Abstract
Purpose
Treating early-onset scoliosis (EOS) with traditional growing rods (TGR) is effective but requires periodic surgical lengthening, risking complications. Alternatives include magnetically controlled growing rods (MCGR) that lengthen noninvasively and the growth guidance system (GGS), which obviate the need for active, distractive lengthenings. Previous studies have reported promising clinical effectiveness for GGS; however the direct medical costs of GGS compared to TGR and MCGR have not yet been explored.
Methods
To estimate the cost of GGS compared with MCGR and TGR for EOS an economic model was developed from the perspective of a US integrated health care delivery system. Using dual-rod constructs, the model estimated the cumulative costs associated with initial implantation, rod lengthenings (TGR, MCGR), revisions due to device failure, surgical-site infections, device exchange, and final spinal fusion over a 6-year episode of care. Model parameters were from peer-reviewed, published literature. Medicare payments were used as a proxy for provider costs. Costs (2016 US$) were discounted 3% annually.
Results
Over a 6-year episode of care, GGS was associated with fewer invasive surgeries per patient than TGR (GGS: 3.4; TGR: 14.4) and lower cumulative costs than MCGR and TGR, saving $25,226 vs TGR. Sensitivity analyses showed that results were sensitive to changes in construct costs, rod breakage rates, months between lengthenings, and TGR lengthening setting of care.
Conclusion
Within the model, GGS resulted in fewer invasive surgeries and deep surgical site infections than TGR, and lower cumulative costs per patient than both MCGR and TGR, over a 6-year episode of care. The analysis did not account for family disruption, pain, psychological distress, or compromised health-related quality of life associated with invasive TGR lengthenings, nor for potential patient anxiety surrounding the frequent MCGR lengthenings. Further analyses focusing strictly on current generation technologies should be considered for future research.
Keywords: early-onset scoliosis, cost analysis, growth guidance system, magnetically controlled growing rod, traditional growing rod
Introduction
Early-onset scoliosis (EOS) is defined as a coronal curvature of the spine exceeding 10° occurring before the age of 10 years, and can be subcategorized as congenital, idiopathic, syndromic, or neuromuscular.1–4 Left untreated, EOS may progress to produce disfigurement and deformity of the chest wall, leading to thoracic insufficiency syndrome characterized by labored breathing, extreme breathlessness/fatigue, and reduced quality of life.3 Treatment options for EOS include observation, casting, bracing, and surgical techniques.3 Ideally EOS treatment would permit correction (partial or complete) of the deformity, maintain the deformity correction, and permit vertical growth of the spine and radial expansion of the rib cage. Fusion surgeries result in iatrogenic limitation of spinal growth with long-term impairment of pulmonary volumes, making these surgeries suboptimal in EOS.5 Hence “growth-friendly” surgeries such as growth guidance system (GGS), magnetically controlled growing rods (MCGR), and traditional growing rods (TGR) have been developed to attempt to satisfy the goals of treatment.
TGR are effective, yet require periodic invasive surgical lengthenings with risk of complications.6 The surgeries inherent with TGR treatment are also associated with considerable socioeconomic, psychological, and health-related quality of life (HRQoL) disadvantages for both patients and their caregivers.7 MCGR have also been shown to be clinically effective and can be lengthened noninvasively, with a hand-held external remote controller, allowing for magnetically controlled continuous elongation (to a set tension), or incremental elongation (to a set distance).7–12 Although device costs for MCGR are higher, several studies have shown that these may be offset by the reduced complications and costs garnered by noninvasive lengthenings.13–16 Both TGR and MCGR are effective for preventing disease progression and facilitating correction of curves.8–10,12,17,18
An alternative construct for EOS, GGS (SHILLA™ Growth Guidance System, Medtronic Spinal & Biologics, Memphis, TN, USA) was cleared for marketing in the United States in July 2014.19 GGS is a new growth-sparing technology that helps provide deformity correction while allowing continued skeletal growth at the proximal and distal construct ends and obviating the need for periodic lengthening procedures. GGS utilizes a unique non-locking set screw that allows the pedicle screws to slide along the rod axis during vertical growth. Once implanted during a surgical procedure similar to TGR and MCGR, GGS has demonstrated clinical effectiveness (in both curve correction and increasing thoracic height) with 6-year follow-up.20,21 Obviating the need for invasive lengthening procedures, GGS would be expected to reduce overall costs per patient in a similar manner to MCGR; however, no economic study of GGS has been published to date. The objective of this research was to estimate – over a 6-year episode of care – the cumulative cost of treating EOS with GGS compared with MCGR and TGR from the US integrated health care delivery system (IDS) perspective.
Materials and methods
Model overview
Similar to the cost analysis by Polly et al14 comparing MCGR with TGR, the present economic model was developed from the IDS perspective. For each treatment using dual-rod constructs, the model assessed the 6-year cumulative costs associated with initial implantation, rod lengthenings (TGR every 6 months; MCGR every 3 months), revisions due to device failure, surgical site infections (SSIs), device exchange (at 3.8 years), and final spinal fusion. Costs are presented in 2016 US$ and, in line with the recommendation of the Congressional Budget Office, were discounted at an annual rate of 3.0%.22 An institutional review board (IRB) exemption was granted given that model parameters were sourced from peer-reviewed, published literature and the present research did not involve human subjects.
For the present study, the cost analysis by Polly et al14 was first reconstructed (from the publicly available paper and technical report) and then updated to reflect the most recent published literature and to include GGS. As such, the model assumptions and parameter values for TGR and MCGR are largely based on Polly et al with the exception of updating the construct type (to 100% dual-rod to reflect current practice), device failure rates, deep SSI rates, time under anesthesia, and reimbursement codes and costs. For completeness, we have summarized the assumptions and data sources in the following section.
Model assumptions and data sources
Table 1 details the model framework created and clinical parameters used, while medical resources are detailed in Table 2. Importantly, the TGR device failure rates (rod breakage rates) were derived from an economic evaluation commissioned by the National Institute for Health and Care Excellence,18,23 while the MCGR and GGS rod breakage rates were obtained from the most recent comparable literature available from multicenter studies.11,20 The device failure rates for TGR and MCGR were corrected using the relative risk of rod breakage for single vs dual-rods to estimate what the rate would be if every construct were a dual-rod construct (constructs were 64% and 85% dual-rod [P Hosseini and J Pawelek, San Diego Spine Foundation, personal communication, April, 2017] in the sources used for TGR and MCGR, respectively).11,18,23,24 The source used for the GGS rod breakage rate already reflected 100% dual-rod construct.20
Table 1.
Model framework and clinical parameters
| Parameter | Base case value (sensitivity analysis [range]) |
Reference |
|---|---|---|
| Model framework | ||
| Time horizon (years) | 6 (1–6) | 14, 20 |
| Size of cohort | Per patient | NA |
| Per 1,000 patients | ||
| Payer mix | ||
| Private payer (%) | 51.5 (0–100) | 25, 26 |
| Medicaid (%) | 48.5 (0–100) | |
| Discount rate (% per annum) | 3.00 (0.00–5.00) | 22 |
| TGR lengthening setting of care | ||
| Hospital outpatient/inpatient (%) | 45.8 (0–100)/54.2 (100–0) | 14 |
| Hospital inpatient 1-day short stay (%) | 55.5 | 14 |
| Hospital inpatient standard ward (%) | 35.2 | |
| Hospital inpatient ICU (%) | 9.3 | 14 |
| MCGR lengthening setting of care | ||
| Physician office (%) | 100.0 | a |
| GGS HCP visit setting of care | ||
| Physician office (%) | 100.0 | a |
| Device failuresb and SSIs | ||
| TGR device failure (% per month) | 0.55 (0.27–1.10) | 18, 23, 24 |
| MCGR device failure (% per month) | 0.56 (0.28–1.13) | 11, 24 |
| GGS device failure (% per month) | 0.61 (0.30–1.21) | 20 |
| Device failure, dual vs single rod (RR) | 0.92 (0.46–1.00) | 24 |
| Device failures requiring complete removal (vs partial) (%) | 5.8 (2.9–11.6) | 14, 24 |
| TGR deep SSI (% per invasive surgery) | 2.99 (1.49–5.97) | 27 |
| MCGR deep SSI (% per invasive surgery) | 1.45 (0.72–2.90) | 11 |
| GGS deep SSI (% per invasive surgery) | 2.24 (1.12–4.48) | 28 |
| SSI: Medicaid patients (vs all other patients) (RR) | 2.06 (1.19–3.58) | 29 |
Notes:
Clinical advisors.
Rod breakage.
Abbreviations: GGS, growth guidance system; HCP, health care professional; ICU, intensive care unit; MCGR, magnetically controlled growing rod; NA, not applicable; RR, relative risk; SSI, surgical site infection; TGR, traditional growing rod.
Table 2.
Resource use
| Parameter | Base case value (sensitivity analysis [range]) |
Reference |
|---|---|---|
| Months between TGR lengthenings | 6.0 (6–12) | 17 |
| Months between MCGR lengthenings | 3.0 (1–6) | 18, 23 |
| Months between GGS HCP visits | 6.0 (3–9) | 30 |
| Years to implant exchange | 3.8 (3–5) | 31, 32 |
| Implantation | ||
| Wedding band use for TGR (% of surgeries) | 28.0 | 14 |
| Tandem connector use for TGR (% of surgeries) | 67.0 | 14 |
| Cross link use for TGR (% of surgeries) | 86.0 | 14 |
| Cross link use for MCGR (% of surgeries) | 86.0 | 14 |
| Cross link use for GGS (% of surgeries) | 100.0 | 30 |
| Partial revision (TGR, MCGR, GGS) | ||
| Pedicle screw/hook replacement (% of surgeries) | 95.0 | 14, 24a |
| Rod set screws replacement (% of surgeries) | 61.0 | 14, 24a |
| All other components (% of surgeries) | 100.0 | 14, 24a |
Note:
Clinical advisors.
Abbreviations: GGS, growth guidance system; HCP, health care professional; MCGR, magnetically controlled growing rod; TGR, traditional growing rod.
The model assumes GGS, MCGR, and TGR are of equal clinical effectiveness and that medical resource use for initial implantations, revisions, and exchanges with GGS, MCGR, and TGR is similar (with the exception of anesthesia time and device cost, where appropriate). The model also assumes that one radiograph is required per insertion, health care professional (HCP) visit (GGS), lengthening procedure (MCGR and TGR), exchange, revision, deep SSI, and final fusion; and treatment of deep SSIs will require intravenous antibiotics and a complete replacement of implants while treatment of superficial infections will require oral antibiotics. As the cost of oral antibiotics would be incurred by the patient (rather than the provider), this has not been included in the analysis; there is also no consideration of pediatric mortality. Using the average observed spinal growth in a child with EOS aged 6 years; the model estimates that all patients will require one surgery to exchange the device at 3.8 years.31,32
The components that require replacement during the course of a partial revision procedure (Table 2) were based on the TGR study by Bess et al and expert clinical advice.14,24 In the absence of such data for GGS and MCGR, these percentages have been assumed to be the same for GGS, MCGR, and TGR. Hence, during a partial removal for GGS, MCGR, or TGR, pedicle screw/hooks were assumed to require replacement in 95% of surgeries, rod set screws in 61% of surgeries, and all other components (including rods and connectors) in 100% of surgeries.
MCGR rod costs were not included for revisions due to MCGR failure within 1 year following an MCGR implantation or MCGR exchange (in the unlikely event of a manufacturing defect); all other costs for the MCGR revisions were included (for example, cross link, hospital facility costs, and professional fees).
Medicare payments were used as a proxy for provider costs (a widely accepted methodology for cost analyses).33 As such, hospital inpatient facility costs were based on Medicare diagnosis-related group (DRG) data, physician professional fees were based on current procedural terminology (CPT) data, and hospital outpatient facility costs were based on ambulatory payment classification (APC) data. As hospital inpatient DRG payments are bundled to include the TGR device cost, such inpatient procedures for GGS and MCGR had the TGR device costs subtracted and the GGS or MCGR device costs added in order to account for the differences in device costs. Table 3 details the total costs used for these procedures in the model, while the Supplementary materials detail the component costs, including all CPT, APC, and DRG codes and costs, as well as anesthesia, intraoperative neurophysiological monitoring, and radiograph codes and costs.
Table 3.
Total costs used in the model (2016 US$)
| Parameter | Base case values (sensitivity analysis [range])
|
||
|---|---|---|---|
| TGR | MCGR | GGSa | |
| Construct | 15,229 (11,421–19,036)c | 47,716 (35,787–59,645)c | 33,456b (25,092–41,820)c |
| Insertiond | 36,653 | 69,140 | 55,054 |
| Lengthening | 6,466e | 270 | NA |
| HCP visit | NA | NAf | 272 |
| Exchanged | 13,519 | 46,007 | 31,746 |
| Complete revisiond | 13,519 | 46,007 | 31,746 |
| Partial revisiond | 12,276 | 44,763 | 30,503 |
| Deep SSId | 13,519 | 46,007 | 31,746 |
| Removal and final fusion | 38,272 | 38,272 | 39,330 |
Notes:
Clinical advisors.
Medtronic Spinal & Biologics.
Construct sensitivity analysis ranges are ±25%.
Construct costs included.
Weighted mean of inpatient and outpatient procedures.
Physician professional fee is included above in MCGR lengthening in the physician office. Data from Polly et al.34
Abbreviations: GGS, growth guidance system; HCP, health care professional; MCGR, magnetically controlled growing rod; NA, not applicable; SSI, surgical site infection; TGR, traditional growing rod.
Sensitivity and scenario analyses were conducted to assess whether the cost analysis results were robust to modifications in the values of important parameters such as device failure rates, time between lengthenings, and construct costs.
Results
Base-case results
For a single patient over the 6-year episode of care, GGS was associated with fewer invasive surgeries than TGR and comparable invasive surgeries to MCGR (GGS: 3.4; MCGR: 3.4; TGR: 14.4). Simulating 1,000 patients with EOS over the 6-year episode of care, deep SSIs were substantially lower for GGS and MCGR than for TGR (GGS: 83; MCGR: 75; TGR: 652), whereas rod breakages per 1,000 patients were slightly lower for MCGR and TGR than for GGS (GGS: 436; MCGR: 406; TGR: 395).
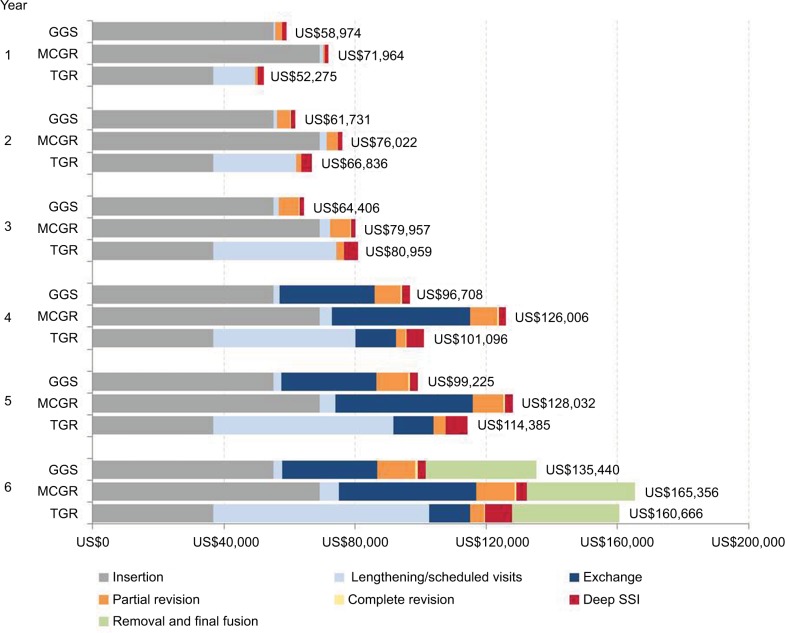
Figure 1 illustrates the cumulative costs for treatment of EOS with GGS compared with MCGR and TGR, detailing the higher cost of initial insertion and exchange (at 3.8 years) for GGS being offset by the cost of frequent TGR surgical lengthenings and associated deep SSIs. From the IDS perspective, the 6-year cumulative cost for GGS was lower than TGR, saving US$25,226.
Figure 1.
Cumulative cost per patient (2016 US$) over six-year episode of care.
Abbreviations: GGS, growth guidance system; MCGR, magnetically controlled growing rods; SSI, surgical site infection; TGR, traditional growing rods.
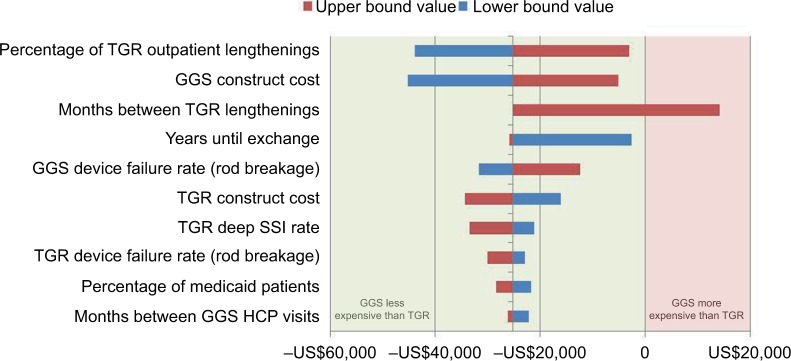
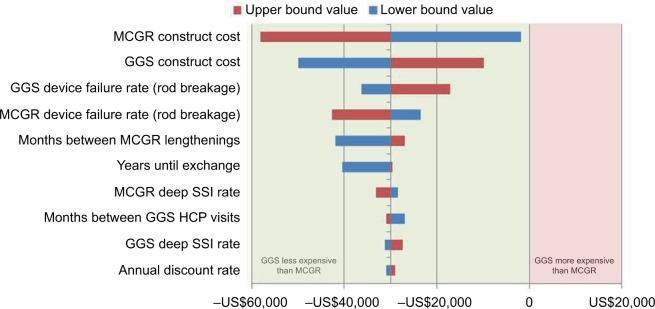
Sensitivity analysis
One-way sensitivity analyses indicated that results were sensitive to changes in construct costs, rod breakage rates, months between lengthenings (TGR and MCGR), and TGR lengthening setting of care (Figures 2 and 3). Only one parameter in the sensitivity analysis (months between lengthenings for TGR) produced a positive budget impact for GGS, suggesting that GGS is likely to be cost saving over a 6-year episode of care from the IDS perspective. Note that GGS becomes cost neutral with TGR if TGR lengthenings occur at approximately every 9 months.
Figure 2.
One-way sensitivity analysis of cumulative cost savings per patient (GGS vs TGR; 2016 US$)
Abbreviations: GGS, growth guidance system; HCP, health care professional; SSI, surgical site infection; TGR, traditional growing rods.
Figure 3.
One-way sensitivity analysis of cumulative cost savings per patient (GGS vs MCGR; 2016 US$)
Abbreviations: GGS, growth guidance system; HCP, health care professional; MCGR, magnetically controlled growing rods; SSI, surgical site infection.
Using clinically realistic scenarios, two-way sensitivity analysis for particularly impactful and less precisely known model parameters, specifically 1) GGS with TGR or MCGR device failure rates, and 2) months between GGS HCP visits with months between TGR or MCGR lengthenings, demonstrated that the cumulative costs varied by relatively little, suggesting that the economic model is robust to plausible parameter values (Tables 1 and 2 show ranges). Only when TGR lengthenings are performed at 9-month or greater intervals is there a positive budget impact, suggesting that GGS is likely to be cost saving over the 6-year episode of care.
Scenario analyses (that is, multi-way sensitivity analyses) were also run, to further assess the device failure rate (rod breakage rate) – first all the rates for all three technologies were set to 0.5493% per month, to reflect the adjusted, dual-rod rate for TGR from The National Institute for Health and Care Excellence (NICE) external assessment report and the longest follow-up for the greatest number of patients.18,23 This had a minimal impact on costs, reducing the 6-year cumulative costs for GGS and MCGR by less than 1%. The second scenario analysis set the values for TGR and MCGR to the lowest found in published literature and the GGS to the highest (GGS: 0.6053% [represents dual-rod construct]; MCGR: 0.3188% [after adjusting for 100% dual-rod construct]; TGR: 0.3905% [after adjusting for 100% dual-rod construct]). This reduced the 6-year cumulative costs for MCGR by approximately 3% and for TGR by 1%; however, GGS remained cost saving compared to both MCGR and TGR.
Discussion
Modeling is a simplified representation of the real world in an analytical framework to help decision-makers (patients, providers, and payers) compare alternative options in terms of their clinical benefit and cost. The present study addresses the growing need to demonstrate how medical technologies fit into the emerging value-based paradigm. To this end, a model was developed to evaluate the clinical-economic value of GGS compared to TGR and MCGR.
The economic model presented in this study demonstrates that the cost impact of GGS due to increased construct cost (vs TGR) and slightly higher revision rate due to device failure (vs TGR and MCGR) is offset by obviating the need for repeated surgeries to lengthen TGR (with associated deep SSIs). The reduction in costs was mainly driven by the absence of inpatient stay, anesthesia, and intraoperative neurophysiological monitoring associated with invasive TGR lengthenings. As seen in Figure 1, GGS becomes cost saving in the second year following implantation and remains so throughout the remainder of the 6-year episode of care. Hence, despite the added expense of the GGS construct compared to the TGR construct, the cost offsets for GGS from obviating the need for repeated surgical lengthenings with risk of complications appear to financially justify use of GGS.
Of note, substituting the original values from the Polly et al paper and technical appendix into this model provides very similar costs to those reported in Polly et al, only differing bŷ1.5%; while the number of deep SSIs, invasive surgeries, and device failures per thousand patients align perfectly.14 This suggests that the model employed is reproducible.
Compared to MCGR, GGS had a similar number of device failures (rod fractures) and deep SSIs; however the reduced construct cost for GGS drove cost savings at implantation and exchange as well as after a device failure or deep SSI. Previous economic analyses showed cost savings or cost neutrality for MCGR vs TGR, which could be reflective of the shorter time horizon with lack of exchange,13,16 or the less expensive single-rod construct used in 15% of patients.14 We believe that our approach is most reflective of current practice with dual-rod construct and represents a realistic 6-year time horizon, considering the average length of treatment.
As a cost analysis, rather than cost-effectiveness analysis, this model did not account for family disruption, pain, psychological distress, implications of multiple anesthetics, or compromised HRQoL associated with invasive TGR lengthenings, nor for patient anxiety surrounding the frequent MCGR lengthenings. Additionally, recent literature has reported that an increased number (eight or more) of invasive surgeries in patients with TGR is significantly correlated with an even higher rate of complications.27 There could therefore be substantial additional direct and indirect cost savings associated with the use of GGS compared to TGR. Further, the model does not include instances where the MCGR rod fails to lengthen (as reported by Choi et al in two of 54 patients), possibly underestimating costs of revision surgery; current recommendations are to reattempt lengthening at a later date and if that fails, replacing the device.11,12 Lastly, due to conflicting views on the necessity of revision for hook dislodgement and screw pull-out complications, these have not been included in the model. While revision costs may therefore be slightly underestimated, they currently only account for 9.1%, 7.5%, and 2.8% of total costs for GGS, MCGR, and TGR, respectively, and slight variations are unlikely to affect the budget impact trend of the model.
Also noteworthy, the Centers for Medicare and Medicaid Services (CMS) approved MCGR for a new technology add-on payment (NTAP) for fiscal year (FY) 2017 in the amount of US$15,750, whereby CMS provides incremental payment (in addition to the DRG payment) for technologies that qualify for NTAP.35 The NTAP payment mechanism is based on the cost to hospitals for the new technology and lasts for 2–3 years until data are available to reflect the cost of the technology in the DRG weights through recalibration. However, NTAP applies only to Medicare patients, of whom <2,000 are under 18 years, meaning that it is unlikely that a Medicare patient would be diagnosed with EOS, a disease that affects fewer than one in 10,000 people.36,37 For this reason, and the fact that CMS is proposing to discontinue NTAP for MCGR for FY 2018, we did not account for the NTAP in this cost analysis.35
Limitations
While the model parameter values were based on the most recent published literature, these reports nevertheless reflect various rod materials and diameters. This is particularly relevant for TGR, for which 3.5, 4.5, and 5.5 mm rods of steel and titanium in both single- and dual-rod constructs were reported in the NICE external assessment report.18,23 This limitation was addressed by adjusting the TGR rod fracture rate using the relative risk of rod fracture for single- vs dual-rod construct reported by Bess et al.24 Further, the data used herein for MCGR represented a mixture of both first- and second-generation devices, whereby the second generation incorporates structural and mechanism improvements intended to reduce device failures. These MCGR data also had a limited length of follow-up (mean of 19.4 months) and a slightly smaller population (54 patients) than that reported for MCGR in the NICE external assessment report (80 patients across eight studies) but was taken from a multicenter study of five centers, rather than a collection of smaller studies and had a higher proportion of dual-rod constructs better reflecting current practice.11,18,23 The relatively short follow-up compared to GGS (6 years) and TGR (4 years) may have inflated the MCGR device failure rate slightly.
Similarly, compared to the original GGS technique that used 3.5 mm rods through 2008, the current GGS technique uses larger rods, deeper screw placement, c-clamps to prevent migration in the event of rod breakage, and O-arm or other image guidance. The rod breakage rate for GGS came from a relatively small sample size (18 patients); however these data were chosen because they are the most reflective of current practice and patients were followed for six years through definitive treatment.20 While the GGS device failure rate represents a key model parameter, to which the cumulative costs are sensitive, it is important to note that the overall trend of the results (a negative budget impact for GGS), does not change in the scenario and sensitivity analysis, when these rates are varied across a clinically relevant set of values.
Conclusion
From the perspective of the US IDS, GGS can be cost saving over the 6-year episode of care by obviating the need for repeated and costly invasive TGR surgical lengthenings and their associated complications, particularly deep SSIs. Compared with MCGR, GGS can be cost saving due to a comparable rod fracture and deep SSI rate and a substantially reduced construct cost. Further analyses focusing strictly on current generation technologies and accounting for HRQoL of children and their caregivers should be considered for future research.
Acknowledgments
This study was sponsored, in part, by Medtronic Spinal & Biologics; however, they did not participate in the data analysis, interpretation of the results, or the writing of this manuscript.
Footnotes
Disclosure
SJA and EMM are consultants to Medtronic through their employment at Covance. (As salaried employees of Covance, no direct compensation was received by these authors; payments were made directly to Covance.) SJL is a consultant to Medtronic and Stryker Spine, Orthopaediatrics, and receives royalties from Lippincott and Globus. DBB has received a research grant from the North American Spine Society, receives speaking fees from Medtronic, and is consultant to Acuity Surgical. REM is a consultant to, and receives royalties from, Medtronic. The authors report no other conflicts of interest in this work.
References
- 1.Cunin V. Early-onset scoliosis: current treatment. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S109–S118. doi: 10.1016/j.otsr.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 2.El-Hawary R, Akbarnia BA. Early onset scoliosis—time for consensus. Spine Deform. 2015;3(2):105–106. doi: 10.1016/j.jspd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher ND, Bruce RW. Early onset scoliosis: current concepts and controversies. Curr Rev Musculoskelet Med. 2012;5(2):102–110. doi: 10.1007/s12178-012-9116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams BA, Matsumoto H, McCalla DJ, et al. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS) J Bone Joint Surg Am. 2014;96(16):1359–1367. doi: 10.2106/JBJS.M.00253. [DOI] [PubMed] [Google Scholar]
- 5.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90(6):1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 6.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136(1):e1–e12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung KM, Cheung JP, Samartzis D, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. 2012;379(9830):1967–1974. doi: 10.1016/S0140-6736(12)60112-3. [DOI] [PubMed] [Google Scholar]
- 8.Akbarnia BA, Cheung K, Noordeen H, Elsebaie H, Yazici M, Dannawi Z, Kabirian N. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976) 2013;38(8):665–670. doi: 10.1097/BRS.0b013e3182773560. [DOI] [PubMed] [Google Scholar]
- 9.Akbarnia BA, Mundis GM, Jr, Salari P, Yaszay B, Pawelek JB. Innovation in growing rod technique: a study of safety and efficacy of a magnetically controlled growing rod in a porcine model. Spine (Phila Pa 1976) 2012;37(13):1109–1114. doi: 10.1097/BRS.0b013e318240ff67. [DOI] [PubMed] [Google Scholar]
- 10.Akbarnia BA, Pawelek JB, Cheung KM, et al. Traditional growing rods versus magnetically controlled growing rods for the surgical treatment of early-onset scoliosis: a case-matched 2-year study. Spine Deform. 2014;2(6):493–497. doi: 10.1016/j.jspd.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Choi E, Yazsay B, Mundis G, et al. Implant complications after magnetically controlled growing rods for early onset scoliosis: a multicenter retrospective review. J Pediatr Orthop. 2017;37(8):e588–e592. doi: 10.1097/BPO.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 12.Teoh KH, Winson DM, James SH, Jones A, Howes J, Davies PR, Ahuja S. Magnetic controlled growing rods for early-onset scoliosis: a 4-year follow-up. Spine J. 2016;16(4 Suppl):S34–S9. doi: 10.1016/j.spinee.2015.12.098. [DOI] [PubMed] [Google Scholar]
- 13.Charroin C, Abelin-Genevois K, Cunin V, et al. Direct costs associated with the management of progressive early onset scoliosis: estimations based on gold standard technique or with magnetically controlled growing rods. Orthop Traumatol Surg Res. 2014;100(5):469–474. doi: 10.1016/j.otsr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Polly DW, Jr, Ackerman SJ, Schneider K, Pawelek JB, Akbarnia BA. Cost analysis of magnetically controlled growing rods compared with traditional growing rods for early-onset scoliosis in the US: an integrated health care delivery system perspective. Clinicoecon Outcomes Res. 2016;8:457–465. doi: 10.2147/CEOR.S113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolton D, Richards J, Nnadi C. Magnetic controlled growth rods versus conventional growing rod systems in the treatment of early onset scoliosis: a cost comparison. Eur Spine J. 2015;24(7):1457–1461. doi: 10.1007/s00586-014-3699-7. [DOI] [PubMed] [Google Scholar]
- 16.Su AW, Milbrandt TA, Larson AN. Magnetic expansion control system achieves cost savings compared to traditional growth rods: an economic analysis model. Spine (Phila Pa 1976) 2015;40(23):1851–1856. doi: 10.1097/BRS.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 17.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005;30(17 Suppl):S43–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 18.Jenks J, Craig J, Higgins J, et al. The MAGEC system for spinal lengthening in children with scoliosis: a NICE medical technology guidance. Appl Health Econ Health Policy. 2014;12(6):587–599. doi: 10.1007/s40258-014-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US FDA . SHILLA growth guidance system 510(k) summary K140750. US FDA; 2014. [Accessed January 19, 2018]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140750.pdf. [Google Scholar]
- 20.Luhmann SJ, Smith JC, McClung A, et al. Radiographic outcomes of Shilla growth guidance system and traditional growing rods through definitive treatment. Spine Deform. 2017;5(4):277–282. doi: 10.1016/j.jspd.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy RE, McCullough FL. Shilla growth guidance for early-onset scoliosis. J Bone Joint Surg Am. 2015;97(19):1578–1584. doi: 10.2106/JBJS.N.01083. [DOI] [PubMed] [Google Scholar]
- 22. CBO.gov [homepage on the Internet] CBO’s 2011 long-term projections for social security: additional information. Congressional Budget Office; 2011. [Accessed January 19, 2018]. [cited June 12, 2017]. Available from: https://www.cbo.gov/publication/41644. [Google Scholar]
- 23. nice.org.uk [homepage on the Internet] MAGEC system for spinal lengthening in children with early onset scoliosis. National Institute for Health and Care Excellence; 2014. [Accessed January 19, 2018]. [cited June 2017] Available from: https://www.nice.org.uk/guidance/mtg18/chapter/8-sources-of-evidence-consideredby-the-committee. [Google Scholar]
- 24.Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. Bone Joint Surg Am. 2010;92(15):2533–2543. doi: 10.2106/JBJS.I.01471. [DOI] [PubMed] [Google Scholar]
- 25. CDC.gov [homepage on the Internet] National Hospital Discharge Survey – Database enquiry 2006–2015. Centers for Disease Control and Prevention; [Accessed January 19, 2018]. [updated September 2, 2015] Available from: http://www.cdc.gov/nchs/nhds.htm. [Google Scholar]
- 26. CDC.gov [homepage on the Internet] National Hospital Ambulatory Medical Care Survey – Database enquiry 2006–2015. Centers for Disease Control and Prevention; [Accessed January 19, 2018]. [updated September 23, 2015] Available from: http://www.cdc.gov/nchs/ahcd.htm. [Google Scholar]
- 27.Kabirian N, Akbarnia BA, Pawelek JB, et al. Deep surgical site infection following 2344 growing-rod procedures for early-onset scoliosis. J Bone Joint Surg Am. 2014;96:e128–e135. doi: 10.2106/JBJS.M.00618. [DOI] [PubMed] [Google Scholar]
- 28.Luhmann SJ, McCarthy RE. A comparison of SHILLA growth guidance system and growing rods in the treatment of spinal deformity in children less than 10 years of age. J Pediatr Orthop. 2017;27(8):e567–e574. doi: 10.1097/BPO.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 29.Manoso MW, Cizik AM, Bransford RJ, et al. Medicaid status is associated with higher surgical site infection rates after spine surgery. Spine (Phila Pa 1976) 2014;39(20):1707–1713. doi: 10.1097/BRS.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medtronic Sofamor Danek USA SHILLA Growth Guidance System. Indications For Use. Jul 17, 2014. [Accessed: Febru-ary 20]. 2018. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140750.pdf.
- 31.Moe JH, Kharrat K, Winter RB, Cummine JL. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Relat Res. 1984;(185):35–45. [PubMed] [Google Scholar]
- 32.McCarthy R, Greer W, Roberson P. A comparison of rod sizes in patients treated with SHILLA growing rods for early onset scoliosis; 9th International Congress on Early Onset Scoliosis; Nov 19–20, 2015; Poster; [Accessed February 26, 2018]. p. 23. Available from: https://www.growingspine.org/User-Files/file/Poster23.ppsx?_sm_au_=iVV1NRsjKGSLSDJq. [Google Scholar]
- 33.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 34.Polly DW, Jr, Ackerman SJ, Schneider K, Pawelek JB, Akbarnia BA. Cost analysis of magnetically controlled growing rods compared with traditional growing rods for early-onset scoliosis in the US: an integrated health care delivery system perspective. Clinicoecon Outcomes Res. 2016;8:457–465. doi: 10.2147/CEOR.S113633. ecollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Medicare and Medicaid Services Prospective payment system proposed policy changes and fiscal year 2018 rates. [Accessed January 19, 2018]. Published in the Federal Register. Federal Register final rule (pages 341–342). Available from: http://www.hhs.com/assets/docs/FY-2018-IPPS-Proposed-Rule-Display-Version-4.14.17.pdf.
- 36. cms.gov [homepage on the Internet] Medicare enrollment section Centers for Medicare & Medicaid Services [updated June 21, 2017] 2015. [Accessed January 19, 2018]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatis-tics/2015/2015_Enrollment.html.
- 37.Debnath UK. Current concepts in the management of early-onset idiopathic scoliosis. Pediatr Health. 2010;4(3):343–354. [Google Scholar]