Short abstract
Introduction
Neuropathic pain is a debilitating condition. The importance of neuroimmune interactions in neuropathic pain has been evidenced by the involvement of different immune cells in peripheral and central sensitization of pathological pain. Macrophages and microglia are the most abundant immune cells activated in injured nerves and spinal cord, respectively. Several lines of evidence showed that macrophage/microglia survival, activation, proliferation, and differentiation require the involvement of macrophage-colony stimulating factor. In this study, we investigated whether blocking macrophage-colony stimulating factor/colony stimulating factor 1 receptor signaling can be effective in relieving neuropathic pain.
Materials and methods
Partial sciatic nerve ligation was performed in mice to induce neuropathic pain behavior. Mice were orally treated with a selective colony stimulating factor 1 receptor inhibitor, PLX5622, daily in both preventive (two days prior to surgery until D14 post-partial sciatic nerve ligation) and reversal paradigms (D28–D33 post-partial sciatic nerve ligation). Animal neuropathic pain behavior was monitored using von Frey hairs and acetone application. Phenotype of macrophages in injured nerves was analyzed at D3 and D33 post-injury using flow cytometry analysis. The effect of PLX5622 on microglia activation in lumbar spinal cord was further examined by immunohistochemistry using Iba-1 antibody.
Results
Significant alleviation of both mechanical and cold allodynia was observed in PLX5622-treated animals, both in preventive and reversal paradigms. PLX5622 treatment reduced the total number of macrophages in injured nerves, it appears colony stimulating factor 1 receptor inhibition affected more specifically CD86+ (M1 like) macrophages. Consequently, the expression of various pro-inflammatory cytokines (TNF-α, IL-1β) was reduced. Microglia activation in dorsal horn of lumbar spinal cord following partial sciatic nerve ligation was significantly inhibited with PLX5622 treatment in both preventive and reversal paradigms.
Conclusion
Macrophages in peripheral nerve and microglia in the spinal cord are required in the generation and maintenance of injury-associated neuropathic pain. Blocking macrophage-colony stimulating factor/colony stimulating factor 1 receptor signaling on these myeloid cells along the pain transmission pathway is an effective strategy to alleviate neuropathic pain.
Keywords: Inflammation, macrophages, microglia, neuropathic pain
Introduction
Neuropathic pain is a debilitating condition. Emerging evidence suggests the importance of neuroimmune interaction in inflammation and abnormal pain sensation in neuropathic pain. In animal models of neuropathic pain where peripheral nerves are injured, various types of immune cells accumulate at the site of injury and become activated. Macrophages which have a crucial role in tissue homeostasis are the most abundant among others. The number of macrophages within injured nerves increases shortly after injury and many macrophages persist at least for three months. In addition, morphological difference among nerve macrophages is detected in which large, foamy macrophages with vacuoles are associated with phagocytosis, whereas small, round macrophages closely localized to the site of injury release inflammatory cytokines.1 Macrophages in injured peripheral nerves also contributes in breakdown of blood-nerve barrier through vascular endothelial growth factor (VEGF) signaling,2 which further mediates inflammatory reactions in injured nerves with an influx of immune cells and molecules.
Interestingly, damage to the peripheral nervous system (for example, injury to the peripheral nerves) can lead to microglia activation in the central nervous system (CNS). Microglia undergo structural and functional modifications in the spinal cord and brain in different animal models of neuropathic pain. In response to peripheral nerve injury, their ability of phagocytosis, antigen presentation, lymphocyte activation, and release inflammatory molecules are significantly enhanced.3 Microglia upregulate cell surface markers and release a variety of inflammatory mediators, including cytokines, chemokines, and neurotropic factors, which impact neurons and other surrounding glial cells, leading to an increased neuronal hyperexcitability.3–5
Macrophage-colony stimulating factor (M-CSF) is one of major factors contributing in the survival, proliferation, activation, and differentiation of macrophages in periphery6,7 and microglia in the CNS.8–10 Upon binding of M-CSF to its receptor, colony stimulating factor 1 receptor (CSF1R) tyrosine kinase, several signaling pathways for cellular activities such as motility, growth, differentiation, survival, and proliferation are activated.11–13 Numerous studies investigate the effect of M-CSF in the pathology of various diseases, including arthritis, cancer, atherosclerosis, vascular injury, and obesity.14 Recently, it has been found that M-CSF released by sensory neurons is a key molecule initiating microglia activation in the dorsal lumbar cords following peripheral nerve damage.15 In this study, we aim to investigate the potential of blocking M-CSF/CSF1R signaling in relieving neuropathic pain. Our findings suggest that both nerve macrophages and spinal microglia are highly associated with the generation/maintenance of hypersensitivity in neuropathic pain through CSF1R signaling pathway. CSF1R could be an effective drug target for treating neuropathic pain.
Materials and methods
Animals
C57BL/6 mice (males, 10–12 weeks old, 20–25 g) were purchased from the Charles River Laboratories. All animals were acclimatized to standard laboratory conditions (14-h light, 10-h dark cycle) and given free access to rodent chow and water. All experiments were approved by the Institutional Animal Care and Use Committee of McGill University and conformed to the ethical guidelines of the International Association for the Study of Pain.
Partial sciatic nerve ligation
Partial sciatic nerve ligation (PSNL) was performed according to the method of Seltzer et al.,16 adapted to mice.17 Briefly, mice were deeply anesthetized with isoflurane, and under aseptic conditions, the left sciatic nerve was exposed at high-thigh level. The dorsum of the nerve was carefully freed from surrounding connective tissues at a site near the trochanter. An 8-0 silk suture was inserted into the nerve with a 3/8 curved, reversed-cutting mini-needle and tightly ligated so that the dorsal 1/3–1/2 of the nerve thickness was trapped in the ligature. The wound was then closed with two to three skin sutures (4-0).
Drug preparation and treatment
PLX5622 is a potent inhibitor of CSF1R tyrosine kinase activity (KI = 5.9 nM) with at least 50-fold selectivity over four related kinases and over 100-fold selectivity against a panel of 230 kinases (Plexxikon Inc.). The molecule has been used to investigate the critical role of microglia/macrophages in many different circumstances.18–21 The dose in the current study was suggested by the company and in accordance with these previous reports where 1200 ppm in chow daily is sufficient to eliminate microglia fully or 300 ppm in chow daily can reduce partially microglia. Our 65 mg/kg dose nominally is close to the 300 ppm chow daily dose which allows us to lower macrophages in nerves. The drug was prepared following manufacturer’s protocol. Briefly, PLX5622 stock was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 130 mg/ml; 2% hydroxypropyl methyl cellulose (Sigma) and 25% polysorbate 80 (Fluka) were prepared to make a diluent. On each dosing day, PLX5622 stock was diluted 20-fold by adding 1 volume of drug stock (130 mg/ml) in 19 volumes of diluent, making a working solution at 6.5 mg/ml. Vehicle solution was prepared with a mix of diluent and DMSO. Mice were treated daily by oral gavage with 100 μl solution (6.5 mg/ml) per 10g body weight (final dose at 65 mg/kg body weight).
To study preventive effect of PLX5622, mice were treated with either a drug or vehicle two days prior to surgery, then daily until D14 post-PSNL. For the reversal effect of PLX5622, treatment started at D28, daily until D33 post-PSNL.
Behavioral analysis
Mice were habituated to the testing environment 1 to 2 h daily for at least two days before baseline testing. Mice were treated with either vehicle or PLX5622 daily in the morning. The investigator was blinded to the treatment conditions.
Mechanical sensitivity was assessed with calibrated von Frey hairs (Stoelting) using the up-down method.22 One hour after the administration of the drug, mice were placed on a metal mesh floor with small Plexiglas cubicle containers and allowed 1 h for habituation before testing. A set of eight calibrated von Frey filaments with increasing stiffness (ranging from 0.008 to 1.40 g of force) were applied to the plantar surface of hind paw until they bent. A positive reaction was recorded if mice exhibited a brisk paw withdrawal reaction from the stimuli. The threshold force required to elicit withdrawal of the paw (50% paw withdrawal thresholds) was determined as the average of two tests separated by at least 1 h.
Acetone test was performed on mice hind paws to evaluate cold allodynia. After von Frey test, mice were put back to their home cages to have a rest with free access to chow and water. Two hours later, they were returned to the same setting described above for the von Frey test, for acetone test. Total duration of acetone-evoked behaviors (flinching, licking, or biting of their hind paws) was counted during 1 min after one drop of acetone (∼25 μl) application to the plantar surface of hind paws as previously described in literature.23
Tissue preparation
For flow cytometry analysis
Mice were deeply anesthetized with a ketamine/xylazine mixture. Anesthetized mice were perfused transcardially with 0.9% NaCl. Both ipsilateral and contralateral nerves were extracted and immediately diced into small pieces with a razor blade.
For intracellular cytokine staining, an in vivo brefeldin A (BFA) protocol was followed.24 Mice were injected with 2 μl/g body weight BFA (Sigma, 2.5 mg/ml in DMSO (EMD Millipore) via tail vein. Five hours later, mice were sacrificed, and nerves were harvested. After tissue digestion, samples were incubated with 1 μl of GolgiPlug containing BFA (BD) added to 800 μl RPMI for 1.5 h at 37°C.
For immunohistochemistry
Mice were deeply anesthetized with a ketamine/xylazine mixture. For histological studies, mice after 0.9% NaCl transcardial perfusion were further perfused with 4% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer at pH 7.4. Lumbar spinal cords were removed and placed in 4% PFA overnight at 4°C and then transferred to 30% sucrose in phosphate buffer for cryoprotection for at least 18 h. Lumbar spinal cords were cut transversely into 25 μm-thick sections on a sliding microtome and collected in an anti-freeze solution (0.05 M sodium phosphate buffer containing 30% ethylene glycol and 20% glycerol, pH 7.3).
Flow cytometry analysis
Cell surface antigen staining
Diced nerves were placed in digestion buffer containing RPMI, collagenase IV (1.6 mg/ml, Sigma), and DNase I (200 units/ml, Sigma), then incubated for 1 h at 37°C. Samples were pressed through a 70-μm nylon filter to remove undigested debris followed by centrifugation with stain buffer (BD). Samples were then incubated with solution containing 2.4G2 antibody to block Fc receptors for 30 min at 4°C, then stained for cell surface antigens with specific fluorochrome-conjugated rat anti-mouse antibodies for 30 min at 4°C.
Intracellular staining
After cell surface antigen staining, samples were fixed with 4% PFA for 15 min at 4°C, then permeabilized for 30 min using fixation/permeabilization kit (BD, 555028). They were then resuspended with 1X perm/wash solution containing antibodies for 30 min at 4°C. Staining specificity was verified with prepared controls in which there was no primary antibody, and correction of spectral overlap was done by using negative and positive compensation beads (BD 552844). Cellular events were acquired using a LSR Fortessa flow cytometer (BD sciences), and data were analyzed using Flow Jo software. Detailed information of antibodies was listed in Table 1. Different combinations of antibodies were used for (a) Figure 2: CD45, CD11b, CD68, CD86, and CD206 and (b) Figure 3: CD45, CD11b, CD68, TNF-α, or IL-1β.
Table 1.
Detailed information of antibodies used for Fluorescence-activated cell sorting (FACS).
| Antibody | Specificity | Clone | Dilution | Company | Catalog no. |
|---|---|---|---|---|---|
| V450 Rat anti-mouse CD45 | Leukocytes common antigen | 30-F11 | 1:50 | BD pharmingen | 560501 |
| PerCP-Cy5.5 Rat anti-mouse CD11b | Mouse integrin alpha M | M1/70 | 1:50 | BD pharmingen | 550993 |
| FITC Rat anti-mouse CD68 | Mouse macrosialin, CD68 | FA-11 | 1:100 | Serotec | MCA1957 |
| PE Rat anti-mouse CD86 (B7.2) | Mouse CD86 (B7.2) | GL1 | 1:50 | eBioscience | 12-0862-85 |
| Alex647 Rat anti-mouse CD206 | Mouse mannose receptor, CD206 | MR5D3 | 1:100 | Serotec | MCA2235 |
| APC Rat anti-mouse TNF-α | Mouse tumor necrosis factor-alpha | MP6-XT22 | 1:50 | eBioscience | 17-7321-82 |
| APC-e780 Rat anti-mouse IL-1β | Pro-form of mouse IL-1 beta | NJTEN3 | 1:50 | eBioscience | 47-7114-80 |
Figure 2.
The effect of CSF1R inhibition on macrophage subsets in injured peripheral nerves. By using flow cytometry analysis, nerve macrophages were identified as CD45CD11b expressing cells. Among them, four distinct macrophage subsets were analyzed by the expression of CD86 and/or CD206. (a) Cells were isolated from peripheral nerves, then macrophages were selected based on the expression of CD45 (x-axis) and CD11b (y-axis). CD45 CD11b positively stained cells were located on upper right corner. From CD45+CD11b+ cells, they were further analyzed for CD68 expression. Among CD45+CD11b+ macrophages, they were analyzed for the expression of CD86 (B7.2) and CD206. (b) CD45+CD11b+ cells in peripheral nerves were significantly increased after PSNL. It peaked at D3 post-PSNL, and then reduced at following time-points (D14 and D28), however, still significantly higher than that of uninjured nerves. Individual macrophage subset based on the expression of CD86 and/or CD206 was shown in different colors (***p < 0.001). Existence of different macrophage subsets in uninjured sciatic nerves was shown in red box. Insert: Example of FACS gating method for CD86 vs CD206 expressing cells. (c) In the preventive study, CD45+CD11b+ macrophages were significantly reduced at D3 post-PSNL in PLX5622-treated animals, compared to vehicle-treated animals (#p < 0.05). More specifically, CD86+CD206− macrophages were reduced with CSR1R inhibition, which did not affect CD206+ macrophages (*p < 0.05). (d) PLX5622 treatment also lowered CD45+CD11b+ macrophages in injured sciatic nerves in a reversal paradigm (##p < 0.01). Accumulation of CD86 expressing macrophages including CD86+CD206+ (**p < 0.01) and CD86+CD206− (*p < 0.05) subsets were reduced with CSF1R inhibition, while CD86−CD206+ macrophages were not affected (n = 7–10/group).
Figure 3.
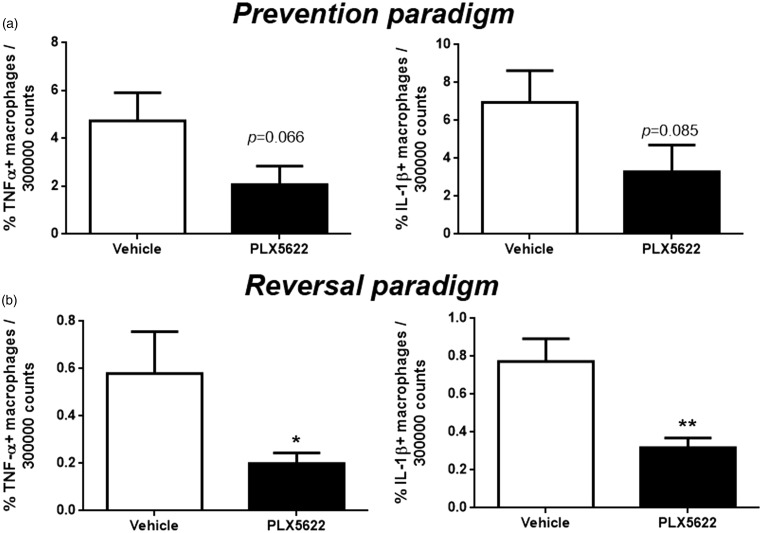
The effect of CSF1R inhibition on macrophage cytokine expression in injured peripheral nerves. Nerve macrophages were first identified by CD45 and CD11b expression by flow cytometry analysis. They were further examined with intracellular staining to detect TNF-α or IL-1β expression. (a) In the prevention paradigm, both TNF-α (p = 0.066) and IL-1β (p = 0.085) expressing macrophages were partially reduced with CSF1R inhibition. (b) In the reversal paradigm, both TNF-α and IL-1β expressing macrophages were sharply decreased (*p < 0.05, **p < 0.01) with CSF1R inhibition (n = 3–6/group).
Quantitative analysis of nerve macrophages using flow cytometry
Cells isolated from sciatic nerves were first analyzed for leukocyte common antigen CD45 and integrin alpha-M beta-2 CD11b expression. CD45 CD11b double positive cells were further gated by CD68 expression. CD45+CD11b+ macrophages were then analyzed for CD86 and/or CD206 expression (Figure 2). The percentage of each macrophage subset was analyzed over 300,000 total counts including debris and other cells. CD86 and/or CD206 expressing macrophages (Figure 2) and TNF-α or IL-1β expressing cells (Figure 3) were also measured over 300,000 counts.
Immunohistochemistry
A standard fluorescent immunohistochemistry protocol was applied to characterize spinal microglia phenotypes. Free-floating sections were incubated in a blocking buffer consisting of 3% goat serum, 1% bovine serum albumin, and 0.25% Triton X-100 in Tris-buffered saline for 1 h at room temperature followed by overnight incubation with rabbit anti-ionized calcium-binding adaptor molecule-1 (Iba-1) polyclonal primary antibody (1:1000; Wako Chemicals) at 4°C. Sections were then incubated with Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody (1:500; Invitrogen) for 1 h at room temperature followed by 5 min incubation of 4′, 6-diamidino-2-phenylindole for nuclear counterstaining (1:100,000; Sigma). Sections were mounted onto slides, then coverslipped with Vectashield mounting medium (Vector Laboratories).
Image processing and analysis
The immunofluorescent staining was examined under microscope. Images were acquired using an Olympus BX51 microscope equipped with a color digital camera (Olympus DP71). Quantitative analysis of the immunofluorescent Iba-1+ cells was performed on images digitized using a constant set of parameters (exposure time, gain and post-image processing) with special attention to avoid signal saturation. Images were acquired using 20X objectives of the microscope, then the area of interest was placed around superficial laminae (laminae I and II) in dorsal horn of lumbar spinal cords, then, Iba-1+ cells were quantified with ImagePro software by tracking Iba-1+ cells co-stained with 4′, 6-diamidino-2-phenylindole+ nuclei. Six to ten sections per animal, three animals/group were included in the quantification.
Statistical analysis
All data were presented as mean ± SEM. Significance of pain behavioral changes (each time point vs. baseline) were analyzed with one-way analysis of variance followed by Dunnett’s test (Figure 1). Unpaired t test was used for the area under the curve to determine the difference between vehicle versus PLX5622-treated animals during the entire treatment period (Figure 1, n = 6/group). Unpaired t tests were performed to measure percentage of each macrophage subset in Figure 2 (n = 7–10/group), Figure 3 (n = 3–6/group), and cell numbers in Figure 4 (n = 3/group). The criterion for statistical significance was p < 0.05.
Figure 1.
The effect of CSF1R inhibition on neuropathic pain behavior. Animals were treated with either vehicle or PLX5622, daily, from two days prior to the surgery, until D14 post-surgery (treatment duration was shown in red line). (a) Compared to vehicle-treated mice, paw withdrawal threshold in PLX5622-treated mice was higher, indicating that PLX5622 prevented the development of injury-associated mechanical allodynia. (b) The withdrawal duration upon acetone application was significantly shorter in mice receiving PLX5622 than those receiving vehicle treatment, suggesting that PLX5622 is also effective in preventing the development of cold allodynia following nerve injury. (c) When the treatment started at D28 when the hypersensitivity was fully established, PLX 5622 increased significantly paw withdrawal threshold, almost to pre-injury level. (d) PLX5622 decreased the paw withdrawal duration in acetone test in a reversal treatment paradigm from D28 to D33. The effect of PLX5622 during entire treatment period was measured by the area under the curve during treatment (inserts in a, b, c, d). (n = 6/group, **p < 0.01, ***p < 0.001).
Figure 4.
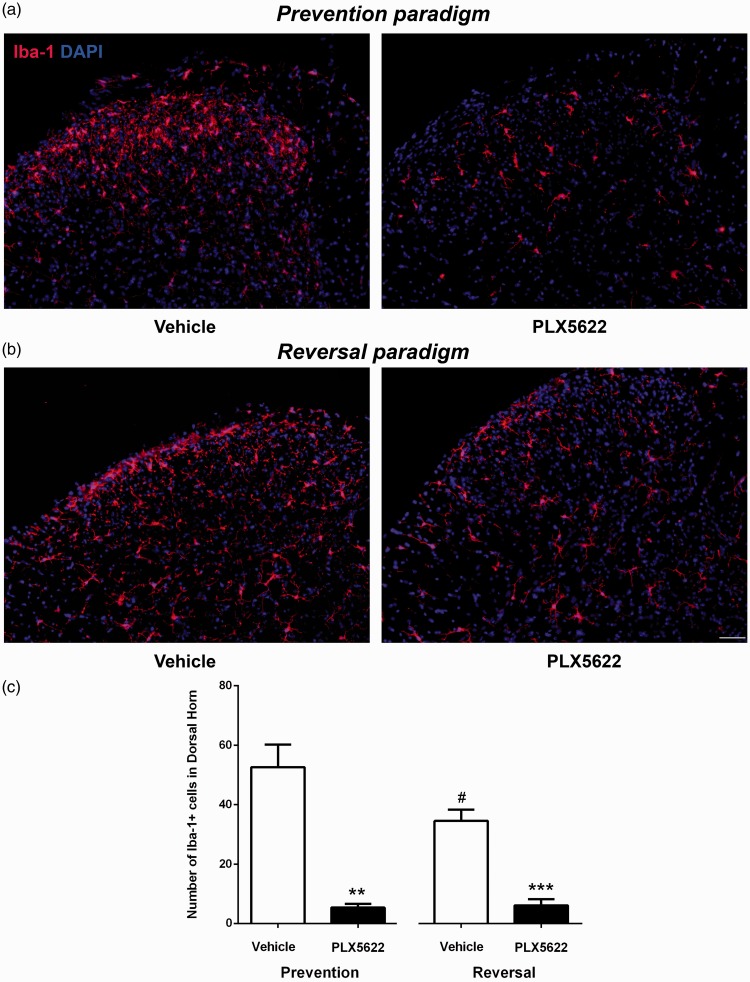
The effect of CSF1R inhibition on spinal microglia. Immunohistochemistry staining revealed that compared to vehicle-treated mice, the density of Iba-1+ microglia in spinal cord dorsal horn reduced in PLX5622-treated mice. Representative Iba-1 staining in the lumbar spinal cord sections of D3 (a) and D33 (b) post-PSNL mice, with vehicle and PLX5622 treatment (Scale bars: 50 μm). (c) The number of Iba-1+ microglia was quantified which was significantly decreased in PLX5622-treated mice in both prevention and reversal groups (**p < 0.01, ***p < 0.001). It is interesting to notice that the number of microglia in vehicle-treated mice peaked at D3 post-PSNL, reduced at D33 post-PSNL (#p < 0.05) (n = 3/group).
Results
Blocking CSF1R signaling effectively attenuates injury-triggered neuropathic pain behavior
Prevention paradigm
Mice were treated with PLX5622, a selective inhibitor of tyrosine kinase in CSF1R, from two days prior to surgery to D14 post-PSNL. Following PSNL, in vehicle-treated mice, paw withdrawal threshold to von Frey hairs significantly decreased shortly after the surgery and remained low until at least D14 post-PSNL, indicating the development of mechanical allodynia. Cold allodynia was also established in these mice since upon application of acetone, paw withdrawal duration increased significantly in vehicle-treated mice. Compared to control mice, the development of both mechanical and cold allodynia was prevented in PLX5622-treated mice. During treatment period (D0–D14), animal paw withdrawal thresholds in PLX5622-treated mice were significantly higher than those in vehicle-treated mice (Figure 1(a)). Moreover, paw withdrawal duration following acetone application (Figure 1(b)) was significantly decreased in PLX5622-treated mice, indicating they became less sensitive to cold stimulation, compared to vehicle-treated mice. All behavioral data were analyzed with two-way analysis of variance with multiple comparisons for each time point and unpaired t test for the entire period based on the area under the curve.
Reversal paradigm
In this experiment, PLX5622 treatment was given to mice from D28 to D33 post-PSNL, when the injury-induced mechanical and cold hypersensitivity was fully established. PLX5622 treatment almost completely reversed injury-triggered mechanical (Figure 1(c)) and cold allodynia (Figure 1(d)). The same statistical analysis used in the prevention experiments was applied in the reversal paradigm.
Blocking CSF1R signaling inhibits macrophage activation in injured nerve
Strategy to analyze macrophages in injured sciatic nerves using flow cytometry analysis
Macrophages accumulate abundantly in injured nerves. As shown in Figure 2(a), cells isolated from sciatic nerves were first analyzed for leukocyte common antigen CD45 and integrin alpha-M beta-2 CD11b expression. The identity of macrophages was further confirmed by staining CD45+CD11b+ positive cells with lysosomal CD68 antibody where over 95% CD45+CD11b+ cells were positive for CD68. CD45+CD11b+ cells in nerves were considered as nerve macrophages. Additional marker was used where over 90% of CD45+CD11b+ cells were found expressing F4/80 (data not shown). Taken together, in the pool of CD45+CD11b+ cells that we analyzed, a clear majority is macrophages, however, a small portion (<10%) could be other types of leukocytes, such as neutrophils. Macrophages were quantified over 300,000 counts which include debris and other cells. CD45+CD11b+ cells were then analyzed for either CD86 and/or CD206 expression. CD86 is also known as B7.2 that provides costimulatory signals necessary for T cell activation, considered a cell surface marker for M1 macrophages. CD206 is a mannose receptor implicated in endocytosis. It is a marker specifically associated with M2 macrophages. The total number of either CD86 or CD206 expressing macrophages was quantified over CD45+CD11b+ cells.
CD86+ and CD206+ macrophage subsets coexist in injured sciatic nerves
Different subsets of macrophages in injured nerve were characterized. PSNL triggered a sharp and dramatic increase of macrophages in injured nerves. At D3 post-PSNL, the percentage of CD45+CD11b+ macrophages increased from 0.37 ± 0.05% (un-injured nerve) to 9.55 ± 0.57%. Although it reduced progressively at later stages (3.23 ± 0.12% at D14 and 2.39 ± 0.39% at D28 post-PSNL), it was still significantly higher than that of uninjured nerves (Figure 2(b)). CD45+CD11b+ macrophages were further analyzed for CD86 and CD206 expression. Macrophage subsets were depicted in different colors in Figure 2(b) including (1) macrophages expressing neither CD86 nor CD206 (gray), (2) macrophages expressing only CD86 (blue), (3) macrophages expressing both CD86 and CD206 (green), and (4) macrophages expressing only CD206 (orange). Four subsets of macrophages co-exist in both un-injured and injured nerves. It is of interest to notice that following PSNL, CD86+ macrophages predominated over CD206+ macrophages at all examined time points. About 54% of CD45+CD11b+ macrophages expressed CD86 at D3 post-PSNL, and then lowered to 40% at D14 post-PSNL which remained until D28 post-PSNL, while there were consistently less than 20% of macrophages in injured nerve expressing CD206 at all time points.
PLX5622 reduces the number of macrophages in injured nerve
We first initiated a prevention paradigm where PLX5622, a selective inhibitor of tyrosine kinase in CSF1R, was administrated two days prior to surgery. At D3 post-PSNL, in injured sciatic nerves of mice treated with PLX5622, the number of CD45+CD11b+ macrophages were significantly reduced (#p < 0.05), compared to vehicle-treated animals (Figure 2(c)). More specifically, CD86+ CD206− cells (shown in blue, Figure 2(c)) were significantly reduced after PLX5622 treatment (*p < 0.05). Interestingly, CD206 expressing macrophages including CD86+CD206+ and CD86−CD206+ subsets were not altered with CSF1R inhibition (Figure 2(c), shown in green and orange, respectively). A reversal paradigm was also applied where PLX5622 treatment started at D28 post-PSNL and lasted for five days. At D33 post-PSNL, the total number of CD45+CD11b+ macrophages significantly reduced with CSF1R inhibition (Figure 2(d), ##p < 0.01). It appears that CSF1R inhibition exerted more prominent effects on CD86 expressing cells including CD86+CD206+ (***p < 0.001, shown in green, Figure 2(d)) and CD86+CD206− macrophages (*p < 0.05, shown in blue, Figure 2(d)), as both macrophage subsets were sharply diminished with PLX5622 treatment. It is also interesting to notice that in vehicle-treated group, CD86+CD206− cells (shown in blue, Figure 2(c) and (d)) were continuously diminished over time after nerve injury, whereas CD86+CD206+ cells (shown in green, Figure 2(c) and (d)) were unchanged.
PLX5622 inhibits nerve macrophage proinflammatory cytokine expression
As macrophages are major source of pro-inflammatory cytokines which have important roles in enhancing neuronal excitability, we used intracellular staining with flow cytometry to determine the ability of nerve macrophage expressing these relevant cytokines upon PLX5622 treatment. In prevention paradigm, PLX5622 treatment reduced partially the number of TNF-α (p = 0.066) and IL-1β (p = 0.085) expressing macrophages isolated from injured nerve (Figure 3(a)). Similar effect was observed in reversal paradigm. TNF-α (*p < 0.05) and IL-1β (*p < 0.01) expressing inflammatory macrophages were also decreased after PLX5622 treatment (Figure 3(b)).
Blocking CSF1R signaling inhibits microglia activation in lumbar spinal cord
M-CSF/CSF1R signaling is not only required for the survival and function of peripheral macrophages but also for CNS microglia. The effect of PLX5622 on spinal microglia which is also an important contributor to neuropathic pain was also examined. Spinal microglia were immunolabeled by an antibody against Iba-1, a calcium-binding protein specifically expressed in microglia. Representative examples of lumbar spinal cord dorsal horns from both preventive and reversal paradigms revealed a significant reduction of Iba-1+ cells with PLX5622 treatment (Figure 4(a) and (b)). Quantitative analysis demonstrated that the number of microglia were higher in vehicle-treated mice at D3 post-PSNL (52.60 ± 7.59, #p < 0.05) than vehicle-treated mice of D33 post-PSNL (34.53 ± 2.17, Figure 4(c)). Inhibition of CSF1R signaling through PLX5622 treatment significantly reduced microglia density in both preventive and reversal paradigms. At D3 post-PSNL, total number of Iba-1+ microglia in dorsal horn of lumbar spinal cord were significantly reduced in PLX5622-treated mice (5.37 ± 0.72, **p < 0.01), compared to vehicle-treated mice (52.60 ± 7.59). In reversal paradigm (D33 post-PSNL), PLX5622 treatment significantly lowered Iba-1+ microglia to 6.10 ± 1.19, compared to vehicle-treated mice (34.53 ± 2.17).
Discussion
Following injury to peripheral nerves, nerve fibers undergo Wallerian degeneration, and animals develop both mechanical and cold allodynia at ipsilateral hind paws. Macrophage/microglia activation is observed in injured peripheral nerves and spinal cord with increased expression of inflammatory molecules. In this study, we revealed that M-CSF/CSF1R signaling pathway plays an important role in regulating both nerve macrophages and spinal microglia activation, thus blocking CSF1R signaling pathway was effective in alleviating neuropathic pain behavior.
Macrophages can be classified into classically activated M1-like pro-inflammatory and alternatively activated M2-like anti-inflammatory or wound healing phenotypes. Upon polarization/activation, they do express different cellular markers, including receptors, enzymes, trophic factors, chemokines, and cytokines.25,26 Usually, M1-like macrophages are considered as neurotoxic while M2-like macrophages promote regeneration in injured axons.27 In this study, we investigated the effect of CSF1R inhibition on macrophage subsets using one of M1 associated markers, CD86 (B7.2) and one of M2 related markers, CD206, on CD45 CD11b labeled macrophages in injured sciatic nerves.
M-CSF is one of the major factors contributing to macrophage survival, proliferation, activation, recruitment, and differentiation.6,7,14,28 In this study, we observed that CSF1R inhibition by PLX5622 reduced macrophage numbers significantly. As tissue macrophages and circulating blood monocytes express CSF1R,29,30 it could be postulated that CSF1R inhibition with PLX5622 could reduce M-CSF-mediated monocyte migration into the injured nerves and alter tissue macrophage differentiation and survival. Moreover, M-CSF could induce VEGF production through MAPK/ERK pathway via the transcription factor Sp1.31 CSF1R inhibition may lead to less VEGF production resulting in reduced local inflammatory reaction which in turn reduces macrophage influx in injured sciatic nerves. Such hypothesis can be supported by the fact that VEGF was found increased in injured nerve, which is critical in maintaining the integrity of blood-nerve barrier.2 Interestingly, it seems that PLX5622 acts more efficiently on CD86 expressing macrophages than CD206 expressing ones. Although underlying mechanisms are still unclear, the lower responsiveness of CD206+ macrophages to M-CSF stimulation has been reported in literature.32 Instead of being modulated by M-CSF, CD206 expression in human macrophages could be regulated by IL-4 and TGF-β stimulation.32 It is also important to consider that when M-CSF stimulated macrophages are exposed to lipopolysaccharide in in vitro studies, they display M2 profiles with an increased production of IL-10.33 Whether blocking CSF1R signaling could enhance anti-inflammatory profile while reducing pro-inflammatory macrophages in injured nerve will need further investigation.
Activation of microglia in the CNS following peripheral nerve injury has been shown to contribute to the development of neuropathic pain in many studies. Microglia undergo structural and functional modifications in the spinal cord and brain in different animal models of neuropathic pain. In response to peripheral nerve injury, microglia became activated. They are prone for enhanced phagocytosis, antigen presentation, lymphocyte activation, and release inflammatory molecules.3 They produce inflammatory molecules such as prostaglandins,34 leukotrienes,35 nitric oxide,36 as well as, pro-inflammatory cytokines, IL-1β,37 IL-6,38 and TNF-α.39 These molecules then modulate spinal neuron excitability.40,41 It has been shown that blocking microglia activation with minocycline can be effective in relieving spinal inflammation and neuropathic pain behaviors. Pre-treatment with minocycline in rats, but not post-treatment, markedly attenuates the release of pro-inflammatory cytokines, oxidative and nitrative stress and, therefore, delays the development of neuropathic pain.42 Administration of minocycline in nerve-injured animals also effectively reduces TLR4 expression, which suppresses microglia activation in spinal cord and hyper-responsive wide dynamic range neurons.43 Minocycline treatment also inhibits the expression of MMP-9 and pro-nociceptive molecules such as IL-6 and IL-18 in the spinal cord while anti-nociceptive molecules (IL-4, IL-10) are unaffected.44 Depletion of spinal microglia with selectively immunotoxin, Mac-1 saporin was able to prevent and reverse neuropathic pain behavior.45 Recently, it has been found that M-CSF released by sensory neurons is a key molecule initiating microglia activation in the dorsal lumbar cords following peripheral nerve damage.15 In the CNS, CSF1R is exclusively expressed by microglia,46 and M-CSF is one of the critical factors for microglia survival, proliferation, activation, and differentiation.8–10 In this study, we showed that CSF1R inhibition through PLX5622 treatment not only prevents macrophage activation in peripheral nerves but also prevents microglia activation in the spinal cord.
Synergistic blocking spinal microglia and nerve macrophages activation could result in significant alleviation of neuropathic pain behaviors, which was evidenced by the effect of CSF1R inhibitor in our study. Peripheral macrophage activation, more specifically inflammatory macrophages expressing CD86, TNF-α, or IL-1β in injured peripheral nerves, is prominently affected by CSF1R inhibition while CD206 expressing macrophages were not significantly altered. Moreover, PLX5622 treatment effectively reduced microglia activation in spinal cord following nerve injury. Although our results provided a proof of concept on the critical role of microglia/macrophages in neuropathic pain, and that CSF1R could be an interesting target for neuropathic pain alleviation, it is however important to bear in mind that blocking M-CSF/CSF1R signaling, especially when the drug is given systemically, it affects not only microglia/macrophage in the nervous system, myeloid cells in other tissues could be most likely affected. Thus, to promote the translation of the current finding, not only more evidence from other animal models of chronic pain are awaited but also thorough analysis of the properties/safety of the compound in other organs is required.
Acknowledgments
The authors are grateful to Plexxikon Inc. for providing the colony stimulating factor-1 receptor (CSF-1R) inhibitor, PLX5622.
Author Contributions
SHL, XQS, and AF performed experiments; BW provided insights and participated in writing; SHL and JZ conceived the study and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by CIHR grants MOP111129 to JZ.
References
- 1.Lee S andZhang J.. Heterogeneity of macrophages in injured trigeminal nerves: cytokine/chemokine expressing vs. phagocytic macrophages. Brain Behav Immun 2012; 26: 891–903. [DOI] [PubMed] [Google Scholar]
- 2.Lim TK, Shi XQ, Martin HC, Huang H, Luheshi G, Rivest S and Zhang J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 2014; 155: 954–967. [DOI] [PubMed] [Google Scholar]
- 3.Calvo M andBennett DL.. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol 2012; 234: 271–282. [DOI] [PubMed] [Google Scholar]
- 4.Vallejo R Tilley DM Vogel L andBenyamin R.. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 2010; 10: 167–184. [DOI] [PubMed] [Google Scholar]
- 5.Austin PJ andMoalem-Taylor G.. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 2010; 229: 26–50. [DOI] [PubMed] [Google Scholar]
- 6.Carenini S, Maurer M, Werner A, Blazyca H, Toyka KV, Schmid CD, Raivich G and Martini R. The role of macrophages in demyelinating peripheral nervous system of mice heterozygously deficient in p0. J Cell Biol 2001; 152: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller M Berghoff M Kobsar I Kiefer R andMartini R.. Macrophage colony stimulating factor is a crucial factor for the intrinsic macrophage response in mice heterozygously deficient for the myelin protein P0. Exp Neurol 2007; 203: 55–62. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto S Nakajima K andKohsaka S.. Macrophage-colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. J Neurochem 2010; 115: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 9.Raivich G Moreno-Flores MT Moller JC andKreutzberg GW.. Inhibition of posttraumatic microglial proliferation in a genetic model of macrophage colony-stimulating factor deficiency in the mouse. Eur J Neurosci 1994; 6: 1615–1618. [DOI] [PubMed] [Google Scholar]
- 10.Kalla R, Liu Z, Xu S, Koppius A, Imai Y, Kloss CU, Kohsaka S, Gschwendtner A, Moller JC, Werner A and Raivich G. Microglia and the early phase of immune surveillance in the axotomized facial motor nucleus: impaired microglial activation and lymphocyte recruitment but no effect on neuronal survival or axonal regeneration in macrophage-colony stimulating factor-deficient mice. J Comp Neurol 2001; 436: 182–201. [PubMed] [Google Scholar]
- 11.Yeung YG Wang Y Einstein DB Lee PS andStanley ER.. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem 1998; 273: 17128–17137. [DOI] [PubMed] [Google Scholar]
- 12.Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann N Y Acad Sci 2006; 1068: 110–116. [DOI] [PubMed] [Google Scholar]
- 13.Dey A, She H, Kim L, Boruch A, Guris DL, Carlberg K, Sebti SM, Woodley DT, Imamoto A and Li W. Colony-stimulating factor-1 receptor utilizes multiple signaling pathways to induce cyclin D2 expression. Mol Biol Cell 2000; 11: 3835–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008; 8: 533–544. [DOI] [PubMed] [Google Scholar]
- 15.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S and Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016; 19: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seltzer Z Dubner R andShir Y.. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43: 205–218. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg AB andBasbaum AI.. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 1998; 76: 215–222. [DOI] [PubMed] [Google Scholar]
- 18.Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL and Green KN. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation 2015; 12: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, Seifert AM, Greer JB, Popow R, Crawley MH, Cohen NA, Green BL, Rossi F, Besmer P, Antonescu CR and DeMatteo RP. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med 2013; 210: 2873–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH and Segall JE. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med 2012; 18: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janova H, Arinrad S, Balmuth E, Mitjans M, Hertel J, Habes M, Bittner RA, Pan H, Goebbels S, Begemann M, Gerwig UC, Langner S, Werner HB, Kittel-Schneider S, Homuth G, Davatzikos C, Volzke H, West BL, Reif A, Grabe HJ, Boretius S, Ehrenreich H and Nave KA. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest 2018; 128: 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaplan SR Bach FW Pogrel JW Chung JM andYaksh TL.. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 23.Lee S Wu Y Shi XQ andZhang J.. Characteristics of spinal microglia in aged and obese mice: potential contributions to impaired sensory behavior. Immun Ageing 2015; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E and McCullough LD. Age- and location-related changes in microglial function. Neurobiol Aging 2015; 36: 2153–2163. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FO Sica A Mantovani A andLocati M.. Macrophage activation and polarization. Front Biosci 2008; 13: 453–461. [DOI] [PubMed] [Google Scholar]
- 26.Martinez FO andGordon S.. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kigerl KA Gensel JC Ankeny DP Alexander JK Donnelly DJ andPopovich PG.. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009; 29: 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JM Griffin JD Rambaldi A Chen ZG andMantovani A.. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol 1988; 141: 575–579. [PubMed] [Google Scholar]
- 29.Byrne PV Guilbert LJ andStanley ER.. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol 1981; 91: 848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V and Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002; 99: 111–120. [DOI] [PubMed] [Google Scholar]
- 31.Curry JM, Eubank TD, Roberts RD, Wang Y, Pore N, Maity A and Marsh CB. M-CSF signals through the MAPK/ERK pathway via Sp1 to induce VEGF production and induces angiogenesis in vivo. PLoS One 2008; 3: e3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D and Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 2005; 142: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleetwood AJ Lawrence T Hamilton JA andCook AD.. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol 2007; 178: 5245–5252. [DOI] [PubMed] [Google Scholar]
- 34.Svensson CI Hua XY Protter AA Powell HC andYaksh TL.. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport 2003; 14: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 35.Okubo M Yamanaka H Kobayashi K andNoguchi K.. Leukotriene synthases and the receptors induced by peripheral nerve injury in the spinal cord contribute to the generation of neuropathic pain. Glia 2010; 58: 599–610. [DOI] [PubMed] [Google Scholar]
- 36.Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P and Colleoni M. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 2008; 137: 81–95. [DOI] [PubMed] [Google Scholar]
- 37.Chauvet N Palin K Verrier D Poole S Dantzer R andLestage J.. Rat microglial cells secrete predominantly the precursor of interleukin-1beta in response to lipopolysaccharide. Eur J Neurosci 2001; 14: 609–617. [DOI] [PubMed] [Google Scholar]
- 38.Shigemoto-Mogami Y Koizumi S Tsuda M Ohsawa K Kohsaka S andInoue K.. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem 2001; 78: 1339–1349. [DOI] [PubMed] [Google Scholar]
- 39.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K and Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem 2000; 75: 965–972. [DOI] [PubMed] [Google Scholar]
- 40.Hanisch UK. Microglia as a source and target of cytokines. Glia 2002; 40: 140–155. [DOI] [PubMed] [Google Scholar]
- 41.Watkins LR Milligan ED andMaier SF.. Spinal cord glia: new players in pain. Pain 2001; 93: 201–205. [DOI] [PubMed] [Google Scholar]
- 42.Padi SS andKulkarni SK.. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol 2008; 601: 79–87. [DOI] [PubMed] [Google Scholar]
- 43.Nazemi S, Manaheji H, Noorbakhsh SM, Zaringhalam J, Sadeghi M, Mohammad-Zadeh M and Haghparast A. Inhibition of microglial activity alters spinal wide dynamic range neuron discharge and reduces microglial Toll-like receptor 4 expression in neuropathic rats. Clin Exp Pharmacol Physiol 2015; 42: 772–779. [DOI] [PubMed] [Google Scholar]
- 44.Rojewska E Popiolek-Barczyk K Jurga AM Makuch W Przewlocka B andMika J.. Involvement of pro- and antinociceptive factors in minocycline analgesia in rat neuropathic pain model. J Neuroimmunol 2014; 277: 57–66. [DOI] [PubMed] [Google Scholar]
- 45.Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo LE, Perez-Sanchez J, Bonin RP, De Koninck Y and Zhang J. Spinal microglia are required for long-term maintenance of neuropathic pain. Pain 2017; 158: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 46.Erblich B Zhu L Etgen AM Dobrenis K andPollard JW.. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PloS One 2011; 6: e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]