Abstract
Objective:
This study aims to investigate the correlations between rapidly accelerated fibrosarcoma/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase signaling pathway and clinicopathological features and prognosis for patients with breast cancer having axillary lymph node metastasis.
Methods:
A total of 118 breast cancer tissues with axillary lymph node metastasis (axillary lymph node metastasis group), 150 breast cancer tissues with non-axillary lymph node metastasis (non-axillary lymph node metastasis group), and 216 normal breast tissues (normal group) were enrolled in this study. The messenger RNA and protein expressions of rapidly accelerated fibrosarcoma, MEK, extracellular signal-regulated kinase, and their phosphorylated (p-) proteins were examined by reverse transcriptase quantitative polymerase chain reaction and immunohistochemistry, respectively. All patients received a 1-year follow-up, and the clinical follow-up data were collected. The multiple factors on the prognosis of patients with breast cancer having axillary lymph node metastasis were tested by Cox regression analysis.
Results:
The messenger RNA expressions of rapidly accelerated fibrosarcoma, MEK, and extracellular signal-regulated kinase and positive rates of rapidly accelerated fibrosarcoma, MEK, phosphorylated MEK, extracellular signal-regulated kinase, and p-extracellular signal-regulated kinase in the axillary lymph node metastasis group were higher than in the non-axillary lymph node metastasis and normal groups (all P < .05). The protein expressions of rapidly accelerated fibrosarcoma, MEK, phosphorylated MEK, extracellular signal-regulated kinase, and p-extracellular signal-regulated kinase were associated with tumor size, clinical stage, and axillary lymph node metastasis number (all P < .05). Rapidly accelerated fibrosarcoma, MEK, and extracellular signal-regulated kinase expressions were significantly correlated with the prognosis of patients with breast cancer (all P < .05). Patients with BC having positive rapidly accelerated fibrosarcoma, MEK, phosphorylated MEK, extracellular signal-regulated kinase, and phosphorylated ERK expressions had a higher survival rate than patients with BC having the negative ones (all P < .05). Rapidly accelerated fibrosarcoma and extracellular signal-regulated kinase protein expressions, clinical stage, pathological grade, and axillary lymph node metastasis number were independent prognostic factors in patients with breast cancer having axillary lymph node metastasis (all P < .05).
Conclusion:
Our study proved that rapidly accelerated fibrosarcoma/MEK/extracellular signal-regulated kinase signaling pathway is significantly correlated with the clinicopathological features and prognosis for patients with BC having axillary lymph node metastasis. Rapidly accelerated fibrosarcoma and extracellular signal-regulated kinase protein expressions are independent prognostic factors for patients with breast cancer having axillary lymph node metastasis.
Keywords: rapidly accelerated fibrosarcoma, mitogen-activated protein kinase (MAPK) kinase, extracellular signal-regulated kinase, breast cancer, axillary lymph node metastasis, clinicopathological features, prognosis
Introduction
Breast cancer (BC) is the most common cancer among women worldwide, and it is also the second leading cause in cancer-related mortality.1 In excess of 1.7 million, women are annually diagnosed with BC around the world, and 35% succumb to the disease and its aftermath.2 Breast cancer is regarded as a complex and heterogeneous disease owing to the lack of understanding related to precise progression mechanisms.3 As a result, the treatment recommendations in cases of BC are continuously varying.4 In recent years, although the relative survival rate for BC has been raised, the 5-year survival rate of patients having metastatic disease remains less than 30%.5 Metastatic spread of tumor tissues to other body organs accounts for the majority of deaths in patients with BC,6 among which axillary lymph node metastasis (ALNM) is the most frequently occurring metastatic disease.7 Currently, a relatively small number of accurate prognostic and predictive factors can be clinically used to manage patients with BC, and it is suggested that gene expression profiling can act as potential prognostic indicator in patients with BC.8 Thus, prognosis determination and identification of new biomarkers in patients with BC having ALNM is trivial for better treatment and higher survival rates.6
Rapidly accelerated fibrosarcoma (Raf)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) has been considered as a highly specific 3-layered kinase cascade consisting of the Ser/Thr kinase Raf (A-Raf, B-Raf, or C-Raf/Raf-1), the highly homologous dual-specificity kinases MEK1/MAP2K1 and MEK2/MAP2K2 (collectively MEK1/2), and the ubiquitously expressed Ser/Thr kinase ERK1/mitogen-activated protein kinase 3 (MAPK3) and its homolog ERK2/MAPK1 (collectively ERK1/2).9 Among the aforementioned 3 Raf proteins, B-Raf has been recognized as the chief MEK activator and displaying a higher affinity for MEK1/2 kinases.10 The highly conserved upstream kinases MEK1/2 can activate ERK1/2 kinases, which are members of the MAPK family.11 The Raf/MEK/ERK signaling pathway has been recognized as a crucial pathway in disease etiology and in the regulation of cell growth, proliferation, differentiation, apoptosis, and survival.12 Attributable to the fact that the Raf/MEK/ERK signaling pathway evolutionarily is a conserved signal transduction module, its significance in tumorigenesis has been emphasized recently. Rapidly accelerated fibrosarcoma/MEK/ERK signaling pathways have been notably involved in multiple tumor entities, including thyroid, malignant melanoma, and colonic and ovarian carcinomas.13 However, correlations between the Raf/MEK/ERK signaling pathway and clinicopathological features and prognosis for patients with BC having ALNM are not fully elucidated. Therefore, we herein assessed the clinical implications of Raf/MEK/ERK expressions and evaluate its therapeutic potential as prognostic determinants for intervention and treatment of patients with BC having ALNM.
Materials and Methods
Ethics Statement
This study was approved by the ethics committee of our hospital. Signed informed consents were obtained from all patients participating in the research.
Study Participants
The study included a total of 268 patients with BC undergoing diagnosis and treatment at our hospital from January 2015 to December 2015, and consequently tissue specimens were collected from the aforementioned patients. The patients were divided into ALNM group (n = 118) and non-ALNM group (n = 150). The ALNM group patients aged between 21 and 70 years (calculated mean age: −50.50 [10.91] years); non-ALNM group patients aged between 24 and 72 years (calculated mean age: −49.81 [9.13] years). The inclusion criteria were as follows: (1) patients pathologically diagnosed with BC before undergoing surgery; (2) patients with complete clinicopathological data, follow-up information, and pathological diagnosis records in our hospital; (3) patients without a history of malignancy in other physiological systems; (4) patients not undergoing other preoperative therapies including radiotherapy, chemotherapy, endocrine therapy, and biological therapy. Additionally, 216 normal breast tissues were collected as the normal group from 216 individuals diagnosed with hyperplasia of mammary glands (age range: 20-70 years; mean age: 49.81 [9.29] years). All study participants were female, of whom the difference in age was insignificant, who were of high comparability. All tissues were fixed in 10% formaldehyde and embedded using conventional paraffin wax.
Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR)
RNA was extracted from tissues using RNA extraction kit (10296010; Invitrogen, Shanghai, China) and reversely transcribed to complementary DNA (cDNA) using PrimeScript RT Reagent Kit (RR014A; Takara Bio, Beijing, China). Rapidly accelerated fibrosarcoma, MEK, and ERK primers were designed by SBS Genetech Co, Ltd (Shanghai, China) according to Raf, MEK, and ERK gene sequences and reverse transcriptase quantitative polymerase chain reaction (PCR) primer design requirements of corresponding references using Primer5 software (PREMIER Biosoft, Palo Alto, California, USA; Table 1). Complementary DNA was used for PCR amplification, and the PCR amplification system settings were as follows: 1 μL cDNA, 1 μL forward and reverse primers, respectively, 1 μL 2 × Taq PCR mix, and 20 μL ddH2O. Subsequently, the reaction liquid was gently shaken and briefly centrifuged. Rapidly accelerated fibrosarcoma reaction condition settings were as follows: 30 predenaturation cycles at 94°C for 5 minutes, 94°C for 45 seconds, 58°C for 30 seconds, 72°C for 30 seconds, and extension at 72°C for 7 minutes. MEK reaction condition settings were as follows: 40 predenaturation cycles at 95°C for 2 minutes, 95°C for 15 seconds, 55°C for 15 seconds, 72°C for 20 seconds, and extension at 72°C for 10 minutes. Extracellular signal-regulated kinase reaction condition settings were as follows: 40 predenaturation cycles at 95°C for 5 minutes, 95°C for 15 seconds, 58°C for 15 seconds, 60°C for 30 seconds, and extension at 72°C for 10 minutes. Glyceraldehyde-3-phosphate dehydrogenase reaction condition settings were as follows: 27 predenaturation cycles at 95°C for 5 minutes, 95°C for 15 seconds, 58°C for 15 seconds, 72°C for 20 seconds, and extension at 72°C for 6 minutes. The prepared gel plate was laid out onto a horizontal electrophoresis system, and diluted 1 × Tris-borate EDTA buffer was poured into the electrophoresis system until gel plate surface was slightly covered. Five microliters of extracted PCR amplification products were loaded onto a 1% agarose gel electrophoresis system. Subsequently, a 100 base-pair DNA ladder was used for horizontal electrophoresis (100 V, 40-50 minutes). The obtained gel was removed and transferred onto a gel imaging system, and amplified fragment lengths were determined by comparisons with Maker bands (100 base-pair DNA ladder). The experimental results were recorded.
Table 1.
Primer Sequences of Raf, MEK, ERK, and GAPDH for RT-qPCR.
| Gene | Sequence |
|---|---|
| Raf | F: 5’-CGAACAGTGAATATTTCCTTTGAT-3’ |
| R: 5’-TCCCTCTCAGGCATAAGGTAA-3’ | |
| MEK | F: 5’-GCTTCTATGGTGCGTTCT-3’ |
| R: 5’-GAGTTGACTAGGATGTTGGA-3’ | |
| ERK | F: 5’-GAACTCCAAGGGCTATACCAAGT-3’ |
| R: 5’-GGAGGGCAGAGACTGTAG GTAGT-3’ | |
| GAPDH | F: 5’-AGAAGGCTGGGGCTCATTTG-3’ |
| R: 5’-GGGCCATCCACAGTCTTC-3’ |
Abbreviations: ERK, extracellular signal-regulated kinase; GAPDH, glyceraldehyde-3-Phosphate Dehydrogenase; F, forward; R, reverse; Raf, rapidly accelerated fibrosarcoma; RT-qPCR, reverse transcriptase quantitative polymerase chain reaction.
Immunohistochemical Staining
The paraffin-embedded specimens were cut into 5 sections of 4 μm thickness. The paraffin sections were conventionally dewaxed using xylene, immersed in 3% hydrogen peroxide (H2O2) at room temperature for 15 minutes, and rinsed with phosphate-buffered saline (PBS) solution (2 × 5 minutes). Citrate buffer (pH 6.0) at high pressure conditions was used to repair antigens (2 minutes) and subsequently cooled off at room temperature with normal goat serum applied overnight (40 minutes), and next, Raf (1:400), MEK (1:400), phosphorylated (p)-MEK (1:400), ERK (1:200), and phosphorylated ERK (p-ERK; 1:200) antibodies (all purchased from SANTA CRUZ Biotechnology, Inc, Santa Cruz, California) were added to incubate paraffin sections at low temperature overnight, respectively. The subsequent day, the sections were rinsed with PBS followed by the addition of biotinylated secondary antibodies and streptavidin into the reaction system. Finally, diaminobenzidine was added in order to color develop the sections. The sections were counterstained, dehydrated, vitrified, and sealed using hematoxylin, gradient ethanol, xylene, and neutral gum, respectively. The positively stained sections were selected as the positive control group, and the negative control group was treated with PBS instead of primary antibodies.
The sections were observed under an optical microscope (Olympus Optical Co, Ltd, Tokyo, Japan), and a semiquantitative analysis was conducted. Ten visual fields under a 40× amplified microscope of each section were selected for further use, and number of positive cells in each visual field were counted and recorded, followed by the calculation of average percentage of positive cells. Positive cells were classified into the following 5 grades based on the percentage of positive cells as follows: less than 5%, 0 point; 5%-25%, 1 point; 26%-50%, 2 points; 51%-75%, 3 points; and 76%-100%, 4 points. The cells were awarded the following 4 grades according to the staining intensity of color as follows: no staining, 0 point; light yellow, 1 point; yellow, 2 points; and brown, 3 points. Eventually, the results were classified into the following 4 scales based on the sum of the aforementioned 2 scores as follows: 0-1 point, negative (−); 2-3 points, weak positive (+); 4-5 points, moderate positive (++); and 6-7 points, strong positive (+++). Rapidly accelerated fibrosarcoma, MEK, phosphorylated MEK (p-MEK), ERK, and p-ERK levels could be seen as positive coloring upon yellow or brownish-yellow cell nucleus and cytoplasm coloration.
Follow-Up
A 1-year follow-up was conducted for all patients via the means of telephone calls, outpatient, or medical record reviews and followed by recording the survival time of patients. The recorded 1-year overall survival rate was regarded as a prognosis factor for analyses. The follow-up period ended on December 31, 2016.
Statistical Analyses
Statistical analyses were performed using the SPSS 20.0 software (IBM Corp, Armonk, New York, USA). Measurement data were demonstrated by mean (standard deviation). Differences between multiple groups were compared by 1-way analysis of variance tests and differences between 2 groups were compared by t tests. Enumeration data were analyzed by χ2 test, and survival rates were calculated by Kaplan-Meier. Comparisons among groups were inspected by the log-rank test. Multivariate analysis of prognostic factors was conducted by Cox regression model. P < .05 was recognized statistically significant.
Results
The mRNA Expression of Raf, MEK, and ERK Among Normal, non-ALNM, and ALNM Groups
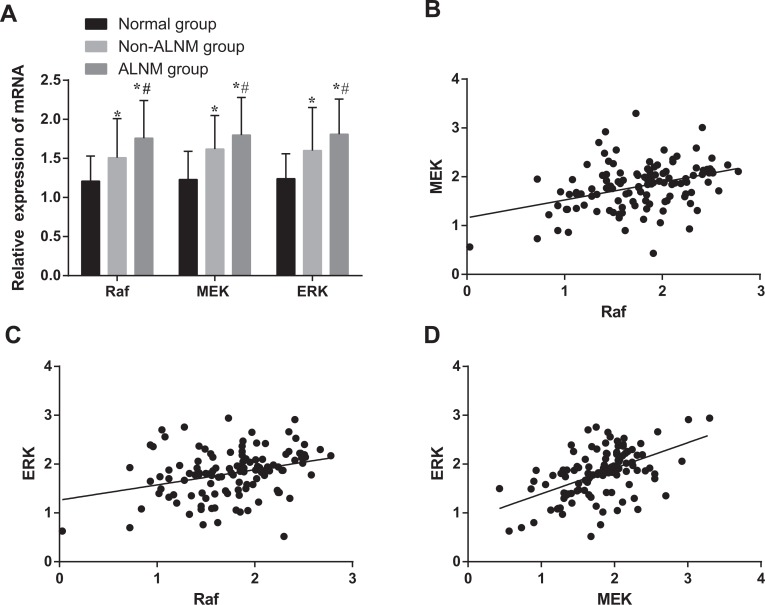
As shown in Figure 1A, messenger RNA (mRNA) expression of Raf, MEK, and ERK were higher in the ALNM group than the non-ALNM group (all P < .05), whereas the non-ALNM group expression was higher than the normal group (all P < .05). As shown in Figure 1B–D, Raf, MEK, and ERK expressions were positively correlated with each other (Raf and MEK, r = 0.37; Raf and ERK, r = 0.31; MEK and ERK, r = 0.51; all P < .05).
Figure 1.
Comparison of mRNA expressions and the correlations among of Raf, MEK, and ERK. A, Comparison of mRNA expressions of Raf, MEK, and ERK; *, compared with the normal group, P < .05; #, compared with the non-ALNM group, P < .05. B, Correlation between Raf and MEK mRNA expressions. C, Correlation between Raf and ERK mRNA expressions. D, Correlation between MEK and ERK mRNA expressions. ALNM indicates axillary lymph node metastasis; BC, breast cancer; ERK, extracellular signal-regulated kinase; mRNA, messenger RNA; Raf, rapidly accelerated fibrosarcoma.
Positive Rate of Raf, MEK, p-MEK, ERK, and p-ERK Proteins Among the Normal, non-ALNM, and ALNM Groups
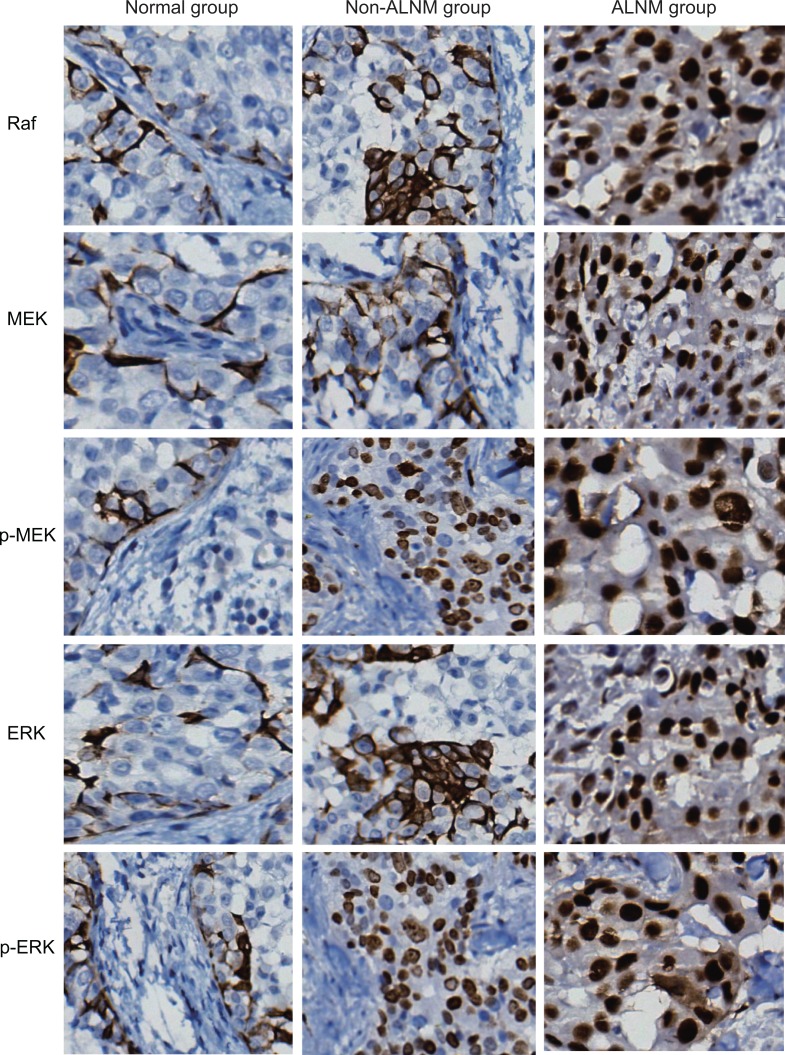
As shown in Figure 2 and Table 2, the positive rates of Raf, MEK, p-MEK, ERK, and p-ERK proteins in the ALNM group were higher (82.2%, 71.2%, 73.7%, 84.7%, and 83.1%, respectively) than the non-ALNM group (70.7%, 63.3%, 68.0%, 74.0%, and 71.3%, respectively; all P < .05), whereas the non-ALNM group positive rates were elevated compared to the normal group (60.2%, 47.7%, 50.5%, 63.9%, 57.9%, respectively; all P < .05).
Figure 2.
Comparison of Raf, MEK, p-MEK, ERK, and p-ERK protein expressions among the normal, non-ALNM, and ALNM groups detected by immunohistochemistry (× 200). ALNM indicates axillary lymph node metastasis; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma.
Table 2.
Comparison of Protein Expressions of Raf, MEK, p-MEK, ERK, and p-ERK Among the Normal, non-ALNM, and ALNM Groups.
| Item | Normal Group (n = 216) | Non-ALNM Group (n = 150) | ALNM Group (n = 118) | P |
|---|---|---|---|---|
| Raf | < .001 | |||
| Negative expression | 86 (39.8%) | 44 (29.3%) | 21 (17.8%) | |
| Positive expression | 130 (60.2%) | 106 (70.7%) | 97 (82.2%) | |
| MEK | < .001 | |||
| Negative expression | 113 (52.3%) | 55 (36.7%) | 34 (28.8%) | |
| Positive expression | 103 (47.7%) | 95 (63.3%) | 84 (71.2%) | |
| p-MEK | < .001 | |||
| Negative expression | 107 (49.5%) | 48 (32.0%) | 31 (26.3%) | |
| Positive expression | 109 (50.5%) | 102 (68.0%)a | 87 (73.7%)a,b | |
| ERK | < .001 | |||
| Negative expression | 78 (36.1%) | 39 (26.0%) | 18 (15.3%) | |
| Positive expression | 138 (63.9%) | 111 (74.0%) | 100 (84.7%) | |
| p-ERK | < .001 | |||
| Negative expression | 91 (42.1) | 43 (28.7%) | 20 (16.9%) | |
| Positive expression | 125 (57.9%) | 107 (71.3%)a | 98 (83.1%)a,b |
Abbreviations: ALNM, axillary lymph node metastasis; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma.
acompared with the normal group, P < 0.05
bcompared with the non-ALNM group, P < 0.05
Correlation Of Protein Expressions of Raf, MEK, p-MEK, ERK, and p-ERK with Clinicopathological Features in Patients With BC Having ALNM
As shown in Table 3, Raf, MEK, and ERK protein expressions were not correlated with patient age, menopausal status, pathological types, and pathological grading (all P > .05). Whereas the positive rates of Raf, MEK, p-MEK, ERK, and p-ERK protein were correlated with tumor size, clinical stages, and ALNM number (all P < .05). The size of the tumor was directly proportional to the positive rates of protein expression of Raf, MEK, p-MEK, ERK, and p-ERK. Similarly, the advancement of tumor clinical stages directly raised protein expression. Likewise, ALNM number was directly proportional to the positive rates of protein expression of Raf, MEK, p-MEK, ERK, and p-ERK.
Table 3.
Correlation of Raf, MEK, p-MEK, ERK, and p-ERK Expressions and Clinicopathological Features in Patients With BC Having ALNM.
| Clinicopathological Feature | N | Raf Positive (%) | P | MEK Positive (%) | P | p-MEK Positive (%) | P | ERK Positive (%) | P | p-ERK Positive (%) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||||
| <50 | 58 | 79.31 | .419 | 77.59 | .131 | 74.14 | .921 | 81.03 | .270 | 81.03 | .566 |
| ≥50 | 60 | 85.00 | 65 | 73.33 | 88.33 | 85.00 | |||||
| Menopause status | |||||||||||
| Premenopausal | 59 | 76.27 | .092 | 66.1 | .223 | 72.88 | .834 | 83.05 | .609 | 83.05 | 1.000 |
| Postmenopausal | 59 | 88.14 | 76.27 | 74.58 | 86.44 | 83.05 | |||||
| Tumor size | |||||||||||
| ≤12 cm | 39 | 69.23 | .010 | 48.72 | <.001 | 48.72 | <.001 | 71.79 | <.001 | 61.54 | <.001 |
| >2 cm | 79 | 88.61 | 82.28 | 86.08 | 91.14 | 93.67 | |||||
| Clinical stage | |||||||||||
| I | 21 | 61.90 | .007 | 33.33 | <.001 | 42.86 | .002 | 52.38 | <.001 | 66.67 | .007 |
| II | 59 | 81.36 | 88.14 | 77.97 | 88.14 | 79.66 | |||||
| III | 38 | 94.74 | 97.37 | 84.21 | 97.37 | 97.37 | |||||
| Pathological grading (%) | |||||||||||
| I | 26 | 80.77 | .578 | 57.69 | .193 | 65.38 | .510 | 76.92 | .510 | 84.62 | .210 |
| II | 41 | 78.05 | 78.05 | 78.05 | 85.37 | 90.24 | |||||
| III | 51 | 86.27 | 72.55 | 74.51 | 88.24 | 76.47 | |||||
| ALNM number | |||||||||||
| 1 ≤ N + ≤ 3 | 70 | 74.29 | .007 | 61.43 | .005 | 64.29 | .005 | 78.57 | .024 | 72.86 | < .001 |
| N + > 3 | 48 | 93.75 | 85.42 | 87.50 | 93.75 | 97.92 | |||||
| Pathological type (%) | |||||||||||
| Noninvasive cancer | 23 | 82.61 | .762 | 52.17 | .061 | 73.91 | .588 | 69.57 | .588 | 78.26 | .333 |
| Early invasive cancer | 28 | 78.57 | 71.43 | 64.29 | 89.29 | 92.86 | |||||
| Specific invasive cancer | 34 | 79.41 | 85.29 | 79.41 | 82.35 | 76.47 | |||||
| Nonspecific invasive cancer | 33 | 87.88 | 69.7 | 75.76 | 93.94 |
Abbreviations: ALNM, axillary lymph node metastasis; BC, breast cancer; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma.
Univariate Analysis of Prognostic Factors for Patients with BC Having ALNM
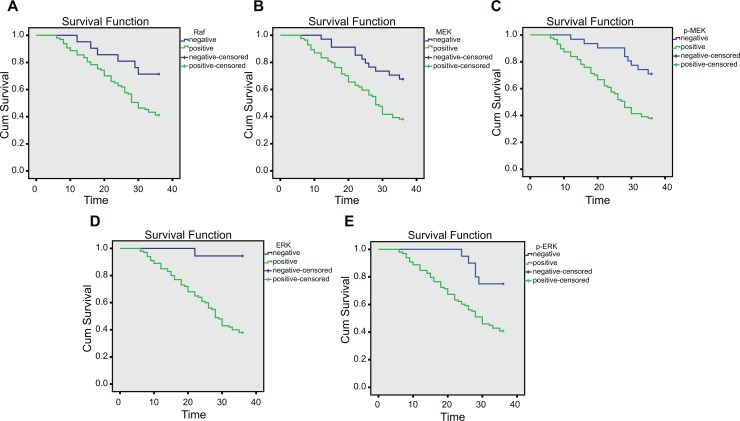
An univariate analysis of prognostic factors affecting the 1-year survival rate of patients with BC having ALNM (Table 4) showed that patient age, menopausal status, and pathological type were unrelated with the prognosis of patients with BC having ALNM (all P > .05). The tumor size (χ2 = 9.417, P = .002), clinical stage (χ2 = 14.730, P < .001), pathological grading (χ2 = 16.240, P < .001), ALNM number (χ2 = 12.400, P < .001), protein expression of Raf (χ2 = 6.323; P = .012; Figure 3A), protein expression of MEK (χ2 = 8.494, P = .004; Figure 3B), protein expression of p-MEK (χ2 = 10.020, P = .002), protein expression of ERK (χ2 = 19.530, P < .001; Figure 3C), and protein expression of p-ERK (χ2 = 5.294, P = .021) were all prognostic factors of patients with BC having ALNM (all P < .05).
Table 4.
Univariate Analysis of Prognostic Factors for Patients With BC Having ALNM.
| Clinicopathological Feature | N, Survival Rate (%) | χ2 | P |
|---|---|---|---|
| Age (years) | |||
| <50 | 58 (51.72) | 1.199 | .274 |
| ≥50 | 60 (41.67) | ||
| Menopause status | |||
| Premenopausal | 59 (54.24) | 2.758 | .097 |
| Postmenopausal | 59 (38.98) | ||
| Tumor size | |||
| ≤ 2 cm | 39 (66.67) | 9.417 | .002 |
| > 2 cm | 79 (36.71) | ||
| Clinical stage | |||
| I | 21 (80.95) | 14.730 | .001 |
| II | 59 (45.76) | ||
| III | 38 (28.95) | ||
| Pathological grading | |||
| I | 26 (80.77) | 16.240 | < .001 |
| II | 41 (41.46) | ||
| III | 51 (33.33) | ||
| Pathological type | |||
| Noninvasive cancer | 23 (47.83) | 0.378 | .945 |
| Early invasive cancer | 28 (50.00) | ||
| Specific invasive cancer | 34 (47.06) | ||
| Nonspecific invasive cancer | 33 (42.42) | ||
| ALNM number | |||
| 1 ≤ N + ≤ 3 | 70 (60.00) | 12.400 | < .001 |
| N + > 3 | 48 (27.08) | ||
| Raf expression | |||
| Negative | 21 (71.43) | 6.323 | .012 |
| Positive | 97 (41.24) | ||
| MEK expression | |||
| Negative | 34 (67.65) | 8.494 | .004 |
| Positive | 84 (38.10) | ||
| p-MEK expression | |||
| Negative | 31 (70.97) | 10.020 | .002 |
| Positive | 87 (37.93) | ||
| ERK expression | |||
| Negative | 18 (94.44) | 19.530 | <.001 |
| Positive | 100 (38.00) | ||
| p-ERK expression | |||
| Negative | 20 (70.00) | 5.294 | .021 |
| Positive | 98 (41.84) |
Abbreviations: ALNM, axillary lymph node metastasis; BC, breast cancer; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma.
Figure 3.
Correlation between protein expressions of Raf, MEK, p-MEK, ERK, and p-ERK and prognosis of patients with BC having ALNM. A, Survival curve of patients with positive and negative protein expression of Raf. B, Survival curve of patients with positive and negative protein expression of MEK. C, Survival curve of patients with positive and negative protein expression of p-MEK. D, Survival curve of patients with positive and negative protein expression of ERK. E, Survival curve of patients with positive and negative protein expression of p-ERK. ALNM indicates axillary lymph node metastasis; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma.
Multivariate Analysis of Prognostic Factors for Patients With BC Having ALNM
A Cox regression model was used for multivariate analyses of risk factors for prognosis of patients with BC having ALNM, and the forward stepwise (likelihood ratio) method was applied for analysis. As shown in Table 5, patient age, menopausal status, pathological type, tumor size, and MEK, p-MEK, and p-ERK protein expression were not independent prognostic factors of BC (all P > .05). Results indicated Raf and ERK protein expression, clinical stage, pathological grading, and ALNM number were independent prognostic risk factors in patients with BC (all P < .05). Patients with BC at more advanced clinical stages, higher pathological grades, larger ALNM numbers, and positive Raf and ERK protein expression had lower survival rates and a worse prognosis of BC.
Table 5.
Multivariate Analysis of Prognostic Factors for Patients With BC Having ALNM.
| Item | B | SE | Wald | Sig. | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|
| Raf | 2.252 | 1.028 | 4.798 | 0.028 | 9.506 | 1.267-71.298 |
| ERK | 2.436 | 1.022 | 5.678 | 0.017 | 11.427 | 1.541-84.741 |
| p-ERK | 0.938 | 0.488 | 3.696 | 0.055 | 2.555 | 0.982-6.648 |
| Clinical stage | 0.801 | 0.255 | 9.876 | 0.002 | 2.229 | 1.352-3.675 |
| Pathological grading | 0.637 | 0.202 | 9.911 | 0.002 | 1.890 | 1.272-2.809 |
| ALNM number | 0.669 | 0.270 | 6.159 | 0.013 | 1.952 | 1.151-3.311 |
Abbreviations: ALNM, axillary lymph node metastasis; B, regression coefficient; BC, breast cancer; CI, confidence interval; ERK, extracellular signal-regulated kinase; Exp (B), constant; p-ERK, phosphorylated ERK; p-MEK, phosphorylated MEK; Raf, rapidly accelerated fibrosarcoma; SE, standard error; Sig, significance; Wald, degree of freedom.
Discussion
Human BC is mostly malignant in women and is characterized by diverse genetic alterations.14 A variety of risk factors concern BC, such as patient age, obesity, race, alcohol intake, density of the breast, personal and family health history, endogenous estrogen levels, use of hormone replacement therapy, and radiation exposure to the breast.15 Furthermore, the most prominent reason for therapy failure in BC is tumor metastasis, including all types of BC metastatic disease, especially ALNM being the most frequent cause of therapy failure.7 A promising way to decrease morbidity and mortality of BC is to search for innovative diagnostic markers for early diagnosis and prognostication.16 Collectively, our study aims to explore the correlation between Raf/MEK/ERK expression and clinicopathological features and prognosis for patients with BC having ALNM.
In our study, Raf, MEK, p-MEK, ERK, and p-ERK mRNA and protein expressions were higher in the ALNM group compared to the non-ALNM group and normal group, suggesting that the Raf/MEK/ERK signaling pathway may be implicated in BC with ALNM. The Raf/MEK/ERK signaling pathway can be constitutively activated via genetic alterations and regulated by cytokines, receptor tyrosine kinases, and heterotrimeric G-protein-coupled receptors.17 The pathway regulation is mediated by a series of kinases, phosphatases, and various exchange proteins.18 Being a well-known pathway, the Raf/MEK/ERK signaling pathway has reportedly been involved in many diseases like leukemia.19 Wu and Singh et al previously reported that the Raf/MEK/ERK signaling pathway could be seen as a survival pathway for melanoma, where the Raf, MEK, and ERK play the role of survival signals and are constitutively activated through activating mutations of NRAS or B-Raf genes.20 According to Liebner et al, the Raf/MEK/ERK signaling pathway is the major signaling pathway implicated in thyroid cancer, wherein it is closely involved in several metabolic processes like promoting cell proliferation, migration, survival and cycle progression, metabolism, angiogenesis, and tumorigenesis.21 Steelman et al demonstrated sensitivity or resistance implications that arise in cases of leukemia therapy due to Raf/MEK/ERK signaling pathway.22 All previous researches on the Raf/MEK/ERK signaling pathway and their correlation with multiple diseases provides us precious insight and motive to explore their values in BC with ALNM.
Furthermore, our results show that protein expression of Raf, MEK, p-MEK, ERK, and p-ERK were positively correlated with clinicopathological features of patients with BC having ALNM, including tumor size, clinical stage, and ALNM number. In addition, our 1-year follow-up analyses also indicated that patients with BC having ALNM demonstrating positive Raf/MEK/ERK expression rates had lower survival rates and a worse prognosis compared to patients with negative Raf/MEK/ERK expression rate. Previous studies demonstrated that the Raf/MEK/ERK signaling pathway possesses prognostic values in various other diseases. Chen et al found that Raf/MEK/ERK signaling pathway components are abnormally expressed in human cancer and concluded that overexpression of Raf could be considered as an independent prognostic biomarker in cases of hepatocellular carcinoma.23 Doyle et al reported that Raf kinase inhibitor protein could provide independent prognostic information for patients with Dukes B colorectal cancer.24 Chang et al previously published a review article stating that ERK plays a significant role in prognosis of tumors.25 A Cox regression model analysis further confirmed that Raf and ERK protein expression, clinical stage, pathological grading, and ALNM number were independent prognostic factors in patients with BC having ALNM, suggesting association between Raf/MEK/ERK signaling pathway and clinicopathological features and poor prognosis for patients with BC having ALNM.
Cell signaling pathways are crucial to understand the trajectory of cancer progression and other life phenomenon, including cell growth regulation, migration and death, and angiogenesis.17 The Raf/MEK/ERK signaling pathway has been extensively studied over the past few decades.18 If a tumor exhibits dependency on the Raf/MEK/ERK pathway, consequently it may be sensitive to Raf and MEK inhibitors. In addition, multiple researches have led to the development of specific inhibitors targeting key components of the Raf/MEK/ERK signaling pathway aiming to ultimately raise patient survival rates or to prevent or impede the development of other diseases such as diabetes, obesity, and premature aging.26 However, the relatively short follow-up period and small sample size included in our study may have influenced the prognostic implications. Therefore, further analyses and investigations employing longer follow-up periods and larger sample sizes will be included in future studies in order to explore the Raf/MEK/ERK signaling pathway for its prognostic implication in patients with BC having ALNM. Henceforth, researching correlations between the Raf/MEK/ERK signaling pathway and clinicopathological features and prognosis for patients with BC having ALNM may provide a novel method to understand BC metastasis and therapy.
Acknowledgments
The authors would like to give their sincere appreciation to the reviewers for their helpful comments on this article.
Abbreviations
- ALNM
axillary lymph node metastasis
- BC
breast cancer
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- p-ERK
phosphorylated ERK
- PBS
phosphate-buffered saline
- Raf
rapidly accelerated fibrosarcoma.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Vandendriessche E, Van De Putte G, Van Den Broecke R, De Jonge E. Improving systemic breast cancer therapy: time to look beyond the primary tumour? Facts Views Vis Obgyn. 2015;7(4):251–256. [PMC free article] [PubMed] [Google Scholar]
- 2. Mistry DA, French PW. Circulating phospholipids as biomarkers of breast cancer: a review. Breast Cancer (Auckl). 2016;10:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falco M, Palma G, Rea D, et al. Tumour biomarkers: homeostasis as a novel prognostic indicator. Open Biol. 2016;6(12):pii: 160254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Downs-Holmes C, Silverman P. Breast cancer: overview & updates. Nurse Pract. 2011;36(12):20–26; quiz 27. [DOI] [PubMed] [Google Scholar]
- 5. Rashid OM, Takabe K. Does removal of the primary tumor in metastatic breast cancer improve survival? J Womens Health (Larchmt). 2014;23(2):184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang LL, Hao S, Zhang S, et al. PTEN/PI3K/AKT protein expression is related to clinicopathological features and prognosis in breast cancer with axillary lymph node metastases. Hum Pathol. 2017;61:49–57. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Ji R, Li J, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010;29:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar R, Sharma A, Tiwari RK. Application of microarray in breast cancer: an overview. J Pharm Bioallied Sci. 2012;4(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park JI. Growth arrest signaling of the Raf/MEK/ERK pathway in cancer. Front Biol (Beijing). 2014;9(2):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sánchez-Torres JM, Viteri S, Molina MA, Rosell R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl Lung Cancer Res. 2013;2(3):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei F, Yan J, Tang D. Extracellular signal-regulated kinases modulate DNA damage response - a contributing factor to using MEK inhibitors in cancer therapy. Curr Med Chem. 2011;18(35):5476–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suojun Z, Feng W, Dongsheng G, Ting L. Targeting Raf/MEK/ERK pathway in pituitary adenomas. Eur J Cancer. 2012;48(3):389–395. [DOI] [PubMed] [Google Scholar]
- 13. Manousaridis I, Mavridou S, Goerdt S, Leverkus M, Utikal J. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol. 2013;27(1):11–18. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Zheng Y, Qiao C, et al. Expression of SATB1 and HER2 in breast cancer and the correlations with clinicopathologic characteristics. Diagn Pathol. 2015;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma S, Patnaik PK, Aronov S, Kulshreshtha R. ApoptomiRs of breast cancer: basics to clinics. Front Genet. 2016;7:175 eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco Targets Ther. 2016;9:5567–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yajima I, Kumasaka MY, Thang ND, et al. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant melanoma progression and therapy. Dermatol Res Pract. 2012;2012:354191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abrams SL, Steelman LS, Shelton JG, et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9(9):1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. [DOI] [PubMed] [Google Scholar]
- 20. Wu S, Singh RK. Resistance to chemotherapy and molecularly targeted therapies: rationale for combination therapy in malignant melanoma. Curr Mol Med. 2011;11(7):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liebner DA, Shah MH. Thyroid cancer: pathogenesis and targeted therapy. Ther Adv Endocrinol Metab. 2011;2(5):173–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steelman LS, Franklin RA, Abrams SL, et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25(7):1080–1094. [DOI] [PubMed] [Google Scholar]
- 23. Chen L, Shi Y, Jiang CY, Wei LX, Wang YL, Dai GH. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2011;37(6):513–520. [DOI] [PubMed] [Google Scholar]
- 24. Doyle B, Hagan S, Al-Mulla F, et al. Raf kinase inhibitor protein expression combined with peritoneal involvement and lymphovascular invasion predicts prognosis in Dukes’ B colorectal cancer patients. Histopathology. 2013;62(3):505–510. [DOI] [PubMed] [Google Scholar]
- 25. Chang YS, Liu JC, Fu HQ, et al. Roles of targeting Ras/Raf/MEK/ERK signaling pathways in the treatment of esophageal carcinoma. Yao Xue Xue Bao. 2013;48(5):635–641. [PubMed] [Google Scholar]
- 26. McCubrey JA, Steelman LS, Chappell WH, et al. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3(9):954–987. [DOI] [PMC free article] [PubMed] [Google Scholar]