Abstract
The rearing method of bison and the nutrient content of the meat may make bison a healthier alternative to beef. We hypothesized that the acute and chronic effects of bison consumption, in comparison to beef, will result in a less perturbed blood lipid panel and a reduced inflammatory and oxidative stress response which will minimize the detrimental effect on vascular function. A double-blind, cross-over randomized trial was employed to examine the consequence of a single 12 oz serving (N=14) and 7 weeks of chronic consumption (N=10) (12 oz per day, 6 days/week) of each meat. Measurements included: blood lipids, interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), c-reactive protein (CRP), oxidized low-density lipoprotein (Ox-LDL), protein carbonyl, hydroperoxides, flow mediated dilation (FMD) and FMD/shear rate. Following a single beef meal: triglycerides (TG) and Ox-LDL were elevated (67±45% and 18±17% respectively), there was a tendency for hydroperoxides to be elevated (24±37%), and FMD/shear rate was reduced significantly (30±38%). Following a single meal of bison: there was a smaller increase in TG (30±27%), and markers of inflammation and oxidative stress and FMD/shear rate were unchanged. Chronic consumption of either meat did not influence body weight, % body fat, or blood lipids. Protein carbonyl (24±45%), PAI-1 (78±126%), IL-6 (59±76%) and CRP (72±57%) were significantly elevated and FMD/shear rate was significantly reduced (19±28%) following 7-weeks of beef consumption, but not bison consumption. Based on our findings, the data suggest that bison consumption results in a reduced atherogenic risk compared to beef.
Keywords: human, vascular disease, endothelium, diet, red meat, oxidative stress, bison
1. Introduction
In recent years consumers have been warned of the negative effects of diets containing large amounts of red meat high in saturated fatty acids (SFA), including elevated cholesterol and the subsequent risk of cardiovascular disease. These negative attributes of beef are partially a product of the relatively recent industrial age, which increased beef production, but from a nutritional standpoint had a negative impact on beef quality: Specifically, two centuries ago most beef cattle were range fed and slaughtered between 4–5 years of age while today approximately 99% of all beef consumed in the United States originates from grain fed cattle which are ready to be slaughtered by 14 months of age. This process results in a greater total fat content and higher omega-6/omega-3 ratio which increases the postprandial inflammatory response leading to endothelial dysfunction and potentially to cardiovascular disease.
The current American diet which consists of highly refined or processed foods and red meats from animals that have spent a greater portion of their life on a feed lot eating corn rather than being range fed and eating grass. As an alternative source of red meat, bison, which are often primarily range fed may offer health advantages, as bison meat not only contains lower total fat content, but also provides a more favorable fatty acid composition compared to beef. Specifically, bison contains an increased ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA), 3–4 times more anti-inflammatory omega-3 PUFA and is particularly high in alpha linolenic acid. In addition, ruminants such as bison are a major contributors of conjugated linoleic acid (CLA) to the human diet, providing significantly more CLA than other non-ruminant meat sources such as pork, fish, chicken, and turkey. The dietary inclusion of a specifically rich source of CLA may be advantageous as CLA is believed to have anti-inflammatory properties, and may have an important role in the prevention of cardiovascular disease and cancer.
To date, there has not been a human clinical trial to test the overall hypothesis that bison is a healthier alternative to beef in terms of vascular health. Of the limited data that do exist, a study comparing the bison and beef hybrid (beefalo), revealed an increase in LDL following beef, but not beefalo consumption. More recently, McAfee et al (2011) reported that consumption of beef and lamb from grass fed cattle contributes to dietary intake of LC n-4 PUFA. Other clinical trials focused upon meat consumption have compared red versus white meat. Indeed, data are lacking on the direct comparison of two red meat sources such as bison meat and comparable cuts of conventional beef cattle meat.
Thus, the objective of the present investigation was, utilizing cross over randomized human trials, to compare the influence of a single meal as well as chronic consumption (7 weeks) of bison and beef on blood lipids, markers of inflammation (IL-6, CRP, TNF-alpha and PAI-1) and markers of oxidative stress (hydroperoxides, protein carbonyl and oxidized LDL). In addition, we also aimed to compare the influence of acute and chronic consumption of bison and beef on vascular function as measured by shear stress-induced brachial artery dilation following the release of a cuff occlusion (flow mediated dilation, FMD). We hypothesized that the acute and chronic effects of bison consumption, in comparison to beef, will result in a less perturbed blood lipid panel and a reduced inflammatory and oxidative stress response. Consequently, we also hypothesized that the consumption of bison, rather than beef, will minimize the detrimental effect of red meat consumption on vascular function, as indicated by a greater brachial artery FMD.
2. Methods and Materials
2.1 Subjects
All methods and procedures utilized in this study were approved by the University of Utah’s Institutional Review Board. A total of 14 healthy male subjects (table 1) volunteered to participate in the acute portion of the study. A subset of 10 participants also took part in the chronic portion of the study. Exclusion criteria included: cholesterol > 240mg/dl, currently on blood pressure or vasoactive medications or the use of tobacco as elevated cholesterol, vasoactive drugs and nicotine all impact vascular function and would blur the results of the treatments.
Table 1.
Subject Characteristics
| Age (yrs) | 34.0 ± 11.3 |
| Height (cm) | 177.4 ± 6.1 |
| Weight (cm) | 82.1 ± 14.9 |
| Body Mass Index (Kg/m2) | 24.7 ± 4.8 |
| Body Fat (%) | 18.3 ± 7.7 |
Data expressed as means ± SD (n = 14).
2.2 Experimental Design
For the acute portion of the study, subjects were randomly assigned two single meals consisting solely of 340 g (12 oz) beef or bison steaks in a crossover design. For the chronic study subjects were randomly assigned beef or bison meat (consisting of steaks and roasts), 340 g (12 oz) per day, 6 days per week for 7 weeks, and instructed to incorporate the meat into their normal diet. Subjects were not specifically asked to maintain an isocaloric diet to prevent weight gain as pilot data indicated that subjects did not gain or lose weight during a similar intervention. Therefore, it was deemed best to minimize interference with the subject’s normal eating habits beyond the meat intervention. A 30-day washout period, during which they returned to their normal diet, was employed prior to a second 7-week period during which subjects adopted the same regime, but with the other meat. To increase meal variation and maintain compliance, subjects were provided nutritional guidance and recipes and were in regular contact with the investigators.
2.3 Beef and bison meat
The beef and bison meat utilized in this investigation consisted of a combination of sirloin steaks and chuck roasts. The beef was typical commercial “USDA Choice” grade, from 19-month old cattle that were feedlot fed (hay, corn silage, and a mixture of grains including wheat, barley and corn). The bison meat was also from 19-month old male bison, which were range fed for 7 months and subsequently 12 months feedlot fed (alfalfa hay, hay silage, corn silage, barley, corn wheat mill run, dry distillers grains, and potato solids).
2.4 FMD Testing
For all FMD tests, subjects reported to the laboratory following an overnight fast and having refrained from caffeine for 24 hours prior to each testing day. Prior to the meal, blood samples were drawn and a fasted FMD test was performed. Previously established guidelines for FMD testing were followed. In brief, subjects lay supine, with a pneumatic cuff (SC5, Hokanson Inc., Bellevue, Washington) placed on the arm immediately proximal to the elbow and distal to the site of the ultrasound Doppler probe. Following a 20-minute rest period, baseline blood velocity and vessel images were recorded using a GE Logiq 7 ultrasound / Doppler machine (General Electric Medical Systems, Milwaukee, WI, USA). The arm cuff was then inflated to supra-systolic pressure (> 250 mmHg) for 5 minutes. Following release of the cuff, blood velocity and brachial arterial images were recorded again for two minutes. The simultaneous measurements of brachial arterial blood velocity and vessel diameter were performed with the Logic 7 utilizing a linear array transducer operating at 14 MHz imaging frequency and 5 MHz Doppler frequency. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Angle-corrected, and intensity weighted mean velocity (Vmean) values were calculated using commercially available software (Logic 7). Electrocardiogram triggering was utilized to capture end-diastolic arterial diameters which were analyzed using automated edge detection Brachial Analysis software (Medical Imaging Application, Coralville, IA, USA).
Each subject then consumed a single meal which consisted solely of 12 ounces of oven-broiled beef steak, with 1/4″ fat trim intact (without additives such as oils, condiments, salt or spices) or an identically prepared 12 oz. bison steak. Subjects were only provided with water to drink, ad libitum. Following the meal, subjects rested quietly in the laboratory for 4 hours, which allowed for the subsidence of the protein/fat effects on insulin concentration. To determine the acute effect of beef or bison consumption, a second blood sample was then drawn followed by a postprandial FMD test. Following each 7-week meat intervention, participants in the chronic study reported back to the laboratory in a fasted state for an additional third (chronic effect) FMD and blood draw.
2.5 FMD Analyses
Vessel diameter and blood velocity were averaged across four second intervals for the first 20 seconds following cuff release and then across ten second intervals for the remainder of the two minute data collection period. Using arterial diameter and Vmean, blood flow in the brachial artery was calculated as: Blood flow = Vmeanπ(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. Shear rate was calculated as: shear rate = (Vmean × 4) / vessel diameter. FMD was expressed as a percent increase in baseline diameter: FMD= (peak hyperemic diameter − baseline diameter)/ baseline diameter. To account for any differences in shear rate, the main stimulus for arterial dilation with this approach, FMD was also expressed relative to shear rate (FMD/shear rate).
2.6 Blood Analyses
Blood samples were centrifuged at 2000 g for 15 minutes, after which serum and plasma were separated and stored at −80 degrees C for future analysis. The blood samples were analyzed for total cholesterol, TG and LDL and HDL concentrations by ARUP Laboratories, University of Utah using a quantitative enzymatic assay on a Roche P-modular platform (Indianapolis, Indiana). The ferric reducing ability of plasma (FRAP) and Ferrous/xylenal orange (FOX) assays were also used to determine total antioxidant capacity and hydroperoxide concentrations, respectively. Oxidized LDL concentrations were measured using an enzyme immunoassay (Mercodia, kit 10-1143-01, Uppsala, Sweden). High sensitivity IL-6, IL-10 and TNF-alpha as well as PAI-1 concentrations were measured using a solid phase sandwich ELISA kits (kits HS600B, D100B, DTA00C, DSE100 respectively, R&D Systems: Minneapolis, MN). Protein carbonyl were measured by a protein Carbonyl ELISA (kit NWK-PCK01, Northwest Life Science Specialities, LLC., Vancouver, Washington). Finally, serum C-reactive protein was also measured using a liquid-phase, double-antibody radioimmunoassay.
2.7 Physical Characteristics and Questionnaires
Three-day food diaries (requiring food type and brand name, volume or mass, and cooking method) and physical activity questionnaires (requiring the type of activity, duration, and intensity based on a Borg RPE scale) were collected from subjects at the beginning and end of each treatment period. In addition, during the chronic investigation subjects were required to complete a daily compliance checklist which allowed us to determine if the subjects were compliant with the meat consumption (greater than 95% compliant). The food records were analyzed using Food Processor nutritional analysis software (Version 8.3, 2004, ESHA Research; Salem, Oregon). In addition at the beginning and end of each 7 week period subjects height, weight and body fat composition (Tanita Body Composition Analyzer Model TBF-300A) were recorded.
2.8 Fatty Acid Analysis of Meat Lipids
A total lipid and fatty acid analysis, including concentrations of saturated, polyunsaturated, omega-6 and omega-3 fatty acids, was performed on the bison and beef steaks and roasts. Fatty acid methyl esters were prepared as described by Murrieta et al.. Briefly, direct transesterification of duplicate 100-mg samples of freeze-dried muscle in 16 mm x 125 mm culture tubes with Teflon-lined caps was accomplished using 2.0 mL of 0.2 M KOH in anhydrous methanol by 60 minutes of incubation at 50° C. Tubes were vortex-mixed three times/ min for about 3 sec to keep tissue in suspension. After reaction, tubes were allowed to cool, and then 3.0 mL of H2O and 2.0 mL of hexane were added to each tube, tubes were capped, and then vortex-mixed for 15 sec. Tubes were centrifuged for 2 min at 2,500 rpm to accelerate phase separation, and the hexane phase transferred to GLC vials containing a 1.0-mm bed of anhydrous Na2SO4. Each tube contained 1.0 mg of glyceryl tritridecanoate (C13:0 as triacylglycerol) as an internal standard initially added to each tube.
Fatty acid methyl esters were separated using a Agilent 6890 GLC (Agilent Technologies, Inc., Wilmington, DE) equipped with a flame ionization detector and a 100-m x 0.25-mm (i.d.) fused silica capillary column (SP-2560, 0.2 micrometer film thickness, Supelco, Bellefonte, PA). Oven temperature was maintained at 175° C for 40 min, and then increased to 240° C at 10° C/min. Injector and detector temperatures were 275° C. Helium was the carrier gas with a split ratio of 50:1 and 0.8 mL/min column flow. Fatty acid peaks were recorded and integrated using Agilent ChemStation software. Fatty acids were identified by comparing retention times with fatty acid methyl ester standards (Nu-Chek Prep, Inc., Elysian, MN and Matreya, Inc., Pleasant Gap, PA).
2.9 Statistical Analyses
Initial sample sizes were based on a power analyses from FMD pilot data, selected as vascular function represents the practical consequence of the meat intervention. Data were analyzed (SPSS version 16.0) using paired t-tests to determine the acute effect (single meal) and chronic effect (7-weeks) of beef or bison consumption. For the acute analysis, pre-meal values (i.e. total cholesterol, LDL, HDL, TG, IL-6, IL-10, PAI-1, CRP, TNF-α, Ox-LDL, protein carbonyl, hydroperoxides, total antioxidant capacity, FMD and FMD/shear) were compared to post-meal values. The chronic effect was determined by comparing fasted values at the beginning to fasted values at the end of the 7-week treatment period. Paired t-tests were also used to determine changes in BMI, body fat (%), body mass, and physical activity as well as diet including carbohydrate, fat, saturated fats, protein total caloric intake between each 7 week period. In addition, independent samples t-test were used to determine differences in total lipid or fatty acids between the beef and bison meat as well as the ratio of n-6/n-3 PUFA and index of atherogenicity. For all tests conducted, the significance level was set at alpha = 0.05. All data are presented as means ± SD.
3. Results
Baseline subject characteristics are presented in Table 1. For the chronic portion of the study, subjects demonstrated excellent compliance as documented in the daily compliance checklist. There was a single subject dropout due to relocation. In addition, there were no differences in any of the dependent variables between the two fasting measurement prior to each 7-week meat intervention, indicating that the 30-day washout period was effective.
3.1 Meat
In brief, these analyses indicated that bison steaks had a significantly lower total fat (P<0.001), saturated fat (P=0.003) and monounsaturated fatty acids (P=0.012), and greater PUFA (P<0.001), omega-3 (P=0.013) and omega-6 (P<0.001) compared to the beef steaks (Table 2). Similarly, the bison roasts (ingested as a component of the chronic meat consumption) had lower total fat (P<0.001) and greater PUFA (P=0.003), omega-3 (P=0.005) and omega-6 (P=0.004) fatty acids compared to the beef roasts as well as a tendency toward lower MUFA (P=0.08). However the saturated fat content did not differ between bison and beef roasts. Based upon the meat composition data, the total fat load ingested in the 12 ounces of beef steaks and roasts were on average 21.8 and 19.0 g of fat respectively, while there were only 9.5 and 8.8 g of fat in the bison steaks and roasts, respectively. Furthermore, while the ratio of n-6/n-3 PUFA were similar between bison and beef, the index of atherogenicity was reduced in the bison roasts and steaks (p=0.036 and 0.008, respectively) compared to beef. In addition to these grouped fatty acid results, there were differences in individual fatty acid content between beef and bison (Table 3).
Table 2.
Fatty acid composition and index of atherogenicity (IA) for beef and bison steaks and roasts
| Steaks | Roasts | |||
|---|---|---|---|---|
| Beef | Bison | Beef | Bison | |
| Total Fat (wt%) | 6.4 ± 1.6* | 2.9 ± 1.7 | 5.6 ± 1.3* | 2.6 ± 0.8 |
| SFA (%) | 44.8 ± 2.8* | 39.3 ± 1.0 | 41.1 ± 4.3 | 38.4 ± 3.1 |
| PUFA (%) | 4.9 ± 0.8* | 17.0 ± 2.4 | 7.2 ± 1.6* | 14.8 ± 2.8 |
| MUFA (%) | 48.1 ± 2.9* | 42.6 ± 2.6 | 49.5 ±2.3 | 44.9 ± 3.8 |
| Omega -3 (%) | 0.3 ± 0.2* | 1.3 ± 0.7 | 0.4 ± 0.2* | 0.9 ± 0.1 |
| Omega -6 (%) | 4.6 ± 0.8* | 15.7 ± 2.1 | 6.8 ± 1.5* | 14.0 ± 2.7 |
| Omega 6/3 ratio | 19.1 ± 10.4 | 15.4 ± 8.3 | 17.2 ± 5.1 | 15.8 ± 2.1 |
| IA | 0.64 ± 0.12* | 0.36 ± 0.02 | 0.57 ± 0.04* | 0.35 ± 0.16 |
Data expressed as means ± SD. Total fat (n=8) is expressed as concentration (g/100g meat) and the remaining variables (n=5) are expressed as a weight% of total fat. SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid; IA, Index of Atherogenicity was calculated as previously described [36].
Indicates a significant difference in that fatty acid between beef and bison for that specific cut of meat, as indicated by independent samples t-test.
Table 3.
Fatty acid composition of beef and bison steaks and roasts
| Fatty Acid | Steaks | Roast | ||
|---|---|---|---|---|
| beef Weight % | bison Weight % | beef Weight % | Bison Weight % | |
| 14:0 | 2.28±0.63* | 1.11± 0.11 | 2.26±0.90* | 1.08±0.08 |
| 14:1 | 0.19±0.07 | 0.14±0.03 | 0.37±0.08* | 0.17 ±0.04 |
| 15:0 | 0.43±0.12 | 0.31±0.05 | 0.39±0.10 | 0.35±0.07 |
| 15:1 | 0.11±0.03* | 0.16±0.01 | 0.11±0.06 | 0.19±0.03 |
| 16:0 | 24.33±1.41* | 16.99±0.65 | 22.48±3.60* | 16.31±1.15 |
| 16:1 | 2.46±0.28* | 1.62±0.22 | 2.57 ± 0.73 | 2.02±0.39 |
| 17:0 | 1.50±0.27* | 1.16±0.12 | 1.19±0.28 | 1.27±0.14 |
| 17:1 | 0.92±0.14* | 0.64±0.10 | 0.80±0.28 | 0.78±0.14 |
| 18:0 | 16.27±1.47* | 19.74±0.58 | 14.74±2.98 | 19.40±2.60 |
| 18:1t9 | 0.39±0.23 | 0.33±0.20 | 0.38±0.25* | 0.79±0.09 |
| 18:1t10 | 0.37±0.13 | 0.58±0.37 | 1.52±2.00 | 0.74±0.16 |
| 18:1t11 | 2.41±2.44 | 1.20±0.65 | 2.856±1.70* | 0.75±0.20 |
| 18:1t12 | 0.15±0.08 | 0.18±0.02 | 0.544±0.38 | 0.41±0.07 |
| 18:1t13 | 0.23±0.13 | 0.25±0.08 | 0.30±0.30 | 0.68±0.14 |
| 18:1c9 | 39.11±5.14 | 35.41±2.81 | 38.07±4.49 | 35.87±3.77 |
| 18:1c11 | 1.53±0.45 | 1.83±0.07 | 1.64±0.44 | 1.98±0.22 |
| 18:1c12 | 0.22±0.05 | 0.20±0.04 | 0.32±0.08* | 0.43±0.02 |
| 18:2n6 | 3.35±0.54* | 11.72±1.51 | 5.19±1.24* | 10.15±1.62 |
| 18:3n3 | 0.18±0.09* | 0.61±0.29 | 0.25±0.08* | 0.49±0.02 |
| CLAc9t11 | 0.36±0.10 | 0.48±0.09 | 0.54±0.28 | 0.25±0.02 |
| 20:4n6 | 0.86±0.36* | 3.52±0.62 | 1.05±0.30* | 3.57±1.14 |
| 20:5n3 | 0.07±0.07* | 0.51±0.34 | 0.11±0.10 | 0.22±0.06 |
| 22:6n3 | 0.02±0.01* | 0.16±0.07 | 0.03±0.01* | 0.14±0.03 |
Weight percentage for fatty acids expressed as mg of fatty acid per 100 mg of total fatty acid. Data expressed as Means ± SD (n=5).
Significantly different than bison meat.
3.2 Comparison of dietary recall, physical activity and physical characteristics during chronic beef and bison consumption
Dietary recall analysis indicated that the addition of 12 oz of beef or bison to the diet of the subjects had no influence on carbohydrate, total dietary fat, or total caloric intake, however protein intake did increase with bison and not beef consumption (Table 4). There was a significant increase in dietary saturated fat as a result of the 7 weeks of beef but not bison consumption. These results are in agreement with the unchanged subject characteristics: body weight, BMI or % body fat (Table 4) following 7 weeks of beef or bison consumption. In addition, physical activity did not change from baseline during either dietary intervention, and was also not different between the two treatments (Table 4).
Table 4.
Dietary recall, physical activity and physical characteristics of subjects before and after each 7 week dietary intervention
| Pre beef | Post beef | Pre bison | Post bison | |
|---|---|---|---|---|
| Total calorie intake (kcal/day) | 2597 ± 770 | 2828±713 | 2647±998 | 2773±708 |
| Protein (g/day) | 130±62 | 138±54 | 124±71 | 184±61* |
| Carbohydrate (g/day) | 360±145 | 321±92 | 348±171 | 268±76 |
| Fat (g/day) | 70±26 | 110±81 | 83±30 | 84±35 |
| Saturated fat (g/day) | 32±26 | 45±33* | 28±21 | 33±23 |
|
| ||||
| Physical activity (minutes) | 52.2±21.2 | 53.9±21.6 | 46.4±17.1 | 47.8±19.7 |
|
| ||||
| Weight (kg) | 80.8 ± 16.9 | 80.0 ±17.4 | 81.0 ± 18.2 | 81.1 ± 17.9 |
| Body Fat (%) | 18.3 ± 7.7 | 17.9 ± 7.3 | 18.1 ± 7.5 | 17.3 ± 8.2 |
| BMI (kg/m2) | 25.6 ± 5 | 25.3 ± 5.1 | 25.6 ± 5.4 | 25.9 ± 5.2 |
Data expressed as means ± SD (N=9).
indicates significant difference from pre to post intervention for that specific meat as indicated by paired samples t-test.
3.3 Blood Analyses
Lipids
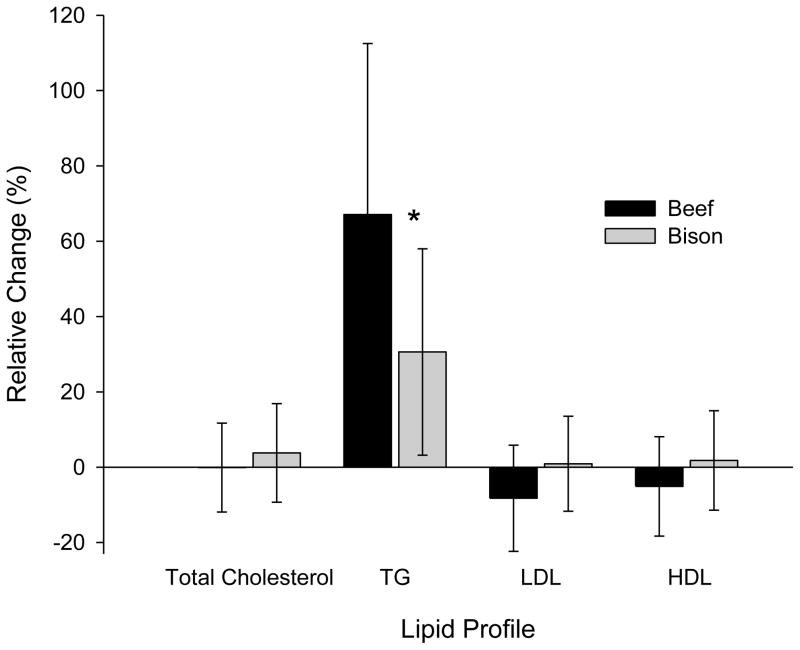
Total cholesterol, LDL and HDL were not significantly influenced by a single meal of either beef or bison (Figure 1). Triglycerides, however, significantly increased by 64.5% (P<0.001) and 30.4% (P<0.001) and following a single meal of beef and bison, respectively. Both the absolute and relative changes in triglyceride levels following beef consumption were significantly greater than the changes following bison consumption. Total cholesterol, Triglycerides, LDL or HDL were not altered by 7 weeks of either beef or bison consumption.
Fig. 1.
Relative change in total cholesterol, triglycerides, LDL and HDL following an acute meal of beef (black bar) and bison (grey bar). Data presented as means ± SD. * indicates a significant difference between beef and bison as determined by paired samples t-test (P>0.05) (N=14).
Antioxidant capacity and oxidative stress
Although total antioxidant capacity (FRAP) and protein carbonyl were not influenced by a single meal of either meat (P>0.26), oxidized LDL levels increased following a single meal of beef (74.64 ± 14.37 to 87.55 ± 18.31 u/L; P=0.026) but not bison (80.8±29.3 to 83.3±37.2 u/L; P=0.57). Likewise hydroperoxides (FOX) tended to increase following a single meal of beef (1.44 ± 0.32 to 1.78 ± 0.66 μm/L; P=0.08) but not bison (1.35 ± 0.35 to 1.36 ± 0.31 μm/L; P=0.77). Neither total antioxidant capacity, hydroperoxides, nor oxidized LDL changed following 7 weeks of consuming either meat. Protein carbonyl, on the other hand, was significantly increased following 7 weeks of beef (0.12 ± 0.12 to 0.30 ± 0.15 nmol/mg; P=0.04), but not bison consumption (0.10 ± 0.13 to 0.24 ± 0.16 nmol/mg; P=0.13).
Inflammation
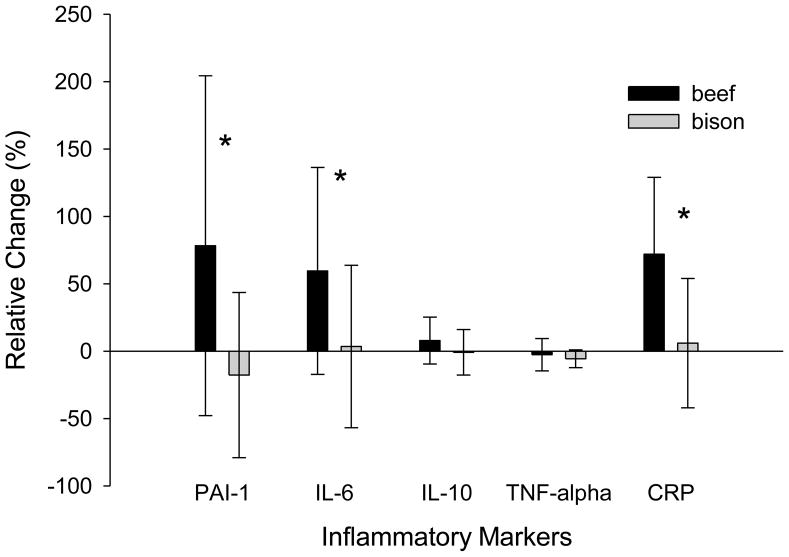
PAI-1 and TNF alpha decreased following a single meal of bison (from 1.09±0.71 to 0.52±0.43 ng/mL, P=0.001; and from 0.29±0.04 to 0.27±0.04 pg/mL, P=0.025, respectively), but were not influenced by a single meal of beef (P=0.17 and 0.46, respectively). IL-10, IL-6, and CRP on the other hand were not influenced by an acute meal of either beef or bison. There was also no significant change in PAI-1, IL-10, IL-6, TNF alpha or CRP following 7 weeks of beef or bison consumption. However, the relative changes in PAI-1 (P=0.02), IL-6 (P=0.04) and CRP (P=.003) following 7 weeks of beef consumption were greater than following 7 weeks of bison consumption (Figure 2).
Fig. 2.
Relative changes in inflammatory markers (PAI-1, IL-6, IL-10, TNF-alpha, CRP) following 7 weeks of beef (black bar) or bison (grey bar) consumption as means ±SD. * indicates a significant difference between beef and bison as determined by a paired samples t-test (p<0.05) (N=9).
3.4 FMD
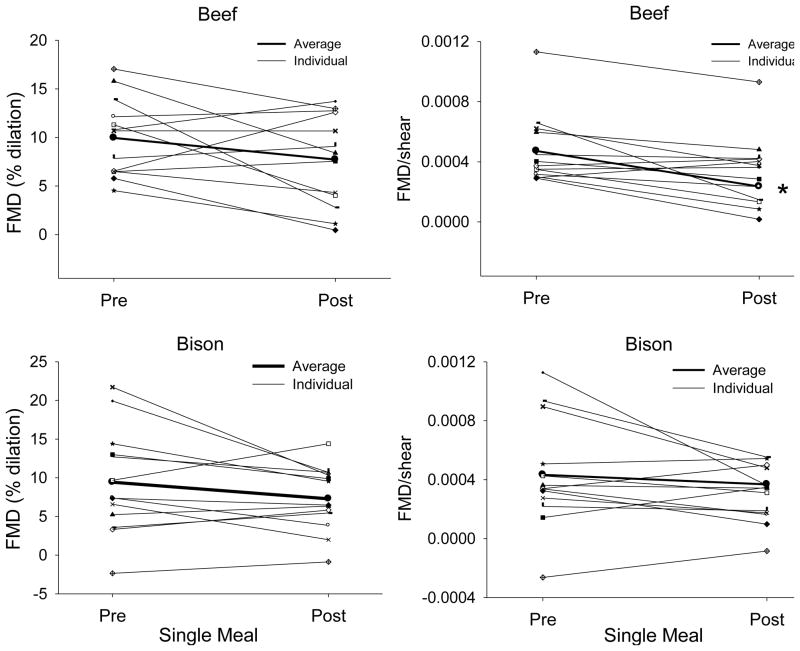
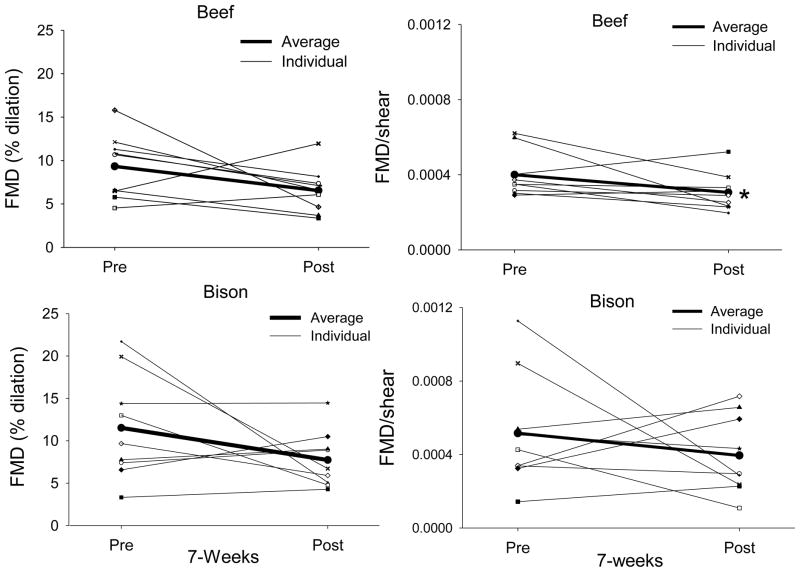
There was tendency for a reduction in FMD following a single meal of beef and bison (p=0.06 for both comparisons). FMD expressed relative to shear was significantly decreased following an acute meal of beef (p=0.004) however, FMD/shear did not change as a result of a single meal of bison (p=0.06) (Figure 3). Similarly to the acute analysis, FMD was not influenced by chronic consumption of beef or bison; however, FMD/shear rate decreased following 7 weeks of beef (P=0.007) but not bison (P=0.19) consumption (Figure 4).
Fig. 3.
FMD (left column) and FMD/shear (right column) rate following a single meal of beef (top) or bison (bottom). The bold line represents the mean values. Note that expressing FMD relative to shear rate accounts for variations in stimulus and reduces the variability in the data. * indicates a significant difference from pre values as determined by a paired samples t-test (N=14).
Fig. 4.
FMD (left column) and FMD/shear rate (right column) pre and post the 7-week intervention of beef (top) and bison meat (bottom). The bold line represents the mean value. Note that the effect of chronic beef consumption on FMD is more consistent than the effect of chronic bison consumption. Furthermore, expressing FMD relative to shear rate reduces the variability in dilation with beef, but not bison. * indicates a significant difference from pre values determined by a paired samples t-test (N=9).
4. Discussion
This study examined the acute (single meal) and chronic (7 weeks) effect of eating beef or bison on blood lipids, inflammatory markers, oxidative stress and endothelial function. Results from this investigation support our hypothesis and indicate that when consumed acutely, bison, in contrast to beef, results in an attenuated increase in triglycerides, no elevation in oxidative stress and does not alter vascular function. Furthermore, in contrast to beef, consuming bison for 7 weeks results in reduced inflammation, lower oxidative stress and a subsequent maintenance of vascular function. In combination, these results suggest that the consumption of bison meat, both acutely and chronically, is associated with lower atherogenic risk than consuming equal portions of beef. Thus, in societies where red meat remains a large percentage of the diet, in terms of vascular health, bison meat appears to provide a healthier alternative.
4.1 Nutritional components of beef and bison meat
Although all meat cuts used in this investigation are considered lean meat (<10% sat fat) based on the American Heart Association guidelines, our results, as well as results from previous investigations, indicate a difference in the fatty acid profile between beef and bison meat. Specifically, bison has one third the total fat of the beef, less SFA and increased PUFA and omega-3. In addition, both bison steaks and roasts had lower Index of Atherogenicity, a modified and inverted ratio of n-6/n-3 PUFA that includes MUFA in the denominator indicating that bison meat is associated with a lower atherogenic risk. These differences are often attributed to the fact that bison are range fed to a greater extent than beef cattle, and agree with McAfee et al (2011) recent report that indicates grass-fed animals have reduced total fat and increased PUFA. Interestingly, although the bison meat consumed in this study originated from 19-month old male bison, which were range fed for only the first 7 months, improvements in the meat fatty acid profile were still present. In fact, previous reports have indicated, even when reared similarly, bison meat is leaner than beef. Despite the reduced fatty acid profile and atherogenic risk of bison compared to beef, it should be noted that the n-6/n-3 ratio in the meat samples used in this investigation were above the recommended level of 4.0 for the prevention of CVD. Previous reports, however, indicate that this ratio is much closer to 4.0 than indicated in the current investigation; these differences might be attributable to differences between specific cuts of meats.
4.2 Inflammation and oxidative stress resulting from beef and bison consumption
It has been reported that triglycerides and saturated fatty acids as well as PUFAs may be the most important modulators of the postprandial immune response. Specifically, triglycerides, SFA and omega-6 promotes inflammation while omega-3 suppresses inflammation. In fact, there is substantial evidence to indicate that the relative concentrations of these specific dietary fatty acids is more important in lowering blood lipid levels and reducing the risk of CVD than the absolute amount of dietary fat.
Considering both beef and bison were not particularly high in total fat, it is not surprising that inflammatory markers did not increase following an acute meal of either meat despite the fatty acid content differences between them. In fact, following acute bison consumption, PAI-1 and TNF-α actually decreased by 44% and 6% respectively. Indeed it is possible that following the single meal of bison, the balance between pro-inflammatory and anti-inflammatory stimuli (CLA, omega-3), in combination with the large amount of protein which has been shown to minimize postprandial lipemia, was tipped in favor of anti-inflammation. However, the repeated inflammatory stimulus produced by the daily consumption of the greater fat containing beef is likely responsible for the observed differences in the inflammatory response between the two meats when consumed chronically.
Recently, numerous investigators have reported that the acute inflammation and therefore increased oxidative stress, following a single high fat meal reduces the vascular response to shear stress. Although not all markers of inflammation and oxidative stress indicated that beef consumption yields greater free radicals, the combination of acute elevations in oxidized LDL and a tendency for hydroperoxides (p=0.08) and the chronic elevation in protein carbonyl with beef, but not bison consumption, suggests that beef does, in fact, lead to greater levels of oxidative stress.
4.3 The effect of beef and bison consumption on FMD and FMD/Shear rate
The FMD test, which quantifies the vascular response to increased shear stress, is a measure of endothelial function/dysfunction and serves as an overall indicator of cardiovascular health. These data indicate that there was only a tendency (p=0.06) for a single meal of beef and bison to reduce FMD. However, more recently our laboratory and others have contended that FMD expressed relative to shear rate (the stimulus for NO release and subsequent dilation) may be a more valid and robust measure of endothelial function as it accounts for variations in the stimulus itself. The current data also support this contention. The variability in the FMD response to a single meal of beef is reduced when FMD is expressed relative to shear rate and reveal that beef, but not bison, decreases vascular function. It is important to note that since shear rate itself was not influenced by the meat consumption (p=0.22), normalizing FMD to shear simply reduced variability in the stimulus rather than accounting for differences in blood flow associated with beef consumption. Likewise, this study also indicates that there was no effect of chronic beef or bison consumption on FMD when expressed simply as a % change in diameter, however when expressed as FMD/shear rate, only chronic beef consumption reduced vascular function. It should also be noted that previous studies of the vascular response to a high fat meal typically employ meals that are relatively high in fat (e.g. 30–50 g of fat/meal) whereas the current meat meals represented a much lower 9–22 g of fat/meal.
4.4 Chronic beef and bison consumption and subject characteristics
Interestingly, there were no significant differences in the anthropometric measurements or blood lipids observed across 7 weeks of beef or bison consumption. Specifically, BMI and percent body fat as well as total cholesterol, TG, LDL and HDL remained unchanged indicating that, in this study, the consumption of large amounts of red meat does not have an effect on several of the factors that are commonly linked to diet. In fact, the meat consumption in this study was nearly double the American Heart Associations recommendations of less than 6 oz of lean meat per day (based on a 2000 calorie diet). Although these findings are initially surprising, there are several possible explanations. First, as previously discussed, although beef had more than twice the amount of total fat compared to bison, neither meat could be considered very high in fat (≈6% and 2% by weight, respectively). This observation adds some credence to the fact that eating lean beef, even in large amounts, may not be overtly unhealthy (see review by Li, Siriamornpun, Wahlqvist et al., 2005), but the current data highlight the atherogenic risks associated with such behavior. Second, satiety resulting from eating the unaccustomed large portions of meat may have lead participants to subconsciously cut down on other meats or foods that are higher in calories and fat resulting in a net balance. Physical activity, which also may be used to help control blood lipids and body composition, can not explain this observation as there was no significant change in physical activity during the course of the study.
4.5 Study Limitations
The current study has several limitations. Specifically, to ensure a significant vascular and metabolic challenge, meat portions were large. However, although large and likely somewhat atypical, it is certainly not extremely uncommon for people to consume such a volume of meat at one sitting in some cultures. In regards to the chronic trial, ideally we would have liked to use smaller portions (8 oz) for a longer duration (12–16 weeks) but we were concerned with subject dropout with a longer intervention period. Although a thorough assessment of meat composition was performed in terms of total fat and fatty acids, protein and carbohydrate concentrations were not determined for the study meat. These values could be approximated based on published USDA nutrient values. Furthermore, physical activity and diet were recorded by survey, an approach which is associated with unavoidable error.
4.6 Summary
This is the first study to compare the cardiovascular health risks associated with the acute and chronic consumption of beef and bison. As a whole, these data suggest that the consumption of bison meat, rather than beef, is associated with a reduced atherogenic risk. Unlike beef, bison consumption did not result in increased inflammation and oxidative stress or decreased vascular function. Thus, in a society which continues to consume large quantities of beef, bison meat appears to provide a healthier red-meat alternative.
Acknowledgments
Funding for portions of this research provided by the National Buffalo Foundation (Westminster, CO), the National Institute of Health grant 1PO1H1091830-01A1 and Veterans Affairs RR&D CDA-2 E7560W.
Abbreviations
- FMD
Flow Mediated Dilation
- IL-6
interleukin-6
- PAI-1
plasminogen activator inhibitor-1
- CRP
c-reactive protein
- Ox-LDL
oxidized low-density lipoprotein
- TG
triglycerides
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- MUFA
monounsaturated fatty acids
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- BMI
body mass index
- IA
index of atherogenicity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine of the National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Washington, DC: The National Academy Press; 2002. Dietary fats: total fat and fatty acids; pp. 335–432. [Google Scholar]
- 2.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. Jama. 2000;284:311–8. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 4.Snowdon DA, Phillips RL, Fraser GE. Meat consumption and fatal ischemic heart disease. Prev Med. 1984;13:490–500. doi: 10.1016/0091-7435(84)90017-3. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169:562–71. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissinen A. Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. The Seven Countries Study Research Group. Eur J Epidemiol. 1999;15:507–15. doi: 10.1023/a:1007529206050. [DOI] [PubMed] [Google Scholar]
- 7.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker JW. Feedlot Empire: beef cattle feeding in Illinois and Iowa, 1940–1900. Ames, IA: The Iowa State University Press; 1975. [Google Scholar]
- 9.Cordain L, Watkins BA, Florant GL, Kelher M, Rogers L, Li Y. Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease. Eur J Clin Nutr. 2002;56:181–91. doi: 10.1038/sj.ejcn.1601307. [DOI] [PubMed] [Google Scholar]
- 10.Rule DC, Broughton KS, Shellito SM, Maiorano G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken. J Anim Sci. 2002;80:1202–11. doi: 10.2527/2002.8051202x. [DOI] [PubMed] [Google Scholar]
- 11.Margioris AN. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:129–37. doi: 10.1097/MCO.0b013e3283232a11. [DOI] [PubMed] [Google Scholar]
- 12.Tyldum GA, Schjerve IE, Tjonna AE, Kirkeby-Garstad I, Stolen TO, Richardson RS, Wisloff U. Endothelial dysfunction induced by post-prandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol. 2009;53:200–6. doi: 10.1016/j.jacc.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–35. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 14.Jarvisalo MJ, Juonala M, Raitakari OT. Assessment of inflammatory markers and endothelial function. Curr Opin Clin Nutr Metab Care. 2006;9:547–52. doi: 10.1097/01.mco.0000241663.00267.ae. [DOI] [PubMed] [Google Scholar]
- 15.Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, Heinrich J, Garn H, Koletzko S, Lack G, et al. Early nutrition and immunity - progress and perspectives. Br J Nutr. 2006;96:774–90. [PubMed] [Google Scholar]
- 16.Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr. 2002;76:504–10. doi: 10.1093/ajcn/76.3.504. [DOI] [PubMed] [Google Scholar]
- 17.Chin SF, Liu W, Storkson JM, Ha YL, Pariza MW. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. Journal of Food Composition and Analysis. 1992;5:185–97. [Google Scholar]
- 18.Butz DE, Li G, Huebner SM, Cook ME. A mechanistic approach to understanding conjugated linoleic acid’s role in inflammation using murine models of rheumatoid arthritis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R669–76. doi: 10.1152/ajpregu.00005.2007. [DOI] [PubMed] [Google Scholar]
- 19.Kritchevsky D, Tepper SA, Wright S, Tso P, Czarnecki SK. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J Am Coll Nutr. 2000;19:472S–7S. doi: 10.1080/07315724.2000.10718950. [DOI] [PubMed] [Google Scholar]
- 20.Lee KN, Kritchevsky D, Pariza MW. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis. 1994;108:19–25. doi: 10.1016/0021-9150(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 21.Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–8. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- 22.Miller A, Stanton C, Murphy J, Devery R. Conjugated linoleic acid (CLA)-enriched milk fat inhibits growth and modulates CLA-responsive biomarkers in MCF-7 and SW480 human cancer cell lines. Br J Nutr. 2003;90:877–85. doi: 10.1079/bjn2003978. [DOI] [PubMed] [Google Scholar]
- 23.Chajes V, Lavillonniere F, Maillard V, Giraudeau B, Jourdan ML, Sebedio JL, Bougnoux P. Conjugated linoleic acid content in breast adipose tissue of breast cancer patients and the risk of metastasis. Nutr Cancer. 2003;45:17–23. doi: 10.1207/S15327914NC4501_2. [DOI] [PubMed] [Google Scholar]
- 24.Towle LA, Bergman EA, Joseph E. Low-fat bison-hybrid ground meat has no effects on serum lipid levels in a study of 12 men. J Am Diet Assoc. 1994;94:546–8. doi: 10.1016/0002-8223(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 25.McAfee AJ, McSorley EM, Cuskelly GJ, Fearon BW, Moss BW, Beattie JAM, Wallace JMW, Bonham MP, Strain JJ. Red meat from animals offered a grass diet increase plasma and platelet n-3 PUFA in healthy conusmers. British Journal of Nutrition. 2011;105:80–9. doi: 10.1017/S0007114510003090. [DOI] [PubMed] [Google Scholar]
- 26.Wolmarans P, Laubscher JA, van der Merwe S, Kriek JA, Lombard CJ, Marais M, Vorster HH, Tichelaar HY, Dhansay MA, Benade AJ. Effects of a prudent diet containing either lean beef and mutton or fish and skinless chicken on the plasma lipoproteins and fatty acid composition of triacylglycerol and cholesteryl ester of hypercholesterolemic subjects. J Nutr Biochem. 1999;10:598–608. doi: 10.1016/s0955-2863(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 27.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods in Enzymology. 1994;233:182–8. [Google Scholar]
- 30.Holvoet P, Macy E, Landeloos M, Jones D, Jenny NS, Van de Werf F, Tracy RP. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem. 2006;52:760–4. doi: 10.1373/clinchem.2005.064337. [DOI] [PubMed] [Google Scholar]
- 31.Dod HS, Bhardwaj R, Sajja V, Weidner G, Hobbs GR, Konat GW, Manivannan S, Gharib W, Warden BE, Nanda NC, et al. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol. 2010;105:362–7. doi: 10.1016/j.amjcard.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–16. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 33.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–7. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 34.Nanjee MN, Crouse JR, King JM, Hovorka R, Rees SE, Carson ER, Morgenthaler JJ, Lerch P, Miller NE. Effects of intravenous infusion of lipid-free apo A-I in humans. Arterioscler Thromb Vasc Biol. 1996;16:1203–14. doi: 10.1161/01.atv.16.9.1203. [DOI] [PubMed] [Google Scholar]
- 35.Murrieta CM, Hess BW, Rule DC. Comparison of acidic and alkaline catalysts for preparation of fatty acid methyl esters from ovine muscle with emphasis on conjugated linoleic acid. Meat Science. 2003;65:523–9. doi: 10.1016/S0309-1740(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 36.Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. 1991;338:985–92. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–91. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 38.Koch RM, Jung HG, Crouse JD, Varel VH, Cundiff LV. Growth, digestive capability, carcass, and meat characteristics of Bison bison, Bos taurus, and Bos x Bison. J Anim Sci. 1995;73:1271–81. doi: 10.2527/1995.7351271x. [DOI] [PubMed] [Google Scholar]
- 39.van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, Castro Cabezas M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res. 2003;44:576–83. doi: 10.1194/jlr.M200419-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Napolitano M, Bravo E. Lipid metabolism and TNF-alpha secretion in response to dietary sterols in human monocyte derived macrophages. Eur J Clin Invest. 2005;35:482–90. doi: 10.1111/j.1365-2362.2005.01523.x. [DOI] [PubMed] [Google Scholar]
- 41.Alipour A, van Oostrom AJ, Izraeljan A, Verseyden C, Collins JM, Frayn KN, Plokker TW, Elte JW, Castro Cabezas M. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:792–7. doi: 10.1161/ATVBAHA.107.159749. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–35. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Bogani P, Galli C, Villa M, Visioli F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis. 2007;190:181–6. doi: 10.1016/j.atherosclerosis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Calder PC, Deckelbaum RJ. Omega-3 fatty acids: time to get the messages right! Curr Opin Clin Nutr Metab Care. 2008;11:91–3. doi: 10.1097/MCO.0b013e3282f449f6. [DOI] [PubMed] [Google Scholar]
- 45.Connor WE. The beneficial effects of omega-3 fatty acids: cardiovascular disease and neurodevelopment. Curr Opin Lipidol. 1997;8:1–3. doi: 10.1097/00041433-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Gardner CD, Kraemer HC. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis. Arterioscler Thromb Vasc Biol. 1995;15:1917–27. doi: 10.1161/01.atv.15.11.1917. [DOI] [PubMed] [Google Scholar]
- 47.Oliver MF. It is more important to increase the intake of unsaturated fats than to decrease the intake of saturated fats: evidence from clinical trials relating to ischemic heart disease. Am J Clin Nutr. 1997;66:980S–6S. doi: 10.1093/ajcn/66.4.980S. [DOI] [PubMed] [Google Scholar]
- 48.Westphal S, Kastner S, Taneva E, Leodolter A, Dierkes J, Luley C. Postprandial lipid and carbohydrate responses after the ingestion of a casein-enriched mixed meal. Am J Clin Nutr. 2004;80:284–90. doi: 10.1093/ajcn/80.2.284. [DOI] [PubMed] [Google Scholar]
- 49.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 50.Gill JM, Al-Mamari A, Ferrell WR, Cleland SJ, Packard CJ, Sattar N, Petrie JR, Caslake MJ. Effects of prior moderate exercise on postprandial metabolism and vascular function in lean and centrally obese men. J Am Coll Cardiol. 2004;44:2375–82. doi: 10.1016/j.jacc.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 51.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 52.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 53.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol. 2007;103:843–51. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–9. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 55.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur J Appl Physiol. 2006;98:256–62. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 57.American Heart Association. [ http://www.americanheart.org/presenter.jhtml?identifier=3040356]
- 58.Li D, Siriamornpun S, Wahlqvist ML, Mann NJ, Sinclair AJ. Lean meat and heart health. Asia Pac J Clin Nutr. 2005;14:113–9. [PubMed] [Google Scholar]