Abstract
Gram-negative bacteria have an outer membrane that serves as a barrier to noxious agents in the environment. This protective function is dependent on lipopolysaccharide, a large glycolipid located in the outer leaflet of the outer membrane. Lipopolysaccharide is synthesized at the cytoplasmic membrane and must be transported to the cell surface. To understand this transport process, we reconstituted membrane-to-membrane movement of lipopolysaccharide by incorporating purified inner and outer membrane transport complexes into separate proteoliposomes. Transport involved stable association between the inner and outer membrane proteoliposomes. Our results support a model in which lipopolysaccharide molecules are pushed one after the other in a PEZ-like manner across a protein bridge that connects the inner and outer membranes.
The cell envelope of Gram-negative bacteria consists of an outer membrane and an inner membrane. The outer membrane has an asymmetric structure with the outer leaflet composed of lipopolysaccharide (LPS) and the inner leaflet composed of phospholipid. LPS is a large glycolipid with six fatty acyl chains and numerous sugars (Fig. 1A) (1). LPS must be transported from its site of synthesis at the inner (cytoplasmic) membrane across the aqueous space between the two membranes (the periplasm) to the cell surface (2–4). Lipid transport is fundamental to cellular physiology; however, it is not known how this membrane-to-membrane LPS transport is accomplished or how transport against a concentration gradient is achieved given there is no ATP in the periplasm (5–9).
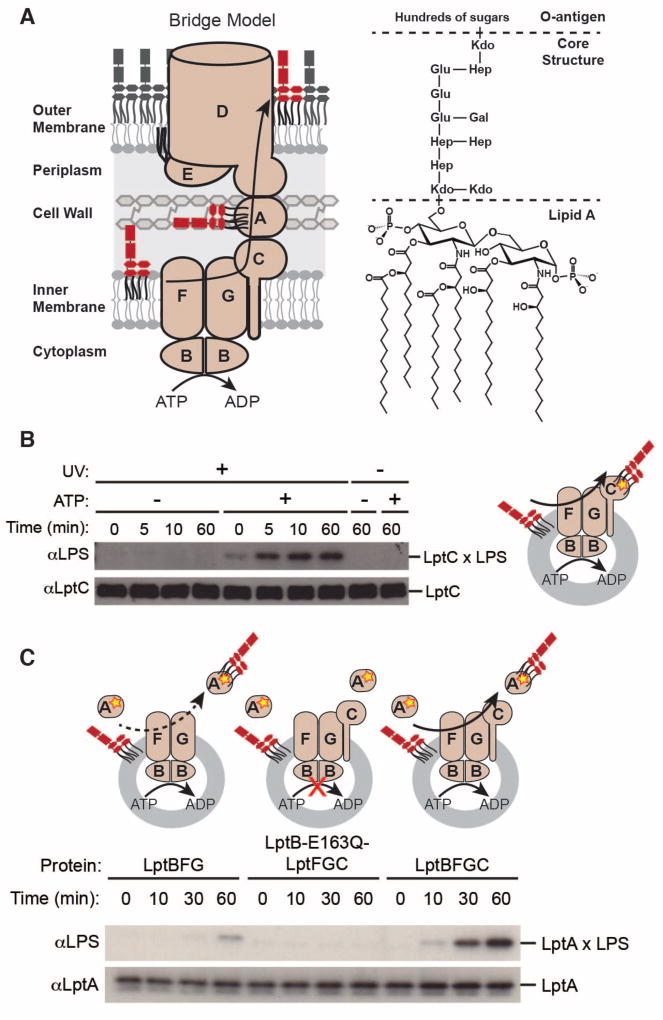
Fig 1. Energy-dependent LPS transport to LptA is stimulated by LptC.
(A) Bridge model of LPS biogenesis and chemical structure of Escherichia coli LPS. The E. coli lipopolysaccharide transport proteins and E. coli LPS were used for all experiments presented here. LptBFG extracts LPS from the inner membrane and transports it to LptC using energy from ATP hydrolysis. Additional energy from ATP hydrolysis is harnessed to push LPS from LptC to LptA. Kdo, 3-deoxy-D-manno-octo-2-ulosonic acid; Hep, L-glycero-D-manno-heptose; Glu, D-glucose; Gal, D-galactose.
(B) LPS photocrosslinks to LptC in an ATP- and time-dependent manner. Assays were initiated by adding 5 mM ATP or buffer (“- ATP”) to proteoliposomes containing LPS and LptBFGC-T47pBPA.
(C) Time-dependence of LPS release to LptA. Assays were initiated by adding 5 mM ATP to proteoliposomes containing LPS and LptBFG, LptB-E163Q-LptFGC, or LptBFGC mixed with soluble LptA-I36pBPA.
In (B) and (C), aliquots were taken at the indicated time points and UV-irradiated. Crosslinking was detected by immunoblotting. Cartoons show experimental designs of the reconstituted systems. Proteins and LPS can be inserted into liposomes in either orientation, but only the productive orientation is shown for simplicity. The yellow star denotes the photo-crosslinking amino acid.
LPS transport requires seven lipopolysaccharide transport (Lpt) proteins (10, 11). At the inner membrane, the heteromeric ATP-binding cassette transporter (LptBFG) associates with a membrane-bound protein (LptC) to form an inner membrane complex. This complex uses ATP hydrolysis to extract LPS from the outer leaflet of the inner membrane and transfer it to LptC and then to a periplasmic protein LptA (11–16). LPS is then transported across the periplasm to an outer membrane translocon, LptDE, a large β-barrel protein with a separate luminal protein plug. LptDE inserts LPS into the outer leaflet of the outer membrane (17–21). Indirect evidence suggests that LptA associates with LptC and LptD to form a protein bridge that spans the periplasm, but the existence of such a bridge has not been established (Fig. 1A) (22–25).
To investigate whether LPS is transported via a protein bridge, we sought to reconstitute LPS transport using purified components. First, to monitor LPS transfer to LptC, we purified LptBFGC with the photocrosslinkable amino acid p-benzoylphenylalanine (pBPA) incorporated in LptC at an LPS binding site (Fig. S1) (24, 26). We incorporated the complex into liposomes containing LPS and confirmed ATPase activity (Fig. S2). We incubated the proteoliposomes with ATP for various lengths of time before exposing them to UV light. We observed increasing crosslinking of LPS to LptC over time and in a manner that was dependent on ATP (Fig. 1B).
Next, we wanted to observe LPS transfer to LptA in our purified system and test whether this transfer depends on LptC. We incubated proteoliposomes containing either LptBFG or LptBFGC with LptA containing pBPA (24). We also prepared liposomes containing the inactive mutant LptB-E163Q in the LptBFGC complex (15). We observed that LPS crosslinked to LptA and the extent of crosslinking was strongly stimulated by LptC (Fig. 1C). This is consistent with earlier in vivo experiments that suggest LPS is transferred from LptC to LptA (24).
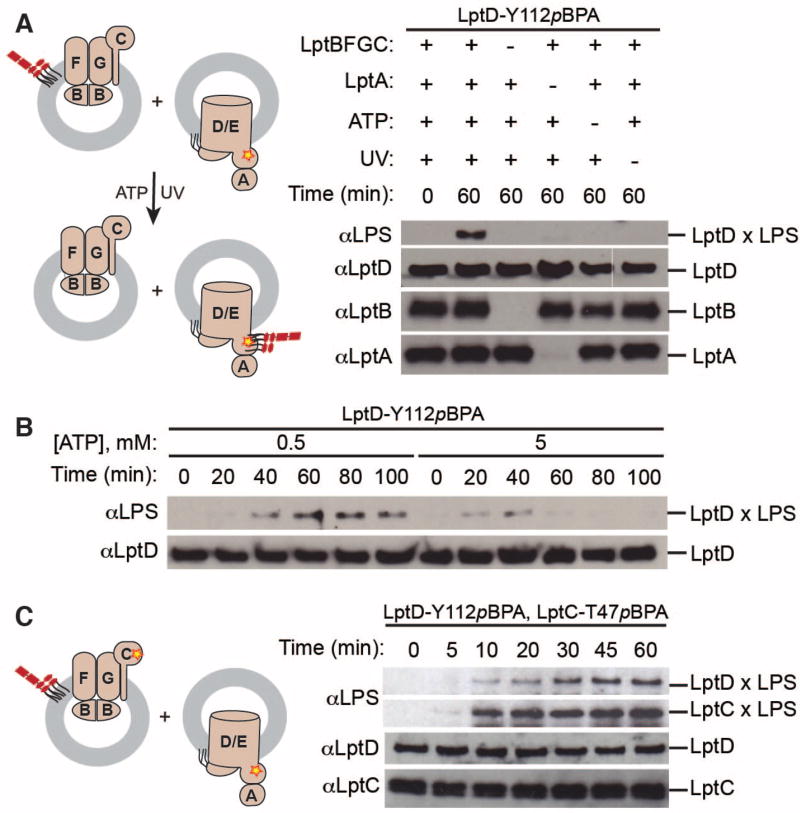
With the goal of achieving transport to a second membrane, we generated proteoliposomes containing the outer membrane LptDE translocon with pBPA incorporated in LptD (Fig. S3) (27). Previous work suggests that LptA preferentially associates with the outer membrane (22). Therefore, we pre-incubated the outer membrane proteoliposomes with excess LptA, isolated the LptA-associated outer membrane proteoliposomes, and incubated them with LptBFGC proteoliposomes containing LPS. LPS crosslinked strongly to LptD in the presence of ATP (Fig. 2A, Fig. S4). We did not observe crosslinking without LptBFGC or LptA. Thus, all seven Lpt proteins and ATP are necessary and sufficient for membrane-to-membrane transport to occur.
Fig 2. Reconstitution of membrane-to-membrane LPS transport.
(A) Seven Lpt proteins and ATP are necessary and sufficient to observe LPS crosslinking to LptD. Proteoliposomes containing LptD-Y112pBPA/LptE and associated LptA were incubated with LPS-containing liposomes with or without LptBFGC. Assays were initiated with 5 mM ATP (or buffer).
(B) LPS transport to LptD depends on time and ATP concentration. Assays were conducted as in (A), initiating with either 0.5 mM ATP or 5 mM ATP.
(C) LPS simultaneously crosslinks to LptC and LptD. Proteoliposomes containing purified LptD-Y112pBPA/LptE with LptA were incubated with proteoliposomes containing LPS and LptBFGC-T47pBPA.
In (A)–(C), aliquots were taken at the indicated time points and UV-irradiated. Crosslinking was detected by immunoblotting. Cartoons show experimental designs of the reconstituted systems. Proteins and LPS can be inserted into liposomes in either orientation, but only the productive orientation is shown for simplicity. The yellow star denotes the photo-crosslinking amino acid.
We tested if the rate of LPS transport was affected by ATP concentration using two concentrations flanking the Km for ATP hydrolysis (Fig. S2). Crosslinking intensity was greater at the low ATP concentration, and persisted for a longer period (Fig. 2B). At high ATP concentrations, the LPS likely moves more quickly through the site where pBPA is incorporated in LptD. This would decrease the probability of crosslink formation because crosslinking efficiency of a ligand to a protein increases with ligand residence time (28). These observations are consistent with a process that is powered by ATP hydrolysis.
We wanted to monitor flux of LPS through the pathway at two sites simultaneously, so we incorporated pBPA into both LptC and LptD and monitored crosslinking over time. Crosslinks between LPS and LptC were observed at the same time as crosslinks between LPS and LptD (Fig. 2C). This suggests that release from the inner membrane is coincident with arrival at LptD. Although this is not direct evidence, this result is consistent with rapid transit across a bridge.
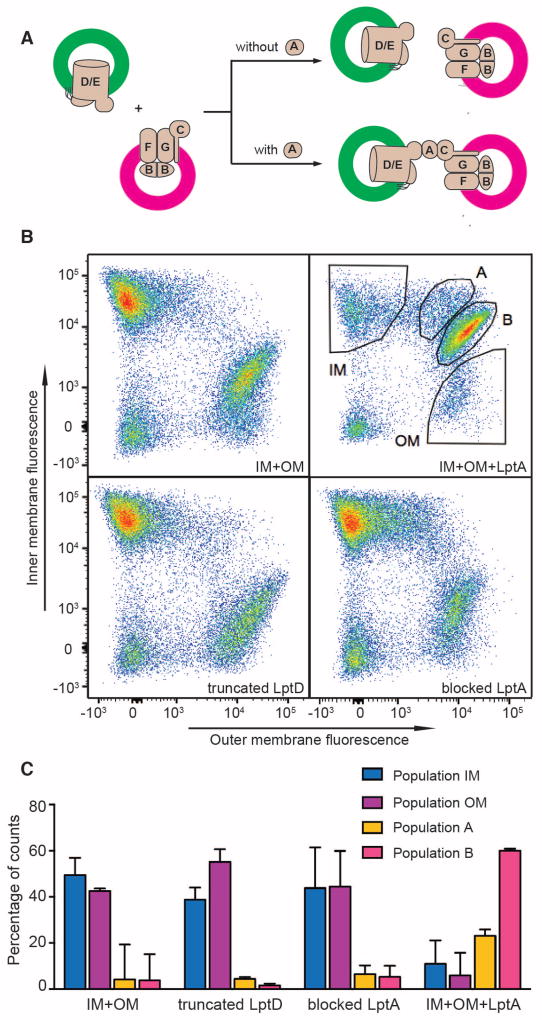
If the bridge model is correct, it should be possible to observe an LptA-dependent association between inner and outer membrane Lpt complexes. To allow analysis by flow cytometry, we labeled inner membrane and outer membrane proteoliposomes with different fluorophores and confirmed that transport activity was maintained (Fig. 3A, Fig. S5, S6, S7) (29, 30). Inner membrane or outer membrane proteoliposomes analyzed alone showed fluorescence only in a single channel, as expected (Fig. S8). Next, we mixed inner and outer membrane proteoliposomes in the presence or absence of LptA. Without LptA, flow cytometry revealed two populations of fluorescent particles corresponding to either inner or outer membrane proteoliposomes (Fig. 3B, first panel). Addition of LptA, in contrast, resulted in a large increase in particles with signal in both fluorescence channels. Most of these two-color particles localized to a distinct population (population B) located approximately along the diagonal between the individual IM and OM populations, but there was also a region of more disperse particles located between the IM population and population B, termed population A (Fig. 3B, second panel).
Fig 3. LptA induces the physical association of inner membrane (IM) and outer membrane (OM) proteoliposomes.
(A) Schematic of predicted proteoliposome states in the presence or absence of LptA. Proteoliposomes containing LptD-Y112pBPA/LptE were labeled with Atto-488 fluorophore and proteoliposomes containing LptBFGC and LPS (not shown for simplicity) were labeled with Atto-565 fluorophore.
(B) Flow cytometric analysis of reaction mixtures containing fluorescent proteoliposomes. Atto-488-labeled proteoliposomes containing LptD-Y112pBPA/LptE with or without pre-incubation with LptA were incubated with Atto-565-labeled proteoliposomes containing LptBFGC and LPS. An N-terminal truncated variant of LptD and an N-terminal blocked LptA were used in separate experiments as controls to substitute the corresponding Lpt components in the reaction mixtures. Samples were incubated as described for crosslinking experiments, initiating with buffer instead of ATP. After incubation, samples were diluted ten-fold and analyzed on a BD FACSAria flow cytometer. Equivalent particle distributions were observed in the presence of ATP.
(C) Distribution of particle counts in gated populations shown in (B). Data were normalized such that percentages of counts represent the portion of the total number of counts in all gated sub-populations. Data represent the averages and standard deviation of triplicate experiments.
We wanted to know whether the two-color particles were the result of either liposome fusion or non-specific association. A fluorescence de-quenching assay showed that fusion did not occur in our system (Fig. S9) (31). Furthermore, we repeated the flow cytometry experiments with a blocked LptA variant containing a protein tag that impedes interaction with LptC or, in a separate experiment, with a truncated LptD variant that is incapable of interaction with LptA. LptA-dependent proteoliposome association was not observed in either case (Fig. 3B, Fig. S8). In toto, these data demonstrate that LptA drives a specific, protein-mediated association between inner and outer membrane proteoliposomes.
We used our flow cytometry system to measure the extent to which LptA stimulates interactions between inner and outer membrane proteoliposomes. We quantified the number of particles in each population (IM, OM, A, and B in Fig. 3B, 3C, Table S1). In the absence of LptA, the majority of particles were in either the inner membrane (IM) population (49.5%) or the outer membrane (OM) population (42.5%), whereas the A and B populations were small (4.2% and 3.8% respectively). Addition of LptA led to a fifteen-fold increase in the particles in the B population (60%) and a five-fold increase in the A population (23.1%). Thus, LptA causes a high proportion of the proteoliposomes to associate with one another.
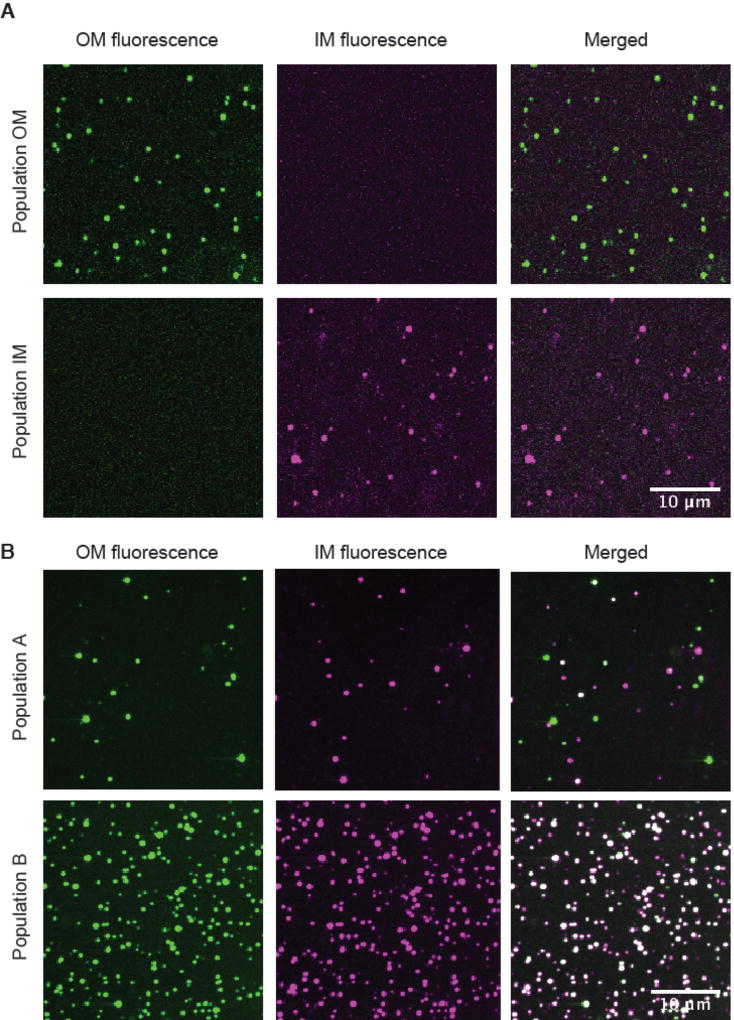
Our final objective was to visualize the LptA-dependent association of inner and outer membrane proteoliposomes using confocal microscopy. We used fluorescence-activated cell sorting (FACS) to isolate particles from the four populations observed by flow cytometry (Fig. 3B, Fig. S10). Images of the single-color populations (IM or OM) confirmed that they contained only inner or outer membrane proteoliposomes (Fig. 4A). In contrast, the B population contained both inner and outer membrane proteoliposomes with the majority colocalized (Fig. S12). This high level of colocalization persisted for at least an hour (Fig. 4B, Fig. S11). The A population also contained inner and outer membrane proteoliposomes but had a low level of colocalization (Fig. 4B). The nature of the A population is unclear, but one possibility is that this population contains proteoliposomes that formed unstable complexes that dissociated following flow cytometry. The bridge and chaperone models of transport can be differentiated by lifetime of the complexes. The LptA-induced association of inner and outer membrane proteoliposomes observed in the B population is not transient which provides clear evidence for the protein bridge. Importantly, these long-lived complexes remain functional. Crosslinks between LptD and LPS were observed when ATP was added to the sorted B population (Fig. S13).
Fig 4. Observation of a long-lived, protein-mediated bridge using confocal microscopy.
(A) Representative confocal microscope images of Population IM and Population OM sorted particles. Atto-488-labeled proteoliposomes containing LptD with associated LptA were incubated with Atto-565-labeled proteoliposomes containing LptBFGC and LPS and were sorted by gating based on fluorescence thresholds using a BD FACSAria flow cytometer, and imaged at 100× magnification. Scale bar: 10 µm.
(B) Representative confocal microscope images of Population A and Population. Atto-488-labeled proteoliposomes containing with associated LptA were incubated with Atto-565-labeled proteoliposomes containing LptBFGC and LPS and sorted by gating based on fluorescence thresholds using a BD FACSAria flow cytometer, and imaged at 100× magnification. Scale bar: 10 µm.
Our biochemical and flow cytometry experiments show that transport of LPS is mediated by a protein bridge. Based on the dimensions of the periplasm, one or two LptAs would be sufficient, together with LptC and the periplasmic domain of LptD, to span the necessary distance. It is unclear whether there is a fixed stoichiometry of LptA in cells, but LptA is synthesized in cells at the same rate as the membrane components of the Lpt pathway (32).
A longstanding question in the field is how energy generated in the cytoplasm can be harnessed to drive LPS molecules to the cell surface against a concentration gradient. The existence of a bridge provides a possible explanation. In our model, LptBFGC extracts LPS molecules from the inner membrane and loads them into the bridge. Each new LPS molecule then pushes molecules already in the bridge towards the outer membrane. This is similar to the action of a PEZ dispenser, in which candies at the top of a stack are pushed out of the top of the dispenser by a spring acting at the base. The PEZ model allows for efficient transport of LPS because movement along a single dimension is faster than movement involving a diffusible chaperone. Millions of LPS molecules must be transported during each round of cell division, and movement along a bridge makes this possible (1).
Supplementary Material
Acknowledgments
The plasmid pSup-BpaRS-6TRN was a generous gift from Professor Peter G. Schultz (The Scripps Research Institute). We thank Dr. Aaron Garner and Dr. Goran Malojčić for preparation of pET23/42-C-LptD-His, Dr. Shinichi Matsuyama (Rikkyo University) for preparation of rabbit anti-LptD, and the Bauer Core Facility at Harvard University for assistance with flow cytometry experiments. Funding: This work was supported by a National Science Foundation Graduate Research Fellowship (to D.J.S. and R.J.T.), the Kavli Institute for Bionano Science and Technology at Harvard University, the Blavatnik Biomedical Accelerator at Harvard University, National Science Foundation grant DMR-0820484 (to D.J.N.), the National Institute of Allergy and Infectious Diseases award numbers AI081059 and AI109764 (to D.K.) and the National Institute of General Medical Sciences award number GM066174 (to D.K.).
Footnotes
Author contributions: D.K., D.J.S., and S.O. conceptualized the pure reconstitution and developed the methodology; D.J.S., S.O., R.X., R.J.T, and A.H.G. performed validation and investigation for the reconstitution. D.K., R.X., R.J.T., and A.H.G. conceptualized flow cytometry of the proteoliposomes. R.X., R.J.T., and A.H.G. developed the flow cytometry methodology, performed validation and investigations. D.K., D.J.N., R.X., R.J.T., and A.H.G. conceptualized the confocal microscopy. R.X., R.J.T., A.H.G., and P.J.F. developed the confocal microscopy methodology, performed validation and investigations. Resources were provided by D.K. and D.J.N. D.K., R.X., R.J.T., D.J.S., and A.H.G. wrote the manuscript and all authors contributed edits. D.K. and D.J.N administered the project and acquired funding.
Competing interests: The authors declare no competing interests.
Data and materials availability: The code used to analyze proteoliposome colocalization is freely available at https://github.com/peterjfoster/Proteoliposome_colocalization. All other data are available in the manuscript or the supplementary materials.
References
- 1.Whitfield C, Trent MS. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annual Review of Biochemistry. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 2.Osborn MJ, Gander JE, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 3.Mulradt PF, Golecki JR. Asymmetrical Distribution and Artifactual Reorientation of Lipopolysaccharide in the Outer Membrane Bilayer of Salmonella typhimurium. The FEBS Journal. 1975;51:343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 4.Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976;15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- 5.Osborn MJ, Gander JE, Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J. Biol. Chem. 1972;247:3973–3986. [PubMed] [Google Scholar]
- 6.Saheki Y, De Camilli P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annual Review of Biochemistry. 2017;86:659–684. doi: 10.1146/annurev-biochem-061516-044932. [DOI] [PubMed] [Google Scholar]
- 7.Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornmann B, Walter P. ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. Journal of Cell Science. 2010;123:1389–1393. doi: 10.1242/jcs.058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murley A, Nunnari J. The Emerging Network of Mitochondria-Organelle Contacts. Molecular Cell. 2016;61:648–653. doi: 10.1016/j.molcel.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nature Reviews Microbiology. 2016;14:337–345. doi: 10.1038/nrmicro.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandeo P, et al. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J. Bacteriol. 2008;190:4460–4469. doi: 10.1128/JB.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperandeo P, et al. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J. Bacteriol. 2007;189:244–253. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5537–5542. doi: 10.1073/pnas.0801196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narita S-I, Tokuda H. Biochemical characterization of an ABC transporter LptBFGC complex required for the outer membrane sorting of lipopolysaccharides. FEBS Letters. 2009;583:2160–2164. doi: 10.1016/j.febslet.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Sherman DJ, et al. Decoupling catalytic activity from biological function of the ATPase that powers lipopolysaccharide transport. Proc. Natl. Acad. Sci. U.S.A. 2014;111:4982–4987. doi: 10.1073/pnas.1323516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran AX, Dong C, Whitfield C. Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli. J. Biol. Chem. 2010;285:33529–33539. doi: 10.1074/jbc.M110.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao S, Luo Q, Zhao Y, Zhang XC, Huang Y. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature. 2014;511:108–111. doi: 10.1038/nature13484. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, et al. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11754–11759. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chng S-S, Ruiz N, Chimalakonda G, Silhavy TJ, Kahne D. Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5363–5368. doi: 10.1073/pnas.0912872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freinkman E, Chng S-S, Kahne D. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2486–2491. doi: 10.1073/pnas.1015617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, et al. Structural basis for outer membrane lipopolysaccharide insertion. Nature. 2014;511:52–56. doi: 10.1038/nature13464. [DOI] [PubMed] [Google Scholar]
- 22.Chng S-S, Gronenberg LS, Kahne D. Proteins Required for Lipopolysaccharide Assembly in Escherichia coli Form a Transenvelope Complex. Biochemistry. 2010;49:4565–4567. doi: 10.1021/bi100493e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freinkman E, Okuda S, Ruiz N, Kahne D. Regulated Assembly of the Transenvelope Protein Complex Required for Lipopolysaccharide Export. Biochemistry. 2012;51:4800–4806. doi: 10.1021/bi300592c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda S, Freinkman E, Kahne D. Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli. Science. 2012;338:1214–1217. doi: 10.1126/science.1228984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tefsen B, Geurtsen J, Beckers F, Tommassen J, de Cock H. Lipopolysaccharide transport to the bacterial outer membrane in spheroplasts. J. Biol. Chem. 2005;280:4504–4509. doi: 10.1074/jbc.M409259200. [DOI] [PubMed] [Google Scholar]
- 26.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Gu Y, Dong H, Wang W, Dong C. Trapped lipopolysaccharide and LptD intermediates reveal lipopolysaccharide translocation steps across the Escherichia coli outer membrane. Sci Rep. 2015;5:11883. doi: 10.1038/srep11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dormán G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 29.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J. Immunol. Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 30.Groot Kormelink T, et al. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A. 2016;89:135–147. doi: 10.1002/cyto.a.22644. [DOI] [PubMed] [Google Scholar]
- 31.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 32.Li G-W, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu Y, Schultz PG. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli. Nature Methods. 2006;3:263–265. doi: 10.1038/nmeth864. [DOI] [PubMed] [Google Scholar]
- 35.Brundage L, Hendrick JP, Schiebel E, Driessen AJ, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 36.Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2000;2:212–218. doi: 10.1038/35008635. [DOI] [PubMed] [Google Scholar]
- 37.Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal. Biochem. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 38.Crocker JC, Grier DG. Methods of Digital Video Microscopy for Colloidal Studies. Journal of Colloid and Interface Science. 1996;179:298–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.