Abstract
African Americans (AA) have a higher incidence of pulmonary hypertension (PH) risk factors. Few studies have examined the racial differences in the prevalence and etiology of PH and direct comparison of invasive hemodynamics between AAs and Caucasians has rarely been reported. In this study, we examined whether racial differences exist in patients referred for right heart catheterization (RHC) and hypothesized that AA race is an independent risk factor for PH and is associated with increased adjusted mortality. We extracted data for AA and Caucasian patients who underwent RHC at Vanderbilt between 1998 and 2014. Clinical information was obtained from Vanderbilt's Synthetic Derivative, a de-identified mirror of our Electronic Medical Record. A total of 4576 patients were analyzed, including 586 (13%) AAs and 3990 (87%) Caucasians. AAs were younger than Caucasians by an average of eight years, but had more prevalent heart failure, features of metabolic syndrome, and higher creatinine. AAs also had higher mean pulmonary artery pressure and pulmonary vascular resistance. After adjusting for relevant co-morbidities, the AA race is associated with 41% increased risk of PH (odds ratio [OR] = 1.41, 95% confidence interval [CI] = 1.12–1.79). Among patients with PH, AA race is associated with 24% increased adjusted mortality (hazard ratio [HR] = 1.24, 95% CI = 1.09–1.45). AAs were younger but had more prevalent cardiometabolic and renal disease and worse pulmonary hemodynamics. The AA race is an independent risk factor for PH. Among patients with PH, the AA race is associated with increased adjusted mortality. Future studies should focus on delineating whether genetic or environmental factors contribute to PH risk in AAs.
Keywords: pulmonary hypertension, racial, ethnic, or social disparities in lung disease and treatment, health disparities
Pulmonary hypertension (PH) is common, especially among women and the elderly, and is associated with increased morbidity and mortality.1–7 PH can develop in isolation or as sequelae to a heterogeneous group of chronic conditions.8–11
Despite growing knowledge about the epidemiology and pathogenesis of PH, few studies have examined racial differences in the prevalence and etiology of PH. African Americans (AAs) have a higher prevalence of risk factors for PH such as heart failure, hypertension, and diabetes and develop these co-morbidities at a younger age.12–15 In addition, AAs experience worse cardiovascular and pulmonary clinical outcomes.1,16–19 It is unknown whether these risk factors translate into a higher prevalence of PH or associated mortality among AAs, as most epidemiologic studies of PH have been performed in racially homogenous populations, precluding direct racial comparisons.20–25 We recently reported higher systolic pulmonary artery pressure by transthoracic echocardiography (TTE) in middle-aged AA participants of the Coronary Artery Risk Development in Young Adults Study (CARDIA) compared to Caucasians.26 However, direct comparison of invasive hemodynamics between AAs and Caucasians referred for right heart catheterization (RHC) has rarely been reported.
In this study, we sought to examine if there were racial differences in PH prevalence, etiology, and prognosis in a large referral population at a tertiary academic medical center. We hypothesized that race is an independent risk factor for PH and that the AA race is associated with more adverse invasive hemodynamics and mortality.
Methods
Study population
The Vanderbilt University Institutional Review Board (no. 140544) approved this study, which consisted of adult patients referred for RHC in both inpatient and outpatient settings at Vanderbilt University Medical Center from 1998 to 2014. The indications for RHC are presented in Suppl. Table 1. All data were extracted from Vanderbilt's Synthetic Derivative, a de-identified mirror image of the Vanderbilt Electronic Medical Record that contains detailed patient information for over 2.5 million unique individuals.27,28 RHC hemodynamic data were extracted from procedural reports and manually validated for accuracy.29–31 For patients who had more than one RHC at Vanderbilt, only data from their first RHC were analyzed. Exclusion criteria and data cleaning procedures for this dataset have been described previously.30,31 In brief, we excluded individuals with shock, hypertensive crisis, or complex congenital heart disease. In addition, patients with insufficient clinical data (absent pulmonary artery or pulmonary artery wedge pressure [PAWP]), previous cardiac (International Classification of Diseases, Ninth Revision [ICD-9] code V42.1) or lung (ICD-9 code V42.6) transplantation, acute myocardial infarction (ICD-9 code 410.*), or chronic pulmonary embolism (ICD-9 code 416.2) were excluded. Non-physiologic values (e.g. negative cardiac output) were systematically deleted and missing data were imputed for the purposes of regression analyses.29–32
PH classification
PH etiology was classified according to contemporary guidelines (Suppl. Table 2).10 Briefly, PH was defined by mean pulmonary artery pressure (mPAP) ≥ 25 mmHg at rest. Pulmonary arterial hypertension (PAH) was defined as PH with mean PAWP ≤ 15 mmHg and pulmonary vascular resistance (PVR) > 3 Wood Units (WU). Individuals who met hemodynamic criteria for PAH underwent manual chart review to confirm or exclude the clinical diagnosis of PAH. PH due to left heart disease included isolated post-capillary PH (Ipc-PH) and combined pre- and post-capillary PH (Cpc-PH), defined as PH with PAWP > 15 mmHg and diastolic pressure gradient of <7 mmHg and ≥7 mmHg, respectively.9 World Health Organization (WHO) group 3 PH was defined as PH with PAWP ≤ 15 mmHg, prevalent chronic obstructive pulmonary disease (COPD) (ICD-9 codes 491.* and 492.*) or interstitial lung disease (ICD-9 codes 516.*), and absence of another etiology on manual review.11
Clinical data
From the Synthetic Derivative, we extracted patient demographics, co-morbidities, medication exposure, laboratory values, and TTE data. Patient demographics such as age, gender, and race were obtained from administrative records. Co-morbidities were defined by a combination of ICD-9 coding and laboratory values or previously validated algorithms and included relevant cardiac, pulmonary, metabolic, and renal disease.33 Medications were restricted to those on an individual's medication list in the six months before RHC to avoid including those prescribed after hemodynamics were obtained. Echocardiographic data were extracted from the TTE performed closest in date to RHC; the median elapsed time between RHC and TTE was one day (interquartile range [IQR] = 2–19 days).34 TTE parameters such as left atrial enlargement, defined as anterior–posterior left atrial diameter > 40 mm, and left ventricular hypertrophy, defined as left ventricular posterior wall thickness ≥12 mm, were determined per established guidelines.35,36 Co-morbidities, laboratory values, and echocardiographic data were restricted to within six months before or after RHC.
Outcomes
The primary outcome was the independent association between race and PH and the secondary outcomes were the independent association of race with PH survival and phenotype. Variables were selected a priori based on clinical knowledge of established or suspected risk factors for PH. For the survival analysis, the Synthetic Derivative is linked to the Social Security Death Index, which is continuously updated and used to determine vital status. Follow-up time was calculated from date of RHC to either date of death or 1 June 2016 (last date of censor).
Statistical analysis
Categorical variables were expressed as absolute values and percentages. Continuous variables were expressed as mean ± standard deviation and effect size of continuous variables were reported based on an increment from the 25th to 75th percentile value to provide more clinical insight than a standard deviation. Baseline characteristics were compared using Chi-squared test for categorical variables and Wilcoxon rank-sum test for continuous variables. To assess the association between race and PH in patients referred for RHC, we used a multivariate logistic regression model, adjusting for age, gender, heart failure, hypertension, diabetes, COPD, interstitial lung disease, body mass index (BMI), creatinine, left ventricular ejection fraction (LVEF), and left ventricular hypertrophy. To determine survival, a Cox proportional hazards model was built. Because heart failure, hypertension, and diabetes violated the proportional hazards assumption, a stratified Cox model was fit with respect to these variables. Prevalent interstitial lung disease, LVEF, and left ventricular hypertrophy were removed in the survival analysis to avoid overfitting based on priority of likely confounders.
Results
Baseline characteristics
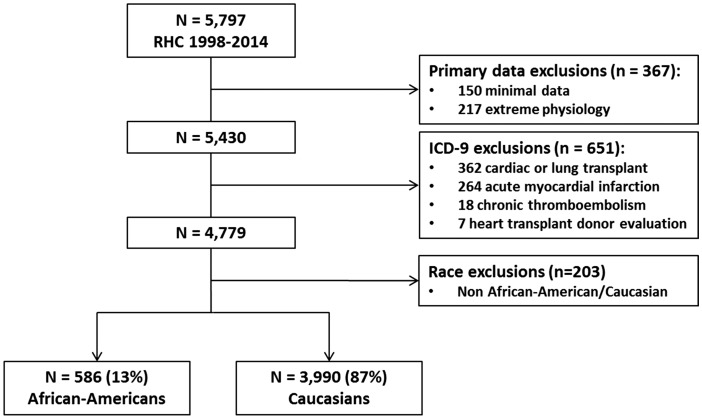
After pre-specified exclusions (n = 1221), the final cohort for analysis included 4576 patients, of which 3990 (87%) were Caucasians and 586 (13%) were AAs (Fig. 1). At the date of first RHC, AAs were younger than Caucasians by an average of eight years (53 ± 14 vs. 61 ± 15, P < 0.001). AAs also had higher rates of heart failure (62% vs. 45%), more features of metabolic syndrome such as hypertension (88% vs. 77%), diabetes (46% vs. 36%), and obesity (39% vs. 33%), and higher creatinine (1.8 ± 2.1 mg/dL vs. 1.2 ± 0.9 mg/dL; Table 1). In addition, AAs were more likely to be prescribed antihypertensive or diuretic medication. AAs and Caucasians did not differ in the prevalence of COPD, interstitial lung disease, or obstructive sleep apnea. There was also no difference in administration of PH-specific mediations between AAs and Caucasians.
Fig. 1.
Flow chart of patients referred for right heart catheterization. A schematic depiction of our study cohort. RHC, right heart catheterization; ICD-9, International Classification of Diseases, 9th Revision.
Table 1.
Baseline characteristics of patients referred for RHC.
| Characteristic | African Americans (n = 587) | Caucasians (n = 3990) | P value |
|---|---|---|---|
| Age (years) | 53 ± 14 | 61 ± 15 | <0.001 |
| Male gender (n (%)) | 272 (46) | 2063 (52) | 0.02 |
| Hypertension (n (%)) | 514 (88) | 3069 (77) | <0.001 |
| Coronary artery disease (n (%)) | 388 (66) | 2894 (73) | 0.002 |
| Heart failure (n (%)) | 366 (62) | 1778 (45) | <0.001 |
| Atrial fibrillation (n (%)) | 122 (21) | 1240 (31) | <0.001 |
| Chronic obstructive pulmonary disease (n (%)) | 73 (12) | 487 (12) | 0.86 |
| Interstitial lung disease (n (%)) | 39 (7) | 247 (6) | 0.66 |
| Obstructive sleep apnea (n (%)) | 64 (11) | 406 (10) | 0.58 |
| Systemic lupus erythematous (n (%)) | 27 (5) | 41 (1) | <0.001 |
| Scleroderma (n (%)) | 2 (2) | 109 (3) | 0.34 |
| Diabetes mellitus (n (%)) | 267 (46) | 1452 (36) | <0.001 |
| Obesity (n (%)) | 229 (39) | 1304 (33) | 0.02 |
| Brain natriuretic peptide (pg/mL) | 948 ± 1254 | 562 ± 861 | <0.001 |
| Creatinine (mg/dL) | 1.8 ± 2.1 | 1.2 ± 0.9 | <0.001 |
| Hemoglobin A1c (%) | 6.4 ± 1.5 | 6.1 ± 1.2 | <0.001 |
| Body mass index (kg/m2) | 31 ± 8 | 30 ± 7 | 0.007 |
| Low density lipoprotein (mg/dL) | 92 ± 35 | 92 ± 39 | 0.72 |
| High density lipoprotein (mg/dL) | 46 ± 19 | 43 ± 17 | 0.003 |
| Triglycerides (mg/dL) | 119 ± 100 | 161 ± 162 | <0.001 |
| Medications (n (%)) | |||
| Any antihypertensive | 459 (78) | 2844 (71) | <0.001 |
| Any diuretic | 398 (68) | 2165 (54) | <0.001 |
| Any anticoagulant | 196 (33) | 1243 (31) | 0.26 |
| Any lipid-lowering agent | 238 (41) | 1998 (50) | <0.001 |
| Pulmonary hypertension medications * | |||
| Prostacyclins | 12 (2) | 95 (2) | 0.62 |
| Endothelin receptor antagonists | 14 (2) | 84 (2) | 0.66 |
| Phosphodiesterase inhibitors | 22 (4) | 107 (3) | 0.14 |
| Any | 36 (6) | 225 (6) | 0.62 |
Medications limited to six months before date of RHC.
Pulmonary hypertension distribution
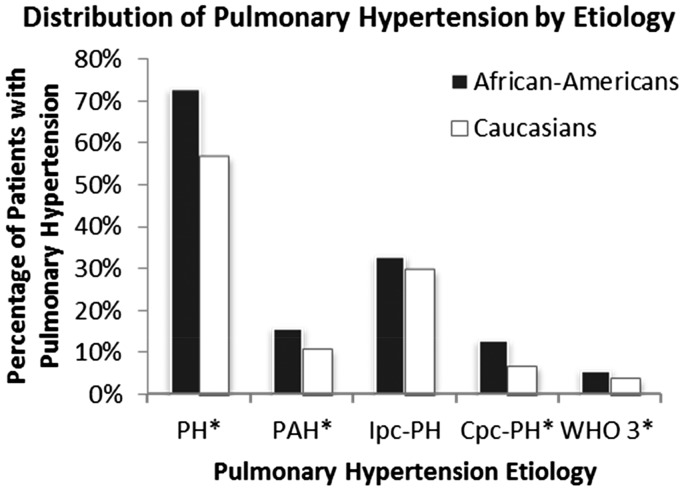
In our referral population, AAs were more likely to have PH than Caucasians (73% vs. 57%, P < 0.001). The distribution of PH by etiology is shown in Fig. 2. Compared to Caucasians, AA patients had increased prevalence of Cpc-PH (13% vs. 7%, P < 0.001), PAH (16% vs. 11%, P < 0.001), and WHO group 3 PH (6% vs. 4%, P = 0.02), whereas there was no difference in the prevalence of Ipc-PH between groups (P = 0.21).
Fig. 2.
Bar graph showing distribution of PH by etiology, expressed as percentage of total number of African Americans (AAs, n = 586) and Caucasians (n = 3990) in our cohort. AAs had higher prevalence of PH (73% vs. 56%), PAH (16% vs. 11%), Cpc-PH (13% vs. 7%), and WHO group 3 PH (6% vs. 4%), but not Ipc-PH (33% vs. 30%). *P < 0.05.
Invasive and hemodynamics and echocardiographic data
AAs had more severe pulmonary hemodynamics compared with Caucasians. AAs had higher right atrial pressure (10 ± 7 mmHg vs. 8 ± 6 mmHg), mPAP (34 ± 14 mmHg vs. 29 ± 14 mmHg), mean PAWP (17 ± 9 mmHg vs. 15 ± 8 mmHg), and PVR (4.0 ± 3.3 WU vs. 3.2 ± 3.3 WU, all P < 0.001; Table 2). AAs also had lower cardiac index (2.8 ± 1.0 L/min/m2 vs. 2.9 ± 1.0 L/min/m2) and mixed venous oxygen saturation (63% ± 11% vs. 68% ± 10%). On TTE, AAs had lower LVEF (40% ± 19% vs. 47% ± 16%) and higher estimated right ventricular systolic pressure (52 ± 23 mmHg vs. 46 ± 21 mmHg), with similar rates of left atrial enlargement and left ventricular hypertrophy.
Table 2.
Invasive hemodynamics and transthoracic echocardiography data.
| All patients (n = 4576) |
Patients with PH (n = 2689) |
|||||
|---|---|---|---|---|---|---|
| AAs (n = 586) | Caucasians (n = 3990) | P value | AAs (n = 428) | Caucasians (n = 2261) | P value | |
| Invasive hemodynamics | ||||||
| RA pressure (mmHg) | 10 ± 7 | 8 ± 6 | <0.001 | 12 ± 7 | 11 ± 6 | 0.002 |
| RV systolic pressure (mmHg) | 52 ± 21 | 45 ± 20 | <0.001 | 60 ± 20 | 57 ± 19 | 0.003 |
| Mean PAP (mmHg) | 34 ± 14 | 29 ± 14 | <0.001 | 40 ± 11 | 38 ± 11 | <0.001 |
| Mean PAWP (mmHg) | 17 ± 9 | 15 ± 8 | <0.001 | 19 ± 9 | 19 ± 8 | 0.79 |
| DPG (mmHg) | 6 ± 9 | 4 ± 9 | <0.001 | 8 ± 10 | 6 ± 11 | <0.001 |
| PVR (Woods units) | 4.0 ± 3.3 | 3.2 ± 3.3 | <0.001 | 4.8 ± 3.5 | 4.3 ± 3.9 | <0.001 |
| Cardiac index* (L/min/m2) | 2.8 ± 1.0 | 2.9 ± 1.0 | <0.001 | 2.6 ± 0.9 | 2.7 ± 0.9 | <0.001 |
| PA oxygen saturation (%) | 63 ± 11 | 68 ± 10 | <0.001 | 61 ± 11 | 64 ± 10 | <0.001 |
| Non-invasive hemodynamics | ||||||
| RV systolic pressure (mmHg) | 52 ± 23 | 46 ± 21 | <0.001 | 56 ± 24 | 51 ± 21 | 0.03 |
| TRV (m/s) | 3.3 ± 1.4 | 3.1 ± 0.8 | <0.001 | 3.5 ± 1.5 | 3.3 ± 0.8 | 0.22 |
| LA enlargement† (n (%)) | 299 (58) | 1911 (58) | 0.85 | 229 (61) | 1263 (65) | 0.15 |
| LV hypertrophy‡ (n (%)) | 168 (33) | 1041 (31) | 0.58 | 126 (33) | 634 (32) | 0.72 |
| LV ejection fraction§ (%) | 40 ± 19 | 47 ± 16 | <0.001 | 40 ± 19 | 45 ± 17 | 0.001 |
By Fick's method.
Anterior-posterior LA diameter > 40 mm.
LV posterior wall thickness ≥ 12 mm.
By biplane Simpson's method.
RA, right atrial; RV, right ventricular; PAP, pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; DPG, diastolic pressure gradient; PVR, pulmonary vascular resistance; AA, African American; PH, pulmonary hypertension; TRV, tricuspid regurgitant velocity; LA, left atrial; LV, left ventricular.
Associations with prevalent PH
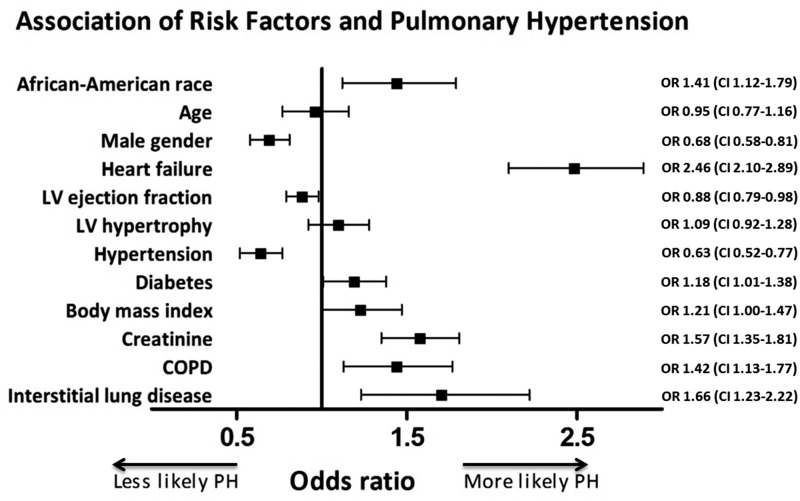
To investigate the association between race and PH diagnosis, we built a logistic regression model incorporating the following clinically relevant variables a priori: age, gender, heart failure, hypertension, diabetes, COPD, interstitial lung disease, BMI, creatinine, LVEF, and left ventricular hypertrophy. In our fully adjusted model, the AA race was independently associated with prevalent PH (odds ratio [OR] = 1.41, 95% confidence interval [CI] = 1.12–1.79), along with heart failure (OR = 2.45, 95% CI = 2.10–2.89), diabetes (OR = 1.18, 95% CI = 1.01–1.38), COPD (OR = 1.42, 95% CI = 1.13–1.77), and interstitial lung disease (OR = 1.66, 95% CI = 1.23–2.22, Fig. 3). In subgroup analysis, the AA race was independently associated with Cpc-PH (P = 0.007), PAH (P = 0.02), and WHO group 3 PH (P = 0.02), but not Ipc-PH (P = 0.11; Table 3). Full multivariable analysis results are reported in Suppl. Table 3.
Fig. 3.
Forest plot of association of risk factors and PH. All continuous variables were reported based on an increment from the 25th to 75th percentile value. AA race, heart failure, diabetes, COPD, interstitial lung disease, and higher BMI and creatinine were risk factors for PH. Male gender and higher LVEF were protective against PH. OR, odds ratio; 95% CI, confidence interval.
Table 3.
Adjusted association of African American race and pulmonary hypertension.
| All PH (n = 2689) | PAH (n = 564) | Ipc-PH (n = 1456) | Cpc-PH (n = 364) | WHO 3 PH (n = 214) | |
|---|---|---|---|---|---|
| Odds ratio | 1.41 | 1.47 | 0.83 | 1.54 | 2.01 |
| Confidence interval | 1.12–1.79 | 1.08–2.02 | 0.66–1.04 | 1.12–2.11 | 1.11–3.64 |
PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; Ipc-PH, isolated post-capillary PH; Cpc-PH, combined pre- and post-capillary PH; WHO 3, World Health Organization group 3.
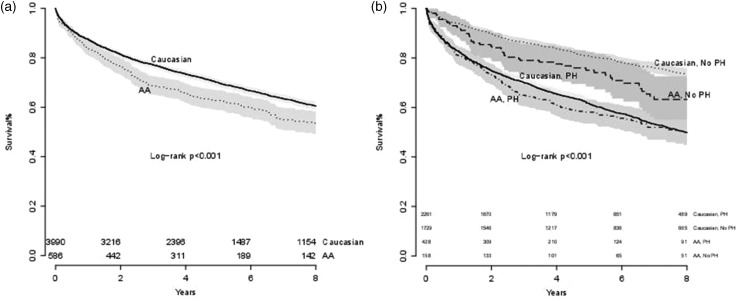
Survival analysis
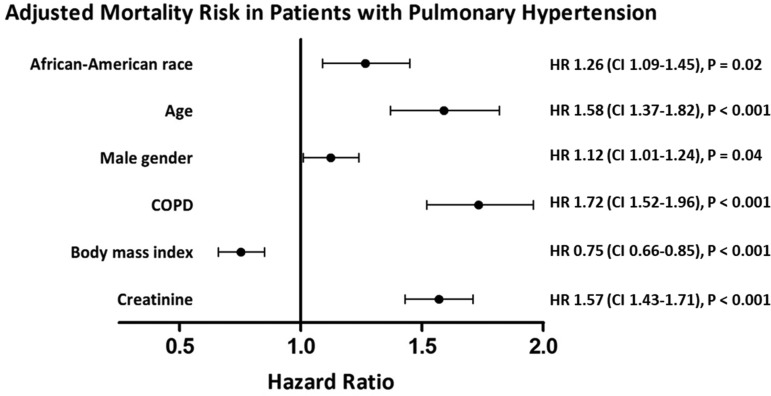
The median follow-up was 4.7 years (IQR = 2.8–8.6 years). In total, 255 (44%) AAs and 1465 (37%) Caucasians died during follow-up. In unadjusted analysis, AA race was associated with decreased survival (log rank test, P < 0.001) in all-comers at eight-year follow-up (Fig. 4a) but not in patients with hemodynamic PH (Fig. 4b). In the fully adjusted, multivariate stratified Cox model of patients with PH, AA race was associated with increased mortality (hazard ratio [HR] = 1.24, 95% CI = 1.09–1.45; Fig. 5). In exploratory analyses, race was not an independent predictor of mortality for any specific PH etiology (Suppl. Table 4).
Fig. 4.
Kaplan-Meier curves for all patients referred for RHC (a) and in patients with and without PH (b). At eight-year follow-up, the AA race was associated with decreased survival (log rank test, P < 0.001) in all-comers but not in patients with PH.
Fig. 5.
Forest plot of association of co-morbidities and mortality in patients with PH using Cox proportional hazards model, stratified by hypertension, heart failure, and diabetes and adjusted for race, age, gender, COPD, BMI, and creatinine. Among patients with PH, the AA race was associated with 26% increased adjusted mortality. HR, hazard ratio; CI, confidence interval.
Discussion
In this study of patients referred for RHC, we found that AAs were younger than Caucasians by an average of eight years but had more prevalent cardiometabolic and renal disease and more severe pulmonary hemodynamics. After adjusting for relevant co-morbidities, AA race was associated with 41% increased risk for PH and 24% increased mortality among patients with PH. Given the higher prevalence of PH and associated mortality among AAs, these findings are important to AAs with PH risk factors and their physicians.
There are limited published data examining racial differences in PH. Retrospective analysis of the National Hospital Discharge Survey showed differential mortality trends between Caucasians and AAs with PH during 2001–2010.1,4 However, they relied on ICD-9 codes for all medical diagnoses which can be inaccurate and lacked invasive hemodynamic data. Contemporary registries tend to focus on patients with PAH, excluding other etiologies of PH.20–23,25,37 The Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management reported decreased survival for Caucasians compared to AAs at five-year follow-up; however, patients with WHO group 2 and 3 PH were not included and invasive hemodynamics between races were not directly compared.38 Two recent studies have drawn attention to the importance of echocardiographic estimates of systolic PAP in AAs. Chaudhry et al. found that 7% of patients in the Jackson Heart Study had echocardiographic evidence of PH, which was associated with increased heart failure hospitalizations.22,39 In the biracial CARDIA study, we found that AAs had higher systolic PAP after adjusting for demographics and left ventricular structure and function.26 The generalizability of the findings from CARDIA is unknown because it consists of relatively healthy individuals, not a clinical referral population. Our study aimed to address some of the current gaps in literature by examining PH prevalence, etiology, and prognosis in a large heterogeneous population referred for invasive hemodynamic evaluation.
We found that AAs had higher PAPs and higher prevalence of PH. Although our study was not designed to uncover the mechanisms behind this observation, several factors may contribute. Jankowich et al. found that higher circulating endothelin-1, a potent vasoconstrictor, is associated with higher systolic PAP and mortality in AA patients of the Jackson Heart Study.40 Compared with Caucasians, AAs with PAH also have reduced response to treatment by endothelin receptor antagonists41 and AAs with systemic hypertension, a risk factor for PH, appear to release more endothelin-1 in response to stress, have almost threefold higher absolute circulating endothelin-1 levels, and have impaired endothelial function and vasodilation.42–44 Therefore, increased endothelin-1 signaling may contribute to higher pulmonary pressure in AAs. Next, we recently have identified shared genetic variants between Caucasian patients with PAH and Cpc-PH.31 Given the much higher prevalence of Cpc-PH among AAs compared to Caucasians in this study, we speculate that AAs may be genetically more predisposed to develop PAH in response to left heart disease. Moreover, lower lung volumes among AAs appear to contribute to higher estimated systolic PAP on echocardiography.26 Finally, socioeconomic status modifies PH risk factors such as heart failure, hypertension, and diabetes and tends to be worse in AAs.12–15 Recently, Parikh et al. reported that AAs had a twofold increased risk of death compared with Caucasians in a smaller cohort (n = 45 AAs) referred specifically for PH evaluation. After adjusting for health insurance status, mortality did not differ between AAs and Caucasians, suggesting that socioeconomic status may influence outcomes.45 Our study built on this work by analyzing a larger sample size including all patients undergoing RHC, regardless of indication, to increase the generalizability of our findings. We also adjusted for additional PH risk factors and echocardiographic measures of LV structure and function. These observations suggest that molecular, genetic, and environmental factors may all predispose AAs to PH and warrant further study to shed insight on PH pathophysiology or to identify at-risk individuals.46
We found that race was not independently associated with Ipc-PH, whereas the prevalence of Cpc-PH among AAs was double that of Caucasians. While AAs had higher prevalence of heart failure and lower mean LVEF, other adverse cardiac remodeling parameters such as left ventricular hypertrophy and left atrial enlargement were similar between races. AAs with PH also had similar PAWP as Caucasians with PH (Table 2), suggesting that more prevalent PH and worse pulmonary hemodynamics seen in AAs are not solely explained by more severe cardiac disease. Consistent with other studies showing pulmonary vascular remodeling in patients with Cpc-PH,47 we speculate that AAs may represent an at-risk population who are more susceptible to pulmonary arterial remodeling in response to left atrial hypertension. Therefore, AAs should be an important subgroup included in clinical trials targeting patients with Cpc-PH and future studies using biological samples are required to test this hypothesis.
Finally, we found that among patients with PH, AA race was independently associated with decreased survival after adjusting for relevant co-morbidities, building upon prior studies that showed increased mortality among AAs with idiopathic PAH.18,19 PH is often diagnosed late in its disease course, due to under-recognition and overlapping symptoms with other conditions.1,2 Studies show that AAs with heart failure have more co-morbidities and higher rates of hospitalization, which could be in part due to concurrent PH.12,14,47–51 Therefore, our observations have important clinical implications regarding the frequency of PH surveillance and the degree of risk factor modification in at-risk AAs. Of note, we did not observe any differences in adjusted mortality in individual PH categories. However, a positive point estimate towards increased mortality was seen in the Ipc-PH and Cpc-PH groups (HR = 1.12, 95% CI = 0.88–1.43 and HR = 1.21, 95% CI = 0.80–1.81, respectively; Suppl. Table 4). In addition to validating these findings in a larger, external cohort, future work should include longitudinal studies to investigate disease trajectory and response to treatment.
Limitations
There are several limitations to this study. First, our cross-sectional, single-centered study involves a referral population, so we are unable to comment on the prevalence and etiology of PH by race in the general community. Second, our dataset does not allow us to comment on socioeconomic or health insurance status, but we acknowledge these are potentially important determinants of outcomes in patients with PH. Third, similar to other large Electronic Medical Record-based studies, co-morbidity information was largely obtained from ICD-9 coding, which can be inaccurate. However, we used algorithms for co-morbidity ascertainment that have high accuracy in our system or have been validated in the Electronic Medical Records and Genomics network.33 Finally, we were unable to review RHC waveforms from the Vanderbilt Synthetic Derivative to confirm hemodynamic measurements, but we have previously shown strong agreement between manually reviewed values and computer generated mean values in our catheterization laboratory.30
Conclusion
AAs referred for invasive hemodynamic evaluation at a large tertiary care center are younger than Caucasians and have a higher co-morbidity burden. The AA race is an independent risk factor for PH and is associated with a more severe pulmonary hemodynamic profile. Finally, among patients with PH, the AA race is associated with increased adjusted mortality. These findings warrant future studies to delineate whether genetic or environmental factors contribute to PH risk in AAs.
Supplemental Material
Supplemental material for Racial differences in patients referred for right heart catheterization and risk of pulmonary hypertension by Bin Q. Yang, Tufik R. Assad, Jared M. O'Leary, Meng Xu, Stephen J. Halliday, Reid W. D'Amico, Eric H. Farber-Eger, Quinn S. Wells, Anna R. Hemnes and Evan L. Brittain in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by the National Institutes of Health grants T32 HL087738-08 (Assad), U01 HL125212-01 (Hemnes), R34 HL141317 (Brittain), the American Heart Association Fellow to Faculty Grant #13FTF16070002 (Brittain), and Gilead Pulmonary Arterial Hypertension Scholars Award Program (Brittain). The dataset used in the analyses were obtained from Vanderbilt University Medical Center's BioVU, which is supported by institutional funding and by the Vanderbilt Clinical and Translational Science Awards grant UL1 TR000445 from National Center for Advancing Translational Sciences/National Institute of Health.
ORCID iD
Bin Q. Yang http://orcid.org/0000-0002-9653-145X
References
- 1.George MG, Schieb LJ, Ayala C, et al. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 2014; 146(2): 476–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyduk A, Croft JB, Ayala C, et al. Pulmonary hypertension surveillance - United States, 1980–2002. MMWR Surveill Summ 2005; 54: 1–28. [PubMed] [Google Scholar]
- 3.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011; 139(1): 128–137. [DOI] [PubMed] [Google Scholar]
- 4.Mehari A, Valle O, Gillum RF. Trends in pulmonary hypertension mortality and morbidity. Pulm Med 2014; 2014: 105864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009; 53(13): 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol 2007; 99(8): 1146–1150. [DOI] [PubMed] [Google Scholar]
- 7.Rezaee ME, Nichols EL, Sidhu M, et al. Combined post- and precapillary pulmonary hypertension in patients with heart failure. Clin Cardiol 2016; 39(11): 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins KW, Thenappan T. World Health Organization group i pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin 2016; 34(3): 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62(Suppl. 25): D100–108. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(Suppl. 25): D34–41. [DOI] [PubMed] [Google Scholar]
- 11.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62(Suppl. 25): D109–116. [DOI] [PubMed] [Google Scholar]
- 12.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360(12): 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loehr LR, Rosamond WD, Chang PP, et al. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101(7): 1016–1022. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168(19): 2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckles G, Chou CF. Disparities in the Prevalence of Diagnosed Diabetes - United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep 2016; 65(45): 1265–1269. [DOI] [PubMed] [Google Scholar]
- 16.Kishi S, Reis JP, Venkatesh BA, et al. Race-ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc 2015; 4(3): e001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens 2004; 17(10): 963–970. [DOI] [PubMed] [Google Scholar]
- 18.Davis KK, Lilienfeld DE, Doyle RL. Increased mortality in African Americans with idiopathic pulmonary arterial hypertension. J Natl Med Assoc 2008; 100(1): 69–72. [DOI] [PubMed] [Google Scholar]
- 19.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005; 95(2): 199–203. [DOI] [PubMed] [Google Scholar]
- 20.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 2008; 83(8): 923–931. [DOI] [PubMed] [Google Scholar]
- 21.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005; 15(Suppl. 6): S6-4–17. [PubMed] [Google Scholar]
- 22.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in African-Americans. PLoS One 2013; 8(12): e84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186(8): 790–796. [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122(2): 156–163. [DOI] [PubMed] [Google Scholar]
- 25.D'Aonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115(5): 343–349. [DOI] [PubMed] [Google Scholar]
- 26.Brittain EL, Nwabuo C, Xu M, et al. Echocardiographic pulmonary artery systolic pressure in the Coronary Artery Risk Development in Young Adults (CARDIA) study: associations with race and metabolic dysregulation. J Am Heart Assoc 2017; 6(4): e005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulley J, Clayton E, Bernard GR, et al. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci 2010; 3(1): 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008; 84(3): 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittain EL and Chan SY. Integration of complex data sources to provide biologic insight into pulmonary vascular disease (2015 Grover Conference Series). Pulm Circ 2016; 6(3): 251–260. [DOI] [PMC free article] [PubMed]
- 30.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ 2016; 6(3): 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol 2016; 68(23): 2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. Circulation 2016; 133(13): 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottesman O, Kuivaniemi H, Tromp G, et al. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med 2013; 15(10): 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells QS, Farber-Eger E, Crawford DC. Extraction of echocardiographic data from the electronic medical record is a rapid and efficient method for study of cardiac structure and function. J Clin Bioinforma 2014; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28(1): 1–39. [DOI] [PubMed] [Google Scholar]
- 36.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23(7): 685–713. quiz 786–688. [DOI] [PubMed] [Google Scholar]
- 37.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173(9): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 38.Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest 2015; 148(4): 1043–1054. [DOI] [PubMed] [Google Scholar]
- 39.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circ Heart Fail 2014; 7(4): 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankowich MD, Wu WC, Choudhary G. Association of elevated plasma endothelin-1 levels with pulmonary hypertension, mortality, and heart failure in African American individuals: The Jackson Heart Study. JAMA Cardiol 2016; 1(4): 461–469. [DOI] [PubMed] [Google Scholar]
- 41.Gabler NB, French B, Strom BL, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest 2012; 141(1): 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campia U, Cardillo C, Panza JA. Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients. Circulation 2004; 109(25): 3191–3195. [DOI] [PubMed] [Google Scholar]
- 43.Treiber FA, Kapuku GK, Davis H, et al. Plasma endothelin-1 release during acute stress: role of ethnicity and sex. Psychosom Med 2002; 64(5): 707–713. [DOI] [PubMed] [Google Scholar]
- 44.Ergul S, Parish DC, Puett D, et al. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension 1996; 28(4): 652–655. [DOI] [PubMed] [Google Scholar]
- 45.Parikh KS, Stackhouse KA, Hart SA, et al. Health insurance and racial disparities in pulmonary hypertension outcomes. Am J Manag Care 2017; 23(8): 474–480. [PubMed] [Google Scholar]
- 46.Talwar A, Garcia JGN, Tsai H, et al. Health disparities in patients with pulmonary arterial hypertension: a blueprint for action. An official American Thoracic Society statement. Am J Respir Crit Care Med 2017; 196(8): e32–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143(3): 758–766. [DOI] [PubMed] [Google Scholar]
- 48.Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J 2011; 161(4): 746–754. [DOI] [PubMed] [Google Scholar]
- 49.Yancy CW, Abraham WT, Albert NM, et al. Quality of care of and outcomes for African Americans hospitalized with heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry. J Am Coll Cardiol 2008; 51(17): 1675–1684. [DOI] [PubMed] [Google Scholar]
- 50.Kamath SA, Drazner MH, Wynne J, et al. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168(11): 1152–1158. [DOI] [PubMed] [Google Scholar]
- 51.Agoston I, Cameron CS, Yao D, et al. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94(8): 1003–1007. [DOI] [PubMed] [Google Scholar]
- 52.Dries DL, Exner DV, Gersh BJ, et al. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340(8): 609–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Racial differences in patients referred for right heart catheterization and risk of pulmonary hypertension by Bin Q. Yang, Tufik R. Assad, Jared M. O'Leary, Meng Xu, Stephen J. Halliday, Reid W. D'Amico, Eric H. Farber-Eger, Quinn S. Wells, Anna R. Hemnes and Evan L. Brittain in Pulmonary Circulation