Abstract
This study sought to test whether the neurobiology of self-processing differentiated depressed adolescents with high suicidality from those with low suicidality and healthy controls (N=119, MAGE= 14.79, SD=1.64, Min=11.3, Max = 17.8). Participants completed a visual self-recognition task in the scanner during which they identified their own or an unfamiliar adolescent face across three emotional expressions (happy, neutral or sad). A 3 Group (HS, LS, HC) by two within subject factors [2 Self conditions (self, other) and 3 Emotions (happy, neutral, sad)] GLM yielded: 1) a main effect of Self condition with all participants showing higher activity in the right occipital, precuneus and fusiform during the self-versus other-face conditions; 2) a main effect of Group where all depressed youth showed higher dorsolateral prefrontal cortex activity than HC across all conditions, and with HS showing higher cuneus and occipital activity versus both LS and HC; and 3) a Group by Self by Emotion interaction with HS showing lower activity in both mid parietal, limbic and prefrontal areas in the Happy self versus other-face condition relative to the LS group, who in turn had less activity compared to HC youth. Covarying for depression severity replicated all results except the third finding; in this subsequent analysis, a Group by Self interaction showed that although HC had similar midline cortical structure (MCS) activity for all faces, LS showed higher MCS activity for the self vs. other faces while HS showed the opposite pattern. Results suggest that the neurophysiology of emotionally charged self-referential information can distinguish depressed, suicidal youth versus non-suicidal depressed and healthy adolescents. Neurophysiological differences and implications for the prediction of suicidality in youth are discussed. General Scientific Summary: Depressed adolescents with high suicidality show less activity in brain areas that support emotional experiences and self-awareness when identifying their own face, suggesting that abnormal self-processing may be associated with greater suicide risk.
Keywords: suicidality, adolescence, self recognition, limbic, cortical midline structures, neuroimaging
Suicide is the second leading cause of death in the United States among youth aged 10–24 years old (Murphy, Xu, & Kochanek, 2010). The adolescent transition in particular is associated with a dramatic increase in suicidality, defined as all suicide related behavior ranging from ideation to non-lethal self-injuries with the intent to die, to actual attempts and completions (Brezo, Paris, & Turecki, 2006; Nock et al., 2013). Although suicide is a preventable cause of death, efforts to reduce its occurrence have had little success (Singh & Yu, 1996), in part due to limited ability to accurately predict suicide risk. Major depressive disorder (MDD) is one of the most well established risk factors for suicidality among youth. Clinically depressed youth are six times more likely to attempt suicide than non-depressed youth (Nock et al., 2013). Given the high rates of suicide attempts and completions among depressed adolescents, identifying predictors of suicidality within this clinical population is essential. Assessment of suicide risk currently relies almost exclusively on self-report interviews and questionnaires, approaches that are limited by the fact that many individuals are poor reporters of their own suicidal behavior (Nock et al., 2010), and many who report suicidal thoughts never attempt suicide (Pokorny, 1983). Clinical neuroscience can begin to address these limitations to suicide diagnosis and prevention by uncovering more objective biomarkers of suicide risk. Identifying key neural circuits distinguishing depressed youth with high suicidal ideation from other depressed youth and psychologically healthy controls may help to better predict suicide risk. Research further suggests that disturbed self-referential processing may contribute to increased risk for suicide (Burke et al., 2015). However, it remains unknown how neural alterations during self-referential processing are associated with suicidality. Thus, the main purpose of this study was to examine neural function during processing of emotional self-faces (versus faces of others) among depressed, suicidal adolescents.
Self-referential Processing Biases and Suicide Risk
Cognitive theories emphasize the role of self-schemas in vulnerability for suicide (Alloy, Abramson, Safford, & Gibb, 2006; Beck, 1961), positing that negative self-schemas increase suicide risk by influencing the perception, encoding, retrieval, and interpretation of self-referential cues (Burke, et al., 2015; Wenzel & Beck, 2008). Evidence also suggests that individuals at risk for suicide are more likely to have negatively biased self-cognitions. For example, suicidal individuals, including adolescents, are more likely to have negative self-appraisals and to make more self-attributions about the causes of negative events compared to non-suicidal controls (Brunstein Klomek et al., 2007; Tang, Wu, & Miao, 2013). Furthermore, depressed individuals, who are typically at higher risk for suicide than psychologically healthy controls, show preferential processing of negative self-referential material such as greater recall of negative self-referential trait adjectives, faster reaction times to negative self-referential stimuli, and preferential attention to negative emotional stimuli, including facial emotional expressions (Fritzsche et al., 2010; Greenberg & Alloy, 1989).
Depressed or depression-prone individuals are also significantly less likely to endorse and correctly recall self-referential positive information, and exhibit greater attentional avoidance to positive facial expressions than psychologically healthy individuals (Alloy, Abramson, Murray, Whitehouse, & Hogan, 1997; Gotlib, Krasnoperova, Yue, & Joormann, 2004; Hankin, Gibb, Abela, & Flory, 2010). A recent study of adolescents showed that youth with suicidal ideation were also more likely to endorse negative self-traits and less likely to endorse positive self-traits compared to adolescents without suicidal ideation during a self-referential processing task, even after controlling for clinical depression (Burke, et al., 2015). Overall the extant research suggests that, in contrast to psychologically healthy individuals, those at risk for suicide show preferential endorsement of negative self-referential information, and avoidance or neglect of positive self-referential material.
Relevance of Self-face Processing for Understanding Suicide Risk
Self-face processing is a discrete modality of self-referential processing, and humans show a distinct response to their own face compared to those of other familiar and stranger faces, as confirmed by preferential processing of self-faces during face recognition paradigms (Ma & Han, 2010). Critically, in healthy adults, self-face recognition activates implicit rewarding and positive self-perceptions typical of normative self-processing (Blackwood et al., 2003; Drake & Seligman, 1989). These positive self-biases facilitate more rapid behavioral responses to the self-face (Ma & Han, 2010). Thus, normative self-face recognition is biased toward positively-valenced information and faster preferential responses to the self-face over the faces of others.
However, evidence supports that one’s self-schema influences the processing of the self-face. For example, low levels of positive self-appraisals contribute to less preferential bias for one’s own face (Ma & Han, 2010) and a negative attributional style is linked to faster reaction times to one’s own sad facial expression (Caudek & Monni, 2013). Given that biased self-schemas and self-referential processing biases are prevalent among depressed and suicidal youth, atypical processing of self-faces may be characteristic of youth at risk for suicide. Prior research has established that attention, perception, and identification biases for affective facial stimuli are robust among youth with emotional disorders and symptoms, including suicidality (Hankin, et al., 2010; Joormann, Gilbert, & Gotlib, 2010; Seymour et al., 2015), although these studies have not focused on self-faces specifically. Emotional facial stimuli are frequently used in these information processing studies, because processing of facial emotional expressions are rapid and automatic (Vuilleumier & Schwartz, 2001), and are believed to function external to awareness (Gotlib & Neubauer, 2000). Taken together, research suggests that identifying one’s own emotional self-face may be a useful and rigorous approach for assessing potential abnormal neurophysiology during emotional self-referential information processing among suicidal youth.
Neurobiology of self-face processing and suicide risk
The neurobiology of self-referential and emotional face processing can inform hypotheses about which alterations in neural circuitry during processing of emotional self-faces versus other-faces may be associated with suicide risk. Neural core components of face perception include the occipital inferior gyrus, lateral fusiform gyrus, and superior temporal sulcus (Stuhrmann, Suslow, & Dannlowski, 2011). Brain regions typically involved in the experience and production of emotions are also implicated in the processing of facial information (Stuhrmann, et al., 2011), including limbic areas such as the amygdala, hippocampus, insula, and thalamus (Kircher et al., 2001; Sugiura et al., 2008; Uddin, Kaplan, Molnar-Szakacs, Zaidel, & Iacoboni, 2005). Finally, and of particular importance, midline cortical structures (MCS) such as the medial prefrontal cortex (MPFC) including BA 9 and 10, anterior cingular cortex (ACC), and posterior cingulate cortex (PCC) are involved in self-face processing and the processing of self-referential or autobiographical information (Hu et al., 2016; Murray, Debbane, Fox, Bzdok, & Eickhoff, 2015) (e.g., self-traits, own name, own voice, etc.). Thus, these structures are identified as key areas in the neural bases of self-processing that are also involved in the processing of the self-face versus other-faces (Araujo, Kaplan, & Damasio, 2013; Fossati et al., 2014). There is also strong evidence that self-face processing involves increased activity localization in the right hemisphere of MCS (Sugiura, 2015).
Less is known about neural alterations in response to self-faces among individuals with emotional disorders who are at high risk for suicide. Individuals with major depression appear to typically exhibit hyperactivity in MCS at rest and during neural tasks compared to psychologically healthy controls, possibly due to persistent high levels of negative, self-focused rumination that characterizes both depression and suicide risk (Nejad, Fossati, & Lemogne, 2013). Research further suggests that depressed individuals may exhibit heightened activity in MCS, and in emotion-related brain areas during self-referential processing in particular (Grimm et al., 2008; Lemogne et al., 2009; Renner et al., 2015). However, while some studies have found hyperactivity in these areas in depressed patients (Lemogne, et al., 2009; Wagner, Schachtzabel, Peikert, & Bär, 2015), others have found hypoactivity (Grimm, et al., 2008; Hooley et al., 2009; Johnson, Nolen-Hoeksema, Mitchell, & Levin, 2009; Renner, et al., 2015). Overall, reviews of the scientific literature suggest the possibility of hyperactive MCS during self-referential processing in depression (Nejad, et al., 2013), but mixed findings may be at least partly due to the limitations of some studies in which the valence of self-referential information was not accounted for in the research design.
In studies examining neural responses to valenced emotional faces, a more reliable pattern of activity is associated with higher risk for suicide. Specifically, depressed individuals exhibit hyperactivation to mood-congruent or negative expressions in unfamiliar faces, but hypoactivity to positive facial expressions, particularly in the amygdala, insula, parahippocampal gyrus, fusiform and putamen, as well as the orbitofrontal cortex (Stuhrmann, et al., 2011). Similarly, Yoshimura and colleagues (2014) showed that depressed patients exhibited a heightened response to negative self-referential traits in the medial prefrontal cortex (MPFC) and ventral anterior cingulate cortex (vACC), compared to healthy controls, but blunted activation in these areas to positive self-traits. This suggests that among depressed populations, biases to more self-relevant emotional expressions (i.e., negative emotions) may involve greater limbic and MCS activity, but blunted activity in these regions to less self-relevant emotions (e.g., happy faces). In one of only three studies examining the neural correlates of suicidality among adolescents, Pan and colleagues (2013) found that depressed adolescent suicide attempters showed greater neural activation to negative expressions in the faces of others (i.e. angry faces) compared to depressed non-attempters or healthy controls, but less activation to happy faces in emotion-related brain areas (e.g., ACC, primary sensory cortex, thalamus). These findings support a possible hyperactivity to negative emotional expressions in structures involved in emotional face processing, and blunted activity to positive facial expressions among depressed and suicidal youth. However, neural reactivity to self-faces specifically has not been specifically examined in this regard.
In sum, although a number of studies demonstrate atypical self-referential processing in depressed youth, researchers have typically not assessed corresponding associations with suicidality in these individuals. However, there is emerging research showing that suicidal individuals have highly biased self-schemas, are more likely to endorse negative self-attributions, and more negative than positive self-traits, even above and beyond the impact of depression (Brunstein Klomek, et al., 2007; Burke, et al., 2015; Tang, et al., 2013). Additionally, there is evidence that youth suicide attempters, relative to depressed non-suicidal youth, exhibit aberrant neural responses to emotional faces in MCS and limbic structures [e.g., ACC; (Pan, et al., 2013)], which are thought to play a key role in the processing of self-information and emotionally-valenced stimuli. Therefore, evidence suggests that depressed, suicidal youth (e.g., youth who endorse intense suicidal thoughts or recent suicide attempts) may demonstrate more intense biases to self-referential information, especially to emotionally-valenced self-information, compared to depressed youth with low levels of suicidality, Yet, it remains unknown how neural function during processing of self-faces is associated with suicidality among youth.
The Current Study
This study is the first to examine the neural substrates of self-referential processing of suicidal individuals. We employed a novel task, based on prior work by Kircher et al, (2001), to assess neural processing of positive and negative self-referential stimuli among clinically referred and healthy adolescents. Specifically, depressed adolescents with high and low levels of suicidality, and psychologically healthy controls viewed images of the self-face versus the face of an unfamiliar adolescent across three different emotional conditions: happy, sad and neutral. We used images of self- and other-emotional faces, given that emotional face processing tasks have proven to be valid and reliable approaches to assess processing biases and atypical neural functioning among individuals with depression and suicidality (Pan, et al., 2013; Stuhrmann, et al., 2011).
This research was guided by five hypotheses. First, we expected higher right sided MCS activity to self-versus other faces regardless of diagnostic group, in addition to greater activity in limbic and other core areas of face processing. Second, we predicted a main effect of diagnostic group whereby depressed youth (both high and low suicidality) would show higher activity in MCS than healthy controls across all conditions (Nejad, et al., 2013). Third, we expected that brain activity in both MCS and limbic structures during the recognition of the happy self-face versus identification of another youth’s happy face would differentiate depressed youth with high versus low suicidality and healthy controls. Specifically, we hypothesized that highly suicidal depressed youth would demonstrate lower activity during happy self-face recognition versus identification of other happy faces. Fourth, we predicted that highly suicidal youth would show greater MCS and limbic activity during recognition of the sad self-face versus identification of another youth’s sad face. Fifth, we expected that the neurobiology of emotional self-versus other-face processing would be affected by high suicidality (as previously hypothesized) even after controlling for effects of self-report measures of explicit self-representation (e.g., attributional style) and for clinical variables such as medication and depression severity.
Methods & Materials
Recruitment Procedures and Clinical Assessments
Participants were recruited in Pittsburgh (Pgh) and Minneapolis (Mpls) from short-term psychiatric inpatients units and during assessments in psychiatric clinic settings, by therapists at school-based mental health clinics, and through flyers and radio ads. All participants underwent psychological evaluations using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS, Kaufman et al., 1997). Depression symptoms were also assessed with the Child Depression Rating Scale (CDRS, Poznanski, Cook, & Carroll, 1979) and subjective experiences of depression symptoms were assessed with the Moods and Feelings Questionnaire (MFQ, Thapar & McGuffin, 1998). Based on these clinical assessments participants were deemed to either have a depressive disorder or be free of present and past diagnoses. Interviews were videotaped and three interviewers recorded the presence of symptoms and diagnoses for 40% of the sample with 93% agreement with regard to symptom presentation and 98% for diagnosis.
Participants’ IQ and puberty status were measured via the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) and Pubertal Development Scale (Petersen, Crockett, Tobin-Richards, & Boxer, 1985), respectively. Parents completed a questionnaire regarding family structure, income and medication their children were taking. Adolescents completed self-report measures of attributional style (Conley, Haines, Hilt, & Metalsky, 2001), which was expected to differ between high and low suicidality and healthy control youth. Total CDRS scores or MFQ (excluding suicidality items to reduce overlap) were used as separate measures of depression severity, and the K-SADS was used to determine past number of years depressed and number of depressive episodes. During the first session, photographs were taken of the participants with happy, sad and neutral expressions. One to two weeks later, neuroimaging scans were completed.
Participants
Right-handed participants were recruited at the Universities of Minnesota (52 females, 32 males, MAge=15.01, SD=1.60) and Pittsburgh (20 females, 15 males; MAge=14.24, SD=1.59). IRBs at both universities approved procedures. Mpls and Pgh participants were similar in average movement parameters, depression severity, chronicity, attributional style, self-esteem, puberty status, IQ, and age, F(1, 116)=3.70–0.38, p=0.06–0.85. Adolescents participants (N=119; see Table 1 for descriptive statistics) were grouped based on depression and current suicidality as either healthy controls (HC), depressed low suicidality (LS), or depressed high suicidality (HS). The suicidality independent variable (suicide ideation and attempts) was calculated by standardizing and averaging suicide items from the K-SADS and CDRS. Specifically, a z-score corresponding to a median-split raw score of 3.20 classified depressed youth as high or low suicidality. The resulting groups differed on clinically meaningful variables such as depression severity (Table 1). Additionally, within depressed youth, the HS group tended to include more attempters than the LS group, χ2(1)=3.20, p=0.06.
Table 1.
Demographics and Clinical Variables: Depressed with Low or High Suicidality and Healthy Control Groups
| Healthy Control | Depressed Low Suicidality |
Depressed High Suicidality |
Comparison Statistic | ||
|---|---|---|---|---|---|
| n = 37 | n = 39 | n = 43 | |||
| Scanning Site | Mpls | 21a | 23a | 40a | χ2 (2) = 16.68*** |
| Pgh | 16a | 16a | 3b | ||
| Suicide attempters within group | n=0a | n=6b | n=16b | χ2 (2) = 17.23*** | |
| Age: M (SD) | 14.48 (1.54) | 14.88 (1.77) | 14.97 (1.59) | F(2, 118) = 0.98 | |
| IQ: M (SD) | 116.89 (12.41)a | 103.03 (13.86)b | 111.70 (18.48)a | F(2, 118) = 7.99*** | |
| Sex | χ2 (2) = 5.72 | ||||
| Male | 20 (54.05%) | 15 (38.46%) | 12 (27.91%) | ||
| Female | 17 (45.95%) | 24 (61.54%) | 31 (72.09%) | ||
| Puberty: M (SD) | F(2, 118) = 3.16* | ||||
| 2.90 (0.62)a | 3.04 (0.61)ab | 3.20 (0.41)b | |||
| Ethnicity | χ2 (12) = 15.65 | ||||
| White | 28 (75.68%) | 22 (56.416%) | 24 (55.81%) | ||
| African American | 1 (2.70%) | 6 (15.38%) | 2 (4.65%) | ||
| Hispanic | 1 (2.70%) | 5 (12.82%) | 5 (11.63%) | ||
| Asian/Asian American | 3 (8.11%) | 1 (2.56%) | 1 (2.33%) | ||
| Native American | 0 | 0 | 1 (2.33%) | ||
| Mixed | 4 (10.81%) | 4 (10.26%) | 8 (18.60%) | ||
| Other | 0 | 1 (2.56%) | 2 (4.65%) | ||
| Family Structure | χ2 (2) = 8.87* | ||||
| Married/Cohabitating | 32 (86.49%)a | 29 (74.36%)a | 25 (58.14%)a | ||
| Single/Separated/Divorced/Widowed | 5 (13.51%)a | 8 (20.51%)b | 18 (41.86%)b | ||
| Income | |||||
| 35K or less | 4 a | 12 b | 16 b | χ2 (4) = 13.60** | |
| 35–75K | 8 a | 13 a | 11 a | ||
| 75K or more | 25a | 11b | 16b | ||
| Maltreatment History | 0a | 16 (41.03%)b | 25 (58.14%)b | χ2 (2) = 30.88*** | |
| Antidepressants | 0a | 12 (30.77%)b | 22 (51.16%)b | χ2 (2) = 25.65*** | |
| Antipsychotics | 0 | 2 (5.13%) | 4 (9.30%) | χ2 (2) = 3.60 | |
| Mood stabilizers | 0 | 0 | 1 (2.33%) | χ2 (2) = 1.78 | |
| Anxiolitic | 0a | 1 (2.56%)b | 5 (11.63%)b | χ2 (2) = 6.36* | |
| Depression Severity: M (SD) | 20.27 (5.76)a | 57.23 (12.97)b | 69.05 (13.46)c | F(2, 118) =192.09*** | |
| Chronicity (Years Depressed) | 0.38 (0.68)a | 3.00 (2.36)b | 3.51 (2.09)b | F(2, 118) = 30.77*** | |
| Attributional Style: M (SD) | 6.73 (5.20)a | 2.26 (6.33)b | −0.95 (6.23)c | F(2, 118) = 16.54*** | |
p < .05.
p < .01.
p < .001.
Note. Comparison cells with different letter subscripts were significantly different from one another. Unless otherwise stated, cell values represent frequencies and percentages.
The Emotional Self-Other Morph-Query (ESOM-Q) task
Stimuli
Photographs of the participants’ faces with three expressions (happy, sad and neutral) were obtained during the intake visit under standardized conditions. The researcher would model the face required for each emotional expression (e.g., smiling for the happy face), and/or instruct the participant to recall a memory associated with the emotion prior to taking the photograph. The researcher also elicited expressions by causing the participant to laugh via jokes, or instructing the participant to intensify the expression (e.g., turning down the corners of the mouth or furrowing the brow). Any or all of these strategies were used as necessary. Participant’s photographs were then mirror transposed and non-facial attributes (hair, ears, large scars, or pimples) were removed. Faces were presented against a black background (Figure 1). A non-familiar teen face (from available photographs of previous participants) of similar gender, race, maturity and attractiveness was paired with each participant. Two experimenters agreed in the selection of the appropriate non-familiar face via visual inspection. The individual faces were then manipulated, “morphed,” with their assigned unfamiliar face, (other participants in the sample) in 5% increments using Abrosoft FantaMorph software (Abrosoft, 2011). This resulted in 21 composites that ranged from 0% self and 100% other to 100% self and 0% other. The purpose was to have several novel but easy to recognize pictures of a face to prevent habituation. Our procedures are similar to those successfully used by Kircher and colleagues (2001) to study the neurophysiology of self versus other face processing.
Figure 1.

Emotional Self Other Morph-Query (ESOM-Q) Task. Participants indicated if the face looked like them or not via button press in response to faces with high or low percentage of self features presented randomly within six blocks across three different expressions (happy, sad, neutral). Minimum self in high self blocks is 65%, and maximum self in high other face blocks is 18%.
The potential influence of diagnosis on the emotional intensity of the stimulus faces shown in the scanner, as well as their fit within a unique category of emotion (e.g. neutral, happy or sad expressions) was examined. Ten trained research assistants, blind to diagnosis, scored the face stimuli for the 100% self-face with neutral, happy and sad expressions for emotional category of expression, with higher scores indicating better fit for the sought emotional category. Specifically, they rated how neutral the neutral faces were on a 1 to 5 scale, and how happy or sad the emotionally-valenced faces were. Additionally, they rated physical attractiveness for the participant’s face regardless of emotion on a 1 to 10 scale. A repeated measures ANOVA (3 groups by 4 ratings) showed that diagnostic groups did not differ in categorical emotional intensity or physical attractiveness of pictures, F(2, 113) = 0.23, p= 0.79.
Experiment
In the scanner, participants were exposed to photographs of faces (Ntotal=150) displaying happy, sad, or neutral expressions with the task of identifying whether or not the picture looked like them by pressing one of two buttons. The experiment consisted of one run and lasted 10 mins 54 secs. It was comprised by six different blocks with faces (self-happy, other-happy, self-sad, other-sad, self-neutral, other-neutral). Instructions presented at the onset of each block lasted 6 sec each. This block design followed similar procedures used by Kircher et al. (2001). Each 70 sec block presented photographs of faces, and at the start, halfway point and end of each block an 18 sec rest period and a fixation cross (i.e. 12 rest periods) was announced by the phrase “rest now.” This provided a respite from the task and established brain activity baselines that were concomitant with the start, center, and end of each self by emotion condition. Faces were displayed for 2 sec followed by a 0.5 sec fixation cross. Each block contained faces of primarily just high or just low degrees of morphing between the self and the other face. This resulted in blocks comprised by self faces or unfamiliar faces within happy, sad or neutral expression groupings, with the following means and standard deviation of morphs: Self blocks: Mself=83%, SD=12%, Minself=65%, Maxself=100%; Other blocks: Mself=18%, SD=12%, Minself=0%, Maxself=35%. Blocks were presented in five counterbalanced task orders and each contained 28 photos of faces presented randomly with regards to morphing percentages within any given self by emotion block. More ambiguous stimuli (i.e. with 40%–60% of self-features) were not used in any block. Within each of the six blocks, four faces were shown of high opposite percentage to the predominant block self condition to avoid response sets and keep the participants engaged (Other block four faces=90% or 80% self; Self block four faces=90% or 80% other) using methods adapted from Kircher et al. (2001). The hemodynamic response function (HRF), upon which neuroimaging is based on, lasts about 12 secs and peaks after approximately 5–8 secs (Kruggel & von Cramon, 1999). Thus, brief exposure (2 sec) to these “opposite” faces is too fast to alter the HRF for the predominant block self condition. Stimuli, accuracy of recognition, and reaction time data were respectively presented and concomitantly recorded with E-prime software (Schneider, Eschman, & Zuccolotto, 2012).
Neuroimaging data acquisition
Neuroimaging data were collected using 3.0 Tesla Siemens Trio MRI scanners in Mpls and Pgh. Structural 3D axial MPRAGE images were acquired in the same session (TR/TE: 2100 ms/3.31 ms; TI: 1050; Flip Angle 8°; FOV: 256×200mm; Slice-Thickness: 1mm; Matrix: 256×200; 176 continuous slices). Mean blood oxygenated level depended activity (BOLD) images were acquired with a gradient echo EPI sequence t=16:05 minutes covering 60 oblique axial slices (2mm; TR/TE=3340/30ms; FOV=200×200mm; matrix=80×80; Flip Angle=90°).
Participants included had imaging data with xyz axis<2 mm (3 translation absolute values) and pitch, roll and yaw rotations <0.587 radians (3 rotation absolute values) shifts. The current sample (N=119) did not include 5 participants who violated the movement parameters. Movement parameters for averaged translation and rotation parameters did not differ between the two sites (Mpls vs. Pgh), F(1, 118)=0.31 p=0.60, or between study groups, F(2, 118)=1.4, p=0.25. However, ART software was used to detect volumes that were outliers (movement > 2mm and rotations<0.587) or that had global signal intensities greater than 9, (Mtrials sensored =4.08, SD=3.2). These volumes were then regressed out of analyses in 1st level GLM analyses described below. The 3 groups were compared in mean numbers of volumes present with high movement and were not statistically different, F(2, 117)=2.12, p=0.12. Frequency of participants with any number of volumes with unacceptable parameters were compared between groups and did not differ, χ2(2, 118)=1.36 p=0.51. Additionally, temporal signal to noise ratios were calculated using the 3dTstats in Afni by dividing the mean baseline estimate (signal) by the standard deviation of the residual time series (noise) for the task. These values were extracted analyzed with a t-test comparing the sites (Pgh vs. Mpls), which yielded no significant differences, t(596)=2.13, p=0.98.
Data Analysis
Neuroimaging data processing
Data were preprocessed and analyzed with Statistical Parametric Mapping software, Version-8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Data for each participant were realigned to the first volume in the time series to correct for head motion. Realigned images were co-registered with the subject’s anatomical image, segmented, normalized to standard Montreal Neurological Institute (MNI) template, and spatially smoothed with a Gaussian kernel of 7mm full-width at half-maximum (FWHW). Trials were modeled with the Canonical Hemodynamic Response Function.
Neuroimaging data analyses
First-level fixed-effect models (GLM) for each subject and condition at each voxel were calculated using a t-statistic producing statistical images for six basic contrasts: self-face (happy-baseline, neutral-baseline and sad-baseline) and other face (happy-baseline, neutral-baseline and sad-baseline). Volumes with high movement parameters were removed during the first level GLM’s using ART software. Groups were then compared via a second level flexible full factorial GLM on the t-contrast images generated by first-level subject level analyses to examine BOLD signal differences between groups during recognition of the self or other face conditions across three emotions. This second level random-effects repeated measure ANOVA with between and within-group factors, as well as subject level effects included the following: a between group factor, Group (HS, LS, HC) and 2 within subjects factors, Self (Self, Other) and Emotions (happy, neutral, sad). This GLM analysis allowed us to test main effects of group and conditions, and group by conditions interactions. To test the direction and meaning of significant interaction effects, follow-up contrasts compared HS to LS to HC for activity during the self condition for the separate emotions. For example, a Group (HS, LS, HC) by Self condition (Self, Other) for just the happy emotional expression would clarify a previous Group by Self interaction. In SPM8, similar ANOVAs with Group (HS, LS, HC), Self condition (Self, Other), and Emotions (happy, sad, neutral) as between and within subjects factors were conducted with and without depression severity as a covariate (clinician report: CDRS or self-report: MFQ), and results are reported accordingly. Results for severity measured by clinician or by self-report yielded similar brain activity differences.
Only whole brain level analyses were conducted and region labeling was confirmed by both the xj-view GUI in SPM8 and Talairach Daemon software. To correct for multiple comparisons, we calculated whole-brain, voxel-wise and cluster extent thresholds via Monte Carlo simulations using 3dClustSim in AFNI and an inherent smoothness of 12.23 15.25 13.93 estimated via the AFNI 3dFWHMx function. This resulted in a voxel-wise threshold of p<0.001 and the following cluster-extent threshold corresponding to p<0.01, FWE-corrected: k>120.7 voxels for analyses without depression severity as a covariate and 130.2 voxels for analyses with depression severity included as a covariate. Only group, condition, and group by condition interaction effects with clusters exceeding those thresholds are reported. Because almost all HS were recruited in Mpls, analyses were repeated only for participants from Mpls (HC=21, LS=23, HS=40) in SPM8. Notably, similar results to the ones reported with the entire sample (both Pgh and Mpls participants) were obtained: Effects of Group: F(2, 486)=21.291, p<0.001; Self Condition: F(1, 486)=29.23 – 27.89, p<0.001; and Group × Self and Group × Self × Emotion Interaction: F(4, 486)=20.23 – 20.89, p<0.001. Given that signal-to-noise ratios were similar between sites and that key findings were similar for just the Mpls site, results are reported with the whole sample across both scanning sites.
Covariate and medication effects
Time series for the first eigenvalues centered in 7 mm spheres for coordinates of significant differences were extracted for use in graphs and to test if differences remained after controlling for covariates that differed between the depressed participants, as well as to examine the role of gender and task order using SPSS software (IBM, 2013). Variables that differed between groups (scanning site, IQ, attributional style) were used as covariates in these follow-up analyses, which were similar as those conducted within SPM8, 3 Group (HS, LS, HC) by within subject factors 2 Self condition (Self, Other) by 3 Emotions (happy, neutral, sad) for the varied brain areas of significant activity differences between groups yielded by SPM8 full factorial analyses. Medicated versus unmedicated depressed youth were also compared in brain activity using SPSS software, which unlike SPM 8, allows to test the significance of covariates in GLM models.
Behavioral response analyses
Analyses were conducted to test whether observed differences in brain activity were accounted for by behavioral differences. Reaction time (RT) and accuracy of self-recognition (0=wrong, 1=right) were examined, and the cut-off for accurate self-face recognition was correct identification for faces that had 65% or more of self features. RT and accuracy were examined with SPSS software and tested with mixed repeated-measures ANOVAs: Group (HS, LS, HC) by Self (self, other) by Emotions (happy, neutral, sad), similar to the analyses conducted for brain function in SPM8.
Results
Neuroimaging Results
Main effect of self condition
Main effects of self condition were found in the right occipital, precuneus and fusiform, F(1, 696)=32.53–24.40, p=0.001–0.42, and in the thalamus, F(1, 696)=24.40, p<0.001 (see Table 2, Figure 2). Follow-up t-test contrasts demonstrated that all participants exhibited higher activity in these areas for the self face more than for the other face, t(1, 696)= 6.75, p<0.001. All effects remained significant when severity of depressive symptoms was covaried in the SPM8 analyses. Furthermore, no other covariates (attributional style, IQ or scanning site) were significantly associated with neural activity in those regions, F(1, 109)= 0.00–0.3, p= 0.11–0.85, and the main effect of the self remained significant when covariates were include in the model, F(1, 105)=22.77, p<0.01. An effect of gender showed that females had more brain activity in these areas than males, F(1, 105)=5.23, p<0.05, yet principal findings held when controlling for gender. There were no effects due to task order, F(3, 105)= 0.24, p= 0.87.
Table 2.
Neural Areas that Distinguish High Suicidality vs. Low Suicidality Depressed vs. Healthy Control Youth during Self and Other
| Group Main Effect | Cluster Size (K) |
p(K) | Hemisphere | MNI | F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | |||||||
| Cuneus, Middle Occipital Gyrus | HS>LS+C | 3544 | 0.000 | Left and Right | −10 | −98 | 16 | 23.34 | 0.000 |
| Inferior Frontal Gyrus, BA9 | HS+LS>C | 121 | 0.000 | Right | 50 | 18 | 26 | 14.41 | 0.036 |
| Self Condition Main Effect | Cluster Size (K) | p(K) | Hemisphere | MNI | F | p | |||
| x | y | z | |||||||
| BA7, Precuneus | Self>Other Face | 414 | 0.000 | Right | 20 | −62 | 46 | 32.53 | 0.001 |
| Middle Occipital Gyrus, Fusiform | 624 | 0.000 | Right | 30 | −86 | 0 | 25.61 | 0.025 | |
| Thalamus | 139 | 0.000 | Right | 10 | −16 | −2 | 24.40 | 0.042 | |
| Group by Self vs. Other Condition by Happy Facial Expression Interaction | Contrast | Cluster Size (K) | p(K) | Hemisphere | MNI | t | p | ||
| x | y | Z | |||||||
| Hippocampus | HS<LS<C | 186 | 0.003 | Right | 28 | −36 | 2 | 5.28 | 0.004 |
| MPFC, BA10 | 144 | 0.006 | Left and Right | 12 | 64 | 8 | 4.76 | 0.045 | |
| Hippocampus, Amygdala | 209 | 0.002 | Left | −16 | −20 | −12 | 4.67 | 0.064 | |
Figure 2.
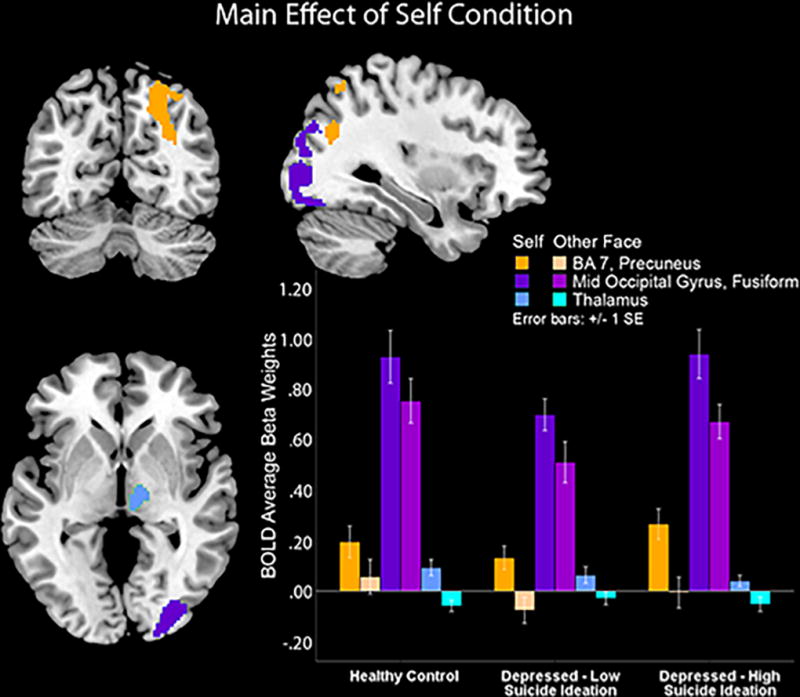
The self face condition is associated with higher posterior midline cortical structures and thalamus activity versus the other face condition across all emotions and groups.
Main effect of group
The omnibus test yielded main effects of group across all self and emotion conditions in a large cluster that encompassed the bilateral cuneus and middle occipital gyrus, F(2, 696)=23.34, p<0.001, and in the right frontal inferior gyrus (BA9), F(2, 696)=14.41, p<0.001 (see Table 2, Figure 3). Follow up t-tests contrasts clarified that this was due to HS youth showing higher activity in the bilateral cuneus and middle occipital gyrus cluster versus LS depressed and HC youth, who did not differ from one another in activity in these occipital areas, t(1, 696)= 6.75, p<0.001. Follow-up t-tests also demonstrated that both depressed groups, HS and LS, showed higher activity versus HC in the right frontal inferior gyrus (BA9) which corresponded to the right dorsolateral prefrontal (DLPFC) area, t(1, 696)= 5.36, p<0.01. The reported main effect of group in occipital areas and DLPFC activity was also significant when severity of depressive symptoms (measured via either the CDRS or the MFQ) was entered as a covariate in SPM8.
Figure 3.
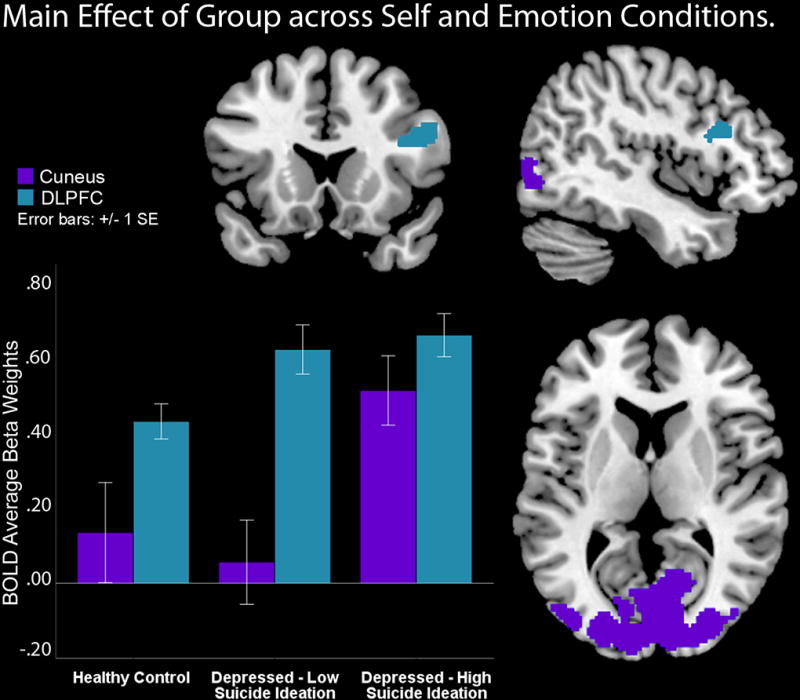
Depressed youth show higher dorsolateral prefrontal cortex (DLPFC) versus controls during all conditions. High suicidality youth showed higher bilateral occipital and cuneus activities versus depressed low suicidality and healthy controls during all conditions.
When other covariates were examined, IQ was not significantly associated with neural activity in the regions specified above, F(1, 104)= 2.61–1.14, p= 0.109–0.853. However, attributional style was a significant covariate, F(1, 104)= 4.26, p<0.05, with a higher values predicting lower cuneus activity left and right, r= −0.21 and −0.34, ps<0.05. The effect of scanning site was also significant (Pgh vs. Mpls), F(1, 104)= 8.26, p<0.01, with higher left and right cuneus activity linked to Mpls participants, r= 0.4 and 0.5, ps<0.01, respectively. However, the reported main effect of Group on cuneus and DLPFC also remained significant, F(2, 104)= 3.65, p<0.05. There were no main effects or interactions due to gender, F(1, 100)= 0.07, p=0.79 or to task order, F(3, 100)= 1.59, p=0.19.
Group by self by emotion interaction
A significant group by self condition by emotion condition interaction showed activation differences in several large clusters in mid parietal and prefrontal cortex areas, F(4, 696)=25.51–22.7, p<0.01. Follow-up contrasts reported in Table 2 revealed that HS depressed youth showed lower activity in both mid parietal and prefrontal areas when recognizing their self-happy face versus identifying another adolescent’s happy face compared to LS depressed youth, who in turn had less activity than HC youth.
Figure 4 shows that HS youth showed lower activity in MCS clusters comprised by the medial prefrontal cortex (BA10), a canonical self-processing area (Sugiura et al., 2005), and a mid-parietal cluster composed by the parahippocampus, hippocampus, and amygdala, t(1, 696)=5.28–4.67, p=.004–0.06, during happy self faces versus happy other faces. Notably, differences were due both to significantly lower activity in response to the happy self faces, and to significantly higher activity in response to the happy other faces in HS versus LS versus HC, which was unique to this emotion. No group by self interaction effects emerged for either sad or neutral faces in the follow-up t-tests. However, when depression severity was included as a covariate in SPM8, the group by self by emotion interaction was no longer significant.
Figure 4.
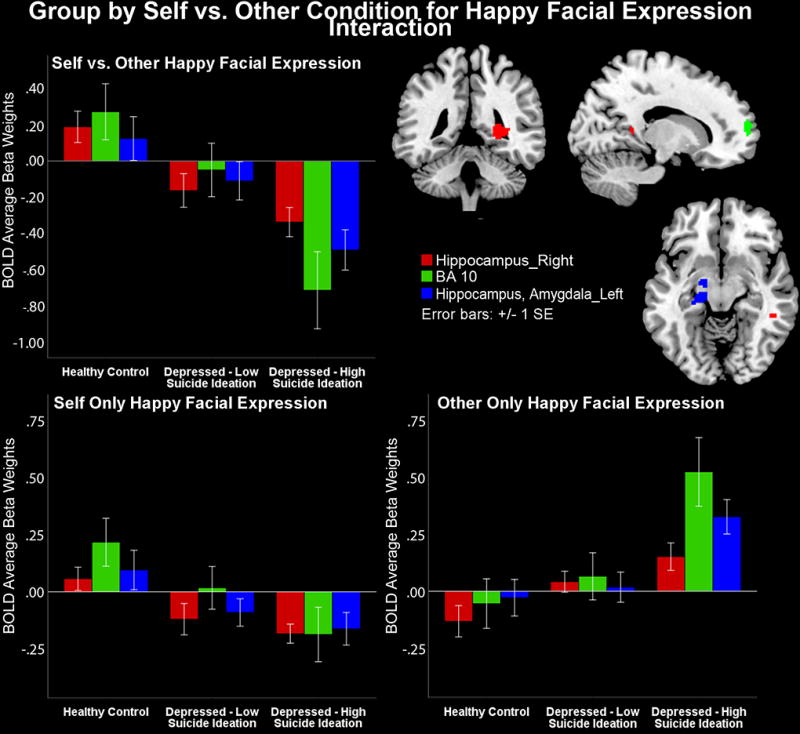
High suicidality youth show lower activity than low suicidality depressed youth who in turn show lower activity than healthy controls in limbic and medial prefrontal cortex (BA10) a canonical self-processing area, during the self face and higher activity during the other face condition for happy faces.
For the brain areas that characterized the group by self by happy emotion interaction, none of the covariates (IQ, site, or attributional style) were significantly associated with the regions specified above, F(1, 110)= 0.08–2.79, p=0.11–0.10, but the reported group by self condition by emotion condition interaction effect remained significant F(2, 110)= 10.7, p<0.00 when covariates were included in the model. There were no main effects or interactions due to task order, F(3, 105)=1.68, p=0.18, or to gender, F(1, 105)=0.22, p=0.63.
Neuroimaging Results covarying for depression severity
Group by self interaction with depression severity covariage
A unique group by self condition interaction emerged (see Table 3). Follow up analyses showed that HC exhibited similar activity to self and other faces in the left cerebellum, bilateral precuneus and PCC as well as in the bilateral ACC (BA 32) and MFPC (BA10) areas. In contrast, LS depressed youth exhibited higher activity in these areas for the self compared to other faces, whereas HS depressed youth demonstrated lower activity in these regions for the self faces and higher activity for other faces (Figure 5). Analyses of the extracted activity for the precuneus and PCC, bilateral ACC and MPFC in SPSS, confirmed the group by self interaction, F(2, 464)= 9.39, p= 0.00. However these analyses also yielded a brain region by self condition by group interaction, F(4, 464)= 2.64, p= 0.04, due to the fact that for the self-face condition HC and LS showed similar (i.e., close to 0) BA10 activity whereas HS depressed youth showed lower (i.e., below 0) BA10 activity; for the other-face condition, both HC and LS showed low and negative BA10 activity. HS depressed, however, showed slightly above zero BA10 activity for the other face (Figure 4). In summary, while HS showed BA10 hypoactivity to self faces and relatively higher BA10 activity to other faces, the other youth (LS and HC) had the opposite pattern.
Table 3.
Neural Areas that Distinguish High Suicidality vs. Low Suicidality Depressed vs. Healthy Control Youth during Self and Other Processing when Severity of Depression is included as a Covariate
| Interaction: Group by Self and Other Condition |
Cluster Size (K) |
p(K) | Hemisphere | MNI | F | p | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Cerebellum Anterior Lobe, Culmen | 379 | 0.000 | Left | −22 | −42 | −26 | 11.49 | 0.349 |
| Precuneus, Posterior Cingulate, BA 31 | 135 | 0.000 | Left and Right | 4 | −56 | 28 | 9.21 | 0.938 |
| Anterior Cingulate and MPFC, BA 32, 10 | 696 | 0.000 | Left and Right | −2 | 54 | −2 | 9.07 | 0.955 |
Note: The effects of group and self on the reported areas in Table 2 were also significant and present when severity of depression was used as a covariate.
Figure 5.
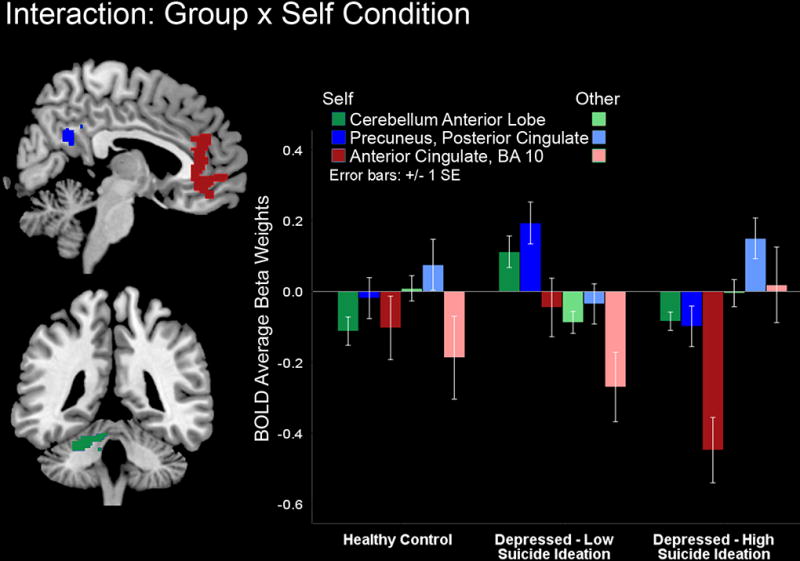
Healthy controls show similar activity in midline cortical structures (MCS) and cerebellum for self and other faces however depressed youth with low suicidality show lower MCS activity to other versus self faces while depressed youth with high suicidality showed lower MCS activity to the self (particularly BA10 and ACC) relative to other faces.
Medication effects
Across all the results, there were no effects of medication when it was included as a 2-category factor (present vs. absent) in analyses for depressed participants [N=82; 2 groups (HS vs. LS)], F(1, 78)=0.39–0.1, p=0.84–0.9.
Behavioral Results
Accuracy
There was an effect of emotion on recognition accuracy, F(2, 208)=10.5, p<0.01, such that all participants regardless of group showed higher accuracy for happy faces, F(2, 208)=8.77, p<0.01. A significant self by emotion interaction, F(2, 208)=10.5, p<0.01, was found, which reflected higher accuracy for the happy self-face. There were no effects of scanning site, task order, group, self, group by self or emotion interactions for accuracy of recognition. Mean accuracy and standard errors by group were as follows, HS: m=0.72, se=0.03; LS: m=0.71, se=0.03; HC: m=0.73, se=0.03.
Reaction Time (RT)
There were no significant differences in RT due to any grouping variable F(2, 106)=134, p=0.27. Mean RT and standard errors by groups were as follows, HS: m=864.80, se=32.40; LS: m=908.57, se=24.71; HC: m=855.49, se=23.74.
Discussion
The current investigation aimed to identify distinct neural signatures during self- and other-face processing across positive and negative emotional valences among HS depressed, LS depressed, and HC youth. First, as expected, findings showed that all youth exhibited distinct neural patterns in response to self-faces versus other faces in right MCS as well as in other brain areas relevant to face processing. Second, as predicted, diagnostic group (i.e., depressed versus healthy controls) showed higher neural activity in the medial inferior frontal gyri (BA 9 portions of the DLPFC) Third, consistent with our main hypothesis, HS youth showed lower activity in MCS (BA10), a key area for self-processing (Deeley et al., 2008; Marsh et al., 2006; Raposo, Vicens, Clithero, Dobbins, & Huettel, 2011; Sheline et al., 2009; Sugiura, et al., 2005), and in limbic structures (hippocampus and amygdala) when recognizing self-happy faces versus other-happy faces, compared to LS who in turn showed lower activity in these regions compared to HC. Unexpectedly, we found no group differences in other key MCS structures (ACC, PCC, precuneus) during self vs. other happy faces. Fourth, when severity of depression was added as a covariate, differences in neural reactivity within limbic structures to emotionally-valenced self-faces no longer distinguished the three groups. Instead, the three groups varied in reactivity within MCS areas only (ACC, PCC, precuneus and MPFC), in response to all self-versus all other-face conditions, regardless of emotional valence. Finally, contrary to our hypothesis, we found no differences among groups in brain activity during recognition of sad self-versus other-faces. Taken together, these findings contribute to existing knowledge of neurobiological correlates of self-referential processing among suicidal youth.
Right Lateral Cortical Structures and Self Processing
Identifying self-faces elicited greater recruitment of the right occipital, fusiform, precuneus, and thalamus regions relative to identifying others’ faces, regardless of group or emotional valence, even after controlling for depression severity. These areas represent regions typically involved in face processing and general processing of self-related information. Higher fusiform activation has been shown to be involved in the neurobiology of face processing, including self-faces (Kircher et al., 2000; Platek et al., 2006; Platek, Wathne, Tierney, & Thomson, 2008), and greater fusiform cortex activity is thought to be linked to increased personal salience of stimuli (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999). In a similar vein, self-face processing has been linked to increased occipital activation relative to recognition of others’ faces (Morita et al., 2008), and occipital regions tend to activate more robustly when morphed faces have more self-relevant facial features than characteristics of others (Uddin, et al., 2005). Similarly, the precuneus is reliably activated by self-related information, including self-descriptive words and self-faces (Kircher, et al., 2000; Northoff et al., 2006). Finally, the observed thalamus activity in response to self-faces is consistent with past postulations suggesting that recruitment in this region is involved when evaluating mental representations of the self and emotional states (David et al., 2006; Seger, Stone, & Keenan, 2004). Altogether, our results provide additional support that self-faces, regardless of affective valence, have unique motivational value and elicit different neurophysiological processes than others’ faces.
Suicidality, Depression and the Neurobiology of Face Processing
Both HS and LS depressed youth exhibited greater activation in the frontal inferior gyri (BA9), specifically the DLPFC, during face-processing across all conditions compared to healthy youth, even after controlling for depression severity. These findings align with prior research on the neural basis of self-processing in depression which shows that depressed patients exhibit hyperactive MPFC and DLPFC during self-processing versus a number of control conditions (Lemogne, et al., 2009; Lieberman, 2007). Furthermore, findings are in line with our own research with a sub-sample of depressed youth (with no history of abuse) who also exhibited heightened DLPFC activity across all self and emotion conditions in this task, compared to the healthy control group (Quevedo et al, under review).
HS depressed youth also exhibited unique neural responses across all conditions compared to both LS depressed and HC youth, specifically greater recruitment of the cuneus and occipital gyrus, which are visual cortex areas typically involved in face processing. Hyperactivation in these areas has been previously linked with affective disorders, including depression (Borchardt et al., 2015; Davidson, Irwin, Anderle, & Kalin, 2003), and with heightened activity in the occipital cortex during face processing is correlated with poor social adjustment and impaired social cognition among patients with psychotic disorders (Taylor, Chen, Tso, Liberzon, & Welsh, 2011). Thus, one possible explanation for our results is that higher occipital activity may reflect maladaptive cognitive and social processes in highly suicidal depressed youth; this is a hypothesis that deserves future inquiry and assessment. Of note, our findings showed that heightened neural activity in the frontal inferior gyri region and visual cortex held even after controlling for depression severity, providing further support that hyperactivity in these regions is linked to high levels of suicidality above and beyond the effects of depression.
Processing of Self versus Other Faces and Suicidality
One of the primary aims of this study was to investigate how suicidality may be associated with neural responses to emotionally valenced self-faces in depressed, suicidal youth. Before controlling for the effects of depressive symptom severity, highly suicidal, depressed youth exhibited hypoactivation of the medial prefrontal cortex (MPFC, BA10), parahippocampus, hippocampus, and amygdala during processing of positively valenced self-face compared to other positive faces. Notably, HS youth showed significantly reduced activity in response to happy self-faces, yet significant heightened activity to others’ happy faces (Figure 4). Although neural circuitry comprised by limbic structures, thalamus, fusiform gyrus and BA10 (an MCS structure involved in self-referential processing) has been repeatedly noted in research of the neurobiology of self-face processing (Kircher, et al., 2001; Stuhrmann, et al., 2011; Sugiura, 2015) it must be noted that this is the first study to show hyporeactivity of the circuitry of self-face processing when recognizing positive self-expressions among suicidal depressed youth. Previous evidence suggests that individuals at higher risk for suicide may exhibit less preferential processing of positive self-referential information (e.g., Burke et al., 2015). Thus, suicidality in depressed individuals may limit the emotional saliency of positively valenced self-relevant information (given low limbic activity), as well as higher order cognitive processes (supported by MPFC, BA10) such as awareness and associative thinking regarding positive self-relevant cues (Kircher, et al., 2001; Kreplin & Fairclough, 2015), which together may in turn disrupt preferential processing for the self and positive self-information that is typical in healthy individuals (Ma & Han, 2010). Finally, prior research has shown that depressive disorders are linked to poor recall of positive autobiographical memories (Haque, Juliana, Khan, & Hasking, 2014; Young, Bellgowan, Bodurka, & Drevets, 2013). (Quevedo et al., under review) also found that depressed youth in this sample (with no comorbid PTSD) showed reduced activity in a right mid-temporal limbic cluster for happy self versus other faces. Thus, low levels of hippocampal and parahippocampal activity during happy self faces in this study (and hyperactivity during other’s happy faces) might reflect deficient encoding and retrieval of positive self-relevant cues in depressed, suicidal youth. Assuming that the current findings replicate, they may suggest that the neurophysiology of encoding and retrieving rewarding personal information is uniquely disrupted due to high suicidality in depressed youth.
Counter to prediction, PCC, precuneus and ACC activity for happy self vs. other faces did not differ between HS, LS and HC groups prior to controlling for depression severity. In addition to self-processing, the ACC and PCC have been implicated in emotion regulation and cognitive control, conflict monitoring, making evaluative judgments, and social cognition (Brewer, Garrison, & Whitfield-Gabrieli, 2013; Lee & Siegle, 2012), which prior studies have implicated in responses to both positive and negative self-information among depressed individuals, or individuals with greater negative affect (Lemogne, et al., 2009; Nejad, et al., 2013). Given that the ACC and PCC are thought to be involved in a number of cognitive and emotional functions relevant to self-processing, it is possible that such functions did not differ during emotionally charged self-processing between the groups studied here. Future work needs to elucidate unique self-processes differentially supported by MPFC and BA10 versus ACC or PCC across emotional conditions to frame these findings.
Interestingly, after controlling for depression severity, results showed that HS youth differed from LS youth in neural activation during self vs. other faces recognition across all emotions in MCS (ACC, PCC, precuneus, and BA10) and the cerebellum, while the effect of suicidality on mid-temporal limbic areas (i.e., the hippocampus, parahippocampus, and amygdala) during the processing of happy self versus other faces was reduced to non-significance. In other words, limbic reactivity to emotionally charged self-information no longer distinguished depressed HS youth from LS youth once the severity of depressive symptoms were controlled. Instead, suicidality was associated with distinct neural patterns within MCS areas in response to self vs. other faces, irrespective of emotional valence (particularly low BA10 activity) for self faces. All together, these findings suggest that suicidality is indeed associated with atypical neural activity in response to self-information. Youth with more severe suicidality and depressive symptoms exhibit blunted neural response to positively-valenced self-referential stimuli, particularly in areas associated with mood and emotional memory (limbic areas), consistent with a number of studies documenting diminished saliency of and memory for processing (including less encoding and retrieval) positive self-information among depressed individuals (Stuhrmann, et al., 2011; Yoshimura, et al., 2014)..Results further suggest that during processing of any self-relevant stimuli regardless of emotional valence, aberrant neural functioning in the MCS may be a predictor of suicidality for youth above and beyond the effects of severity of depression.
These findings build on prior studies which demonstrate that depression and suicidality are linked to MPFC and limbic hypoactivity in response to happy emotional faces (Henderson et al., 2014; Pan, et al., 2013). Given that MPFC activity enables reward motivated planning (Gerlach, Spreng, Madore, & Schacter, 2014; Pochon et al., 2002), suicidal youth might be more likely to fail to integrate rewarding self-relevant experiences toward future behavior (Auerbach, Millner, Stewart, & Esposito, 2015), thereby further increasing suicide risk in these adolescents. Future research needs to elucidate whether impaired reward-motivated behavior is associated with lower limbic and MPFC function during positive-self-referential information.
Self-referential Processing of Negative Self-Faces and Suicidality
Contrary to our hypotheses, we found no group by self condition interaction effects on neural function during sad or neutral face recognition, both before and after controlling for depression severity. These results conflict with prior findings showing that depression and suicidality are associated with limbic hyperactivation to negative stimuli, including negative emotional faces (Henderson, et al., 2014; Thomas et al., 2011). However, the tasks adopted in prior research entailed emotion-perception or facial emotion discrimination of unfamiliar faces (e.g., matching facial expressions, identifying facial emotions), which contrast the demands of the task adopted in the current investigation, which required participants to discriminate between self- vs other-faces. Thus, it is possible that our task demands primarily elicited recruitment of neural networks involved in self-processing, which may have contributed to a different pattern of results among suicidal youth. An alternative explanation for our own lack of findings for negative self-faces versus other-faces across diagnostic groups is that depressed individuals, or individuals at higher risk for suicide, may be more likely to exhibit hyperactivity in brain areas involved in emotional and self-referential processing even at baseline, as suggested by prior research showing neural hyperactivation across many different task conditions (Nejad, et al., 2013). Therefore, it may be more challenging to find differences when comparing neural response to negative self-referential information versus other information, given that both conditions might elicit higher levels of activity in relevant brain areas. Finally, all participants were more accurate for identifying happy self-faces, suggesting that happy self faces are more emotionally salient and easier to recognize.
Limitations
Despite the number of strengths inherent in the current study, such as the use of a rigorous research design, a large sample size, an adolescent sample, and a novel task, this investigation also has some limitations to note. First, most of the high suicidality adolescents were recruited from a different site (Mpls site) than youth in other diagnostic groups, and thus the effects of scanning site cannot be entirely decoupled from the effects of suicidality, particularly for findings involving the occipital and cuneus activity. However analyzing only Mpls data in SPM8 yielded similar effects to those found for the whole sample, and main effects of group and group by condition interactions remained significant when controlling for scanning site. Second, although prior research has found RT differences during self-referential or emotional face processing tasks between groups at higher risk for suicide (e.g., depressed individuals) and low risk/psychologically healthy groups, we did not find differences in accuracy or RT among clinical groups in this study. Thus, it is possible that our task may be lower in cognitive demand or difficulty level, and that our neuroimaging findings should be interpreted with this concern in mind. However, it should be noted that the lack of behavioral findings is likely due to important methodological differences in this study, notably that the task was deliberately designed to enhance identification accuracy of emotional self- and other-emotional faces and to reduce variation in accuracy by eliminating more ambiguous faces (e.g., faces with 40–60% self features) and by using clear emotional expressions in all presented images. This was to ensure that any neural differences detected were not confounded by misidentification of self vs. other faces or of emotion type. Thus, similar reaction times and accuracy across groups suggest that the task is equally feasible for high suicidal, low suicidal and control youth, and likely valid for other psychiatrically disordered populations. It is also important to note that this is the first study to examine self-referential processing among depressed, suicidal youth using both positive and negative self- and other-faces. In order to better understand how emotionally charged self-processing may relate to suicide, it will be essential to use a variety of tasks in future research that can assess multiple aspects of self-referential processing, such as attention, perception, identification, and recall of emotional self-referential stimuli.
Conclusions and Direction for Future Research
In sum, the current findings suggest atypical neural functioning among suicidal, depressed youth, particularly blunted emotional reactivity and awareness in response to positively-valenced self faces and self faces in general. These findings advance knowledge regarding the neural correlates of suicidality during adolescence, when vulnerability to suicide is especially high. Moreover, these neural findings are consistent with existing evidence that depressed youth with high suicidality, compared to depressed youth with low suicidality, may have more negative or disturbed self-schemas that influence the automatic information processing of emotionally charged self-information (Burke, et al., 2015). Thus, findings suggest the possibility that dysfunctional processing of emotional self-related information, particularly that of positive valence, may contribute to increased risk for suicide among depressed youth. Findings from this study are also consistent with research showing that suicide attempters have more impaired prefrontal cortex function within the broad network of midline cortical structures compared to depressed or healthy individuals during decision making tasks (Vanyukov et al., 2016). In fact, in addition to sub-serving self-referential processing, areas such as the MPFC, DLPFC, and ACC are also thought to be involved in executive functions such as planning, decision making, and performance monitoring (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Sripada, Gonzalez, Luan Phan, & Liberzon, 2011; Wingenfeld et al., 2009). Research suggests that suicide attempters are deficient in these executive functions, leading to greater impulsivity and poor problem solving which increase suicide attempt risk (Annweiler et al., 2013). Thus, overlap in neural circuitry (i.e., MCS) involved in both automatic self-referential processing and executive function might help explain why depressed youth with disturbed self-schemas are more likely to engage in suicidal acts. In order to further explore this possibility, future research should carefully investigate how MCS function during self-referential processing of emotional information is associated with distinct aspects of suicidality, such as unplanned/impulsive suicide attempts vs. planned suicide attempts or suicidal ideation in both adults and young patients. This research may potentially help identify targets for suicide interventions, such as more effective cognitive control strategies which could be used in response to self-relevant emotional information.
Our findings also emphasize the importance for future research to examine the neural underpinnings of biased self-processing among suicidal youth with and without depression. We focused on suicidality among clinically depressed youth given the alarming rates of suicidal behavior and completed suicides among this group. However, the highly suicidal group also had more severe depressive symptoms, suggesting the possibility that aberrant neural response to positively valenced self-related information and self cues in general may be associated with severe depression, which may in turn contribute to or be associated with higher suicidality. Longitudinal and cross-diagnostic research is needed to test this hypothesis. In addition, it is important to recognize that suicidality is a transdiagnostic behavior that is also observed among youth with many other forms of psychopathology. When depression severity effects were controlled, findings strongly suggested that neural activity in MCS regions were particularly predictive of more severe suicidality. Along these lines, previous research with this same sample by Quevedo et al. (under review) found that although non-maltreated depressed youth also exhibited altered mid-temporal limbic activity (hippocampus, parahippocampus and amygdala) to positively valenced self-stimuli, they did not show blunted activity in MPFC (specifically BA10) observed among depressed suicidal youth in this study, in analyses with and without severity as a covariate. This suggests that suicidal youth with a past history of maltreatment may be especially likely to exhibit disruptions in the higher order cognitive processing of self-relevant information supported by MPFC/BA10 or that functional abnormalities in these areas are common to both suicidal, severely depressed, and maltreated youth. Future research examining links between suicidality, child maltreatment and disrupted MCS during self-processing is thus warranted. Finally, it will be important for future research to focus on MCS areas to understand how disturbed self-processing may predict suicide risks across diagnostic groups.
Acknowledgments
A K01MH092601 from NIMH and a NARSAD to the first author, NSF Graduate Research Fellowship (00039202) to the second author, and a Social Sciences and Humanities Research Council of Canada Postdoctoral Fellowship to the fourth author supported this work. Thanks to Drs. Emily Pisetsky, Tom Zeffiro for assistance in writing, analyses and training. Gratitude to Drs. Kathleen Thomas, Mary Phillips, and Ronald Dahl: mentors of the first, second and sixth authors.
Footnotes
Disclosures: No conflicts of interest.
References
- Abrosoft. Abrosoft FantaMorph. 2011 http://www.fantamorph.com/index.html.
- Alloy LB, Abramson LY, Murray LA, Whitehouse WG, Hogan ME. Self-referent information-processing in individuals at high and low cognitive risk for depression. Cognition & Emotion. 1997;11(5–6):539–568. [Google Scholar]
- Alloy LB, Abramson LY, Safford SM, Gibb BE. In: The Cognitive Vulnerability to Depression (CVD) Project: Current Findings and Future Directions. Alloy Lauren B, Riskind John H., editors. 2006. [Google Scholar]
- Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. Journal of Alzheimer’s Disease. 2013;37(1):147–171. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- Araujo HF, Kaplan J, Damasio A. Cortical midline structures and autobiographical-self processes: an activation-likelihood estimation meta-analysis. Frontiers in human neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Millner AJ, Stewart JG, Esposito EC. Identifying differences between depressed adolescent suicide ideators and attempters. J Affect Disord. 2015;186:127–133. doi: 10.1016/j.jad.2015.06.031. S0165-0327(15)30182-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. A systematic investigation of depression. Compr Psychiatry. 1961;2:163–170. doi: 10.1016/s0010-440x(61)80020-5. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, ffytche DH, Simmons A, Murray RM, Howard RJ. Self-responsibility and the self-serving bias: an fMRI investigation of causal attributions. Neuroimage. 2003;20(2):1076–1085. doi: 10.1016/S1053-8119(03)00331-8. S1053811903003318 [pii] [DOI] [PubMed] [Google Scholar]
- Borchardt V, Krause AL, Starck T, Nissilä J, Timonen M, Kiviniemi V, Walter M. Graph theory reveals hyper-functionality in visual cortices of Seasonal Affective Disorder patients. The World Journal of Biological Psychiatry. 2015;16(2):123–134. doi: 10.3109/15622975.2014.966144. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Front Hum Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezo J, Paris J, Turecki G. Personality traits as correlates of suicidal ideation, suicide attempts, and suicide completions: a systematic review. Acta Psychiatr Scand. 2006;113(3):180–206. doi: 10.1111/j.1600-0447.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- Brunstein Klomek A, Zalsman G, Apter A, Meged S, Har-Even D, Diller R, Orbach I. Self-object differentiation in suicidal adolescents. Compr Psychiatry. 2007;48(1):8–13. doi: 10.1016/j.comppsych.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Burke TA, Connolly SL, Hamilton JL, Stange JP, Abramson LY, Alloy LB. Cognitive Risk and Protective Factors for Suicidal Ideation: A Two Year Longitudinal Study in Adolescence. Journal of Abnormal Child Psychology. 2015:1–16. doi: 10.1007/s10802-015-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudek C, Monni A. Do you remember your sad face? The roles of negative cognitive style and sad mood. Memory. 2013;21(8):891–903. doi: 10.1080/09658211.2013.765893. [DOI] [PubMed] [Google Scholar]
- Conley CS, Haines BA, Hilt LM, Metalsky GI. The Children’s Attributional Style Interview: Developmental tests of cognitive diathesis-stress theories of depression. J Abnorm Child Psychol. 2001;29(5):445–463. doi: 10.1023/a:1010451604161. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160(1):64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly EM, Azuma R, Surguladze S, Giampietro V, Brammer MJ, Murphy DG. Changes in male brain responses to emotional faces from adolescence to middle age. Neuroimage. 2008;40(1):389–397. doi: 10.1016/j.neuroimage.2007.11.023. S1053-8119(07)01058-0 [pii] [DOI] [PubMed] [Google Scholar]
- Drake RA, Seligman ME. Self-serving biases in causal attributions as a function of altered activation asymmetry. Int J Neurosci. 1989;45(3–4):199–204. doi: 10.3109/00207458908986232. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fritzsche A, Dahme B, Gotlib I, Joormann J, Magnussen H, Watz H, von Leupoldt A. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychological medicine. 2010;40(05):815–826. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2(6):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc Cogn Affect Neurosci. 2014;9(12):1942–1951. doi: 10.1093/scan/nsu001. nsu001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of abnormal psychology. 2004;113(1):127. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Neubauer DL. Information-processing approaches to the study of cognitive biases in depression. Stress, coping, and depression. 2000:117–143. [Google Scholar]
- Greenberg MS, Alloy LB. Depression versus anxiety: Processing of self-and other-referent information. Cognition & Emotion. 1989;3(3):207–223. [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63(4):369–376. doi: 10.1016/j.biopsych.2007.05.033. doi: S0006-3223(07)00610-5 [pii] [DOI] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JR, Flory K. Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. Journal of abnormal psychology. 2010;119(3):491. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S, Juliana E, Khan R, Hasking P. Autobiographical memory and hierarchical search strategies in depressed and non-depressed participants. BMC Psychiatry. 2014;14:310. doi: 10.1186/s12888-014-0310-z. s12888-014-0310-z [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Vallejo AI, Ely BA, Kang G, Krain Roy A, Pine DS, Gabbay V. The neural correlates of emotional face-processing in adolescent depression: a dimensional approach focusing on anhedonia and illness severity. Psychiatry Res. 2014;224(3):234–241. doi: 10.1016/j.pscychresns.2014.09.006. S0925-4927(14)00237-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun-Todd DA. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Research: Neuroimaging. 2009;171(2):106–119. doi: 10.1016/j.pscychresns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Hu C, Di X, Eickhoff SB, Zhang M, Peng K, Guo H, Sui J. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci Biobehav Rev. 2016;61:197–207. doi: 10.1016/j.neubiorev.2015.12.003. S0149-7634(15)30092-0 [pii] [DOI] [PubMed] [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: 2013. [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4(4):313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. Journal of Child Psychology and Psychiatry. 2010;51(5):575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Present and Lifetime (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, David AS. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10(1–2):133–144. doi: 10.1016/s0926-6410(00)00036-7. doi: S0926-6410(00)00036-7 [pii] [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, David AS. Recognizing one’s own face. Cognition. 2001;78(1):B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Kreplin U, Fairclough SH. Effects of self-directed and other-directed introspection and emotional valence on activation of the rostral prefrontal cortex during aesthetic experience. Neuropsychologia. 2015;71:38–45. doi: 10.1016/j.neuropsychologia.2015.03.013. S0028-3932(15)00118-9 [pii] [DOI] [PubMed] [Google Scholar]
- Kruggel F, von Cramon DY. Temporal properties of the hemodynamic response in functional MRI. Human brain mapping. 1999;8:259–271. doi: 10.1002/(SICI)1097-0193(1999)8:4<259::AID-HBM9>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Soc Cogn Affect Neurosci. 2012;7(5):521–534. doi: 10.1093/scan/nsp001. nsp001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehéricy S, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4(3):305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han S. Why we respond faster to the self than to others? An implicit positive association theory of self-advantage during implicit face recognition. J Exp Psychol Hum Percept Perform. 2010;36(3):619–633. doi: 10.1037/a0015797. 2010-10162-007 [pii] [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Human brain mapping. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of general psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N. The role of the right prefrontal cortex in self-evaluation of the face: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20(2):342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Xu JQ, Kochanek KD. Deaths: Final data for 2010. National vital statistics reports. CDC/NCHS, National Vital Statistics System; Vital statistics of the United States. 2010;61(4) [PubMed] [Google Scholar]
- Murray RJ, Debbane M, Fox PT, Bzdok D, Eickhoff SB. Functional connectivity mapping of regions associated with self- and other-processing. Hum Brain Mapp. 2015;36(4):1304–1324. doi: 10.1002/hbm.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in human neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry. 2013;70(3):300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. Measuring the suicidal mind implicit cognition predicts suicidal behavior. Psychological science. 2010 doi: 10.1177/0956797610364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pan L, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol Med. 2013;43(10):2129–2142. doi: 10.1017/S0033291712002966. S0033291712002966 [pii] [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Tobin-Richards M, Boxer A. Measuring pubertal status: Reliability and validity of a self-report measure. University Park: Pennsylvania State University; 1985. [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Langleben DD. Neural substrates for functionally discriminating self-face from personally familiar faces. Hum Brain Mapp. 2006;27(2):91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Wathne K, Tierney NG, Thomson JW. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. doi: S0006-8993(08)01664-8 [pii] [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99(8):5669–5674. doi: 10.1073/pnas.082111099. 99/8/5669 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny AD. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry. 1983;40(3):249–257. doi: 10.1001/archpsyc.1983.01790030019002. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64(4):442–450. [PubMed] [Google Scholar]
- Quevedo K, Harms M, Scott H, Thomas KM, Sauder M, Smyda G. The Neurobiology of Self-face Processing among Depressed Adolescents. doi: 10.1016/j.jad.2017.12.023. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others and relations. [Research Support, N.I.H., Extramural] Social cognitive and affective neuroscience. 2011;6(3):260–269. doi: 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner F, Siep N, Lobbestael J, Arntz A, Peeters FP, Huibers MJ. Neural correlates of self-referential processing and implicit self-associations in chronic depression. Journal of affective disorders. 2015;186:40–47. doi: 10.1016/j.jad.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh: Psychology Software Tools, Inc; 2012. [Google Scholar]
- Seymour KE, Jones RN, Cushman GK, Galvan T, Puzia ME, Kim KL, Dickstein DP. Emotional face recognition in adolescent suicide attempters and adolescents engaging in non-suicidal self-injury. European Child and Adolescent Psychiatry. 2015 doi: 10.1007/s00787-015-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. 0812686106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Yu SM. Trends and differentials in adolescent and young adult mortality in the United States, 1950 through 1993. Am J Public Health. 1996;86(4):560–564. doi: 10.2105/ajph.86.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: Contributions of subjective value, stimulus type, and trait impulsivity. Human brain mapping. 2011;32(10):1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M. Three faces of self-face recognition: potential for a multi-dimensional diagnostic tool. Neurosci Res. 2015;90:56–64. doi: 10.1016/j.neures.2014.10.002. S0168-0102(14)00225-9 [pii] [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Jeong H, Horie K, Sato S, Kawashima R. Face-specific and domain-general characteristics of cortical responses during self-recognition. Neuroimage. 2008;42(1):414–422. doi: 10.1016/j.neuroimage.2008.03.054. S1053-8119(08)00302-9 [pii] [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. Neuroimage. 2005;24(1):143–149. doi: 10.1016/j.neuroimage.2004.07.063. doi: S1053-8119(04)00451-3 [pii] [DOI] [PubMed] [Google Scholar]
- Tang J, Wu S, Miao D. Experimental test of escape theory: accessibility to implicit suicidal mind. Suicide and Life-Threatening Behavior. 2013;43(4):347–355. doi: 10.1111/sltb.12021. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J Psychiatr Res. 2011;45(4):526–538. doi: 10.1016/j.jpsychires.2010.08.004. S0022-3956(10)00245-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. Validity of the shortened Mood and Feelings Questionnaire in a community sample of children and adolescents: a preliminary research note. Psychiatry Res. 1998;81(2):259–268. doi: 10.1016/s0165-1781(98)00073-0. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, Anderson IM. Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med. 2011;41(9):1845–1855. doi: 10.1017/S0033291711000043. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage. 2005;25(3):926–935. doi: 10.1016/j.neuroimage.2004.12.018. doi: S1053-8119(04)00766-9 [pii] [DOI] [PubMed] [Google Scholar]
- Vanyukov P, Szanto K, Hallquist M, Siegle G, Reynolds C, Forman S, Dombrovski A. Paralimbic and lateral prefrontal encoding of reward value during intertemporal choice in attempted suicide. Psychological medicine. 2016;46(02):381–391. doi: 10.1017/S0033291715001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56(2):153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schachtzabel C, Peikert G, Bär KJ. The neural basis of the abnormal self-referential processing and its impact on cognitive control in depressed patients. Human brain mapping. 2015 doi: 10.1002/hbm.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Texas: American Psychological Association; 1999. [Google Scholar]
- Wenzel A, Beck AT. A cognitive model of suicidal behavior: Theory and treatment. Applied and Preventive Psychology. 2008;12(4):189–201. [Google Scholar]
- Wingenfeld K, Rullkoetter N, Mensebach C, Beblo T, Mertens M, Kreisel S, Woermann FG. Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology. 2009;34(4):571–586. doi: 10.1016/j.psyneuen.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social Cognitive and Affective Neuroscience. 2014;9(4):487–493. doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. Behavioral and neurophysiological correlates of autobiographical memory deficits in patients with depression and individuals at high risk for depression. JAMA psychiatry. 2013;70(7):698–708. doi: 10.1001/jamapsychiatry.2013.1189. 1688033 [pii] [DOI] [PubMed] [Google Scholar]


