Abstract
Background and Purpose
Because falls can have deleterious consequences, it is important to understand the influence of fatigue and medications on balance in Parkinson disease (PD). Thus, the purpose was to investigate the effects of fatigue on balance in individuals with PD. Because brain-derived neurotrophic factor (BDNF) has been shown to be related to motor performance, we also explored its role.
Participants
27 individuals (age=65.4±8.1; males=14, females=13) with neurologist-diagnosed PD with 13 genotyped for BDNF as Val66Val, 11 as Val66Met, 2 as Met66Met (one refused).
Methods
Participants were tested both on and off medication, one week apart. On both days, they completed a pre and post-test interspersed by a fatiguing condition. Factorial ANOVAs were performed for the following balance domains: 1. anticipatory postural response; 2. adaptive postural responses; 3. dynamic balance; 4. sensory orientation; and, 5. gait kinematics. For BDNF, t-tests were conducted comparing genotype for the pre and post difference scores in both the on and off medication states.
Results
There were no interactions between time (pre and post) and medication for any of the domains (ps≥0.187). Participants with BDNF Met alleles were not significantly different from Val66Val participants in balance (ps≥0.111) and response to a fatiguing condition (ps≥.070).
Discussion and Conclusions
Fatigue does not appear have a detrimental effect on balance and there was not a differential effect of medication in individuals with PD. These results also indicate that individuals with a BDNF Met allele did not have a greater decay in function after a fatiguing condition.
Keywords: BDNF, gait, postural instability, fall risk
INTRODUCTION
Parkinson disease (PD) is a chronic neurodegenerative movement disorder that results in slowness of movement, tremors, stiffness in the limbs and trunk, and impaired balance. It is estimated that there are currently between 4.1 and 4.6 million individuals over the age of 50 with idiopathic PD in the world’s 10 most populous nations.1 Although PD itself is not fatal, serious complications such as falls have been associated with increased mortality and morbidity and can lead to increased dependency and risk of nursing home admission.2–4 While the role of the cardinal motor symptoms of the disease (bradykinesia, resting tremor, rigidity, and postural instability) have been linked to fall risk in this population, the role of its many associated non-motor symptoms, is still misunderstood. For instance, Shulman et al found that depression, anxiety, fatigue, and sleep disorders occur commonly in PD and these non-motor symptoms may reduce the function of individuals with PD.5 Additionally, Martinez-Martin et al6 found that non-motor symptoms strongly contribute to a decline in quality of life in individuals with PD, perhaps even more than motor symptoms. Moreover, non-motor symptoms as a whole may be the most important predictor of quality of life in individuals with PD. Of the non-motor symptoms, fatigue, either as the cause or effect of inactivity and its physiologic consequences, may be an important limiting factor in the physical function and capability of individuals with PD.7
Fatigue is a common complaint among individuals with PD. In a nine-year follow up study, Friedman and Friedman8 found that approximately 50% of individuals with PD experience fatigue. People with PD may have fatigue associated with deconditioning, generalized lack of energy, or decline in force generation or in speed of repetitive movements, as well as fatigue due to sleep disturbance, decreased motivation, slowed mental processes, or depression.9 While there is no clear distinction as to whether PD is more closely associated with peripheral fatigue (localized muscle fatigue) or central fatigue (central nervous system fatigue), the fatigue-causing factors mentioned above suggest that a combination of both is highly plausible. However, most consider it a non-motor PD symptom which may support the concept the PD fatigue has a larger central fatigue component. Unfortunately, to date there has been little research done to investigate PD fatigue on motor function and in particular the role that fatigue plays on one of cardinal signs of PD, postural instability.
The lack of information about the effect of fatigue on balance leaves a critical gap in our knowledge and understanding of fall risk in PD. In fact, multiple studies have already established links between both peripheral and central fatigue on balance and postural stability in other populations. For example, research from Helbostad et al suggest a correlation between physical (peripheral) fatigue and fall risk in older persons.10 Additionally, researchers in a separate study found that central fatigue also decreased balance and stability in young athletes.11 These researchers suggested that because balance depends on the processing of three central nervous system sensory systems (visual, vestibular, and somatosensory), any fatigue-related alterations in their function could affect one's balance.11 This research suggests that even mentally fatiguing tasks may potentially further reduce balance ability in individuals who have an increased fall risk. Likewise, because the neuromotor system is responsible for executing the response of the three sensory systems, it may also be affected by fatigue resulting in balance decrement.
Another factor that theoretically may contribute to fatigue’s effect on balance and gait in PD is the brain-derived neurotrophic factor (BDNF) genotype. BDNF is an activity-dependent neurotrophin that plays an important role in the growth, survival, and neuronal cell differentiation in the central nervous system12 and has been shown to increase in response to acute exercise.13 It is considered an important factor in neuroprotection against diseases like PD and AD. The genotype for BDNF (Val/Val, Val/Met, or Met/Met) affects the amount of trafficking of this important neurotrophin with those carrying the met allele having lower levels of BDNF expression.14 The met allele is associated with decreased motor performance,15 brain motor system function, and greater error in motor learning.16 Moreover, motor learning is considered a critical element for training automatic postural responses necessary for fall prevention and is less pronounced in individuals with PD.17 The met allele may also negatively affect motor rehabilitation training in individuals with neurologic disorders18 and has been shown to predict poor outcomes in PD.19 Because BDNF is an important motor-related neurotrophin whose expression is affected by genotype, it stands to reason that it could affect motor capacity in gait and balance and warrants exploration. If individuals with PD have the met allele that may mean that their motor performance capacity may be suboptimal. Therefore, it is also plausible that a fatiguing condition may expose or uncover latent impairment in motor capacity in balance and gait.
It seems logical that fatigue would negatively impact balance in PD and that fatigue may result in higher fall risk during periods of fatigue; however, this has not been vetted in the literature. Therefore, the primary aim of this pilot study was to investigate the role that fatigue plays on balance and gait in individuals with PD. We hypothesized that different aspects of balance performance (anticipatory, adaptive, and dynamic) and gait would decrease after a condition that induced fatigue. A secondary exploratory aim was to investigate the differential effects of BDNF polymorphisms (Val/Met, Val/Val, and Met/Met) on balance and gait performance due to a fatiguing condition. Based on the literature, we hypothesized that Met allele carriers would have poorer balance performance and a greater decay in balance function after fatigue than the Val/Val genotype. Another secondary aim was to explore the role of PD-medication (on and off) on the balance response to a fatiguing condition. Although there is evidence that postural instability may be refractory to dopaminergic treatment,20 we wanted to determine if balance- and gait-related performance as a result of fatigue was differentially affected by PD medication. Since the literature suggests that PD medications do not affect postural instability, we hypothesized that there would be no difference in response to fatigue in either the on or off medication conditions.
METHODS
Participants
The inclusion criteria for the study included the following: neurologist-diagnosed idiopathic PD, Hoehn and Yahr21 stages 1–4, willingness to be tested in the on and off PD medication states, and 45–80 years of age. The exclusion criteria were those with moderate-to-severe dementia (Montreal Cognitive Assessment Score ≥ 17),22 inability to stand or walk for more than 10 minutes (self-report), or other significant co-morbidities (self-report) that would be contraindicated for the fatiguing condition in this study (i.e., atrial fibrillation, chronic obstructive pulmonary disease, poorly controlled or unstable cardiovascular disease). The reason to exclude those with other significant co-morbidities was to minimize the impact of potential compounding factors that would limit physical activity participation. The sample size range was estimated using PASS 13.0 ((NCSS, LLC. Kaysville, Utah, USA, www.ncss.com/software/pass) and powered based on Primary Aims 1 and 2, with an interaction effect and main effects for each within variable (time: pre and post; medication: on and off). A repeated measures design with no between factors and 2 within factors (time: pre and post; medication: on and off) was conservatively estimated to be between 28 and 33 subjects. This design would achieve 80% power to test the interaction and main effects with a 5% significance level and with a Cohen’s f effect size range of 0.50 to 0.55 based on the opinion of the investigators.
The study protocol was approved by the University of Nevada, Las Vegas Biomedical Institutional Review Board and all participants provided written consent. Participant recruitment methods included the following: snowball recruitment, flyer distribution through local PD support groups, social media through PD-specific websites, the Michael J. Fox Trial Finder (https://foxtrialfinder.michaeljfox.org), local neurologists’ offices specializing in movement disorders, and previous PD research participant lists.
Recruitment yielded 27 total participants (mean age = 65.4±8.1; males = 14, females = 13) with neurologist-diagnosed PD (mean months since diagnosis = 59.7±42.1, mean Levodopa Equivalent Daily Dose (LEDD) = 442.4±240.2) (Table 1). Participants ranged from 1 to 4 on the Hoehn and Yahr Scale (median and mode = 2). Of the 27 participants, 2 participants chose not to participate in the off-medication day, and 1 participant did not take PD medications so this individual was only tested during the off-medication day. For the BDNF genotype groupings, 13 were genotyped as Val/Val, 11 as Val/Met, and 2 as Met/Met. One participant refused the genotyping.
Table 1.
Descriptive details of the participants.
| General | Age | Mean = 65.4±8.1 |
| Gender | Males = 14, Females = 13 | |
| PD specific | Months since diagnosis | Mean = 59.7±42.1 |
| Hoehn and Yahr Scale | Mean = 2, Mode = 2
|
|
| Levodopa Equivalent Daily Dose | Mean = 442.4±240.2 | |
| Cognition | Montreal Cognitive Assessment | Mean = 25.7±3.5 |
| Balance and falls | Falls in the last month | Mean = 5.3±18.0 |
| Falls in the last year | Mean = 58.0±212.9
|
|
| Fall injuries in the last year | Mean = 0.7±1.9
|
|
| Activities-Specific Balance Confidence Scale | Mean = 78.1±17.6 | |
| Modified Fear of Falling Avoidance Behavior Questionnaire | Mean = 28.3±12.5 | |
| Physical activity levels | Number of minutes in vigorous activity per day | Mean = 27.6±43.0 |
| Number of minutes in moderate activity per day | Mean = 31.4±46.4 | |
| Number of minutes in walking per day | Mean = 49.4±42.1 | |
| Number of minutes sitting per day | Mean = 342.2±190.1 | |
| Parkinson’s Fatigue Scale | Mean = 2.7±1.0 |
Study Design
This study was a pre- and post-test design in both the on and off PD medication states. After screening, demographic data collection, and testing, blood samples were taken to determine the BDNF genotype which was used to categorize the participants by BDNF polymorphism (Val/Met, Val/Val, Met/Met). All participants that agreed to be tested off medication were tested on two separate days, one on-medication and one off-medication, separated by at least 5 days to prevent carryover effects from delayed onset muscle soreness and activity-related fatigue. The two participants that chose not to participate in the off-medication day and the one participant that did not take PD medications were tested only once. All test evaluators were blinded to genotype.
Data collection occurred at the University of Nevada, Las Vegas Physical Therapy Gait and Balance Laboratory from September 2015 to June 2016. Participants were instructed to eat a similar breakfast, including any caffeine consumption, on both testing days, to avoid any strenuous activity on the day of and the days leading up to the test days, and were encouraged to get a restful sleep. Participants were also instructed to avoid eating protein with breakfast on the testing day as research has shown that protein can interfere with the therapeutic effects of PD medications and can result in fluctuations of motor symptoms, especially bradykinesia.23
On the first day of testing, participants were instructed to take their PD medications 30 minutes prior to arrival as research shows that Levodopa has a peak onset time of 1 hour.24 The additional 30 minutes before peak onset were allotted to allow for the collection of the following data: 1. Demographics - age, gender, fall history (last year, last month, and injurious falls in last year), cognitive level (Montreal Cognitive Assessment),25 physical activity level (International Physical Activity Questionnaire),26 balance confidence (Activities-Specific Balance Confidence Scale),27 and fear of falling avoidance behavior (Fear of Falling Avoidance Behavior Questionnaire – modified);28 and, 2. PD characteristics - year of PD diagnosis, PD medication usage (Levodopa Equivalent Dose),29 Hoehn and Yahr Scale,21 and The Parkinson’s Fatigue Scale.30
Next, participants completed a battery of balance and gait tests, followed by a fatiguing condition, and lastly, the same balance and gait tests (see Appendix, Supplemental Digital Content 2) for test descriptions and evidence for reliability and validity). The pre and post-tests consisted of the same tests and measures with the exception of a 3 minute rest period between tests during the pre-test phase to ensure that participants were not fatigued when performing the tests. During the post-test, the 3 minute rest periods were replaced with a 30-second sit-to-stand exercise to maintain the sense of fatigue throughout the second half of testing. The following balance and gait tests were used:
Anticipatory postural responses. The anticipatory postural response is how one responds to a balance challenge that the person knows about and is associated with the activation of postural muscles prior to an expected balance perturbation. Anticipatory postural responses are mediated by supraspinal centers, including premotor and cerebellar systems.31 This type of balance response was assessed using the anticipatory postural response subsection on the mini-Balance Evaluation Systems Test (mini-BESTest),32–34 and Functional Reach Test (FRT)35 (quantified using VirtuBalance technologyi). Collectively, these tests allowed inference about the effects of the fatigue condition on anticipatory (supraspinal) contributions to balance.
Adaptive postural responses. The adaptive or compensatory postural response is how one responds to a balance perturbation that occurs without knowledge and is associated with the reflex activation of postural muscles after an unexpected balance perturbation. Because these postural responses occur without knowledge, the latency period of an adaptive postural response is longer than a stretch reflex but shorter than voluntary reaction time suggesting that they are mostly mediated by spinal cord reflex circuitry and do not typically have a large supraspinal contribution.36 This type of balance response was assessed using the Bertec Balance Systemii motor control test which quantifies postural sway as a result of unexpected movements of the balance platform (i.e. forward/backward translation). Additionally, the reactive postural response subsection of the Mini-BESTest was also utilized. These tests allowed inference about the effect of the fatigue condition on adaptive or spinal cord (reflex circuitry) contributions to balance.
Balance sensory orientation. Balance sensory orientation testing helps to determine the specific contribution of three different balance sensory systems to static balance: visual, vestibular, and somatosensory.37 Quantification of the contribution of these three sensory systems was done using the Bertec Balance System Sensory Organization Test (SOT).38 The SOT allowed inference about what postural sensory system (i.e., visual, vestibular, somatosensory) was most influenced by the fatigue condition.
Dynamic balance. Gait was assessed by the 20 Feet Walk Test, Timed Up and Go test (TUGT),39 and Timed Up and Go Cognitive (TUGTcognitive ),40 gait component of the mini-BESTest using the Protokineticsiii Zeno instrumented walking mat.41 The walking mat was used to quantify the following gait characteristics during those tests: gait speed, step length, step length coefficient of variation, stride length, stride length coefficient of variation, stride velocity, and stance percentage.
Collectively, these balance tests allowed inference about the effects of the fatigue condition on supraspinal (premotor), and spinal cord (reflex circuitry) contributions to balance, as well as determined which postural sensory system (i.e., visual, vestibular, somatosensory) was most influenced by the fatigue condition.
To achieve fatigue, participants performed a 30-second sit-to-stand exercise followed by the Modified Bruce Treadmill Test (mBTT)42 until they reported a 7 out of 10 score using the Visual Analogue Scale for Fatigue (VAS-F).43. We chose to use of the VAS-F as fatigue has both central and physical components and this self-report assessment seemed well-suited to address both components and to rapidly assess fatigue in a quantitative manner. In addition, the VAS-F has been used in PD studies in the past.8,44 The use of a more lengthy testing process to measure fatigue would have allowed participants to rest, which would have impacted their balance in the post-test assessments. Lee et al reported evidence for the reliability and validity of the VAS-F for assessing fatigue quantitatively.43 In their work, they utilized Pearson correlations to establish the concurrent validity of the final VAS-F instrument with both the Profile of Mood States (POMS) and the Stanford Sleepiness Scale (SSS) in a population of healthy individuals. The VAS-F has also been found to be valid for assessing the impact of fatigue in other populations, such as in people with multiple sclerosis.45 Additionally, the level of 7 out of 10 was selected as it balances the safety of a fatigue-inducing test with a moderate-severe self-report level of fatigue.
The mBTT was selected because it was progressively and physically challenging and would likely induce fatigue in a relatively short amount of time. Cycling protocols to induce fatigue were considered; however, it was felt that a treadmill protocol would more closely approximate typical, day-to-day fatigue inducing conditions in people with PD and also would more likely induce fatigue in relevant neuromotor pathways. Moreover, the mBTT protocol was selected over the standard Bruce Treadmill protocol because it starts at a lower workload than the standard test and has been shown to be safe in people with PD.46 During the mBTT, the participants’ heart rate, oxygen saturation, fatigue level, speed, and incline were monitored and recorded (Table 2). On a few occasions, participants were not able to safely or comfortably walk at the required mBTT speed and incline progression. In those cases, the progressions were modified to participants’ tolerance and safety until reaching the 7 out of 10 threshold. Participants were instructed to walk without using their hands on the railing; however, in some cases it was allowed for safety reasons. In all cases, participants were able to reach the fatigue threshold, with and without safety modification. Immediately after reaching the fatigue threshold (operationally defined as 7 out of 10), participants then performed the balance and gait tests again, except the rest periods were replaced with the 30-second sit-to-stand exercise so as to maintain fatigue. On the second testing day, participants were tested after having not taken their PD medications for at least 12 hours. The same balance and gait pre-testing, fatiguing condition, and balance and gait post-testing protocol was conducted the same as Day 1.
Table 2.
Means and standard deviations (on and off medication) when the Modified Bruce Treadmill Test (mBTT) was stopped due to achievement a perceived 7 out of 10 fatigue. P values represent the t-test comparisons of the two conditions.
| ON medication | OFF medication | P value | |
|---|---|---|---|
| mBTT level | 2.6 (1.5) | 3.2 (2.7) | P=.123 |
| Test duration (minutes) | 10.6 (5.2) | 11.9 (8.7) | P=.309 |
| Gait speed (meters per second) | 1.10 (0.55) | 1.01 (0.49) | P=.318 |
| Treadmill incline (degrees) | 11.0 (5.0) | 10.8 (5.6) | P=.783 |
| Heart rate (beats per minute) | 110.0 (23.5) | 109.3 (18.4) | P=.900 |
| Oxygen saturation (%) | 96.4 (2.0) | 96.2 (2.3) | P=.754 |
| Fatigue rating (VAS-F, 0–10) | 7.1 (0.3) | 7.1 (0.6) | P=770 |
BDNF Genotyping
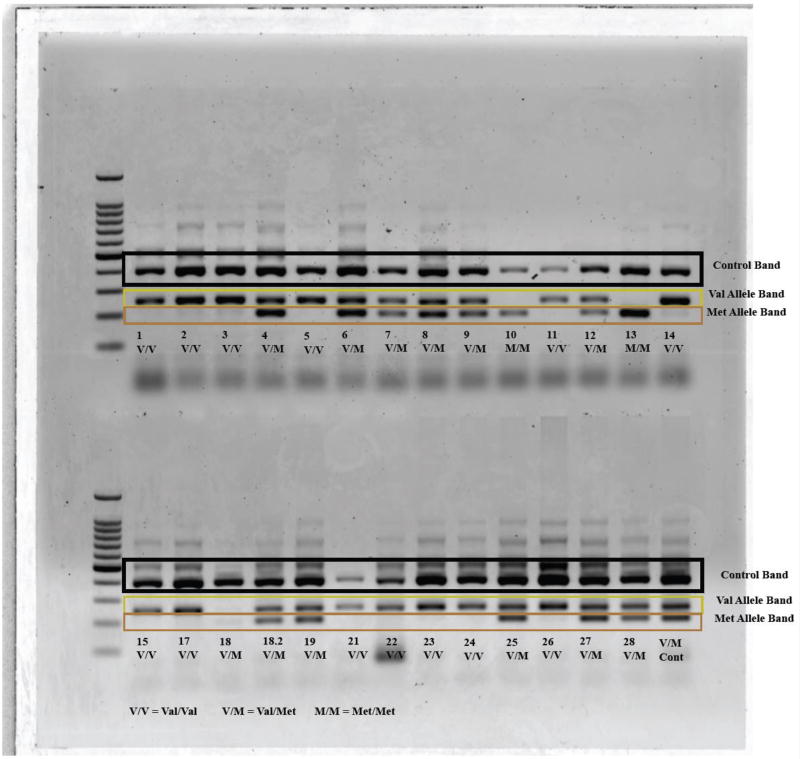
During the first test day, a sample of blood (600 µl) was collected via finger-sick into an anticoagulant tube (Multivette 600 LH, Sarstedt, Fisher Scientific, Pittsburgh, PA). DNA isolation was performed using a commercially available kit (Wizard Genomic DNA Purification Kit, Promega, Madison, WI). DNA concentration (ng/mL) was determined using an Epoch microplate reader with the Take3 System (Biotek U.S., Winooski, VT). BDNF gene region rs6265 was amplified using polymerase chain reaction (PCR) as described by Sheikh et al (Sheikh et al., 2010). Three amplicons, two allele specific amplicons, 253 bp (val) and 201 bp (met) along with the 401 bp amplicons (entire res6265 region used as an internal control) were distinguished with four primers: P1 (forward) CCTACAGTTCCACCAGGTGAGAAGAGTG, P2 (reverse) (TCATGGACATGTTTGCAGCATCTAGGTA), P3 (G allele specific) CTGGTCCTCATCCAACAGCTCTTCTATAAC, and P4 (A allele specific) ATCATTGGCTGACATTTCGAACCCA. The PCR reaction consisted of a total of 12 µL containing: 1X Kapa Hotstart Genotyping Mix (Kapa Biosystems), 0.5 µM of each of the four primers (P1, P2, P3, P4) and 20 ng of genomic DNA. Thermocycling conditions were as follows: denaturation at 94°for 3 min followed by 30 cycles of at 95° for 45 sec, 65° for 60 sec, and 72° for 60 sec, followed by a final extension of 72° for 2 minutes. DNA 1000 bp ladder (Promega, USA) and 10 µL PCR products were loaded onto a 2% agarose gel and electrophoresed at 100 V for 90 min. Based on the following banding patterns, samples were classified as Val/Val (253/253 bp), Val/Met (253/201 bp), and Met/Met (201/201 bp) with all of them having the rs6265 internal control (401 bp) band (Figure 2). Each sample was genotyped from at least two independent polymerase chain reactions to ensure fidelity.
Figure 2.
Characterization of BDNF genotype using banding patterns on a DNA ladder.
Data Analysis
All data were analyzed using SPSS version 22.0 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). Data with non-normal distributions were transformed. To address the primary aim of the study, a 2 (condition: pre and post) X 2 (medication: on and off) factorial ANOVA was performed for each outcome variable in each of the following balance domains: 1. anticipatory postural response (mini-BESTest - Anticipatory subsection, FRT); 2. adaptive postural responses (Bertec Balance System motor control test, mini-BESTest - Reactive Postural Control); 3. Dynamic balance (TUGT, TUGTcognitive, mini-BESTest – Dynamic Gait subscale); 4. sensory orientation (Bertec Balance System SOT); and, 5. gait characteristics. The secondary aim of the study was to compare the difference in balance function between the two BDNF genotypes, Val/Val and those with a Met allele (Val/Met and Met/Met) and also to see if there was a difference between them on response to a fatiguing condition. To compare the difference in balance function between the two genotypes an independent t-test was conducted for the pre-test scores, both on and off medication, across all of the aforementioned balance domains. To compare the differences in response of the two groups to a fatiguing condition, independent t-tests were used to compare the difference between the pre- and post-tests across all of the aforementioned balance domains. Missing data were inputted using the last observation carried forward method. The other secondary aim of PD medication was analyzed using the main effect of medication on the primary factorial ANOVAs. To control against errors originating from multiplicity, the Benjamini-Hochberg procedure47,48 was used to reduce the number of false positives from the 21 total comparisons. Benjamini-Hochberg adjusted alpha values were then compared to the p values from the results. These will only be discussed in the results section if the Benjamini- Hochberg level changed the interpretation
RESULTS
Anticipatory postural responses
For the mini-BESTest – Anticipatory subscale, there was not a statistically significant interaction between time (pre and post) and medication state (on and off), p=.131 (Tables 3 and 4). There were no statistically significant differences for the main effects of time (p=.942) and medication (p=.698). Likewise, for the FRT, there was not a statistically significant interaction between time and medication state, p=.785. There were no statistically significant differences for the main effects of time (p=.054) and medication (p=.813).
Table 3.
Means and standard deviations for pre and post outcome variables across each of the balance domains based on ON and OFF PD medication state.
| Balance domain |
Outcome variable | ON PD medications |
OFF PD medications |
||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Anticipatory postural response (n=24) | mini-BESTest - Anticipatory subsection (0–6 points) | 4.83 (1.17) | 4.67 (1.09) | 4.75 (1.19) | 4.75 (1.36) |
| Functional Reach Test (inches) | 9.62 (3.19) | 9.22 (2.55) | 9.61 (2.81) | 9.10 (2.91) | |
| Adaptive postural responses (n=24) | Motor control test - backward latency (msec) | 130.04 (7.72) | 129.90 (8.14) | 128.92 (10.02) | 128.17 (8.67) |
| Motor control test - backward amplitude (deg/sec) | 7.56 (2.94) | 7.13 (3.05) | 7.77 (3.51) | 7.17 (2.77) | |
| Motor control test - forward latency (msec) | 128.63 (10.40) | 129.04 (12.00) | 124.69 (10.17) | 126.29 (11.03) | |
| Motor control test - forward amplitude (deg/sec) | 6.56 (2.36) | 6.48 (2.50) | 6.58 (2.42) | 6.54 (2.48) | |
| mini-BESTest - Reactive Postural Control subsection (0–6 points) | 4.33 (1.95) | 4.13 (2.07) | 4.21 (1.93) | 4.17 (1.81) | |
| Dynamic gait (n=24) | TUGT (sec) | 10.16 (3.13) | 9.44 (2.82) | 10.12 (4.33) | 9.30 (2.79) |
| TUGTcognitive (sec) | 11.85 (6.39) | 11.73 (7.88) | 12.03 (9.01) | 12.21 (12.01) | |
| mini-BESTest – Dynamic Gait subscale (0–10 points) | 8.92 (1.18) | 8.54 (1.41) | 8.67 (1.69) | 8.71 (1.65) | |
| Sensory orientation (n=24) | SOT composite (%) | 66.42 (9.08) | 67.00 (8.45) | 71.17 (8.59) | 69.50 (9.85) |
| SOT visual (%) | 70.67 (12.69) | 75.00 (12.52) | 76.79 (11.79) | 76.88 (10.37) | |
| SOT somatosensory (%) | 97.25 (5.27) | 97.12 (9.75) | 98.08 (2.93) | 98.13 (3.29) | |
| SOT vestibular (%) | 63.00 (11.35) | 63.96 (13.95) | 68.04 (12.55) | 63.08 (19.99) | |
| Gait characteristics (n=23) | Gait speed (cm/sec) | 112.78 (24.20) | 118.66 (25.00) | 113.26 (25.58) | 118.09 (27.52) |
| Step length (cm) | 61.72 (11.00) | 63.43 (11.69) | 60.76 (12.51) | 61.91 (12.73) | |
| Step length coefficient of variation (%) | 14.25 (3.94) | 13.92 (5.41) | 15.23 (7.95) | 14.90 (6.19) | |
| Stride length (cm) | 123.73 (22.33) | 126.63 (23.33) | 121.75 (25.48) | 123.85 (25.50) | |
| Stride length coefficient of variation (%) | 11.74 (3.17) | 12.09 (4.07) | 13.44 (6.16) | 13.14 (4.65) | |
| Stride velocity (cm/sec) | 113.30 (23.95) | 119.15 (24.76) | 113.73 (25.56) | 118.55 (27.40) | |
| Stance percent (%) | 64.57 (2.95) | 64.34 (3.11) | 64.87 (3.00) | 64.53 (2.97) | |
Table 4.
P values, effect size measurements, and power values for the time by medication interactions and main effects of medication and time (pre and post) for each of the balance domains.
| Balance domain |
Outcome variable | Interaction | Main effect of medication |
Main effect of time |
|
|---|---|---|---|---|---|
|
| |||||
| Anticipatory postural response (n=24) | Mini-BESTest - Anticipatory | P=.131 | p=.698 | p=.942 | |
|
|
|
|
|||
| Power=.137 | Power=.050 | Power=.103 | |||
|
| |||||
| Functional Reach Test | P=.785 | p=.813 | p=.054 | ||
|
|
|
|
|||
| Power=.058 | Power=.056 | Power=.495 | |||
|
| |||||
| Adaptive postural responses (n=24) | Motor control test - backward latency | P=.694 | p=.182 | p=.699 | |
|
|
|
|
|||
| Power=.066 | Power=.261 | Power=.066 | |||
|
| |||||
| Motor control test - backward amplitude | P=.702 | p=.452 | p=.041 | ||
|
|
|
|
|||
| Power=.066 | Power=.113 | Power=.543 | |||
|
| |||||
| Motor control test - forward latency | P=.494 | p=.011 | p=.334 | ||
|
|
|
|
|||
| Power=.102 | Power=.759 | Power=.157 | |||
|
| |||||
| Motor control test - forward amplitude | P=.887 | p=.741 | p=.734 | ||
|
|
|
|
|||
| Power=.052 | Power=.062 | Power=.063 | |||
|
| |||||
| mini-BESTest - Reactive Postural Control | P=.840 | p=.205 | p=.280 | ||
|
|
|
|
|||
| Power=.128 | Power=.053 | Power=.206 | |||
|
| |||||
| Dynamic gait (n=24) | TUGT | P=.984 | p=.457 | p<.001 | |
|
|
|
|
|||
| Power=.052 | Power=.058 | Power=.872 | |||
|
| |||||
| TUGT cognitive | P=.968 | p=.406 | p=.049 | ||
|
|
|
|
|||
| Power=.107 | Power=.068 | Power=.050 | |||
|
| |||||
| mini-BESTest – Dynamic Gait | P=.183 | p=.672 | p=.259 | ||
|
|
|
|
|||
| Power=.256 | Power=.053 | Power=.188 | |||
|
| |||||
| Sensory orientation (n=24) | SOT composite | P=.277 | p=.009 | p=.524 | |
|
|
|
|
|||
| Power=.187 | Power=.780 | Power=.095 | |||
|
| |||||
| SOT visual | P=.208 | p=.015 | p=.208 | ||
|
|
|
|
|||
| Power=.237 | Power=.714 | Power=.237 | |||
|
| |||||
| SOT somatosensory | P=.945 | p=.479 | p=.961 | ||
|
|
|
|
|||
| Power=.051 | Power=.106 | Power=.050 | |||
|
| |||||
| SOT vestibular | P=.210 | p=.369 | p=.496 | ||
|
|
|
|
|||
| Power=.236 | Power=.142 | Power=.102 | |||
|
| |||||
| Gait characteristics (n=23) | Gait speed | P=.500 | p=.983 | p<.001 | |
|
|
|
|
|||
| Power=.101 | Power=.050 | Power=1.000 | |||
|
| |||||
| Step length | P=.355 | p=.191 | p=.001 | ||
|
|
|
|
|||
| Power=.148 | Power=.252 | Power=.955 | |||
|
| |||||
| Step length coefficient of variation | P=.998 | p=.229 | p=.373 | ||
|
|
|
|
|||
| Power=.050 | Power=.220 | Power=.140 | |||
|
| |||||
| Stride length | P=.511 | p=.213 | p=.003 | ||
|
|
|
|
|||
| Power=.098 | Power=.233 | Power=.890 | |||
|
| |||||
| Stride length coefficient of variation | P=.429 | p=.036 | p=.925 | ||
|
|
|
|
|||
| Power=.120 | Power=.569 | Power=.051 | |||
|
| |||||
| Stride velocity | P=.502 | p=.972 | p<.001 | ||
|
|
|
|
|||
| Power=.100 | Power=.050 | Power=1.000 | |||
|
| |||||
| Stance percent | P=.654 | p=.252 | p=.007 | ||
|
|
|
|
|||
| Power=.072 | Power=.203 | Power=.816 | |||
Adaptive postural responses
There were no statistically significant interactions between time and medication state for amplitude (ps≥.702) and latency (ps≥.494) for both forward and backward on the motor control test (Tables 3 and 4). The main effect of medication was statistically significant only for forward latency (p=.011) but was not for the others (ps≥.452). The main effect of time was statistically significant only for backward amplitude (p=.041) but not for the others (ps≥.182). There was no interaction for the mini-BESTest – Reactive Postural Control subscale, p=.840, or for either of the main effects, ps≥.205.
Dynamic balance
For the TUGT, TUGTcognitive, and mini-BESTest – Dynamic Gait subscale, there were no statistically significant interactions between medication and time, ps≥.183 (Tables 3 and 4). There were no main effects of medication, ps≥.406. Of the three outcome variables, the TUGT and TUGTcognitive had statistically significant main effects of time (ps≤=0.049).
Sensory orientation
There were no statistically significant interactions between time and medication on the SOT composite and the three sensory balance systems (visual, somatosensory, vestibular), ps≥.210 (Tables 3 and 4). There were no main effects of time, ps≥.208; however, two of the four sensory orientation outcomes, composite (p=.009) and visual (=.015), were statistically significant.
Gait characteristics
There were no statistically significant interactions between time and medications for any of the 7 gait characteristic outcomes, ps≥.355 (Tables 3 and 4). Of the 7 outcomes, the only statistically significant main effect of medication was stride length coefficient of variation, p=.036. All but two (step length coefficient of variation (p=.373) and stride length coefficient of variation (p=.925)) of the 7 outcomes were statistically significant for the main effect of time, ps≤.007.
BDNF genotype
In comparing those with a Met allele (Val/Met and Met/Met) and those without (Val/Val) in both the on and off state of PD medication, there were no statistically significant differences across the following gait and balance categories on their pre-fatigue assessment: anticipatory postural responses (ps≥.474), adaptive postural responses (ps≥.262), dynamic balance (ps≥.299), sensory orientation (ps≥.166), and gait characteristics (ps≥.111). Likewise, there were no differences between the two BDNF groups (with and without Met), on their response to a fatiguing condition across all of the balance domains (ps≥.070).
DISCUSSION
In this pilot study, individuals with PD, who experienced a fatiguing condition, did not demonstrate significant decrements in anticipatory balance responses, adaptive balance responses, sensory organization, dynamic gait, or gait characteristics. Moreover, the BDNF polymorphism did not influence balance and gait or their response to a fatiguing condition. Lastly, the use of PD medications did not improve balance and gait in individuals with PD. From a clinical prospective, these results suggest that treadmill exercise to the point of fatigue, as defined in this study, may not increase balance and gait dysfunction and, logically, may not increase one’s risk for a fall. Our results also suggest that PD medications are not sufficient for improving postural instability; thus, clinicians should seek the use of other evidence-based treatment approaches to address this problem. As a pilot study, we hope that these results can inform future researchers of the effects of fatigue on balance and the challenges related to study design as well as inducing and measuring fatigue.
It is possible that the reason for the lack of change was that the fatiguing condition on the treadmill used in our study was not sufficiently fatiguing to decay balance and gait. Thus, it is possible that balance and gait do indeed decay with fatigue but the fatiguing condition chosen for this trial did not fatigue enough to see the true effect. On the other hand, it is possible that the fatiguing condition was sufficiently fatiguing but that balance and gait systems are resilient to it. Alternatively, as fatigue in PD is widely considered non-motor, the present results may support the notion that fatigue in PD is related to more central, non-motor mechanisms and the present design may not have sufficiently addressed this. In light of our findings, we cannot make a definitive conclusion about which line of reasoning is more likely. In retrospect, the treadmill-based fatiguing condition may have not been a good design choice because it may have primed, warmed up, or entrained the lower extremities and postural muscles and neuromotor systems, thereby improving posture and gait which may have counteracted any fatigue effects. It is also possible that balance performance did not decrease because participants were experiencing transient asthenia rather than actual fatigue.
The mBTT Protocol used may not have been an appropriate method to induce fatigue to impair balance in individuals with PD. At the time of this study, there were few studies relating a fatigue condition and balance and gait. However, recently published research has suggested that there may be more appropriate activities to induce sufficient fatigue. Hamacher et al successfully used an incremental exercise test on a cycle ergometer to elicit a reduction in stability during gait in older individuals.49 In retrospect, cycle ergometry may have been a better choice for the fatigue-inducing modality since it would not have likely recruited the same neuromuscular circuitry for balance and gait as the treadmill did and, thus, may have mitigated a potential priming effect.
As mentioned above, the treadmill may have primed the nervous system, which may have enhanced balance performance even after the fatiguing condition. Previous research has demonstrated that treadmill walking promotes a faster and a more stable walking pattern in people with PD.50 Moreover, the concept of movement-51 and sensory-based52 priming53 have received recent attention in the literature. The validity of the priming notion may be evidenced by the fact that participant TUGT times were significantly faster, regardless of medication usage, during the post-fatigue testing. These results are also consistent with previous research by Lambourne et al that has suggested that, moderate steady-state exercise can enhance motor performance by increasing central nervous system arousal resulting in improved motor response time to sensory stimuli.54 A study published by Koo et al also found evidence to suggest that treadmill training has a positive effect on neurotransmitters as well, specifically dopamine, which could logically improve motor function.55
To date, there is also no clear, widely-accepted definition for fatigue. Most previous studies did not state how they defined fatigue and instead, used participant’s self-report. With no explicit description of what fatigue is, it is possible that individuals may not actually be fatigued, but rather be experiencing other closely-related, overlapping states. For example, Egerton discussed the definition of fatigue as well as a related synonym, asthenia. “(1) fatigue--the state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli, and (2) asthenia—clinical sign or symptom manifested as debility or lack or loss of strength and energy.”56 Although our study was concerned with fatigue as defined above, it is possible that participants may have misinterpreted their loss of strength and energy, or their asthenia, for fatigue. As the definition for asthenia does not include decreased work capacity and reduced response to stimuli, this could result in better preservation of balance performance after a fatiguing condition, than if “fatigue” had been achieved. It should also be noted that in PD, the distinction between fatigue and asthenia is not clear. It is possible that the latter is a closer approximation of the “PD fatigue” and, based on the above definition, may not be exacerbated by a fatiguing condition. This is an important distinction and warrants more attention by researchers. Additionally, our study design likely induced a more “peripheral” than “central” fatigue although in reality it is difficult to disentangle them. Future researchers should consider investigating the impact of different tasks that are more centrally or peripherally fatiguing. Additionally, according to Dobkin, the concept of “fatigue” is the more central component, that often arises from “fatigability,” the more peripheral component.57 Dobkin describes “fatigability” as a demonstrable decline in muscle strength as the result of repetitive activation of specific muscle groups, but notes that it may be difficult to localize because the boundary between the central and peripheral components of motor reserve and endurance is unclear.57 Dobkin also states that because most individuals have difficulty separating psychological manifestations of fatigue from the neuromuscular mechanisms, the use of subjective fatigue rating scales, such as the VAS-F, may not be sufficient enough to specifically measure peripheral fatigue. On the other hand, it is more likely that people with PD have a more central fatigue component than a peripheral fatigability component and the method of self-report used in this study was indeed appropriate. However, perhaps the treadmill condition did not sufficiently challenge the central component. Again, this may have been a mismatch of theory. Future research should explore these relationships.
This is one of the first published studies to explore the relationship between the BDNF polymorphism and balance. Our results suggest that the BDNF polymorphism has no effect on balance and gait in individuals with PD and does not affect the response to a fatiguing condition. This latter result is support by research from Lotrich et al who reported that the BDNF polymorphism was not associated with a self-report of fatigue in euthymic individuals assessed for depression.58 Additionally, although this study was underpowered for this aim, the raw data also does not suggest any trends in the relationship. Some research has suggested that BDNF polymorphism may be more involved in motor learning and plasticity than actual motor performance. As the present study did not explore motor learning or plasticity, the effect explored was motor performance. Since motor performance did not appear to be affected in this study, it can be concluded that this polymorphism may not play a large role in balance and gait in PD. In support of this, Svetel et al also found that the Val66Met polymorphism does not modify motor and non-motor features in people with PD.59
Of the four cardinal signs of PD, postural instability is the only sign shown to be mostly unresponsive to PD medications. Our results are in line with previous research that has suggested that postural instability is refractory to dopaminergic therapies. In support of this, Di Guilio et al suggest that postural instability in PD is caused by disruption to non-dopaminergic systems.60 Muller et al has suggested that the neurotransmitter acetylcholine may possibly be implicated in postural instability.61 Likewise, Bohnen et al found that thalamic acetylcholine activity was significantly reduced in individuals with PD with a history of falls as compared to non-fallers, even when there was no difference in nigrostriatal dopaminergic activity between the two groups.62 For this reason, dopaminergic therapies may not have a positive impact on postural instability in PD.60 Because postural instability is associated with higher fall risk,63 it is important to educate individuals with PD that balance performance is not improved by medication usage and stress the importance of evidence-based therapies like strengthening and balance training to decrease fall risk.64,65
There are a few additional limitations that warrant discussion. First, our recruitment methods did not meet our sample size estimations within the funding time frame. Due to this limitation, our study may have been underpowered for our primary aims. Because we fell short of our sample size estimations, we decided to run an interim analysis to determine if we should submit a request for an extension and additional funding. The interim analysis demonstrated that our interaction effect sizes were too small to justify an extension and additional funds. A sample size re-estimation (10% drop out rate and using the interaction effect sizes from Table 4) revealed that between 114 and 868 participants (including the participants already collected) would be needed to have sufficient power to identify statistically significant interaction. Additionally, because our sample size was small we were unable to see if PD subgroups (eg, postural instability – gait difficulty, tremor dominan) reacted differently to the fatiguing condition. Additionally, the subjective nature of our fatigue, even though it theoretically incorporates both central and peripheral fatigue, may have introduced variability in the actual fatigue levels. Also, while the VAS-F has been used in PD populations, there is still insufficient data on the clinimetric properties in PD.66 Because the majority of our participants (Table 1) were classified as Hoehn and Yahr 1–2 (pre-clinically relevant balance dysfunction), the results may not be an accurate representation of individuals with PD who are more likely to be referred to physical therapy clinics for balance impairment. Lastly, the balance and gait assessment was conducted by researchers who were not blinded to the study aims.
CONCLUSIONS
Fatigue resulting from treadmill training may not negatively impact static or dynamic balance responses in individuals with PD. Likewise, it also does not negatively impact gait characteristics and may actually improve gait speed immediately following cessation of the treadmill session. Clinicians can feel confident in inducing moderate fatigue as defined in the present study in their patients with PD using a treadmill with the intensity and duration used in the present study without increasing risk for falls. Clinicians should also be sure to educate these individuals on the limitations of an effect of PD medication on postural instability. Furthermore, the BDNF polymorphism appears to be unrelated to balance performance or balance responses following a fatiguing condition.
Supplementary Material
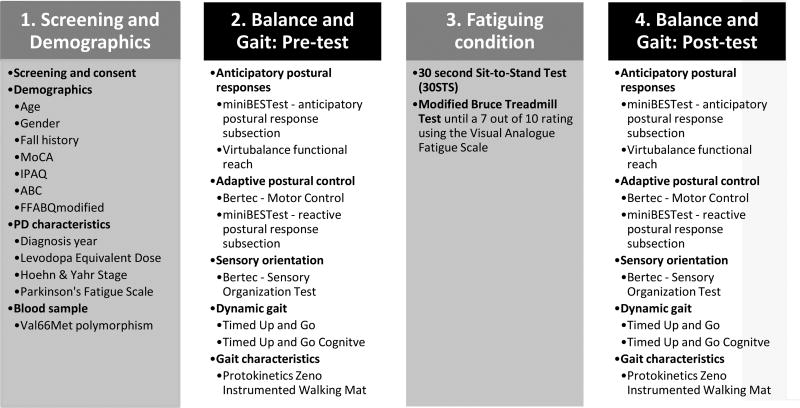
Figure 1.
Participant screening and clinical balance and gait testing.
Acknowledgments
FUNDING: Research reported in this publication was supported by a University of Nevada, Las Vegas Student Opportunity Research Award and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM109025.
Footnotes
Presented at:
Baer M, Klemetson B, Scott D, Navalta J, Murtishaw A, Kinney J, Landers MR. The effects of fatigue on balance in individuals with Parkinson’s disease: influence of medication and brain-derived neurotrophic factor genotype. APTA Combined Sections Meeting, San Antonio, Texas, February 15–18, 2017.
Video Abstract available for more insights from the authors (see Video, Supplemental Digital Content 1)
VirtuSense Technologies, Peoria Next Innovation Center 801 W Main St, Suite B216 Peoria, IL 61606, (309)495-7325
Bertec Corporation, 6171 Huntley Rd, Suite J Columbus, OH 43229, (614) 543-8099
ProtoKinetics LLC, 60 Garlor Dr., Havertown, PA 19083, (610) 449-4879
Contributor Information
Michael Baer, Physical Therapist, Spring Valley Hospital, 5400 S Rainbow Blvd, Las Vegas, NV 89118, michael-baer@att.net.
Bradley Klemetson, Meier and Marsh Professional Therapies, 2356 N 400 W #101, Tooele, UT 84074, bradnkenzie@gmail.com.
Diana Scott, Physical Therapist, Centennial Hills Hospital, 6900 North Durango Drive, Las Vegas, NV 89149, dcscott325@yahoo.com.
Andrew S. Murtishaw, PhD Candidate, Department of Psychology, University of Nevada, Las Vegas, 4505 Maryland Parkway, Box 455030, Las Vegas, Nevada, USA, 702-895-5523, andrew.murtishaw@unlv.edu.
James W. Navalta, Associate Professor, Department of Kinesiology, University of Nevada, Las Vegas, 4505 Maryland Parkway, Box 453034, Las Vegas, Nevada, USA, 702-895-2344, james.navalta@unlv.edu.
Jefferson W. Kinney, Associate Professor, Department of Psychology, University of Nevada, Las Vegas, 4505 Maryland Parkway, Box 455030, Las Vegas, Nevada, USA, 702-895-4766, jefferson.kinney@unlv.edu.
Merrill R. Landers, Chair and Professor, Department of Physical Therapy, University of Nevada, Las Vegas, 4505 Maryland Parkway, Box 453029, Las Vegas, Nevada, USA, 702-895-1377, merrill.landers@unlv.edu.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Morris JG, Traficante R, Reid WG, O'Sullivan DJ, Williamson PM. The sydney multicentre study of Parkinson's disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry. 1999;67(3):300–307. doi: 10.1136/jnnp.67.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson's disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996;61(1):70–74. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Martin P. An introduction to the concept of "quality of life in Parkinson's disease". J Neurol. 1998;245(Suppl 1):S2–6. doi: 10.1007/pl00007733. [DOI] [PubMed] [Google Scholar]
- 5.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2002;8(3):193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, Group NV. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord. 2011;26(3):399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 7.Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology. 2003;60(7):1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JH, Friedman H. Fatigue in Parkinson's disease: a nine-year follow-up. Mov Disord. 2001;16(6):1120–1122. doi: 10.1002/mds.1201. [DOI] [PubMed] [Google Scholar]
- 9.Lou JS. Physical and mental fatigue in Parkinson's disease: epidemiology, pathophysiology and treatment. Drugs Aging. 2009;26(3):195–208. doi: 10.2165/00002512-200926030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Helbostad JL, Leirfall S, Moe-Nilssen R, Sletvold O. Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci. 2007;62(9):1010–1015. doi: 10.1093/gerona/62.9.1010. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins JC, Valovich McLeod TC, Perrin DH, Gansneder BM. Performance on the Balance Error Scoring System Decreases After Fatigue. J Athl Train. 2004;39(2):156–161. [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 13.Dinoff A, Herrmann N, Swardfager W, Lanctot KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. The European journal of neuroscience. 2017;46(1):1635–1646. doi: 10.1111/ejn.13603. [DOI] [PubMed] [Google Scholar]
- 14.Foltynie T, Lewis SG, Goldberg TE, et al. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson's disease. J Neurol. 2005;252(7):833–838. doi: 10.1007/s00415-005-0756-5. [DOI] [PubMed] [Google Scholar]
- 15.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience. 2006;9(6):735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 16.McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20(5):1254–1262. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson DS, Dijkstra BW, Horak FB. Postural motor learning in people with Parkinson's disease. J Neurol. 2016;263(8):1518–1529. doi: 10.1007/s00415-016-8158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93(12):1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foltynie T, Cheeran B, Williams-Gray CH, et al. BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009;80(2):141–144. doi: 10.1136/jnnp.2008.154294. [DOI] [PubMed] [Google Scholar]
- 20.Grimbergen YA, Munneke M, Bloem BR. Falls in Parkinson's disease. Curr Opin Neurol. 2004;17(4):405–415. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- 21.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Alzheimer's Disease Neuroimaging I. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC geriatrics. 2015;15:107. doi: 10.1186/s12877-015-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincus JH, Barry K. Influence of dietary protein on motor fluctuations in Parkinson's disease. Arch Neurol. 1987;44(3):270–272. doi: 10.1001/archneur.1987.00520150026014. [DOI] [PubMed] [Google Scholar]
- 24.Hsu A, Yao HM, Gupta S, Modi NB. Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet((R))), sustained-release carbidopa-levodopa (Sinemet((R)) CR), and carbidopa-levodopa-entacapone (Stalevo((R))) J Clin Pharmacol. 2015;55(9):995–1003. doi: 10.1002/jcph.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 28.Landers MR, Durand C, Powell DS, Dibble LE, Young DL. Development of a scale to assess avoidance behavior due to a fear of falling: the Fear of Falling Avoidance Behavior Questionnaire. Phys Ther. 2011;91(8):1253–1265. doi: 10.2522/ptj.20100304. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson MH, Bladh S, Hagell P. Fatigue in Parkinson's disease: measurement properties of a generic and a condition-specific rating scale. J Pain Symptom Manage. 2013;46(5):737–746. doi: 10.1016/j.jpainsymman.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 2009;164(2):877–885. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2011;35(2):90–97. doi: 10.1097/NPT.0b013e31821a620c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King LA, Priest KC, Salarian A, Pierce D, Horak FB. Comparing the Mini-BESTest with the Berg Balance Scale to Evaluate Balance Disorders in Parkinson's Disease. Parkinson's disease. 2012;2012:375419. doi: 10.1155/2012/375419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King L, Horak F. On the mini-BESTest: scoring and the reporting of total scores. Phys Ther. 2013;93(4):571–575. doi: 10.2522/ptj.2013.93.4.571. [DOI] [PubMed] [Google Scholar]
- 35.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45(6):M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 36.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5. McGraw Hill; 2013. [Google Scholar]
- 37.Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions--a conceptual model. Brain Res Brain Res Rev. 1998;28(1–2):118–135. doi: 10.1016/s0165-0173(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 38.Ford-Smith CD, Wyman JF, Elswick RK, Jr, Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76(1):77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 39.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 40.Hofheinz M, Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin Rehabil. 2010;24(9):831–842. doi: 10.1177/0269215510367993. [DOI] [PubMed] [Google Scholar]
- 41.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait & posture. 2004;20(1):20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 42.Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Phys Ther. 2000;80(8):782–807. [PubMed] [Google Scholar]
- 43.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 44.Friedman J, Friedman H. Fatigue in Parkinson's disease. Neurology. 1993;43(10):2016–2018. doi: 10.1212/wnl.43.10.2016. [DOI] [PubMed] [Google Scholar]
- 45.Kos D, Nagels G, D'Hooghe MB, Duportail M, Kerckhofs E. A rapid screening tool for fatigue impact in multiple sclerosis. BMC neurology. 2006;6:27. doi: 10.1186/1471-2377-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryant MS, Jackson GR, Hou JG, Protas EJ. Treadmill exercise tests in persons with Parkinson's disease: responses and disease severity. Aging Clin Exp Res. 2016;28(5):1009–1014. doi: 10.1007/s40520-015-0498-x. [DOI] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 48.Landers MR, Lopker M, Newman M, Gourlie R, Sorensen S, Vong R. A Cross-sectional Analysis of the Characteristics of Individuals With Parkinson Disease Who Avoid Activities and Participation Due to Fear of Falling. J Neurol Phys Ther. 2017;41(1):31–42. doi: 10.1097/NPT.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 49.Hamacher D, Torpel A, Hamacher D, Schega L. The effect of physical exhaustion on gait stability in young and older individuals. Gait & posture. 2016;48:137–139. doi: 10.1016/j.gaitpost.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Herman T, Giladi N, Hausdorff JM. Treadmill training for the treatment of gait disturbances in people with Parkinson's disease: a mini-review. J Neural Transm (Vienna) 2009;116(3):307–318. doi: 10.1007/s00702-008-0139-z. [DOI] [PubMed] [Google Scholar]
- 51.Stoykov ME, Corcos DM, Madhavan S. Movement-Based Priming: Clinical Applications and Neural Mechanisms. J Mot Behav. 2017;49(1):88–97. doi: 10.1080/00222895.2016.1250716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Field-Fote EC. Exciting recovery: augmenting practice with stimulation to optimize outcomes after spinal cord injury. Prog Brain Res. 2015;218:103–126. doi: 10.1016/bs.pbr.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Stoykov ME, Madhavan S. Motor priming in neurorehabilitation. J Neurol Phys Ther. 2015;39(1):33–42. doi: 10.1097/NPT.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambourne K, Audiffren M, Tomporowski PD. Effects of acute exercise on sensory and executive processing tasks. Med Sci Sports Exerc. 2010;42(7):1396–1402. doi: 10.1249/MSS.0b013e3181cbee11. [DOI] [PubMed] [Google Scholar]
- 55.Koo JH, Cho JY, Lee UB. Treadmill exercise alleviates motor deficits and improves mitochondrial import machinery in an MPTP-induced mouse model of Parkinson's disease. Exp Gerontol. 2017;89:20–29. doi: 10.1016/j.exger.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Egerton T. Self-reported aging-related fatigue: a concept description and its relevance to physical therapist practice. Phys Ther. 2013;93(10):1403–1413. doi: 10.2522/ptj.20130011. [DOI] [PubMed] [Google Scholar]
- 57.Dobkin BH. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabilitation and neural repair. 2008;22(2):105–110. doi: 10.1177/1545968308315046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lotrich FE, Albusaysi S, Ferrell RE. Brain-derived neurotrophic factor serum levels and genotype: association with depression during interferon-alpha treatment. Neuropsychopharmacology. 2013;38(6):985–995. doi: 10.1038/npp.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svetel M, Pekmezovic T, Markovic V, et al. No association between brain-derived neurotrophic factor G196A polymorphism and clinical features of Parkinson's disease. Eur Neurol. 2013;70(5–6):257–262. doi: 10.1159/000352033. [DOI] [PubMed] [Google Scholar]
- 60.Di Giulio I, St George RJ, Kalliolia E, Peters AL, Limousin P, Day BL. Maintaining balance against force perturbations: impaired mechanisms unresponsive to levodopa in Parkinson's disease. Journal of neurophysiology. 2016 doi: 10.1152/jn.00996.2015. jn 00996 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller ML, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. 2013;13(9):377. doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson's disease. Clin Neuropharmacol. 1989;12(2):98–105. doi: 10.1097/00002826-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Klamroth S, Steib S, Gassner H, et al. Immediate effects of perturbation treadmill training on gait and postural control in patients with Parkinson's disease. Gait & posture. 2016;50:102–108. doi: 10.1016/j.gaitpost.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26(9):1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 66.Friedman JH, Alves G, Hagell P, et al. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson's disease. Mov Disord. 2010;25(7):805–822. doi: 10.1002/mds.22989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.