake.22 Maximal voluntary strength (one-repetition maximum [1-RM]) was assessed for the chest press, latissimus pulldown, knee extension, and knee flexion using the 10-repetition maximum method (Tuff Stuff, Pomona, CA).23 Results were used to quantify fitness and to prescribe exercise intensity for the intervention.
Exercise Intervention
The exercise program aligned with American College of Sports Medicine/American Cancer Society (ACSM/ACS) exercise guidelines for survivors of cancer—150 minutes of aerobic exercise and 2 to 3 days of resistance exercise training per week.24,25 Participants received three supervised one-on-one exercise sessions per week. Days 1 and 3 endorsed resistance and aerobic exercise of approximately 80 minutes, and day 2 included approximately 50 minutes of aerobic exercise. All sessions were led by a certified ACSM/ACS cancer exercise trainer. Participants wore a Polar heart monitor (Polar, Lake Success, NY) during each exercise session. The trainer documented attendance and minutes of exercise per session. Reasons for missing sessions were documented, with make-up sessions allowable up to 18 weeks. Details of the exercise intervention are provided in the Appendix (online only).
Usual Care Group
Participants who were randomly assigned to usual care were asked to log and maintain their current level of physical activity throughout the 16-week study period26 and to wear an accelerometer daily during this period. At the completion of the 16-week study period, participants were offered the identical exercise intervention.
Statistical Analyses
At the time of project initiation, metabolic syndrome as a collective entity had not been investigated in survivors of breast cancer; thus, sample size was calculated by using changes in insulin27 with a 16-week exercise intervention among survivors of breast cancer. Enrollment of 100 women provided 80% statistical power (α = .05) to detect a 2.6-μU/mL (SD = 4.0 μU/mL) difference in mean insulin levels, assuming 20% dropout using a two-group t test.
Within-group differences in mean changes for individual outcomes measured at 4 months and at 3-month follow-up (exercise group only) were evaluated by using general linear models repeated-measures analyses of variance. Between-group differences in mean changes for individual outcomes measured at 4 months were evaluated by using mixed-model repeated-measures analysis. A priori covariates, including the type of treatment (chemotherapy, radiation, or both), surgery type, medication use (eg, antihypertensives and hyperglycemia medications), BMI, total kilocalorie intake, diet quality, and macronutrient distribution, were explored in models as a result of their possible associations with outcomes, but none modified results. Post hoc analyses included stratification by menopausal status at time of diagnosis.28 Analyses were performed by using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC).
RESULTS
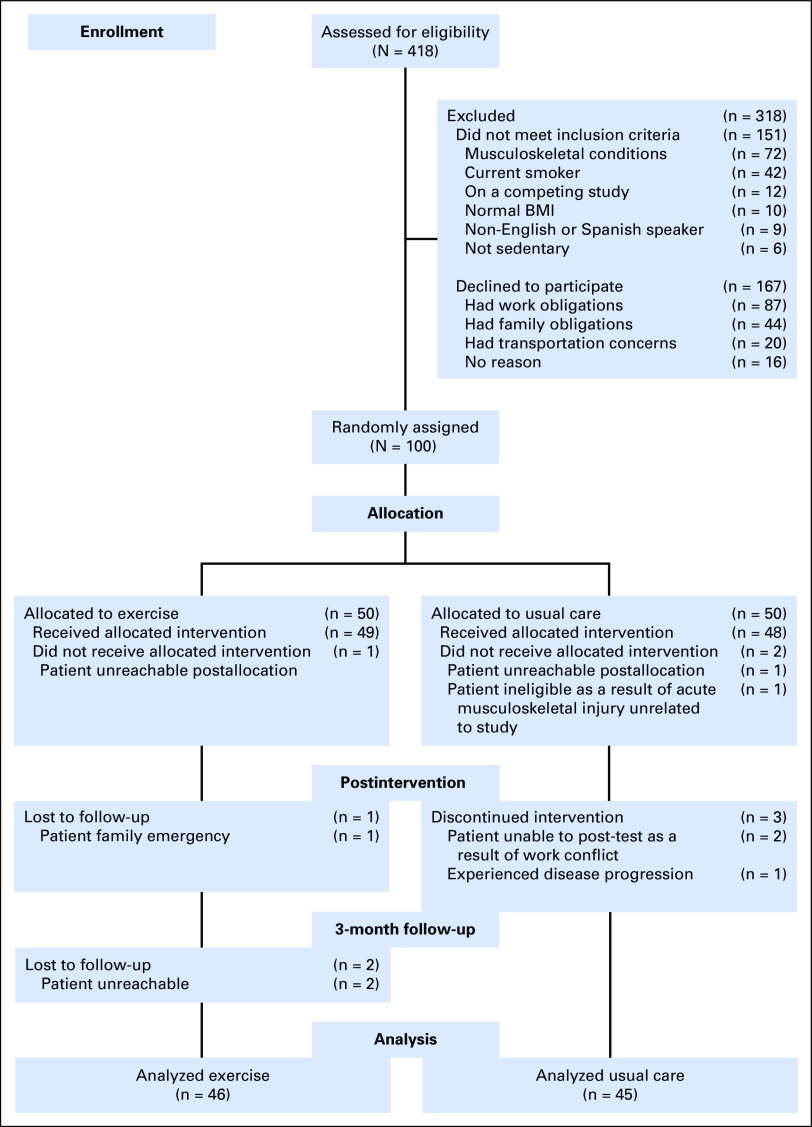
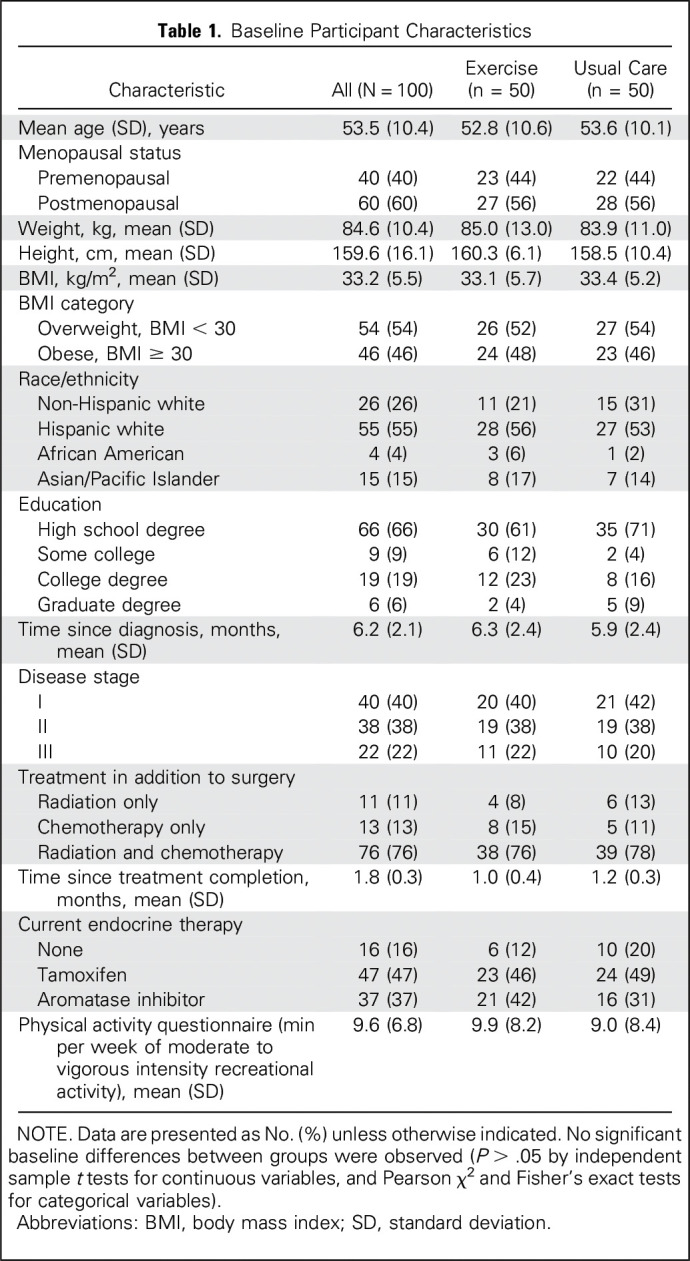
Figure 1 depicts the study schema. Baseline characteristics were similar across the two groups (Table 1). Attendance of 96%—average, 46 of 48 sessions—and adherence of 95% with aerobic and resistance exercises were observed in the exercise group. No adverse events were reported. Self-reports and accelerometry data suggested that the usual care group did not alter their physical activity levels over the study period. Results of our main outcomes did not vary by menopausal status (data not shown).
Fig 1.
CONSORT diagram of the metabolic syndrome trial. BMI, body mass index.
Table 1.
Baseline Participant Characteristics
Changes in Metabolic Syndrome
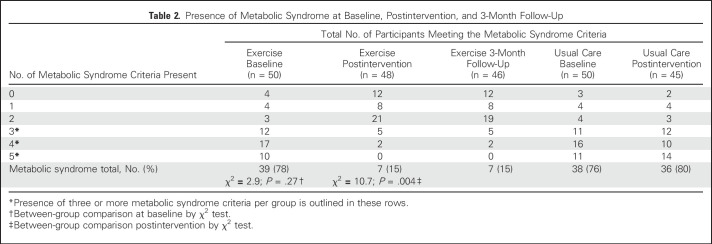
At baseline, 77% of participants had metabolic syndrome (Table 2). Postintervention, 15% of the exercise group and 80% of the usual care group had metabolic syndrome (χ2 = 10.7; P = .004).
Table 2.
Presence of Metabolic Syndrome at Baseline, Postintervention, and 3-Month Follow-Up
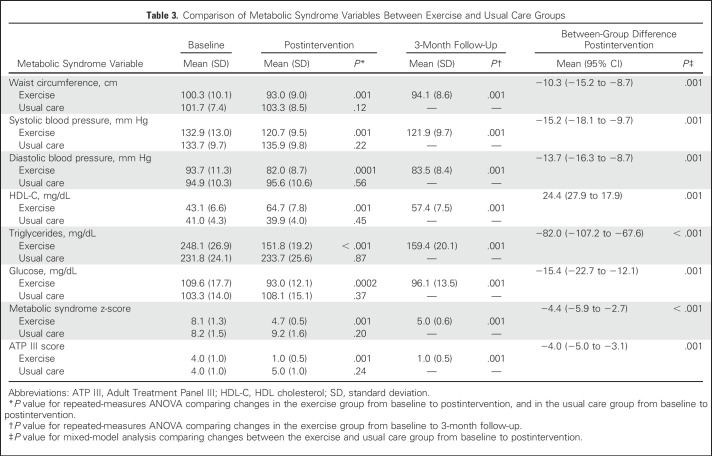
Table 3 lists the baseline, postintervention, and 3-month follow-up (exercise only) changes in metabolic syndrome variables by study group. The exercise group experienced significant improvements in all metabolic syndrome variables, including metabolic syndrome z-scores and ATP III scores compared with baseline (P < .01) and the usual care group (P < .001). At follow-up, all metabolic syndrome variables remained significantly improved in the exercise group compared with baseline (P < .001).
Table 3.
Comparison of Metabolic Syndrome Variables Between Exercise and Usual Care Groups
Changes in Sarcopenic Obesity and Body Composition
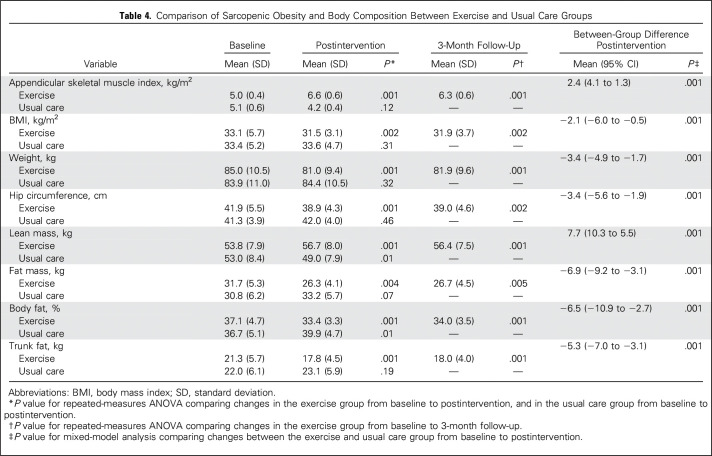
At baseline, 95% of our study population presented with sarcopenic obesity (data not shown). Postintervention, biomarkers of sarcopenic obesity, appendicular skeletal muscle index, and BMI were significantly reduced in the exercise group compared with baseline (P ≤ .01) and the usual care group (P < .001; Table 4). The exercise group experienced significant decreases in body weight and all indicators of adiposity compared with baseline (P ≤ .01) and the usual care group (P < .001). Lean mass increased significantly in the exercise group compared with baseline (P ≤ .01) and the usual care group (P < .001). At follow-up, all body composition variables remained significantly improved in the exercise group compared with baseline (P < .001).
Table 4.
Comparison of Sarcopenic Obesity and Body Composition Between Exercise and Usual Care Groups
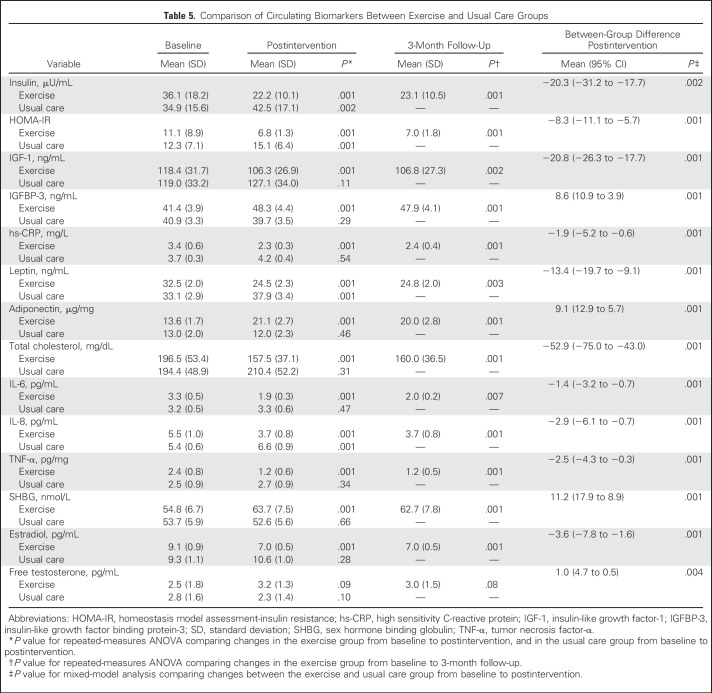
Changes in Circulating Biomarkers
Table 5 lists baseline, postintervention, and 3-month follow-up changes in circulating biomarker variables by group. The exercise group experienced significant reductions in all biomarkers, including those that are related to insulin resistance, proinflammatory markers, and estradiol, compared with baseline (P < .01) and the usual care group (P < .001), whereas insulin, HOMA-IR, leptin, and IL-8 were significantly increased in the usual care group (P < .01). At follow-up, all biomarkers, with the exception of free testosterone, remained significantly improved in the exercise group compared with baseline (P < .01).
Table 5.
Comparison of Circulating Biomarkers Between Exercise and Usual Care Groups
DISCUSSION
A supervised 16-week aerobic and resistance exercise intervention led to significant improvements in metabolic syndrome, sarcopenic obesity, and circulating biomarkers that were maintained at 3-month follow-up among ethnically diverse, sedentary, and overweight or obese survivors of breast cancer. This is the first study, to our knowledge, to significantly improve all components of metabolic syndrome with a structured exercise intervention in survivors of breast cancer. This work has the potential to change practice through the promotion of the ACSM/ACS exercise guidelines for survivors of cancer. Our work demonstrates the impact of these guidelines on cardiometabolic risk factors in a diverse population and may identify predictive biomarkers for additional study. As our intervention targeted women who had recently completed treatment, our results support the implementation of a structured exercise intervention early in the survivorship continuum.
The prevalence of metabolic syndrome in this sample (77%) exceeds that of previous studies that reported a prevalence of 55.4%13 and 59.2%29; however, our results were similar to our previous observational study that reported 72.5% prevalence after 3 to 4 months of chemotherapy.30 It is possible that this higher prevalence is a result of the large proportion of Hispanic white women (55%), who have a greater prevalence of metabolic syndrome compared with non-Hispanic whites,31 as well as inclusion criteria that targeted sedentary and overweight or obese survivors of breast cancer. The necessity to establish the prevalence of metabolic syndrome in a larger cohort of survivors of breast cancer to plan for future personalized interventions is apparent.
The magnitude of benefit observed in the current study is impressive and parallels the findings of a pilot study by Nuri et al,32 who found that a 15-week supervised, combined moderate-intensity exercise intervention significantly improved metabolic syndrome variables, with the exception of waist circumference, among 29 sedentary, overweight, postmenopausal survivors of breast cancer—changes that occurred in the presence of an approximate 1-kg decrease in body weight. In contrast, Thomas et al13 and Guinan et al33 did not find significant improvements in metabolic syndrome after aerobic exercise in survivors of breast cancer, possibly because of lower adherence13 and the inclusion of an active study population.33
Our large, exercise-induced effects on metabolic syndrome also may be a result of the high rate of metabolic syndrome, high adherence, a supervised environment, and the inclusion of resistance exercise. Our adherence rate of 96% exceeds the 70% to 80% noted in other trials34-36 and could be attributed to flexible session timing (5 am to 8 pm, 7 days per week), one-on-one supervision, and the provision of parking permits or bus passes to overcome transportation barriers. In addition, we conducted the intervention in a controlled clinical setting under direct supervision to ensure exercise safety and dose intensity needed to offset metabolic dysregulation.38
Resistance exercise was included in our intervention to elicit an impact on lean mass and glucose metabolism. Aerobic exercise has traditionally been viewed as the main mode of exercise that is effective at reducing waist circumference, fasting glucose, HDL-C, and TGs13,33,39; however, aerobic exercise combined with resistance exercise alleviates metabolic syndrome and promotes functional improvements in muscular strength after the treatment of breast cancer.7-9 Resistance exercise induces changes in insulin sensitivity by preserving and/or increasing lean body mass, increasing glucose storage, facilitating glucose clearance from circulation, and reducing the amount of insulin that is required to maintain normal glucose tolerance in obese adults.40 Resistance exercise combined with aerobic exercise decreases metabolic syndrome in dyslipidemic men and women7,15 and obese postmenopausal women,41 similar to our study. Thus, combining resistance and aerobic exercise may improve metabolic health-related outcomes more than aerobic exercise alone, although no randomized controlled trials have directly addressed this question in survivors of breast cancer.
Sarcopenic obesity is an independent predictor of cancer survival.42 In postmenopausal women, sarcopenic obesity is associated with elevated proinflammatory mediators—that is, C-reactive protein, TNF-α, and IL-643—which is consistent with the high prevalence of sarcopenic obesity and heightened proinflammatory state at baseline that was observed in our study population. This is the first study, to our knowledge, to report the prevalence of sarcopenic obesity in survivors of breast cancer (95%), which is higher than that previously reported in postmenopausal women without a history of cancer (25%)44; however, we recognize that our screening criterion—for example, waist circumference > 88 cm—predisposed our sample to sarcopenic obesity and metabolic syndrome. As no other previous investigations have examined combined exercise-induced changes in sarcopenic obesity in survivors of breast cancer, a direct comparison of past studies is lacking. Of relevance, Adams et al45 reported a significant improvement in sarcopenia after a resistance exercise intervention for survivors of breast cancer who underwent chemotherapy, and Schmitz et al46 found attenuated appendicular skeletal muscle mass in a 12-month weight lifting intervention among survivors of breast cancer. These results are in agreement with our study and support the potential of resistance exercise to elicit positive changes in lean mass in this high-risk population.
The 4-kg weight loss observed in this study parallels the approximate 5-kg loss reported in another study that also included diet counseling among survivors of breast cancer.47 As dietary intake, diet quality, and macronutrient composition remained unchanged, the significant weight loss that resulted from our exercise-alone intervention is possibly a result of the increased energy expenditure from a controlled exercise stimulus within this sedentary population. We estimated that each combined exercise session resulted in an approximate 500-kcal energy expenditure; therefore, it is possible that this induced a large enough caloric deficit over the entire study period to result in weight loss.
The intervention also resulted in reductions in insulin resistance and proinflammatory and hormonal biomarkers hypothesized to mediate the relationship between obesity and breast cancer.48,49 These effects are consistent with the extant literature on exercise interventions in survivors of breast cancer, which has been recently summarized by two systematic reviews with meta-analysis.50,51 These meta-analyses found that exercise decreased the serum concentrations of IL-6, TNF-α, and IL-2.50
Hyperinsulinemia/insulin resistance is associated with increased cancer recurrence and mortality,52,53 presumably as a result of the promotion of the survival and growth of residual cancer cells; thus, the decreases in circulating insulin and HOMA-IR with exercise that were observed in the current study represent an additional mechanism that might translate into survival benefits. Whereas a variety of exercise approaches have been shown to reduce insulin levels in survivors of cancer,51 it is unclear which exercise methodology will provide optimal results. Future studies that evaluate exercise type, setting (clinic, home based, with or without supervision), frequency, intensity, time, and intervention period on insulin sensitivity are needed to best advise clinical practice and patient care.
Overall, our work provides support for the adoption of the ACSM/ACS exercise guidelines for survivors of breast cancer. This work also underscores the need for future fully powered investigations that examine the effects of exercise and other energy balance–derived interventions—that is, diet and diet plus exercise—on physiologic and cancer outcomes in survivors of breast cancer. Ongoing interventions, such as the Breast Cancer Weight Loss study,54 aim to address the impact of weight loss on cancer recurrence; however, the question remains as to whether an exercise intervention alone will benefit cardiometabolic and cancer outcomes.
On the basis of our findings and past studies, future work requires designing investigations to examine the effects of precision-derived—that is, varying modes and intensity for each patient—exercise interventions on metabolic syndrome; designing and implementing home- or community-based exercise interventions that are more easily disseminated; and assessing the potential effect that the attenuation of metabolic syndrome has on mortality rates—our ongoing follow-up analysis will provide preliminary data to address this point. We are currently testing a novel exercise intervention that is designed to elicit greater benefits on cardiometabolic factors in survivors of breast cancer (ClinicalTrials.gov identifier: NCT03284346) that may progress the current exercise paradigm for survivors of cancer.
Strengths of our study include a focus on high-risk survivors of breast cancer with high rates of metabolic syndrome, inactivity, and obesity; the ethnically diverse sample; the comprehensive assessment of metabolic syndrome components and markers; use of a continuous metabolic syndrome score that replicated that used in cancer13 and noncancer populations16; the randomized controlled trial design; the high adherence rate; and the modest loss-to-follow-up rate. Limitations include possible recruitment bias, a lack of intervention reproducibility with high adherence outside of a supervised setting (eg, home-based or virtually coached exercise interventions), lack of an attention control group, and limited frequency of dietary recall information.
In summary, our findings support supervised aerobic and resistance exercise as an effective strategy to attenuate metabolic syndrome, body composition, and serum biomarkers in survivors of breast cancer. Our findings support the incorporation of supervised clinical exercise programs into breast cancer treatment and survivorship care plans.
ACKNOWLEDGMENT
We acknowledge the Clinical Investigations Support Office of the Norris Comprehensive Cancer Center for their support of this investigation, the extraordinary generosity of our study participants—without their participation, our study would not have been possible—and the exercise training staff for delivering an engaging and uniform intervention.
Appendix
Participants, Recruitment, and Random Assignment.
The principal investigator (C.M.D.-C.) approached newly diagnosed survivors of breast cancer in person during clinic hours to present the study. Women self-referred via study brochures at the University of Southern California Physical Therapy Associates Clinic and the Keck Medicine of University of Southern California Pasadena, and contacted the principal investigator by phone.
Exercise Intervention.
All exercise sessions took place at the Integrative Center for Oncology Research in Exercise in the Division of Biokinesiology and Physical Therapy at the University of Southern California . Each session began with a 5-minute aerobic exercise warm-up at 40% to 50% of estimated VO2max, followed by a bout of resistance exercise, including leg press, lunges, leg extension, leg flexion, chest press, seated row, triceps extension, and biceps curl. Resistance exercise was performed in a circuit training fashion with no formal rest period between each exercise. The circuit was performed as follows: leg press ↔ chest press → lunges ↔ seated row → leg extension ↔ triceps extension → leg flexion ↔ biceps curl, where ↔ indicates the two exercises between which the participants alternated until all sets were completed, then the following pair of exercises was performed. Initial resistance was set at 80% of the estimated one-repetition maximum for lower-body exercises and 60% estimated one-repetition maximum for upper-body exercises. Weight was increased by 10% upon completion of two consecutive sessions in which three sets of 10 repetitions were performed at a set weight. Repetitions increased from 10 (week 4) to 12 (week 8) to 15 (week 12) every 4 weeks to safely build muscular endurance. Participants were required to wear prescribed compression garments during exercise sessions.
Resistance exercise was followed by the self-selected aerobic exercise session of the participant’s choosing, including treadmill walking/running, rowing machine, or stationary bicycle. Heart rate was monitored throughout the aerobic sessions to maintain a heart rate at 65% to 80% of maximum. Aerobic exercise was increased from 30 minutes (week 1) to 50 minutes (week 16) as cardiorespiratory fitness increased. Participants ended each session with a 5-minute cool down at 40% to 50% estimated VO2max.
Footnotes
Supported by Grants No. K07-CA160718 from the National Cancer Institute and UL1-TR001855 and UL1-TR000130 from the National Center for Advancing Translational Science.
Clinical trial information: NCT01140282.
AUTHOR CONTRIBUTIONS
Conception and design: Christina M. Dieli-Conwright, Kerry S. Courneya, Wendy Demark-Wahnefried, Thomas A. Buchanan, Darcy V. Spicer, Debu Tripathy, Joanne E. Mortimer
Collection and assembly of data: Christina M. Dieli-Conwright, Nathalie Sami, Kyuwan Lee
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Christina M. Dieli-Conwright
No relationship to disclose
Kerry S. Courneya
No relationship to disclose
Wendy Demark-Wahnefried
Employment: Advanced Tear Diagnostics (I)
Stock or Other Ownership: Advanced Tear Diagnostics (I)
Nathalie Sami
No relationship to disclose
Kyuwan Lee
No relationship to disclose
Thomas A. Buchanan
No relationship to disclose
Darcy V. Spicer
No relationship to disclose
Debu Tripathy
Consulting or Advisory Role: Novartis, Pfizer, Nektar
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Nektar
Leslie Bernstein
No relationship to disclose
Joanne E. Mortimer
Honoraria: Novartis (I)
Consulting or Advisory Role: Puma Biotechnology
Speakers' Bureau: Novartis
REFERENCES
- 1.de Haas EC, Oosting SF, Lefrandt JD, et al. : The metabolic syndrome in cancer survivors. Lancet Oncol 11:193-203, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Russo A, Autelitano M, Bisanti L: Metabolic syndrome and cancer risk. Eur J Cancer 44:293-297, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Capasso I, Esposito E, Pentimalli F, et al. : Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther 10:1240-1243, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Agnoli C, Berrino F, Abagnato CA, et al. : Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case-control study. Nutr Metab Cardiovasc Dis 20:41-48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasanisi P, Berrino F, De Petris M, et al. : Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer 119:236-238, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Calip GS, Malone KE, Gralow JR, et al. : Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat 148:363-377, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earnest CP, Johannsen NM, Swift DL, et al. : Aerobic and strength training in concomitant metabolic syndrome and type 2 diabetes. Med Sci Sports Exerc 46:1293-1301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell KL, Neil SE, Winters-Stone KM: Review of exercise studies in breast cancer survivors: Attention to principles of exercise training. Br J Sports Med 46:909-916, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Winters-Stone KM, Dobek J, Bennett JA, et al. : The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: A randomized controlled trial. J Cancer Surviv 6:189-199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieli-Conwright CM, Mortimer JE, Schroeder ET, et al. : Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: Rationale, design, and methods. BMC Cancer 14:238, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, Punyanitya M, Chen J, et al. : Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring) 14:727-736, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein S, Allison DB, Heymsfield SB, et al. : Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 85:1197-1202, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Thomas GA, Alvarez-Reeves M, Lu L, et al. : Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int J Endocrinol 2013:168797, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean PS, Higgins JA, Wyatt HR, et al. : Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297:R793-R802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman LA, Slentz CA, Willis LH, et al. : Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise—STRRIDE-AT/RT). Am J Cardiol 108:838-844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JL, Slentz CA, Houmard JA, et al. : Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 100:1759-1766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. : Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 24:e13-e18, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G, Alberiche M, et al. : Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57-63, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN: Body composition in healthy aging. Ann N Y Acad Sci 904:437-448, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kriska AM, Knowler WC, LaPorte RE, et al. : Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13:401-411, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Ebbeling CB, Ward A, Puleo EM, et al. : Development of a single-stage submaximal treadmill walking test. Med Sci Sports Exerc 23:966-973, 1991 [PubMed] [Google Scholar]
- 23.Brzycki M: Strength testing—Predicting a one-rep max from repetition-to-fatigue. J Phys Ed Rec Dance 64:88-90, 1993 [Google Scholar]
- 24.Rock CL, Doyle C, Demark-Wahnefried W, et al. : Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62:243-274, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Schmitz KH, Courneya KS, Matthews C, et al. : American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409-1426, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Irwin ML, Tworoger SS, Yasui Y, et al. : Influence of demographic, physiologic, and psychosocial variables on adherence to a yearlong moderate-intensity exercise trial in postmenopausal women. Prev Med 39:1080-1086, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ligibel JA, Campbell N, Partridge A, et al. : Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol 26:907-912, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Irwin ML, McTiernan A, Bernstein L, et al. : Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 14:2881-2888, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porto LA, Lora KJ, Soares JC, et al. : Metabolic syndrome is an independent risk factor for breast cancer. Arch Gynecol Obstet 284:1271-1276, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Dieli-Conwright CM, Wong L, Waliany S, et al. : An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer 122:2646-2653, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ervin RB: Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Rep 13:1-7, 2009 [PubMed] [Google Scholar]
- 32.Nuri R, Kordi MR, Moghaddasi M, et al. : Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. J Cancer Res Ther 8:238-242, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Guinan E, Hussey J, Broderick JM, et al. : The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—A pilot study. Support Care Cancer 21:1983-1992, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Courneya KS, Segal RJ, Gelmon K, et al. : Predictors of supervised exercise adherence during breast cancer chemotherapy. Med Sci Sports Exerc 40:1180-1187, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Arem H, Sorkin M, Cartmel B, et al. : Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: The Hormones and Physical Exercise (HOPE) study. J Cancer Surviv 10:654-662, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz KH, Ahmed RL, Hannan PJ, et al. : Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 14:1672-1680, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Reference deleted. [Google Scholar]
- 38.Bakker EA, Lee DC, Sui X, et al. : Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc 92:1214-1222, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairey AS, Courneya KS, Field CJ, et al. : Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: A randomized controlled trial. Cancer Epidemiol Biomarkers Prev 12:721-727, 2003 [PubMed] [Google Scholar]
- 40.Ivy JL: Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med 24:321-336, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Cuff DJ, Meneilly GS, Martin A, et al. : Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 26:2977-2982, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Prado CM, Lieffers JR, McCargar LJ, et al. : Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 9:629-635, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Dutra MT, Avelar BP, Souza VC, et al. : Relationship between sarcopenic obesity-related phenotypes and inflammatory markers in postmenopausal women. Clin Physiol Funct Imaging 37:205-210, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Batsis JA, Mackenzie TA, Jones JD, et al. : Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999-2004 National Health and Nutrition Examination Survey. Clin Nutr 35:1472-1483, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams SC, Segal RJ, McKenzie DC, et al. : Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res Treat 158:497-507, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Brown JC, Schmitz KH: Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat 151:385-392, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrigan M, Cartmel B, Loftfield E, et al. : Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) study. J Clin Oncol 34:669-676, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hursting SD: Obesity, energy balance, and cancer: A mechanistic perspective. Cancer Treat Res 159:21-33, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Irwin ML: Weight loss interventions and breast cancer survival: The time is now. J Clin Oncol 32:2197-2199, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Meneses-Echávez JF, Correa-Bautista JE, González-Jiménez E, et al. : The effect of exercise training on mediators of inflammation in breast cancer survivors: A systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev 25:1009-1017, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Kang DW, Lee J, Suh SH, et al. : Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 26:355-365, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Goodwin PJ, Ennis M, Pritchard KI, et al. : Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol 20:42-51, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Barone BB, Yeh HC, Snyder CF, et al. : Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 300:2754-2764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ligibel JA, Barry WT, Alfano C, et al. : Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): Study design. NPJ Breast Cancer 10.1038/s41523-017-0040-8 [epub ahead of print on September 21, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]