Abstract
Shift work is a risk factor for inflammation, hypertension and cardiovascular disease. This increased risk cannot be fully explained by classic risk factors. Shift workers’ behavioral and environmental cycles are typically misaligned relative to their endogenous circadian system. However, there is little information on the impact of acute circadian misalignment on cardiovascular disease risk in shift workers, independent of differences in work stress, food quality and other factors that are likely to differ between night and day shifts. Thus, our objectives were to determine the independent effect of circadian misalignment on 24-h high-sensitivity C-reactive protein (hs-CRP; a marker of systemic inflammation) and blood pressure levels—cardiovascular disease risk factors—in chronic shift workers. Chronic shift workers undertook two 3-day laboratory protocols that simulated night work comprised of 12-hour inverted behavioral and environmental cycles (circadian misalignment) or simulated day work (circadian alignment), using a randomized, crossover design. Circadian misalignment increased 24-h hs-CRP by 11% (P<0.0001). Circadian misalignment increased 24-h systolic blood pressure (SBP) and diastolic blood pressure (DBP) by 1.4 mmHg and 0.8 mmHg, respectively (both P≤0.038). The misalignment-mediated increase in 24-h SBP was primarily explained by an increase in SBP during the wake period (+1.7 mmHg; P=0.017), whereas the misalignment-mediated increase in 24-h DBP was primarily explained by an increase in DBP during the sleep opportunity (+1.8 mmHg; P=0.005). Circadian misalignment per se increases hs-CRP and blood pressure in shift workers. This may help explain the increased inflammation, hypertension and cardiovascular disease risk in shift workers.
Keywords: circadian misalignment, hypertension, inflammatory markers, night work, shift work
Introduction
Fifteen percent of the US workforce undertakes shift work (Bureau of Labor Statistics, 2005). Shift work is a risk factor for inflammation, elevated blood pressure, hypertension and cardiovascular disease, even after controlling for traditional risk factors (Morikawa et al., 1999; Sakata et al., 2003; Suwazono et al., 2008; Lieu et al., 2012; Vyas et al., 2012). Shift workers frequently undergo circadian misalignment (i.e., misalignment between the endogenous circadian system and 24-h environmental/behavioral cycles). This misalignment has been proposed to explain, in part, why shift work has been reported to be a risk factor for inflammation, elevated blood pressure, hypertension and cardiovascular disease (Morris et al., 2012b).
Humans—as well as most other life on Earth—possess an endogenous circadian system that optimally synchronizes physiology and behavior to the solar day (for a review, see Morris et al., 2012a). The mammalian endogenous circadian system consists of the suprachiasmatic nucleus in the hypothalamus and circadian oscillators in virtually all peripheral tissues and organs (Morris et al., 2012a). At the molecular level, transcriptional-translation feedback loops are involved in generating intrinsic circadian rhythms (Morris et al., 2012a). Inflammatory markers strongly predict cardiovascular disease risk and have been shown to be elevated in shift workers compared to day workers (Libby, 2006; Sookoian et al., 2007; Burgueno et al., 2010; Puttonen et al., 2011). We and others have shown that circadian misalignment increases levels of numerous inflammatory markers including high-sensitivity C-reactive protein (hs-CRP; a marker of systemic inflammation), tumor necrosis factor (TNF)-α (pro-inflammatory), interleukin (IL)-6 (pro- and anti-inflammatory), IL-10 (anti-inflammatory) and resistin (pro-inflammatory) in non-shift workers (Leproult et al., 2014; Wright et al., 2015; Morris et al., 2016a). Moreover, we have also shown that circadian misalignment increases blood pressure in non-shift workers (Scheer et al., 2009; Morris et al., 2016a). Whether circadian misalignment also influences inflammatory markers and blood pressure in chronic shift workers is unknown.
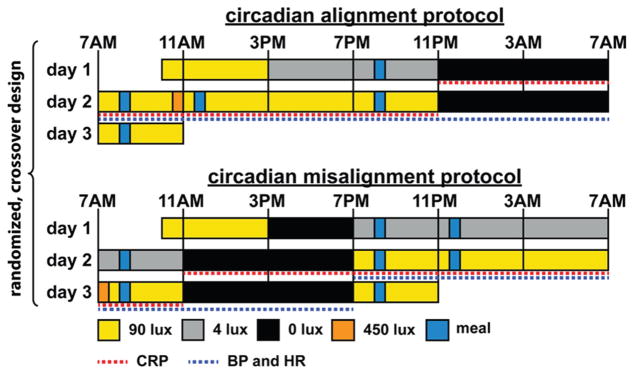
Thus we tested the impact of circadian misalignment, similar to that experienced by real-life shift workers, on 24-h levels of hs-CRP and blood pressure, which are risk factors for cardiovascular disease (Stamler et al., 1993; Libby, 2006; Kaptoge et al., 2010). To do so, we measured—using a randomized, within-participant, crossover design—hs-CRP and blood pressure levels across the 24-h behavioral and light/dark cycles when the behavioral and environmental cycle (including sleep/wake, fasting/feeding, rest/activity, and dark/light cycles) was aligned and misaligned (following a rapid 12-h shift of the behavioral cycle) with the endogenous circadian system (Figure 1).
Figure 1. Circadian alignment protocol (top panel) and circadian misalignment protocol (bottom panel).
On day 1 of both protocols, participants received an ad libitum lunch at approximately 12PM. Light levels indicated are in the horizontal angle of gaze: ~90 lux, to simulate typical room light intensity, 30-min periods of ~450 lux to simulate the morning commute both preceding the day work shift (circadian alignment protocol) and following the night work shift (circadian misalignment protocol), ~4 lux to permit assessment of the dim-light melatonin levels, 0 lux during scheduled sleep episodes. Light blue bars represent meals.
Methods
Other aspects of this study—which was designed to test separate hypotheses—have previously been published (Morris et al., 2016b).
Experimental design
Each participant underwent two 3-day laboratory protocols, according to a crossover design, to test the effect of circadian misalignment on hs-CRP and blood pressure levels (Figure 1). One protocol included a simulated day shift (circadian alignment protocol) and the other a simulated night shift (circadian misalignment protocol). The visits were separated by three to eight weeks (mean±SD: 5±2 weeks). Minimization, a type of adaptive stratified sampling, was used to minimize imbalance—according to age, gender and body mass index (BMI)—in the order of laboratory visits (five participants undertook the circadian alignment protocol first and four participants undertook the circadian misalignment protocol first) (Altman and Bland, 2005). That our circadian misalignment protocol caused circadian misalignment between the central circadian pacemaker and the 24-h environmental and behavioral cycles was confirmed by dim light melatonin data (a phase marker of the central circadian pacemaker), as published before (Morris et al., 2016b).
Participants
Nine, healthy, non-smoking, drug- and medication-free (except for oral contraceptives) shift workers completed this study (mean±SD [range] age, 34±8 y [24–48 y]; BMI, 24.2±3.4 kg/m2 [19.3–29.3 kg/m2]; 3 males). Health status of participants was established by medical history, physical examination, electrocardiography, standard blood and urine analysis and interview by a clinical psychologist. All participants were currently employed shift workers (≥12 months of consecutive shift work), who had ≥5 night shifts per month (mean±SD [range], 12±3 night shifts/month [6–18 night shifts/month]; a night shift was defined as at least 6 h of work between 10PM and 8AM, with no shift duration >12 h). Participants’ mean±SD consecutive shift work experience was 4.5±7.7 y (range, 1.1–25.1 y). Participants’ mean±SD lifetime cumulative shift work experience was 5.3±7.7 y (range, 1.1–25.1 y). Two participants were rotating shift workers and seven participants were permanent night shift workers. Eight participants worked in healthcare (4 registered nurses, 1 patient care assistant, 1 certified nursing assistant, 1 emergency department transporter, and 1 medical dispatcher) and 1 participant was a network engineer. Participants’ cumulative nap duration—measured by self-report before study enrollment and actigraphy before each laboratory visit (see below for details)—whilst working a single night shift was <1 h. Participants were excluded if they maintained their night shift sleep/wake schedule (i.e., sleeping during the day and awake at night) whilst working day shifts and/or on days off from work. The Partners Human Research Committee approved this research, which was conducted in the Center for Clinical Investigation (CCI) at Brigham and Women’s Hospital (Boston, MA). This study was performed in accordance with the declaration of Helsinki. All participants provided written informed consent.
As previously reported (Morris et al., 2016b), two participants’ dim light melatonin onset (DLMOn) or dim light melatonin offset (DLMOff) showed a phase difference >4 h between the alignment and misalignment protocols. These two participants (one was a registered nurse and the other was a patient care assistant, both were females aged 24 y with a BMI of 19.3 or 27.5 kg/m2) were excluded from all subsequent data analysis because they had an unstable timing of their central circadian clock that would otherwise have interfered with assessing the effect of the scheduled circadian alignment versus misalignment. For the remaining 7 participants (mean±SD [range] age, 37±7 y [30–48 y]; BMI, 24.4±3.1 kg/m2 [21.0–29.3 kg/m2]; night work frequency, 12±4 night shifts/month [6–18 night shifts/month]; consecutive shift work experience, 5.3±8.8 y [1.3–25.1 y]; lifetime cumulative shift work experience, 6.3±8.5 y [1.5–25.1 y]; 2 rotating shift workers and 5 permanent night workers; 3 males), there was no significant difference in DLMOn between alignment conditions (mean±SD [range], −0.80±0.47 h [−2.82 to +0.80 h]; P=0.14, paired samples t-test). On day 1 of the alignment protocol in dim light, mean±SD (range) DLMOn was 8:11PM±54 min (6:34PM to 1:22AM) and on day 1 of the misalignment protocol in dim light, mean±SD (range) DLMOn was 7:23PM±34 min (5:54PM to 22:33PM).
Pre-inpatient study conditions
For ≥14 days (mean±SD: 19±5 days) before each laboratory visit, participants wore an Actiwatch Spectrum (Philips-Respironics, Murrysville PA), recorded their bedtimes, wake times and work schedules in a diary, and reported the information to a time-stamped voicemail system. Participants were instructed to sleep between 11PM and 7AM on the night preceding each inpatient admission to reduce possible sleep debt before entering the laboratory.
Inpatient study conditions
On the first day of each 3-day laboratory protocol, participants were admitted to the CCI at approximately 10:00AM to undertake either the circadian alignment protocol or circadian misalignment protocol, in a crossover design (Figure 1). Participants remained in a private laboratory room throughout each laboratory protocol to allow strict control of environmental conditions. Participants were not permitted to exercise while in the laboratory. In the circadian alignment protocol, participants’ sleep opportunity occurred from 11PM until 7AM for days 1–3. In the circadian misalignment protocol, on day 1 the participants’ sleep/wake cycle was inverted by 12 h by including an 8-h wake episode (beginning at the scheduled time of awakening of 7AM on day 1 at home) plus a 4-h sleep opportunity between 3PM and 7PM. The subjects then stayed awake for 16 h until their next 8-h sleep opportunity that occurred from 11AM until 7PM. This sleep/wake cycle was maintained until the end of the protocol (day 3). In each protocol, participants completed standardized, scheduled computerized cognitive tests in order to simulate work conditions during wake periods. In both conditions, on ‘test days’ (wake period 2 in the alignment protocol and wake period 3 in the misalignment protocol), computerized cognitive testing began 55 min following schedule wake time, and continued at approximately hourly intervals until approximately 15 h following scheduled wake time. Light levels during both protocols are shown in Figure 1.
Diet
Participants were given an ad libitum lunch around noon on the first day of each laboratory protocol, after which they received an isocaloric calculated diet throughout the rest of the protocol, calculated according to the Harris-Benedict equation with an activity factor of 1.4. The diet consisted of 45–50% carbohydrate, 30–35% fat, and 15–20% protein, 150 mEq Na+ (±20%), 100 mEq K+ (±20%), and at least 2.5 liters of water per 24 h. Participants were instructed to consume all food provided (verified by checking their food trays). Because the circadian misalignment protocol was 12 hours longer (to enable a 12 h slam shift of the behavioral cycle) than the circadian alignment protocol, participants were given additional prorated food and water in the circadian misalignment protocol (50% of calculated 24 h calorie and water requirements). This additional food and water was distributed across breakfast (8:00 PM) and lunch (11:30 PM) on day 1 (wake period 2) of the circadian misalignment protocol. Other than the abovementioned additional food and water, the diet was identical within each participant between laboratory visits. In each protocol, following the final sleep opportunity (day 3), participants consumed an ad libitum breakfast before leaving the laboratory.
Blood sampling for hs-CRP
Two intravenous cannulae (one serving as a back-up) were placed between 12:35PM and 4:35PM on wake period 1 in the circadian alignment protocol and between 12:35PM and 2:35PM on wake period 1 in the circadian misalignment protocol. Blood samples were drawn every 4 h for hs-CRP assessment, starting shortly after bedtime in the circadian alignment protocol (sleep opportunity 1 and wake period 2) and in the circadian misalignment protocol (sleep opportunity 2 and wake period 3). Serum hs-CRP levels were measured by turbidimetry (Roche Diagnostics, Indianapolis, IN) with a dynamic range of 0.1 to 20 mg/l, an intra-assay coefficient of variation (CV) of 0.6 to 1.3%, and an inter-assay CV of 2.2 to 3.5%.
Blood pressure and heart rate
Twenty-four-hour blood pressure and heart rate measurements started shortly after scheduled wake time until scheduled wake time 24 h later, i.e., between 7AM–7AM in the circadian alignment protocol (wake and sleep period 2) and between 7PM–7PM in the circadian misalignment protocol (wake and sleep period 3). Measurements were obtained with a Spacelabs 90217 ambulatory blood pressure monitor (Spacelabs Medical, Inc., Snoqualmie, WA), validated according to the Association for the Advancement of Medical Instrumentation’s standards (Baumgart and Kamp, 1998). Sleep opportunity-associated blood pressure and heart rate dipping were calculated as the decrease from the wake period to the sleep opportunity as a percentage of the wake period.
Heart rate variability
For assessment of heart rate variability, three-lead electrocardiogram was recorded on a Vitaport (TEMEC Instruments, Kerkrade, Netherlands) between 10:40PM–10:35PM in the circadian alignment protocol (sleep opportunity 1 and wake period 2) and 10:40AM–10:35AM in the circadian misalignment protocol (sleep opportunity 2 and wake period 3). Participants underwent 7 min of voluntary paced breathing (12 breaths/min) at 10:20AM and 10:20PM in the circadian alignment protocol (wake period 2) and at 10:20PM and 10:20AM in the circadian misalignment protocol (wake period 3). For the analysis of heart rate variability, cardiac inter-beat intervals were obtained by using a QRS wave detector based on amplitude and first derivative of electrocardiogram waveform and verified by a trained technician to ensure that only “normal-to-normal” R waves times were included (Fraden and Neuman, 1980). Power spectra were calculated by fast Fourier transform of 3.41-Hz cubic spline re-sampled data across time windows with at least two, 2.5 min periods of stable signal. Root mean square differences of consecutive heartbeat intervals (RMSSD) and percentage of consecutive heartbeat intervals differing by 20 and 50 ms (pNN20 and pNN50) were obtained as cardiac vagal markers. We focused on pNN20 in addition to the classical pNN50 because pNN20 is superior for distinguishing between sleep/wake states and groups such as young vs. old people and healthy individuals vs. congestive heart failure patients (Mietus et al., 2002). Technical difficulties precluded heart rate variability assessments in 1 participant.
Polysomnography and actigraphy
Sleep was recorded by polysomnography (PSG; Vitaport; TMEC Instruments, Kerkrade, Netherlands)—in accordance with the American Academy of Sleep Medicine recommendations (Iber et al., 2007)—during sleep opportunity 1 in the circadian alignment protocol and during sleep opportunity 2 in the circadian misalignment protocol, as detailed previously (Morris et al., 2016b). PSG recordings, included electroencephalography (EEG), left and right electrooculography (EOG), bipolar submental electromyography (EMG), and bipolar electrocardiography (ECG). EEG was recorded from F3, C3, and O1 referenced to M2, and from F4, C4, and O2 referenced to M1. Digitized EEG, EOG, EMG, and ECG data were inspected using Vitascore software (TEMEC Instruments, Kerkrade, Netherlands). PSG recordings were scored visually in 30-s epochs according to the conventional criteria of the American Academy of Sleep Medicine by one registered polysomnographic technologist (Iber et al., 2007).
We used actigraphy data to estimate total sleep time (TST) for sleep periods in which PSG assessments did not occur. Participants wore the Actiwatch Spectrum (Philips-Respironics, Murrysville, PA) device on their non-dominant wrist with integrated activity data recorded at 1-min intervals. Automatic sleep/wake scoring was performed with Actiwatch software (Actiware version 5.71.0, Philips-Respironics, Murrysville, PA; sensitivity set at medium) between lights-off and lights-on. Total sleep time was objectively estimated by this analysis. Technical difficulties precluded actigraphic estimates of TST in 1 participant.
Statistics
We tested the fixed effects of condition (circadian misalignment vs. circadian alignment), time into the behavioral cycle, and their interaction on hs-CRP, blood pressure and heart rate (separate models for 24-h, sleep and wake blood pressure and heart rate data) levels and cardiac vagal markers by using linear mixed models (random intercepts for participants, time into the behavioral cycle and condition as categorical fixed factors). We also tested the effect of condition on the sleep opportunity-associated dipping in blood pressure and heart rate and actigraphic estimates of total sleep duration by employing linear mixed models. Alignment condition effects on hs-CRP 24-h rhythms were also assessed with a linear mixed model cosine analysis, with condition (aligned vs. misaligned) and a 24-h cosine curve according to time since scheduled awakening included as fixed factors. This analysis was also performed separately for each condition (i.e., without an alignment condition fixed factor) to test whether significant 24-h hs-CRP rhythms were present in each condition. Correlations were tested by Spearman’s rank correlation coefficients. Where appropriate, Bonferroni-adjusted multiple comparisons were performed. Where necessary, analysis was performed on transformed values (logged or within-participant percent mean data). The above statistical procedures were performed using IBM SPSS version 21 (IBM Corporation, Armonk, NY). For hs-CRP, values >2.5 SDs from the mean within each participant were excluded from analysis in order to remove spurious outliers—this resulted in the total exclusion of 2.4% (2 data points) of hs-CRP values. Statistical significance was accepted as P<0.05. Data are presented as mean±SEM, unless otherwise indicated.
Results
Circadian misalignment increased hs-CRP (Figure 2 and Supplemental Figure 1)
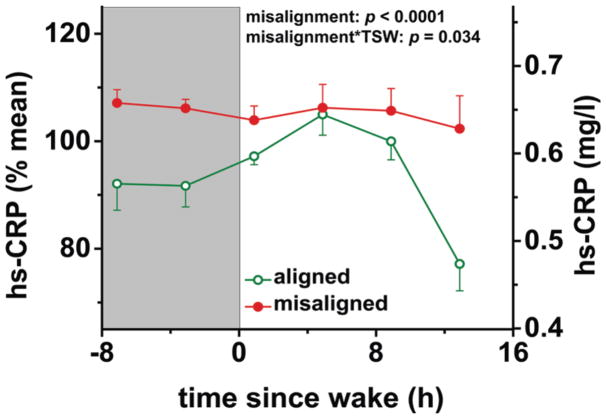
Figure 2. Effects of circadian misalignment on high-sensitivity C-reactive protein levels.
hs-CRP, high-sensitivity C-reactive protein; TSW, time since wake; grey bar, sleep opportunity. Data are represented as mean±SEM.
Circadian misalignment, as compared to circadian alignment, increased 24-h hs-CRP by 11% (P<0.0001). This effect was dependent on time into the behavioral cycle (P=0.034), reflecting that hs-CRP was higher in the misalignment than alignment condition during the entire sleep opportunity and at the end of the wake period (all P≤0.012). Cosine analysis also indicated that circadian misalignment increased 24-h hs-CRP levels (P<0.0001). Cosine analysis revealed an interaction between alignment condition and the 24-h rhythm for hs-CRP (P=0.013); in the alignment condition the 24-h peak-to-trough difference in hs-CRP was 19% (P=0.001) whereas in the misalignment condition there was no significant 24-h rhythm for hs-CRP (P=0.79).
Circadian misalignment increased blood pressure (Figures 3 and 4)
Figure 3. Effects of circadian misalignment on blood pressure and heart rate levels.
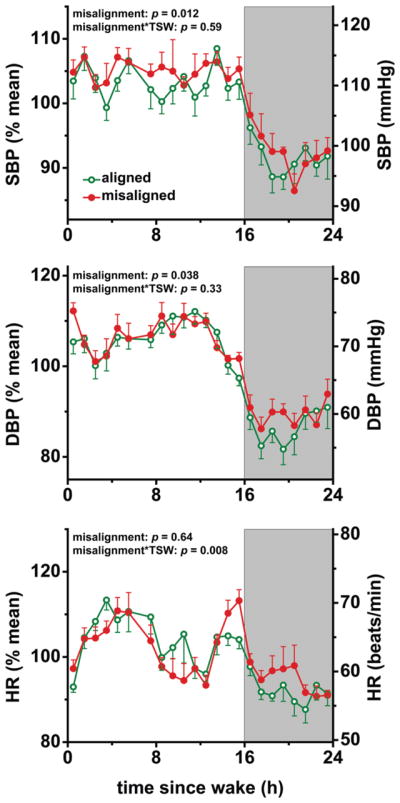
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; TSW, time since wake; grey bar, sleep opportunity. Probability values from 24-h analyses. Data are represented as mean±SEM.
Figure 4. Effects of circadian misalignment on sleep opportunity-associated dipping in blood pressure and heart rate.
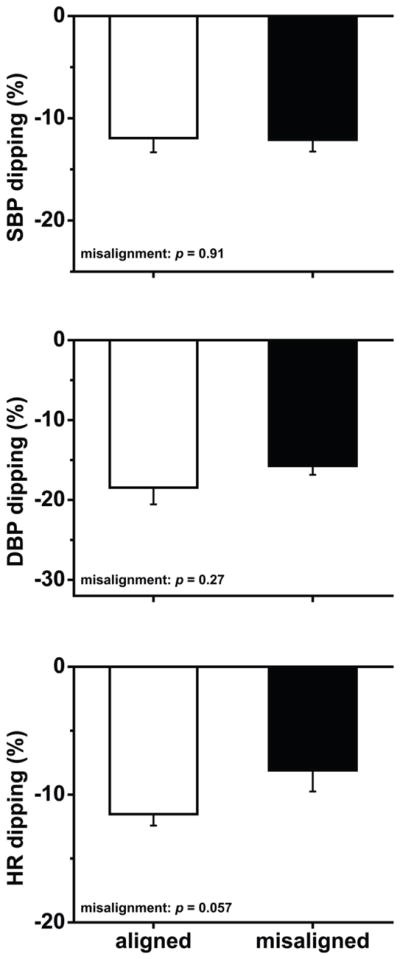
Dipping was calculated as the decrease from the wake period to the sleep opportunity as a percentage of the wake period. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate. Data are represented as mean±SEM.
Circadian misalignment increased 24-h systolic blood pressure (SBP) by 1.4 mmHg and 24-h diastolic blood pressure (DBP) by 0.8 mmHg (both P≤0.038). The 24-h SBP difference seemed primarily explained by circadian misalignment increasing SBP during the wake period by 1.7 mmHg (P=0.017), because circadian misalignment had no significant effect on sleep opportunity SBP (P=0.19). The 24-h DBP difference appeared primarily explained by circadian misalignment increasing DBP during the sleep opportunity by 1.8 mmHg (P=0.005), because circadian misalignment had no significant effect on wake period DBP (P=0.94). Circadian misalignment had no significant effect on sleep opportunity-associated SBP or DBP dipping (both P≥0.27).
Circadian misalignment increased sleep opportunity heart rate (Figure 3 and 4)
There was no significant effect of circadian misalignment on 24-h heart rate or wake period heart rate (both P≥0.17). Circadian misalignment increased sleep opportunity heart rate by 1.8 beats/min (P=0.029). We found no significant circadian misalignment effect on sleep opportunity-associated heart rate dipping (P=0.057).
No effect of circadian misalignment on markers of cardiac vagal modulation (Figure 5)
Figure 5. Effects of circadian misalignment on wake-period cardiac vagal modulation (n=6).
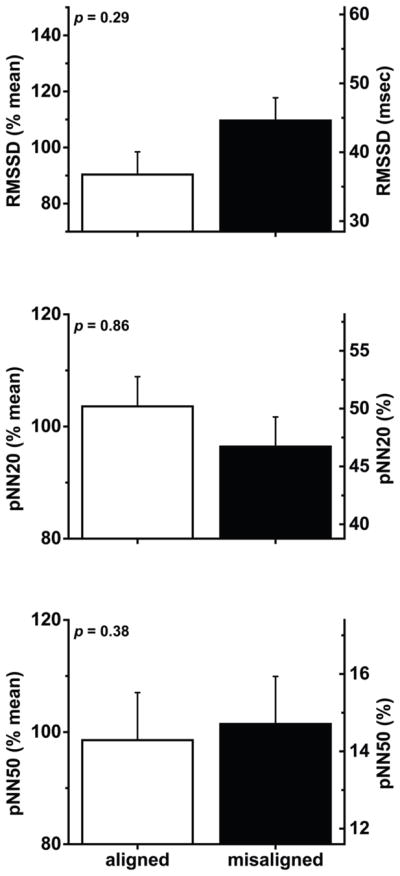
Data were collected during paced breathing (see methods for details). RMSSD, root mean square differences of consecutive heartbeat intervals; pNN20, percentage of consecutive heartbeat intervals differing by >20 ms; pNN50, percentage of consecutive heartbeat intervals differing by >50 ms. Data are represented as mean±SEM.
There was no significant effect of circadian misalignment on RMSSD, pNN20 or pNN50 data collected during paced breathing (all P≥0.29; n=6).
Relationship of shift work experience and circadian misalignment-mediated changes in sleep duration with circadian misalignment-mediated changes (i.e., alignment data subtracted from misalignment data) in cardiovascular disease risk markers
There were no significant correlations between cumulative lifetime shift work experience and circadian misalignment-mediated changes in 24-h hs-CRP (rho, −0.54), 24-h SBP (rho, 0.64) or 24-h DBP (rho, 0.57), wake SBP (rho, 0.43), sleep DBP (rho, 0.43) or sleep HR (rho, −0.32; all P≥0.18)—those outcome variables that showed an effect of circadian misalignment.
We have previously reported that circadian misalignment decreased polysomnography PSG-assessed TST by 123 min in the current protocol (Morris et al., 2016b). To investigate to what degree the observed circadian misalignment-mediated effects on cardiovascular disease risk markers were related to circadian misalignment-mediated effects on TST, we used PSG sleep recordings and actigraphic sleep recordings. We assessed whether PSG TST (during sleep period 1 in the alignment protocol and sleep period 2 in the misalignment protocol) was correlated with hs-CRP (24-h and sleep only data) or SBP measured during the subsequent wake period. We also tested whether actigraphic estimates of TST (during sleep period 2 in the alignment protocol and sleep period 3 in the misalignment protocol, with misalignment decreasing actigraphy-estimated TST by 39 min, P=0.020) were correlated with the increase in DBP and heart rate measured during the same sleep opportunities (PSG was not assessed simultaneously with the blood pressure and heart rate assessments). There was no significant correlation between circadian misalignment-mediated changes in 24-h or sleep hs-CRP, or wake period SBP and circadian misalignment-mediated changes in PSG-assessed TST (all P≥0.22). There was also no significant correlation between circadian misalignment-mediated changes in sleep opportunity DBP (P=0.072; n=6) or heart rate (P=0.70; n=6) and circadian misalignment-mediated changes in actigraphy-assessed TST.
Discussion
We found that short-term circadian misalignment, resulting from a rapid 12-h inversion of the behavioral and environmental cycle (including the sleep/wake, rest/activity, fasting/feeding, and dark/light cycle) and which is typical in shift workers, increased 24-h hs-CRP in chronic shift workers. hs-CRP is a marker of systemic inflammation and it is well-accepted that inflammation has an important role in the development of cardiovascular disease (Libby, 2006). Moreover, epidemiological studies have shown chronically elevated hs-CRP to predict cardiovascular disease (Kaptoge et al., 2010). We also found that circadian misalignment modestly increased 24-h blood pressure in chronic shift workers. The small circadian misalignment-induced increase in blood pressure may be clinically important considering there is an increased risk of cardiovascular disease with progressive elevations in blood pressure, beginning at normal blood pressure levels (Stamler et al., 1993). Epidemiologic studies convincingly show an increased prevalence of cardiovascular disease in night workers versus day workers, and field studies in shift workers have shown increased inflammatory markers and blood pressure during or following night work compared to day work or days off (Chau et al., 1989; Adams et al., 1998; Kitamura et al., 2002; Fialho et al., 2006; Su et al., 2008; Khosro et al., 2011). However, these studies cannot definitively distinguish a possible causal role of circadian misalignment in the observed adverse health effects of shift work versus that of differences in other factors, such as work stressors, dietary habits, physical activity, etc. In this highly-controlled, experimental, within-participant protocol we could determine the independent influence of circadian misalignment on cardiovascular risk factors in shift workers, while controlling these other potential factors in the laboratory. Our finding that circadian misalignment per se increases hs-CRP and blood pressure in chronic shift workers provides evidence for circadian misalignment as an underlying mechanism to explain why shift work is a risk factor for inflammation, elevated blood pressure, hypertension and cardiovascular disease (Morikawa et al., 1999; Sakata et al., 2003; Suwazono et al., 2008; Lieu et al., 2012; Vyas et al., 2012).
Possible mechanisms involved in circadian misalignment-mediated increases in hs-CRP and blood pressure
Short-term sleep restriction increases inflammatory markers, including hs-CRP (Meier-Ewert et al., 2004; Haack et al., 2007). We found no significant correlation between circadian misalignment-mediated changes in PSG-assessed TST and circadian misalignment-mediated changes in 24-h hs-CRP levels. This may be a result of insufficient statistical power. Animal studies have shown that circadian disruption by jet lag simulation exaggerates inflammatory responses that were also not explained by sleep loss or stress measures; these inflammatory responses were suggested to be related to altered clock gene expression in the central circadian pacemaker and peripheral clocks (Castanon-Cervantes et al., 2010).
Short-term sleep restriction increases daytime and nighttime blood pressure in normotensive and hypertensive humans (Lusardi et al., 1996; Tochikubo et al., 1996; Lusardi et al., 1999; Meier-Ewert et al., 2004). However, we found no significant correlation between circadian misalignment-mediated changes in PSG-assessed TST and circadian misalignment-mediated changes in wake period SBP levels (there was no misalignment effect on wake period DBP). Because no PSG recordings were performed concurrently with blood pressure recordings, we also estimated TST via actigraphy during the sleep opportunities in which blood pressure was measured. Circadian misalignment-mediated changes in actigraphy-estimated TST were not significantly correlated with circadian misalignment-mediated changes in sleep-opportunity DBP levels (there was no misalignment effect on sleep-opportunity SBP). The lack of correlation between changes in TST and blood pressure maybe because of insufficient statistical power.
In humans, cortisol and melatonin administration increases and decreases blood pressure, respectively (Connell et al., 1987; Scheer et al., 2004; Cagnacci et al., 2005). We found that circadian misalignment had no effect on mean 24-h serum cortisol levels, as previously reported (Morris et al., 2016b). Thus, the circadian misalignment-mediated increase in 24-h blood pressure levels may not be related to any misalignment effect on 24-h cortisol levels. We have previously reported that simulated night work (circadian misalignment) decreases melatonin levels (Morris et al., 2015). In this study, we did not measure melatonin levels concurrently with blood pressure measurements. However, it is likely that the 90-lux light exposure during wakefulness in the misalignment protocol significantly reduced melatonin production, as we have previously reported with the same light conditions in non-shift workers (Morris et al., 2015). If so, this may help explain why circadian misalignment increased blood pressure in the current study (Scheer et al., 2004; Cagnacci et al., 2005).
The endogenous circadian system causes blood pressure to gradually rise from the biological morning to the evening and fall across the biological night (Shea et al., 2011). During our circadian misalignment protocol, participants attempted to sleep across the biological morning, afternoon and evening, when the circadian system is promoting an increase in blood pressure. This circadian effect on blood pressure may help explain the increased sleep-opportunity DBP during circadian misalignment that we observed.
Comparisons with previous research
Epidemiological studies have shown inflammatory markers to be higher in shift workers than day workers (Sookoian et al., 2007; Burgueno et al., 2010; Puttonen et al., 2011). We and others have shown that circadian misalignment (independent of shift work-induced differences in stress, diet, etc.) increases levels of the inflammatory markers hs-CRP, TNF-α, IL-6, IL-10 and resistin in non-shift workers (Leproult et al., 2014; Wright et al., 2015; Morris et al., 2016a). Here, for the first time, we show that circadian misalignment per se increases hs-CRP levels in chronic shift workers. Different forms of circadian disruption (e.g., nighttime light exposure, shifting the light/dark cycle) have also been shown to alter inflammatory markers in rodents (Castanon-Cervantes et al., 2010; Fonken et al., 2013). We found a 24-h rhythm in hs-CRP in the alignment but not in the misalignment condition. Diurnal variation in hs-CRP has been reported in people with cardiovascular disease but not in healthy individuals (Meier-Ewert et al., 2001; Dominguez-Rodriguez et al., 2006).
In chronic shift workers, blood pressure is higher and the day/night blood pressure rhythm is blunted whilst working night shifts compared to day shifts (Chau et al., 1989; Adams et al., 1998; Kitamura et al., 2002; Fialho et al., 2006; Su et al., 2008). These prior studies could not isolate the independent effect of circadian misalignment on blood pressure. We have previously shown that circadian misalignment increases blood pressure in non-shift workers (Scheer et al., 2009; Morris et al., 2016a). Here, we show that circadian misalignment increases 24-h blood pressure in chronic shift workers. Manipulating the light/dark cycle and circadian clock genes has also been reported to affect blood pressure in rodents (Zhang et al., 2000; Doi et al., 2010).
Strengths, limitations and future directions
Strengths of our study include the highly controlled laboratory protocol, which was able to determine for the first time the independent impact of circadian misalignment—similar to that typically experienced by shift workers—on 24-h hs-CRP and blood pressure levels in chronic shift workers. Limitations of the study should also be considered. First, our participants were healthy. The effect of circadian misalignment on inflammatory markers and blood pressure may be different in chronic shift workers with cardiovascular disease. Second, our sample size was small, owing to the arduous task of recruiting currently employed chronic shift workers to undertake two 3-day in-laboratory stays. Thus, we were very likely statistically underpowered to detect some effects. Third, we investigated the effect of circadian misalignment on only one inflammatory marker and a selection of variables related to cardiovascular function. Fourth, our study cannot determine whether chronic shift workers respond differently to circadian misalignment than non-shift workers. Moreover, we cannot determine whether differential responses to our protocol are present between permanent and rotating shift workers. Fifth, in our protocol, the behavioral and light/dark cycles were shift by 12 hours in attempt to cause significant circadian misalignment. Future studies should determine whether smaller (e.g., 8 hours) behavioral and light/dark cycles shifts, which likely result in a smaller degree of circadian misalignment, causes the same or different findings to those reported here. Sixth, we assessed the impact of short-term circadian misalignment on cardiovascular disease risk factors; longer-term studies are required.
Summary
We demonstrate that circadian misalignment (typical in shift workers) per se increases hs-CRP and blood pressure levels in chronic shift workers. Thus, our findings may help explain why shift work is a risk factor for inflammation, elevated blood pressure, hypertension, and cardiovascular disease.
Supplementary Material
Acknowledgments
We thank the research volunteers and Brigham and Women’s Hospital’s CCI staff. This study was supported by NHLBI Grant R01 HL094806 to F.A.J.L.S. C.J.M. was supported by the NSBRI through NASA Grant NCC 9-58, NHLBI Grant R01 HL094806, NIDDK Grant R01 DK099512, and NHLBI Grant R01 HL118601. F.A.J.L.S. was supported by NHLBI Grant R01 HL094806, NIDDK Grant R01 DK099512, and NHLBI Grant R01 HL118601. K.H was supported by NHLBI R00 HL102241, NIA R01AG048108-01A1 and NIA P01AG009975. The project described was supported by Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources.
Footnotes
Conflict of interest: None.
References
- Adams SL, Roxe DM, Weiss J, Zhang F, Rosenthal JE. Ambulatory Blood Pressure and Holter Monitoring of Emergency Physicians before, during, and after a Night Shift. Academic Emergency Medicine. 1998;5:871–877. doi: 10.1111/j.1553-2712.1998.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart P, Kamp J. Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3:303–307. [PubMed] [Google Scholar]
- Bureau of Labor Statistics US. WORKERS ON FLEXIBLE AND SHIFT SCHEDULES IN MAY 2004. U.S. Department of Labor; Washington, DC: 2005. [Google Scholar]
- Burgueno A, Gemma C, Gianotti TF, Sookoian S, Pirola CJ. Increased levels of resistin in rotating shift workers: a potential mediator of cardiovascular risk associated with circadian misalignment. Atherosclerosis. 2010;210:625–629. doi: 10.1016/j.atherosclerosis.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. American Journal of Hypertension. 2005;18:1614–1618. doi: 10.1016/j.amjhyper.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of Inflammatory Responses by Chronic Circadian Disruption. The Journal of Immunology. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau NP, Mallion JM, de Gaudemaris R, Ruche E, Siche JP, Pelen O, Mathern G. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- Connell JMC, Whitworth JA, Davies DL, Lever AF, Richards AM, Fraser R. Effects of ACTH and Cortisol Administration on Blood Pressure, Electrolyte Metabolism, Atrial Natriuretic Peptide and Renal Function in Normal Man. Journal of Hypertension. 1987;5:425–434. [PubMed] [Google Scholar]
- Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Garcia-Gonzalez M, Abreu-Gonzalez P, Ferrer J, Kaski JC. Relation of nocturnal melatonin levels to C-reactive protein concentration in patients with ST-segment elevation myocardial infarction. The American journal of cardiology. 2006;97:10–12. doi: 10.1016/j.amjcard.2005.07.120. [DOI] [PubMed] [Google Scholar]
- Fialho G, Cavichio L, Povoa R, Pimenta J. Effects of 24-h Shift Work in the Emergency Room on Ambulatory Blood Pressure Monitoring Values of Medical Residents. American Journal of Hypertension. 2006;19:1005–1009. doi: 10.1016/j.amjhyper.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim Light at Night Exaggerates Weight Gain and Inflammation Associated With a High-Fat Diet in Male Mice. Endocrinology. 2013;154:3817–3825. doi: 10.1210/en.2013-1121. [DOI] [PubMed] [Google Scholar]
- Fraden J, Neuman MR. QRS wave detection. Med Biol Eng Comput. 1980;18:125–132. doi: 10.1007/BF02443287. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Americal Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosro S, Alireza S, Omid A, Forough S. Night work and inflammatory markers. Indian J Occup Environ Med. 2011;15:38–41. doi: 10.4103/0019-5278.82996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Onishi K, Dohi K, Okinaka T, Ito M, Isaka N, Nakano T. Circadian rhythm of blood pressure is transformed from a dipper to a non-dipper pattern in shift workers with hypertension. J Hum Hypertens. 2002;16:193–197. doi: 10.1038/sj.jhh.1001328. [DOI] [PubMed] [Google Scholar]
- Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. The American Journal of Clinical Nutrition. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African-Americans. J Hypertens. 2012;30:61–66. doi: 10.1097/HJH.0b013e32834e1ea3. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a Restricted Sleep Regimen on Ambulatory Blood Pressure Monitoring in Normotensive Subjects. American Journal of Hypertension. 1996;9:503–505. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. American Journal of Hypertension. 1999;12:63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am CollCardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert K, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of c-reactive protein concentrations in healthy human subjects. Clinical Chemistry. 2001;47:426–430. [PubMed] [Google Scholar]
- Mietus JE, Peng CK, Henry I, Goldsmith RL, Goldberger AL. The pNNx files: re-examining a widely used heart rate variability measure. Heart. 2002;88:378–380. doi: 10.1136/heart.88.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Ishizaki M, Tabata M, Nishijo M, Higashiguchi K, Yoshita K, Sagara T, Kido T, Naruse Y, Nogawa K. Relationship between shift work and onset of hypertension in a cohort of manual workers. Scandinavian journal of work, environment & health. 1999;25:100–104. doi: 10.5271/sjweh.411. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012a;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences. 2016a;113:E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016b;101:1066–1074. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FAJL. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012b;199:337–358. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttonen S, Viitasalo K, Harma M. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. 2011;28:528–535. doi: 10.3109/07420528.2011.580869. [DOI] [PubMed] [Google Scholar]
- Sakata K, Suwazono Y, Harada H, Okubo Y, Kobayashi E, Nogawa K. The relationship between shift work and the onset of hypertension in male Japanese workers. J Occup Environ Med. 2003;45:1002–1006. doi: 10.1097/01.jom.0000085893.98441.96. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Van Montfrans GA, Van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an Endogenous Circadian Blood Pressure Rhythm in Humans That Peaks in the Evening. Circulation Research. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Fernández Gianotti T, Burgueño A, Alvarez A, González CD, Pirola CJ. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. Journal of Internal Medicine. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Archives of Internal Medicine. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- Su TC, Lin LY, Baker D, Schnall PL, Chen MF, Hwang WC, Chen CF, Wang JD. Elevated blood pressure, decreased heart rate variability and incomplete blood pressure recovery after a 12-hour night shift work. J Occup Health. 2008;50:380–386. doi: 10.1539/joh.l7056. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Nogawa K. Shift Work Is a Risk Factor for Increased Blood Pressure in Japanese Men: A 14-Year Historical Cohort Study. Hypertension. 2008;52:581–586. doi: 10.1161/HYPERTENSIONAHA.108.114553. [DOI] [PubMed] [Google Scholar]
- Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012:345. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B-L, Zannou E, Sannajust F. Effects of photoperiod reduction on rat circadian rhythms of BP, heart rate, and locomotor activity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;279:R169–R178. doi: 10.1152/ajpregu.2000.279.1.R169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.