Abstract
Pulmonary arterial hypertension (PAH) is a syndrome characterized by progressive lung vascular remodelling, endothelial cell (EC) dysfunction, and excessive inflammation. The primary cilium is a sensory antenna that integrates signalling and fine tunes EC responses to various stimuli. Yet, cilia function in the context of deregulated immunity in PAH remains obscure. We hypothesized that cilia function is impaired in ECs from patients with PAH due to their inflammatory status and tested whether cilia length changes in response to cytokines. Primary human pulmonary and mouse embryonic EC were exposed to pro- (TNFα, IL1β, and IFNγ) and/or anti-inflammatory (IL-10) cytokines and cilia length was quantified. Chronic treatment with all tested inflammatory cytokines led to a significant elongation of cilia in both control human and mouse EC (by ∼1 µm, P < 0.001). This structural response was PKA/PKC dependent. Intriguingly, withdrawal of the inflammatory stimulus did not reduce cilia length. IL-10, on the other hand, blocked and reversed the pro-inflammatory cytokine-induced cilia elongation in healthy ECs, but did not influence basal length. Conversely, primary cilia of ECs from PAH patients were significantly longer under basal conditions compared to controls (1.86 ± 0.02 vs. 2.43 ± 0.08 µm, P = 0.002). These cilia did not elongate further upon pro-inflammatory stimulation and anti-inflammatory treatment did not impact cilia length. The missing length modulation was specific to cytokine stimulation, as application of fluid shear stress led to increased cilia length in the PAH endothelium. We identified loss of cilia length regulation upon cytokine stimulation as part of the endothelial dysfunction in PAH.
Keywords: primary cilia, pulmonary endothelium, inflammation, chemokines and cytokines
Introduction
Pulmonary arterial hypertension (PAH) represents a group of lung diseases characterized by high pulmonary artery pressure (PAP) (> 25 mmHg) eventually leading to right heart failure.1,2 Blood vessels in the lungs of PAH patients are highly remodeled due to genetic changes, altered cellular signaling, metabolic changes, aberrant pressures, and chronic inflammation.2–4
PAH patients have a high inflammatory status both in the systemic circulation and in the lung vasculature.4 Vascular inflammation involves various cytokines, including the pro-inflammatory TNFα, IL1β, and IFNγ, as well as the anti-inflammatory cytokine IL10. Under the influence of pro-inflammatory cytokines, endothelial cells (ECs) express proteins, such as cell adhesion molecules, for the recruitment of blood borne inflammatory cells into the vessel wall.5,6 Chronic or dysregulated inflammation leads to EC dysfunction involving a number of factors, such as loss of barrier integrity.5,7
As with most mammalian cell types, ECs carry primary cilia. The main structural part of the cilium is the rigid axoneme consisting of 9 + 0 microtubule doublets, which protrudes from the cellular membrane into the lumen or extracellular space.8 The base of the cilium, the transition zone, functions as an active barrier for both the cytoplasmic and membrane content preventing free exchange and contributing to a unique subcellular environment.9 The endothelial primary cilium is a highly regulated and specified antenna that senses and orchestrates responses to chemical and mechanical cues from the flowing blood.10,11 Emerging data further identify the primary cilium as a specialized organelle involved in intracellular signaling processes from hedgehog proteins, growth factors, calcium, and others.12–19 Furthermore, EC primary cilia are important for vascular integrity and homeostasis, since absence of primary cilia has been shown to promote endothelial-to-mesenchymal transition (EndoMT).20 In accordance, primary cilia were found in areas of disturbed flow and are therefore suggested to protect against shear-induced EndoMT.21,22
Cilia length is instrumental for cilia function and is controlled by intraflaggelar transport (IFT). Cilia length itself can regulate cargo loading of the IFT particles (e.g. with receptors), suggesting that signaling directly links to cilia length.23 In addition, many signaling pathways have been shown to influence cilia length.24–26 As such, cilia length increases in response to pro-inflammatory cytokines and was thereby proposed to mediate inflammatory responses.27
In diseases with dysregulated vascular inflammation, such as PAH, ECs are chronically exposed to vast amounts of pro-inflammatory cytokines. Hence, we hypothesized that the loss of cytokine-induced cilia length control is part of the EC dysfunction in PAH and tested whether cytokines can affect cilia length of human pulmonary microvascular EC from patients.
Methods
Cell culture
Cells were grown on 0.1% gelatin coated eight-chamber slides (BD Biosciences) until confluency. Ciliated mouse embryonic endothelial cells (MEC)28 were cultured as previously described.20 Primary human pulmonary microvascular endothelial cells (MVEC) were obtained from end-stage PAH patients and healthy tissues of lobectomy donors, as described before.29 The tissue harvest and MVEC isolations were approved by the IRB of the VU University Medical Center (VUmc, Amsterdam, The Netherlands) and consent was given. MVEC were cultured in complete ECM medium supplemented with 1% pen/strep, 1% endothelial cell growth supplement, and 5% FCS (ScienceCell). Shear stress was applied, as previously described,29 by culturing cells on µ-slides I Luer (ibidi) and applying medium flow at 15 dyn/cm2 over the adherent cells with the ibidi pump system for five days.
Treatments
Treatments were performed in starvation medium with 1% FCS and pen/strep. Stimuli were provided in fresh medium for 24 h. The following concentrations were used: TNFα 10 ng/mL, IL1β 10 ng/mL, IFNγ 100 U/mL, and IL10 10 ng/mL. Forskolin (FK) was used in a concentration of 100 µM (Sigma-Aldrich). H89 and Gö6983 (Sigma-Aldrich) were applied at 10 µM and 2 µM, respectively. The NFκB inhibitor BAY 11-7085 (Cayman Chemicals) was applied at 1 µM final concentration.
Cilia immunostaining
Cells were fixed in 4% paraformaldehyde (Merck) in PBS for 10 min at room temperature (RT). Fixed cells were permeabilized with 0.05% Tween 20 (Merck) in PBS. Incubation with the primary antibody against acetylated-α-tubulin (6-11B-1, 1:2000, Sigma-Aldrich) was performed overnight at 4℃. This was followed by 1 h incubation with secondary Cy3-labeled goat-anti-mouse antibody (1:500, Vector Laboratories) and DAPI nuclear counterstaining (1:1000, Molecular Probes) for 5 min at RT. Samples were mounted in Prolong Gold (Molecular Probes).
Cilia length measurements
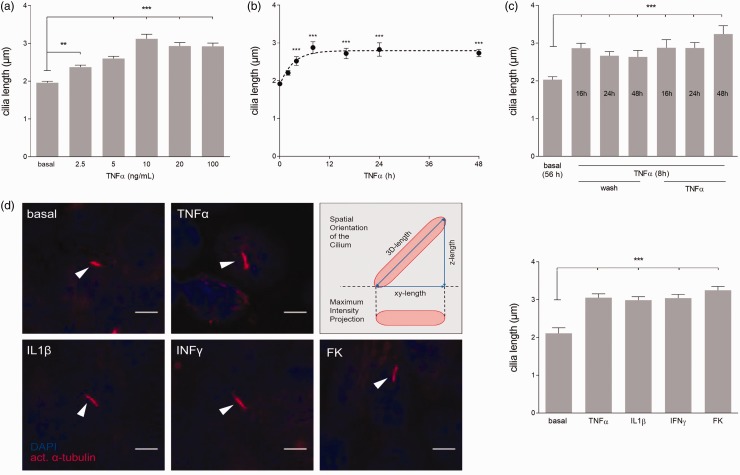
Confocal z-stacks were taken with a fixed step distance of 0.25 μm using a SP5 confocal microscope (Leica). Image acquisition and cilia length measurements were performed as described previously.30 In short, a random population of at least ten cilia per condition were measured using the Pythagoras (PyT) method. Herefore, cilium length was determined in the xy- as well as in z-direction with ImageJ (NIH) and the three-dimensional length was calculated based on the Pythagorean theorem a2+b2 = c2, with a being the xy-length based on a maximum intesity projection (MIP) and b the z-length (Fig. 1, schematic). With the PyT method, the spatial orientation of the cilium is accounted for, wherefore selection bias and standard deviation are minimized.
Fig. 1.
Various pro-inflammatory cytokines elongate primary cilia permanently. (a) TNFα was applied to MEC in different concentrations for 24 h (n ≥ 100 per concentration). (b) Time course of cilia elongation was quantified in MEC after addition of 10 ng/mL TNFα (n ≥ 43 cilia per time point). (c) Cilia length was determined after an initial trigger of 8h TNFα (10 ng/mL) followed by additional stimulation or an alternative wash step after 16 h, 24 h, or 48h. All conditions were fixed and quantified after 56 h (n ≥ 34 cilia per condition). (d) Maximum intensity projections (MIP) of representative (flat) cilia (arrow heads) upon 24 h treatment with TNFα (10 ng/mL), IL1β (10 ng/mL), IFNγ (100 U/mL), or the positive control FK (100 μM) (scale bar = 5 μm). Schematic depicts differences between MIP used to determine xy-length and three-dimensional (3D) length calculation applied for cilia length analysis and statistics. Quantification of average 3D cilia length is shown to the right (n ≥ 53 per condition).
Statistics
Experiments in MEC were performed in duplicate and repeated three times, experiments with MVEC were performed in duplicate in at least three donors. Statistics were calculated based on the averaged cilia length per donor with the total number of donors used as independent n. Data visualization and statistics were generated with GraphPad Prism 7. Data are presented as mean ± SEM. Samples were tested for Gaussian distribution by D'Agostino-Pearson omnibus normality test. If not otherwise indicated, significance was determined by one-way ANOVA with Kruskal–Wallis test and Dunn's post hoc test. *P = 0.033, **P = 0.002, ***P < 0.001
Results
TNFα induces sustained cilia elongation
We sought to determine whether TNFα influences cilia length dose dependently. Therefore, different concentrations of TNFα were tested on MEC (Fig. 1a). A low concentration of 2.5 ng/mL TNFα induced significant cilia elongation compared to unstimulated controls (1.96 ± 0.04 to 2.37 ± 0.05 µm, P = 0.002). Cilia length plateaued at an average length of approximately 3 µm with a concentration of 10 ng/mL or higher (P < 0.001). An additional increase in TNFα concentration did not further increase cilia length.
To examine the time course of cilia elongation upon TNFα stimulation, cilia length was measured at various time points (Fig. 1b). Indeed, cilia elongation upon TNFα stimulation was time-dependent. At 2 h after treatment, cilia elongation was visible, although not significant. Length was significantly increased 4 h after treatment (P < 0.001) and reaches a plateau after 8 h. The stimulation with TNFα caused sustained elongation and no significant differences in cilia length were found between 8 h and 48 h after treatment.
Additionally, we tested whether the removal of TNFα would reverse cilia length (Fig. 1c). To our surprise, washing steps after 16 h, 24 h, or 48 h did not alter cilia length. Moreover, additive treatment after 16 h, 24 h, or 48 h with TNFα did not show an extra effect on cilia length. Taken together, 8 h of TNFα treatment with a concentration of 10 ng/mL is sufficient to reach TNFα-induced maximal average cilia length and retain cilia elongation for at least 48 h.
Various pro-inflammatory cytokines stimulate cilia elongation
To investigate whether various pro-inflammatory cytokines stimulate cilia elongation, MEC were exposed to either TNFα, IL1β, or IFNγ (Fig. 1d). In general, cilia length significantly increased from approximately 2 µm to approximately 3 µm after stimulation (P < 0.001). FK was used as a positive control and did show the same average cilia elongation as the inflammatory cytokines.
Since cells responding to stimulation have considerable cilia length variations, their frequency distribution was analyzed (Suppl. Fig. 1). The cytokine and FK stimulated samples showed a shift in frequency distribution towards longer cilia compared to controls. Cilia of 2 µm were still present, indicative for cells that did not respond to stimulation, but the majority elongated to approximately 3 µm and some individual cilia even up of 7 µm.
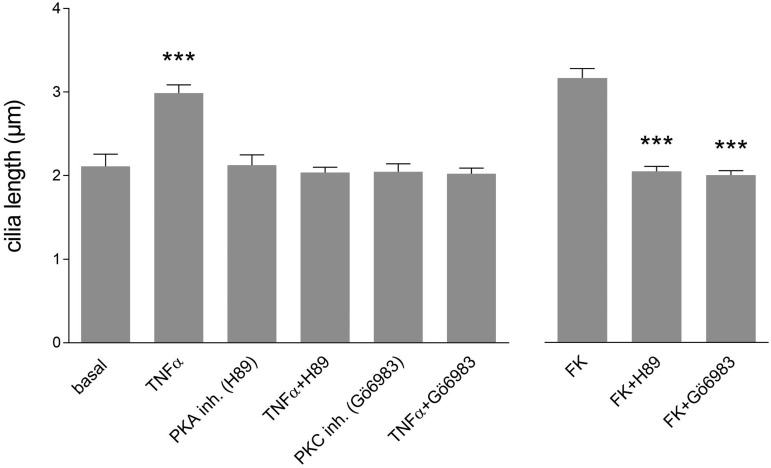
Cytokine-induced cilia elongation is dependent on PKC-dependent/PKC signaling
It has been shown that primary cilia elongate upon direct stimulation of cyclic AMP with concomitant activation of PKC-dependent.31 Involvement of PKC-dependent/PKC signaling in ciliary extension upon TNFα was tested by chemical inhibition with H89 or Gö6983 (Fig. 2). H89 and Gö6983 alone did not alter cilia length in MEC compared to basal condition. In agreement with the previous experiments, cilia length significantly increased to an average of approximately 3 µm upon TNFα stimulation (P < 0.001). Importantly, inhibition of PKA and PKC prevented TNFα-induced elongation. Similar results were obtained with IL1β, IFNγ, or the direct PKA activator FK (Suppl. Fig. 2A). In the presence of the inhibitors, none of the tested stimuli altered cilia length suggesting that cilia elongation by inflammatory cytokines is transduced through PKA/PKC dependent signaling.
Fig. 2.
Cilia elongation upon inflammatory cytokines is PKA/PKC-dependent. MEC were stimulated for 24 h with different combinations of TNFα (10 ng/mL) and/or the PKA inhibitor H89 (10 µM) or PKC inhibitor Gö6983 (2 µM) (n ≥ 43 cilia per condition). The PKA activator FK was used as positive control.
To confirm the importance of PKC signaling more specifically, PKC knockdown was performed by shRNA. The knockdown was sufficient to prevent TNFα-induced elongation (Suppl. Fig. 2B). The use of the lentiviral construct resulted in viable cells with a 60% decrease in PKC mRNA levels (Suppl. Fig. 2C).
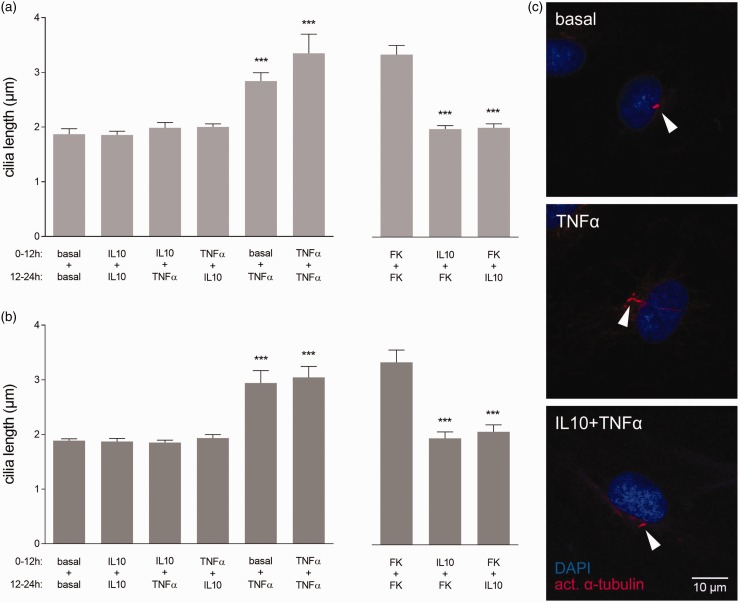
IL10 blocks and reverses cilia elongation upon cytokine stimulation
We reasoned that when cells react to inflammatory cytokines by elongating their primary cilia, anti-inflammatory stimuli might counteract this response. Therefore, we tested whether IL10 prevents and reverses pro-inflammatory cytokine-induced cilia elongation.
MEC were treated with basal medium, TNFα, or IL10 alone for 12 h. Afterwards either IL10 or TNFα were added on top of the previous stimulus for additional 12 h (Fig. 3a). In agreement with our previous experiments, 12 h or 24 h of TNFα stimulation alone showed significant cilia elongation to ∼3 µm (P < 0.001). IL10 alone did not influence cilia length compared to basal condition. Importantly, application of IL10 to TNFα blocked cilia elongation and IL10 added after TNFα reversed cilia length back to basal levels. The effect of IL10 on cilia length was not exclusive to TNFα, but identical upon IL1β, IFNγ, and IL10 blocked the effect of direct PKA activation by FK stimulation (Suppl. Fig. 3A). Interestingly, its inhibitory function was dependent on the actual presence of IL10. Pre-treatment with IL10 followed by FK without IL10 in the medium was not sufficient to prevent elongation (Suppl. Fig. 3B). In conclusion, IL10 blocked and reversed cilia elongation upon stimulation with various inflammatory cytokines.
Fig. 3.
IL10 blocks and reverses cilia elongation upon inflammatory cytokine stimulation in mouse and human endothelial cells. (a) MEC were either incubated with IL10 (10 ng/mL), TNFα (10 ng/mL), or left untreated for 12 h. Subsequently, different combinations of TNF or IL10 were added directly into the previous conditions and incubated for another 12 h (n ≥ 26 cilia per condition). (b) The combination treatments were repeated in human pulmonary MVEC of healthy individuals (donor = 3, n ≥ 28 cilia per condition). (c) MIP of representative cilia (arrowheads) on MVEC under the different conditions.
Cilia cytokine responses are similar in mouse and human endothelial cells
Primary cilia are highly conserved among species, wherefore their mechanisms might be as well. To examine, whether the primary cilia of human lung EC react in a similar manner to the pro-inflammatory TNFα and anti-inflammatory IL10 as mouse EC, we repeated the combination treatments in MVEC from healthy control lungs (Fig. 3b and c). Indeed, 12 h and 24 h of TNFα showed a significant cilia elongation to approximately 3 µm (P < 0.001) compared to basal length of ∼2 µm. Again, IL10 alone had no effect on basal cilia length. When combined with TNFα, IL10 blocked cilia elongation, while IL10 applied 12 h after TNFα reversed cilia length back to basal levels.
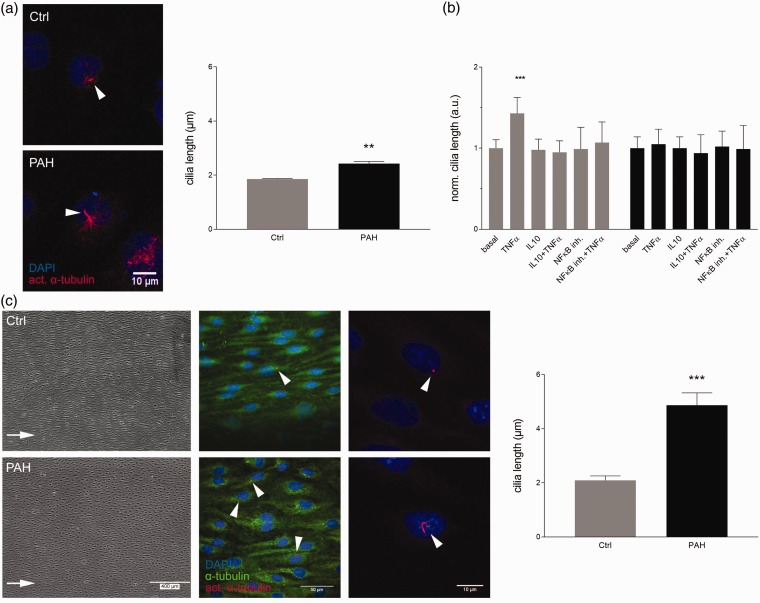
Endothelial cells of PAH patients display elongated cilia
To test, whether cilia length differs between patient and control MVEC, cilia were quantified in samples of three control and three donors with PAH (Fig. 4a). Under basal conditions the average length of cilia on EC from PAH patients was significantly increased to 2.43 ± 0.08 µm compared to 1.86 ± 0.02 µm of the controls (P = 0.002).
Fig. 4.
PAH patient-derived endothelial cells exert elongated cilia that are non-responsive to pro- and anti-inflammatory treatment but respond to fluid shear stress. (a) MVEC from controls and PAH lungs were grown to confluency and did not receive additional treatment. Cilia length was quantified. Samples were normally distributed and significance was calculated using an unpaired student's t-test on their average lengths (donor = 3, n ≥ 10 per donor). (b) Healthy controls (gray) and PAH-derived MVEC (black) were stimulated with TNFα, IL10 (both 10 ng/mL), NFκB inhibitor (1 µM) alone, or a combination of TNFα and a inhibitor. Cilia length was normalized to intra-experimental controls (basal) (donor ≥ 3, n ≥ 10 per donor and condition). (c) Control and PAH MVEC were subjected to high fluid shear stress (15 dyn/cm2) for five days and cilia length was quantified. Representative phase-contrast and fluorescence staining are shown. Arrows indicate direction of flow. Cilia length was quantified and differences were calculated with an unpaired student's t-test.
TNFα and IL10 do not affect cilia length in PAH cells
Healthy MEC and MVEC showed cilia elongation upon stimulation with inflammatory cytokines to ∼3 µm. IL10 could shorten cilia and block the effects of TNFα. PAH patient-derived MVEC exerted elongated cilia under basal conditions already (Fig. 4a), wherefore we tested cilia length responses in these patient cells (Fig. 4b). To our surprise, treatment with TNFα had no additional effect on cilia length and IL10 treatment of PAH MVEC did neither reduce basal cilia length nor affected the response to TNFα. To answer whether specifically the response to IL10 was lost or if cilia of PAH cells are generally unresponsive to anti-inflammatory treatment, the NFκB inhibitor BAY 11-7085 was tested. BAY 11-7085 showed similar responses like IL10 blocking the effects of TNFα in controls but leaving elongated basal PAH cilia unchanged.
The sheared PAH endothelium showes increased cilia length
To further elucidae, if PAH MVEC have completely lost their cilia length regulation ability, fluid shear stress was tested as trigger (Fig. 4c). Primary cilia of PAH MVEC were found significantly elongated to 4.87 ± 0.46 µm compared to 2.09 ± 0.17 µm of controls (P < 0.001) after five days of high shear stress. Additionally, while cilia of the sheared controls were localized towards the leading edge of the cells in the direction of flow and migration, cilia of PAH MVEC were found randomly positioned around the nucleus.
Discussion
Microvascular EC from lungs of patients with PAH display elongated cilia. These cells are incapable to adapt their cilia length in response to pro- and anti-inflammatory cytokine stimulation.
Primary cilia play a pivotal role in vascular integrity and homeostasis. Cilia dysfunction is implicated in several pathologies, such as atherosclerosis and developmental diseases. Among the variety of ciliopathies, cilia can be elongated, truncated, less present, or completely absent.26 In the Joubert syndrome, patients show less and shortened cilia.32 In contrast, the phenotype of Meckel-Gruber syndrome is elongated cilia. Most patients with Bardet-Biedl syndrome show truncated cilia,26 although patients with one specific subtype have elongated renal epithelial cilia.33 In addition to variations in cilia form across different ciliopathies, there is considerable heterogeneity in cilia function dependent on host cell and vascular bed. Renal cilia play a part in repair processes; they elongate upon renal injury and decrease in length during renal repair.34 In chondrocytes, cilia shorten upon mechanical loading to minimize cell sensitivity to prolonged activation.35 In mesenchymal stem cells, cilia elongation has been shown important for differentiation.36 Taken together, cells change their cilia length in response to environmental cues. When this process is dysfunctional, initial adaptation is disturbed and homeostasis and repair impaired.
PAH is a fatal group of diseases with a high inflammatory status.4 Cilia length increases in response to pro-inflammatory cytokines and was thereby proposed to mediate inflammatory responses.27 Hence, we tested the pro-inflammatory cytokines TNFα, IL1β, and IFNγ on healthy EC and all three cytokines provoked a similar increase in average cilia length to ∼3 µm. Thereby, not only the increase in length but also the length distribution was comparable between the various cytokines. This indicates a generic mechanism for cilia elongation upon inflammatory stimuli, since the cytokines themselves act through unique receptors and signaling pathways.5 Moreover, similar effects were observed between mouse and human ECs, which point towards a conserved mechanism among species.
Chondrocytes have been shown to elongate cilia upon IL1β stimulation in a PKA/PKC-dependent manner.27 In line with this finding, we show that PKA and PKC inhibition prevented cilia elongation after stimulation with the inflammatory cytokines. Interestingly, PKA/PKC signaling was not necessary to express cilia and basal length was not affected by PKA or PKC inhibition. Therefore, PKA/PKC signaling is predominantly needed for cilia elongation and might present a common integrator for various stimuli.
To consider, the individual stimuli might cause subtile differences in absolute cilia length. Using the PyT methode to determine average cilia length keeps selection bias minimal. However, subtile length differences <0.2 µm might be underestimated.30 What controls maximal cilia length and what absolute minimal change of length is functionally important remains to be resolved.
To reverse cilia length, removing the inflammatory stimulus was not sufficient, but application of the anti-inflammatory cytokine IL10 was needed to shorten the extended cilia back to basal levels demonstrating the need for active cues to switch EC from a pro- to anti-inflammatory state. In addition, IL10 (or NFκB inhibition) could block cilia elongation upon inflammatory cytokines and FK. Here, the effect was direct and reversible and left basal cilia length unaffected. Interestingly, the effect of IL10 was general for all tested cytokines and conserved in mouse and human EC. The precise mechanism of IL10 regulating cilia length remains to be determined. A direct effect of IL10 on PKA/PKC was not yet shown, while PKA itself is involved in IL10 production.37 Recent literature showed that the effects of TNFα, INFγ, and IL10 might, at least in part, be regulated independent of PKA via the SOCS (suppressor of cytokine signaling) pathway.37 The authors found a synergistic activation of SOCS-3 when combining IL10 and cyclic AMP treatment that was independent from PKA activation.
When repeating the stimulations in MVEC from patients with PAH, we found that cilia were already elongated under basal conditions compared to controls. Therefore, we assumed that treatment with IL10 would reduce cilia length. To our surprise, PAH ECs did neither respond to pro- nor anti-inflammatory cytokines with a variation of cilia length. The basal elongation and loss of TNFα response might be an adaptation to the excessive amounts of pro-inflammatory cytokines produced by the diseased cells and the chronic state of inflammation in patient lungs.4 However, the failure of IL10 to reduce cilia length might alternatively indicate that the basal elongation is independent from inflammatory signaling but rather due to metabolic changes.38 Of interest, longer cilia are known to be associated with decreased proliferation39 and thereby might be the cells attempt to counteract the hyper-proliferative phenotype characteristic to the PAH endothelium.40 In accordance with this line of reasoning, PAH patients with high levels of IL10 have a worse prognosis.41 On the contrary, higher IL10 levels are also suggested as compensatory mechanism in more advanced stages of the disease. In addition, administration of IL10 prevented development of PH in the monocrotaline rat model a model characterized by severe inflammation.42
Application of fluid shear stress (instead of cytokines) significantly altered cilia length of PAH cells, wherefore the loss of cilia length control seems specific to cytokine stimulation. Nevertheless, cilia of sheared PAH cells were longer than of controls and randomly localized around the nucleus, although they should be oriented towards the leading edge of the shear adapted cells, such as seen in the controls. This indicates defective mechano-responses and cell polarity in the sheared PAH cells, which might be a consequence of the defective shear sensing and delayed morphological adapation that we reported earlier.29 However, mono-motile cilia are linked to cell polarity, directed migration, and wound repair43 pointing towards a generally dysfunctional PAH endothelial responsiveness to micro-environmental cues that might manifest through or, at least partly, be caused by the dysfunctional cilium.
To summarize, longer cilia are an inherent feature of PAH MVEC. The sustained elongation of primary cilia and a loss of length regulation function upon cytokine stimulation might represent an adaptive response to the chronic inflammation and act as a rescue mechanism to prevent further recruitment of blood-borne inflammatory cells into the vessel wall of patient lungs and decrease EC proliferative rates. However, the functional consequences of cilia non-responsivness on intra-endothelial signaling and the surrounding tissue remain subject to further studies. Here, investigating defective cilia elongation in response to inflammatory cytokines might reveal downstream cellular changes contributing to disease progression. Additionally, restoring cilia responses to anti-inflammatory treatment in ECs from PAH patients might decelerate disease progression by maintaining the EC phenotype.
Supplemental Material
Supplemental material, Supplemental Figures for Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation by Anneloes Dummer, Nina Rol, Robert Szulcek, Kondababu Kurakula, Xiaoke Pan, Benjamin I. Visser, Harm Jan Bogaard, Marco C. DeRuiter, Marie-José Goumans and Beerend P. Hierck in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Methods for Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation by Anneloes Dummer, Nina Rol, Robert Szulcek, Kondababu Kurakula, Xiaoke Pan, Benjamin I. Visser, Harm Jan Bogaard, Marco C. DeRuiter, Marie-José Goumans and Beerend P. Hierck in Pulmonary Circulation
Acknowledgments
The authors acknowledge the support for CVON-PHAEDRA from the Netherlands CardioVascular Research Initiative, including the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Science.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This project is funded by the Phaedra consortium (CVON no. 2012-08).
ORCID iD
Robert Szulcek http://orcid.org/0000-0003-0450-7338
References
- 1.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endor. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Guignabert C, Dorfmuller P. Pathology and pathobiology of pulmonary hypertension. Semin Respir Crit Care Med 2013; 34: 551–559. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson MG, Kowalski PS, Bartelds B, et al. A critical role for Egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res 2014; 103: 573–584. [DOI] [PubMed] [Google Scholar]
- 4.Groth A, Vrugt B, Brock M, et al. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014; 15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindt TTJ, Goldsby RAR, Osborne BA. Kuby Immunology, 6th ed New York: W.H. Freeman, 2007. [Google Scholar]
- 6.Carbone F, Montecucco F. Inflammation in arterial diseases. IUBMB Life 2015; 67: 18–28. [DOI] [PubMed] [Google Scholar]
- 7.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009; 78: 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Heiden K, Groenendijk BCW, Hierck BP, et al. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn 2006; 235: 19–28. [DOI] [PubMed] [Google Scholar]
- 9.Takao D, Verhey KJ. Gated entry into the ciliary compartment. Cell Mol Life Sci 2016; 73: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Praetorius HA. The primary cilium as sensor of fluid flow: new building blocks to the model. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am J Physiol Cell Physiol 2015; 308: C198–208. [DOI] [PubMed] [Google Scholar]
- 11.Poelmann RE, Van der Heiden K, Gittenberger-de Groot A, et al. Deciphering the endothelial shear stress sensor. Circulation 2008; 117: 1124–1126. [DOI] [PubMed] [Google Scholar]
- 12.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci 2010; 123: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement CA, Ajbro KD, Koefoed K, et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 2013; 3: 1806–1814. [DOI] [PubMed] [Google Scholar]
- 14.Christensen ST, Clement CA, Satir P, et al. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 2012; 226: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia 2012; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 2007; 69: 377–400. [DOI] [PubMed] [Google Scholar]
- 17.Egorova ADD, Van der Heiden K, Van de Pas S, et al. Tgfβ/Alk5 signaling is required for shear stress induced klf2 expression in embryonic endothelial cells. Dev Dyn 2011; 240: 1670–1680. [DOI] [PubMed] [Google Scholar]
- 18.Basten SG, Giles RH. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia 2013; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mick DU, Rodrigues RB, Leib RD, et al. Proteomics of primary cilia by proximity labeling. Dev Cell 2015; 35: 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egorova AD, Khedoe PPSJ, Goumans M-JTH, et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 2011; 108: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Heiden K, Hierck BP, Krams R, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 2008; 196: 542–550. [DOI] [PubMed] [Google Scholar]
- 22.Dinsmore C, Reiter JF. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep 2016; 17: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wren KN, Craft JM, Tritschler D, et al. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol 2013; 23: 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keeling J, Tsiokas L, Maskey D. Cellular Mechanisms of Ciliary Length Control. Cells 2016; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi K, Kasahara K, Miyazaki I, et al. Factors that influence primary cilium length. Acta Med Okayama 2011; 65: 279–285. [DOI] [PubMed] [Google Scholar]
- 26.Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation 2012; 83: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wann AKT, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci 2012; 69: 2967–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauli SM, Kawanabe Y, Kaminski JJ, et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008; 117: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szulcek R, Happé CM, Rol N, et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension. role of platelet endothelial cell adhesion molecule-1 cleavage. Am J Respir Crit Care Med 2016; 193: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dummer A, Poelma C, DeRuiter MC, et al. Measuring the primary cilium length: improved method for unbiased high-throughput analysis. Cilia 2016; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Besschetnova TY, Kolpakova-Hart E, Guan Y, et al. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 2010; 20: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malicdan MCV, Vilboux T, Stephen J, et al. Mutations in human homologue of chicken talpid3 gene (KIAA0586) cause a hybrid ciliopathy with overlapping features of Jeune and Joubert syndromes. J Med Genet 2015; 52: 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mokrzan EM, Lewis JS, Mykytyn K. Differences in renal tubule primary cilia length in a mouse model of Bardet-Biedl syndrome. Nephron Exp Nephrol 2007; 106: e88–96. [DOI] [PubMed] [Google Scholar]
- 34.Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20: 2147–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGlashan SR, Knight MM, Chowdhury TT, et al. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int 2010; 34: 441–446. [DOI] [PubMed] [Google Scholar]
- 36.Dalbay MT, Thorpe SD, Connelly JT, et al. Adipogenic differentiation of hMSCs is mediated by recruitment of IGF-1r onto the primary cilium associated with cilia elongation. Stem Cells 2015; 33: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasperini S, Crepaldi L, Calzetti F, et al. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur Cytokine Netw 2002; 13: 47–53. [PubMed] [Google Scholar]
- 38.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Zaghloul NA, Bubenshchikova E, et al. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol 2011; 13: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 2009; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Okada T, Miyashita H, et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 2007; 101: 734–741. [DOI] [PubMed] [Google Scholar]
- 43.Veland IR, Lindbæk L, Christensen ST. Linking the primary cilium to cell migration in tissue repair and brain development. Bioscience 2014; 64: 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Figures for Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation by Anneloes Dummer, Nina Rol, Robert Szulcek, Kondababu Kurakula, Xiaoke Pan, Benjamin I. Visser, Harm Jan Bogaard, Marco C. DeRuiter, Marie-José Goumans and Beerend P. Hierck in Pulmonary Circulation
Supplemental material, Supplemental Methods for Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation by Anneloes Dummer, Nina Rol, Robert Szulcek, Kondababu Kurakula, Xiaoke Pan, Benjamin I. Visser, Harm Jan Bogaard, Marco C. DeRuiter, Marie-José Goumans and Beerend P. Hierck in Pulmonary Circulation