Abstract
No study has evaluated the mutagenicity of atmospheres with a calculated air quality health index (AQHI). Thus, we generated in a UV-light-containing reaction chamber two simulated atmospheres (SAs) with similar AQHIs but different proportions of criteria pollutants and evaluated them for mutagenicity in three Salmonella strains at the air-agar interface. We continuously injected into the chamber gasoline, nitric oxide, and ammonium sulfate, as well as either α-pinene to produce SA-PM, which had a high concentration of particulate matter (PM): 119 ppb ozone (O3), 321 ppb NO2, and 1007 μg/m3 PM2.5; or isoprene to produce SA-O3, which had a high ozone (O3) concentration: 415 ppb O3, 633 ppb NO2, and 55 μg/m3 PM2.5. Neither PM2.5 extracts, NO2, or O3 alone, nor non-photo-oxidized mixtures were mutagenic or cytotoxic. Both photo-oxidized atmospheres were largely direct-acting base-substitution mutagens with similar mutagenic potencies in TA100 and TA104. The mutagenic potencies [(revertants/h)/(mgC/m3)] of SA-PM (4.3 ± 0.4) and SA-O3 (9.5 ± 1.3) in TA100 were significantly different (P < 0.0001), but the mutation spectra were not (P = 0.16), being ~54% C → T and ~46% C → A. Thus, the AQHI may have some predictive value for the mutagenicity of the gas phase of air.
Keywords: mutagenicity, smog, air pollution, mutation spectra, ozone, PM
INTRODUCTION
Urban air pollution, a known environmental health hazard, is often referred to as smog and is generated by the photolysis and subsequent reactions of urban air pollutants such as nitrogen oxides and hydrocarbons.1, 2 The International Agency for Research on Cancer (IARC) has classified outdoor air pollution as a group 1 (known) human lung carcinogen.3 In addition to cancer, a wide variety of other health effects are associated with air pollution, including cardiovascular disease, asthma, chronic obstructive pulmonary disease, low birth weight, upper-respiratory infections in children, and premature mortality.4–8
The use of in vitro studies for characterizing the relative changes between exposure conditions has become increasingly important due to the cost and technical complexity of in vivo methods. In addition, the National Academy of Sciences has called for eliminating the use of animals for future studies.9 The mutagenicity of air has been studied in the Salmonella (Ames) mutagenicity assay for 40 years, starting in 1977.10, 11 Since then, more than 250 studies in Salmonella alone have characterized the mutagenicity of air worldwide, leading to a remarkable number of insights.3 However, nearly all of these studies have involved the evaluation of organic extracts of PM; only ~12 have evaluated organic extracts of the gas phase of polluted air via extracts of volatile compounds captured on XAD-2 resin or polyurethane foam.3, 12, 13
Extracting organics from either PM or the gas phase of polluted air can alter the pollutant’s physical and chemical characteristics.14–16 In addition, evaluating the mutagenicity of these extracts does not permit the study of the effects of gases or vapors directly. For example, exposure of cultured mammalian cells directly to an air pollutant mixture at the air-liquid interface permits the determination of the relative toxicity of multi-pollutant mixtures containing both particle- and gas-phase components that interact with each other and produce secondary reaction products.17 A similar exposure method with Salmonella involving an air-agar interface has been used extensively over the years for cigarette smoke,18–21 various volatile agents,22 and atmospheres generated by smog chambers.12, 13, 22–31 However, such an approach cannot evaluate the PM of air because, as discussed in the Discussion, the bacteria do not take up the PM. Among the smog chamber studies, all have used a single chemical to initiate the reactions leading to the formation of mutagenic secondary reaction products.23–31 To extend those studies, we have used an environmentally relevant complex mixture, gasoline, to initiate the reaction and determined the mutations induced by the resulting gas phase of the atmosphere.
We investigated the mutagenicity and mutation spectra of two chemically different atmospheres: one rich in PM (SA-PM) and the other rich in ozone (SA-O3) but that were otherwise equivalent in calculated health risk based on a well-established index. Because no air mutagenicity index exists, we used Health Canada’s Air Quality Health Index (AQHI) for comparative purposes.32 The AQHI is a no-threshold index developed to evaluate health risk (mortality) on acute pulmonary exposures at ambient concentrations based on the combination of PM2.5, O3, and nitrogen dioxide (NO2) concentrations measured. Thus, we generated two atmospheres with nearly identical AQHI values but where the secondary reaction products were primarily in the particulate phase in one (SA-PM) and in the gas phase in the other (SA-O3).
Along with chemical analyses reported elsewhere,33 we used three strains of the bacterial Salmonella (Ames) mutagenicity assay to evaluate the mutagenicity of (a) an aqueous and various organic extracts of the PM2.5 from the atmosphere high in PM (SA-PM) via the plate-incorporation method, and (b) of the gas phase of both atmospheres via the air-agar interface method. The goals of this study were to determine the mutagenic potencies and mutation spectra in Salmonella strain TA100 of the two simulated atmospheres so that we could compare the mutation spectra to that of (a) the well-studied gas-phase pollutant peroxyacetyl nitrate (PAN), (b) PM from polluted air and cigarette smoke, and (c) the P53 gene of lung tumors from smokers and nonsmokers. We also wanted to see if the AQHI would reflect the mutagenic potencies and mutation spectra of the two atmospheres. A matrix of the various experiments we performed to address these questions is shown in Table S1.
EXPERIMENTAL METHODS
Generation of Simulated Atmospheres
The details of the generation of the atmospheres and criteria pollutants are described elsewhere.33 Briefly, we used EPA’s Mobile Reaction Chamber (MRC) (Figure 1A) to generate two simulated atmospheres (SA-PM and SA-O3) that contained different concentrations of O3, PM2.5, and NO2 but that had similar AQHI values. Atmospheres generated with the UV lights on in the MRC are photo-oxidized atmospheres, where most of the primary reactants have been converted to secondary reaction products. Atmospheres generated with the UV lights off in the MRC are non-photo-oxidized atmospheres composed of only the untransformed, primary reactants.
Figure 1.
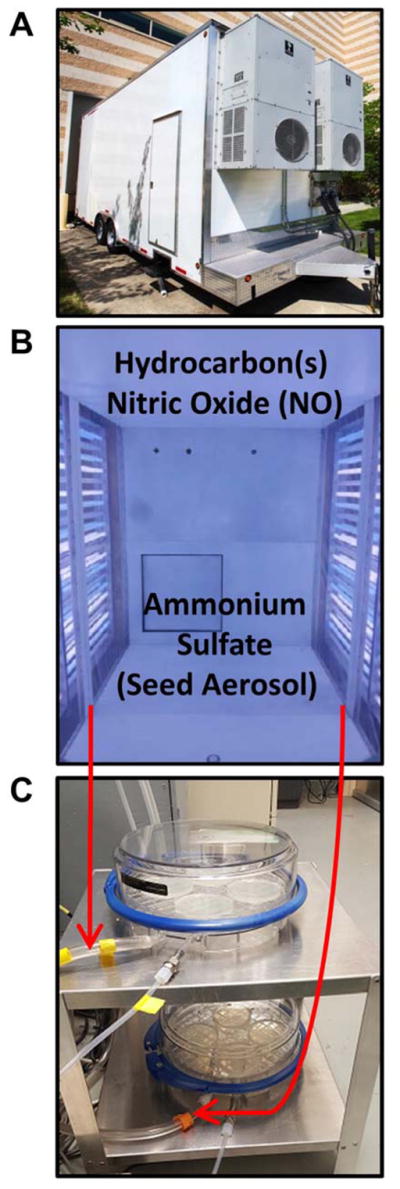
(A) The Mobile Reaction Chamber (MRC) is housed in a 24-ft trailer containing the smog chamber in the back and the control room in the front. The control room had various air-monitoring equipment to characterize the generated atmospheres. (B) View inside the smog chamber with the UV lights (300–400 nm) on to simulate solar radiation. (C) The in vitro exposure chambers (MIC) held 4 Petri dishes each and permitted direct sampling of the generated atmospheres from the MRC into the MIC at various flow rates.
The MRC is a 24-ft trailer containing a 14.3-m3 Teflon-lined environmental irradiation chamber operated as a continuous-stirred tank reactor by which the reactants were added continuously. Environmental irradiation chambers are sophisticated systems that can be used to generate realistic, reproducible, and controlled urban-like atmospheres. Photochemistry in the MRC was catalyzed by 120 fluorescent bulbs mixed evenly with black light bulbs and UV bulbs (300–400 nm), which simulated solar radiation in urban atmospheres.
SA-PM had high levels of PM2.5 and relatively low levels of gases (O3 and NO2) so that the AQHI was driven by the PM concentration. Alternatively, SA-O3 had relatively low levels of PM2.5 and high levels of gases (O3 and NO2) so that the AQHI was driven by the O3 and NO2 concentrations. Because the atmospheres had similar AQHI values, we considered them equivalent even though their chemical compositions differed in terms of the distribution of their compounds in the gas versus the particulate phase. Details of the calculations of the AQHI values are described elsewhere.33
For SA-PM, we injected continuously into the MRC a mixture of 6 parts per million carbon (ppmC) α-pinene, 24 ppmC gasoline, and 500 ppb nitric oxide (NO) into the MRC along with nebulized ammonium sulfate (2 μg/m3) to provide a nucleation base for photochemical reaction products. For SA-O3, we injected continuously into the MRC a mixture of 6 ppmC isoprene, 9 ppmC gasoline, 900 ppb NO, and nebulized ammonium sulfate (2 μg/m3). As described elsewhere,33 we measured the concentrations of criteria pollutants, aromatics, peroxyacetyl nitrate (PAN), paraffins, olefins, and other volatile organics produced from the photochemical reactions.
Briefly, we measured continuously the concentrations of NO and oxides of nitrogen (NOx) with a Model 42i chemiluminescent NOx analyzer (Thermo Scientific, Fitchburg, WI). We monitored O3 with a Model 49i Ozone Analyzer (Thermo Scientific, Fitchburg, WI). We determined the concentration of secondary organic aerosol (SOA) by filter collection and assessed the aerosol size using a scanning mobility particle sizer (SMPS, TSI Inc., Shoreview, MN). We analyzed hydrocarbons using gas chromatography combined with flame ionization detection (GC/FID) on a Hewlett-Packard Model 5890 (Palo Alto, CA). We identified compounds by column retention time location using a CALTABLE that contained more than 300 compounds; we verified these with a mass spectra detection system (GC/MS); details in.33
Generation of Criteria Pollutants O3, NO2, and Aqueous and Organic Extracts of PM2.5
SA-O3 had higher concentrations of O3 and NO2 than did SA-PM, as described elsewhere.33 We generated an equivalent O3 concentration as that measured during SA-O3 experiments using the Thermo Scientific Model 49i-PS Ozone Primary Standard. The generated O3 flow was delivered to a sampling manifold where 1 L/min was sampled using a vacuum pump through the in vitro exposure chambers described below.
Similarly, we used a Thermo Scientific Model 146i Dynamic Gas Calibrator to generate an atmosphere of NO2. The NO2 was generated using reverse gas phase (ozone) titration of NO in nitrogen. Briefly, an Airgas Primary Standard tank of NO (50 ppm) supplied the gas to the Dynamic Gas Calibrator, and inside the calibrator, O3 titrated the NO stream to produce the NO2. The NO2 was then mixed with dilution air to produce the desired exposure concentration; a slight excess of NO (15%) remained in the gas test stream, indicating that no O3 remained based on the stoichiometry of the reaction. The clean air used to dilute the NO2 to the appropriate concentration was supplied using the Thermo Scientific Model 111 Zero Air Supply that was metered by mass flow controllers. The final NO2 atmosphere was delivered to the in vitro exposure chambers at 1 L/min using a vacuum pump.
Collection and Extraction of PM
We collected PM2.5 on polytetrafluorethylene (PTFE) Teflon™ filters as described33 and extracted the PM2.5 as described34 with the following modifications. Briefly, we cut each filter into five approximately equal pieces, weighed each piece, covered each piece with 5 ml of one of five different solvents for 2.5 h, and then sonicated each piece for 45 min. The solvents were LC grade high-purity water, methanol (MeOH), dichloromethane (DCM):MeOH (1:1), hexane:isopropanol:benzene (40:20:40, HIB), and DCM.
We filtered each extract through a 0.2-μm Anotop filter, rinsed the filter with 2 ml of the respective solvent, and brought the total volume of each extract to 10 ml. We determined the percentage of extractable organic material (EOM) by gravimetric analysis as described elsewhere,35 and we also performed this analysis with blank filters, which we used as the basis for calculating the %EOM. Based on the %EOM, we solvent-exchanged a portion of each organic extract into dimethyl sulfoxide (DMSO) for bioassay with stock solutions at 2 mg EOM/ml DMSO. This was done by evaporating the solvent under nitrogen at 37°C until nearly dry, adding the appropriate amount of DMSO, and continuing evaporation until the volume equaled that of the added DMSO.
Mutagenicity Assays and S9 Mix
We used the Salmonella (Ames) mutagenicity assay36 in two different ways to evaluate the mutagenicity of either organic extracts of PM2.5 (by the plate-incorporation method) or the gas phase of the simulated atmospheres (by the air-agar-interface method). We used three strains of Salmonella. Strain TA100 detects base-substitution mutations in DNA at Guanine:Cytosine (GC) sites, TA98 detects frameshift mutations, and TA104 detects base-substitution mutations at both GC and Adenine:Thymine (AT) sites, the latter potentially being due to oxidative mutagens. Dimethyl sulfoxide (DMSO) was the negative control, and the positive controls are noted in footnotes in Tables S2–S11.
The metabolic activation consisted of S9 mix36 that contained Aroclor-induced Sprague-Dawley rat liver (Moltox, Boone, NC) such that 500 μl of S9 mix/plate gave a concentration of 1 mg of S9 protein/plate. We also prepared heat-inactivated (HI) S9 by heating the S9 mix at 60°C for 15 min to eliminate enzymatic activity. We used HI S9 to determine if the S9 was metabolizing the mutagens in the atmospheres or if the S9 protein served only as a protective layer to prevent desiccation of the cells under the air-agar interface conditions.
Methods for Evaluating the Mutagenicity of the PM2.5 Extracts and the Atmospheres
We evaluated the PM2.5 extracts by the plate-incorporation method36 by testing the extracts at 5 to 400 μg EOM/plate in strain TA100 +/− metabolic activation (S9). We evaluated the O3, NO2, and the gas phase of SA-PM and SA-O3 atmospheres by adding 100 μl of overnight culture of strain TA100, TA98, or TA104 +/− S9 to molten top agar and pouring the suspension onto VBME bottom agar in glass Petri dishes, permitting us to expose the cells at the air-agar interface as described below.
We exposed the cells in plates with the lids off for 0–14 h that we had placed inside Modular Incubator Chambers (MIC) (Billups-Rothenberg, MIC-101TM, Del Mar, CA) (Figure 1C). The atmosphere within the MRC, either unreacted (UV lights off) or photo-oxidized (UV lights on) was drawn into the MIC via a vacuum pump at a flow rate of 3.5 or 1.0 L/min. Each MIC contained 4 plates, and exposures were done in duplicate at least twice. These MICs have been described previously in detail for their use as a Gas In Vitro Exposure System (GIVES) in combination with a smog chamber.37 We used two identical modules for all experiments, which permitted us to expose different sets of strain/S9 combinations simultaneously. The same exposure method was used for O3 and NO2 except that the MIC was connected directly to the source of these gases, which did not pass through the smog chamber and were not exposed to UV lights. After both testing methods, we incubated the plates at 37°C for 72 h, after which we counted the colonies (revertants, rev) with an automatic colony counter (AccuCount 1000, Manassas, VA).
Statistical Analyses
We first combined the primary data (rev/plate) from replicate experiments and calculated a linear regression of the resulting time-response curve (rev/h) for each condition using Prism (GraphPad, San Diego, CA). We considered an atmosphere to be mutagenic if it produced a slope (rev/h) that was significantly (P < 0.05) different from zero. For those atmospheres that were mutagenic, we then normalized the mutagenic potencies (slopes) to (rev/h)/(mgC/m3) because, by definition, all the organic secondary reactants contained carbon. We then compared these normalized mutagenic potencies by unpaired, 2-tailed t-tests with P < 0.05 to assess the relative mutagenic potencies of the two atmospheres in various strains, at various flow rates of exposure, and in the presence or absence of metabolic activation.
Targeted Next-Generation DNA Sequencing
We purified 100 revertants of strain TA100 from each of a control and exposed plate from SA-PM and SA-O3 at a flow rate of 1 L/min into the MIC, for a total of 400 revertants. We streaked each revertant onto minimal medium supplemented with excess biotin but no histidine. This purified the revertants from the background (non-revertant) cells because only revertants can grow without histidine. After incubating for 48 h, we touched each streak with a sterile toothpick and inoculated this into 1 ml of liquid minimal medium containing excess biotin but no histidine in 24-well plates; 1 rev/well. The cultures grew overnight so that the cell concentration reached stationary phase (109 cells/ml), and then we sampled 100 μl from each culture, combining 50 revertants into a 50-ml centrifuge tube. We froze the cells at −80°C until the DNA was isolated for sequencing using standard procedures for next-generation amplicon sequencing with slight modifications as described in Supporting Information.
RESULTS
Chemical Analysis of Atmospheres
We measured the concentrations of criteria pollutants, aromatics, PAN, paraffins, olefins, and other volatile organics produced from the photochemical reactions, and the results are reported elsewhere.33 Although there were likely many products in the photo-oxidized atmospheres, only 13 carbonyls were quantitated.33 The photo-oxidized SA-PM had 119 ppb O3, 321 ppb NO2, and 1070 μg/m3 PM2.5, which corresponded to an AQHI value of 103. The photo-oxidized SA-O3 had 415 ppb O3, 633 ppb NO2, and 55 μg/m3 PM2.5, which corresponded to an AQHI value of 97. These AQHI values were calculated based on 1-min average concentrations of the three pollutants during the duration of the exposure period; thus, they are a subset of the average AQHI values presented elsewhere.33
The two photo-oxidized atmospheres had similar AQHI values; however, because of the different biogenic precursors and different HC:NOx values, the atmospheres had different magnitudes and distributions of carbonyl compounds.33 Due to the high SOA yield coefficient for α-pinene, SA-PM formed much higher concentrations of PM than did SA-O3, where the relatively small SOA yield coefficient for isoprene resulted in a limited production of PM and a higher percentage of carbon remaining in the gas-phase.33
Mutagenic Potencies of Gas Phase of Atmospheres
The primary data (rev/plate) for the air-agar-interface exposure experiments are shown in Tables S2–S8, and time-response curves of the photo-oxidized (UV lights on) atmospheres constructed from the primary data are shown in Figure S1; time-response curves of non-photo-oxidized (UV lights off) atmospheres are not shown. The mutagenic potencies (rev/h) calculated from the linear regressions shown in Figure S1 were normalized relative to carbon concentration by incorporating the data from Table S9 to calculate the normalized mutagenic potencies, (rev/h)/(mgC/m3), shown in Table 1. Using these normalized mutagenic potencies (Table 1), we constructed the histograms in Figure 2 to illustrate statistical comparisons between the mutagenic potencies of the two atmospheres between strains TA100 and TA104 and between the two flow rates.
Table 1.
Mutagenic Potencies of Photo-oxidized Atmospheres
| Strain | Flow rate (L/min) | S9 | Rev/h ± SEa | (Rev/h)/(mgC/m3)b | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SA-PM | SA-O3 | SA-PM | SA-O3 | |||
| TA98 | 3.5 | − | 0 | Toxicc | 0 | Toxicc |
| + | 0 | Toxicc | 0 | Toxicc | ||
| HI | 0 | No Datad | 0 | No Datad | ||
| TA100 | − | 17.6 ± 2.1 | Toxicc | 4.8 ± 0.6 | Toxicc | |
| + | 20.3 ± 1.2 | 43.1 ± 5.8 | 5.4 ± 0.3 | 13.8 ± 1.9 | ||
| HI | 11.9 ± 2.6 | Toxicc | 3.2 ± 0.7 | Toxicc | ||
| TA104 | − | 0 | Toxicc | 0 | Toxicc | |
| + | 49.7 ± 16.9 | Toxicc | NATCe | NATCe | ||
| HI | 9.6 ± 2.6 | No Datad | 3.7 ± 1.0 | No Datad | ||
| TA100 | 1.0 | − | 8.8 ± 0.8 | 5.7 ± 1.4 | NATCe | 2.0 ± 0.5 |
| + | 11.3 ± 1.0 | 27.5 ± 3.7 | 4.3 ± 0.4 | 9.5 ± 1.3 | ||
| HI | 9.7 ± 0.9 | No Datad | 3.7 ± 0.3 | No Datad | ||
| TA104 | − | No Datad | 10.3 ± 2.8 | No Datad | 3.6 ± 1.0 | |
| + | 8.4 ± 2.1 | 28.5 ± 3.0 | 3.2 ± 0.8 | 9.8 ± 1.0 | ||
| HI | 6.1 ± 1.7 | No Datad | 2.3 ± 0.7 | No Datad | ||
Mutagenic potencies are the slopes of the linear regressions in Figure S1.
Mutagenic potencies are the calculated values shown in Table S9.
Toxic refers to a reduced number of rev/plate at some time points, indicating that the exposure was cytotoxic; such results generally prevented the calculation of a linear regression because there were only 2 time points instead of the necessary 3 points needed to calculate the regression.
No Data means that the experiment was not done.
NATC = not able to calculate; this was because insufficient engineering or chemical data were available to permit the conversion of rev/h to (rev/h)/(mgC/m3).
Figure 2.
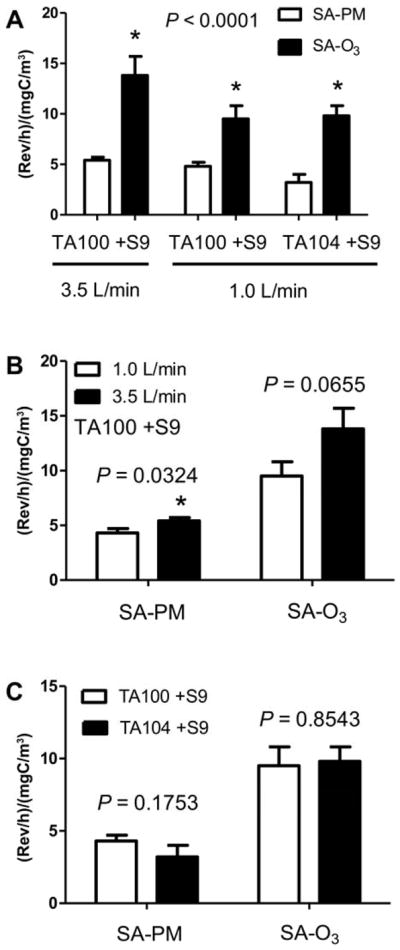
Comparisons of the mutagenic potencies of the two atmospheres: (A) in TA100 at a flow rate of 3.5 L/min, and in TA100 and TA104 at a flow rate of 1.0 L/min; (B) between two flow rates; and (C) between two strains of Salmonella.
When the UV lights were off, the unreacted components of SA-PM injected into the MRC and then delivered to the MIC at either 3.5 or 1 L/min were neither cytotoxic nor mutagenic (Tables S2–S4). In contrast, when the UV lights were on, the resulting photo-oxidized atmospheres were either cytotoxic, especially at a flow rate of 3.5 L/min, or mutagenic (in strains TA100 and TA104) under various conditions (Figure S1A). After expressing the mutagenic potencies as (rev/h)/(mgC/m3), there were sufficient data to compare the two atmospheres under three conditions: TA100 +S9 at 3.5 L/min, and TA100 and TA104 at 1 L/min (Table 1).
SA-O3 was 2–3 times more mutagenic than was SA-PM under all 3 conditions (Figure 2A). SA-PM was more mutagenic at the higher flow rate than at the lower one, but the mutagenicity of SA-O3 was similar when delivered to the cells at either flow rate (Figure 2B). The mutagenic potency of SA-PM was similar in strains TA100 and TA104, and the same was true for SA-O3 (Figure 2C). Thus, both atmospheres induced base substitutions at GC sites, but likely not at AT sites because they were not more mutagenic in strain TA104 relative to TA100.
Using the data in Table 1, we made comparisons to assess the role of metabolic activation (Table 2) that showed that 4/5 comparisons for SA-PM found no influence of metabolic activation, indicating that most of the mutagenic activity of SA-PM was direct-acting. The one comparison possible for SA-O3 showed that the mutagenicity of this atmosphere was increased by S9, suggesting that most of its mutagenicity was indirect-acting.
Table 2.
Role of Metabolic Activation on Mutagenicity
| Comparisons of mutagenic potencies | P-valuea |
|---|---|
| TA100 3.5 L/min SA-PM | |
| −S9 (4.38 ± 0.6) v +S9 (5.4 ± 0.3) | 0.3211 |
| −S9 (4.8 ± 0.6) v HI S9 (3.2 ± 0.7) | 0.0912 |
| +S9 (5.4 ± 0.3) v HI S9 (3.2 ± 0.7) | 0.0014* |
| TA100 1 L/min SA-PM | |
| +S9 (4.3 ± 0.4) v HI S9 (3.7 ± 0.3) | 0.2864 |
| TA104 1 L/min SA-PM | |
| +S9 (3.2 ± 0.3) v HI S9 (2.3 ± 0.7) | 0.2499 |
| TA104 1 L/min SA-O3 | |
| −S9 (3.6 ± 1.0) v +S9 (9.8 ± 1.0) | 0.0002* |
Comparisons were made by conducting an unpaired, 2-tailed
t-test, (P < 0.05) of mutagenic potencies are from Table 1.
Mutagenicity of PM2.5 Extracts and Criteria Pollutants
The %EOM values of the extracts of the PM2.5 relative to that of the filter blanks were 93.7% for MeOH, 92.1% for DCM/MeOH, 77.6% for water, 80.1% for HIB, and 74.8% for DCM. Exposure of TA100 +/− S9 to 5 to 400 μg EOM/plate showed that none of these aqueous or organic extracts of the PM2.5 were mutagenic or cytotoxic (Table S10). Neither O3 at 415 ppb or NO2 at 633 ppb, which were the levels observed in SA-O3, were mutagenic or cytotoxic (Table S11). Thus, the mutagenic activities of the SAs were due solely to other secondary reaction products and not to the criteria pollutants used to calculate the AQHI values for each atmosphere.
Mutation Spectra
After subtracting the control from the exposed mutation spectra (Tables S12 and S13), the mutation spectra induced by SA-PM was 49% C → T and 51% C → A, and that induced by SA-O3 was 59% C → T and 41% C → A (Table S14). The two mutation spectra were not significantly different (P = 0.16), resulting in an average mutation spectra of 54% C → T and 46% C → A for the SAs. However, there are two adjacent Cs in TA100 at which the mutations reported here can be recovered, and the proportion and types of mutations induced by SA-PM and SA-O3 at these two sites were distinctly different (P < 0.001) (Table S15). The mutation spectra of control revertants from HI S9 plates, +S9 plates, or from our historical control38 were not significantly different (P = 0.58 to 0.94), supporting our comparison of the control HI S9 revertants to the +S9 exposed revertants of SA-PM and our use here of targeted next-generation DNA sequence analysis compared to Sanger di-deoxy sequencing that we used historically.
DISCUSSSION
Features of Atmospheres
Although SA-PM had a high concentration of PM and low concentrations of O3 and NO2, and SA-O3 had the reverse, the two atmospheres were similar in many ways, as reflected by their similar AQHI values. In the absence of photo-oxidation, the primary chemical components (NO, ammonium sulfate, gasoline, and α-pinene or isoprene) used to produce the atmospheres were not mutagenic in Salmonella, nor were any of the criteria pollutants, including PM2.5, O3, or NO2. With photo-oxidation, neither atmosphere induced frameshift mutations or mutations at AT sites, but both induced similar proportions of mutations at GC sites via largely a direct-acting mechanism in strain TA100.
The primary differences were that SA-O3 was 2–3 times more mutagenic and cytotoxic than was SA-PM, and these differences were likely due to the difference in distribution of the organics, which were largely distributed in the gas phase in SA-O3 but mostly in the PM in SA-PM. Support for this is found in the chemical analysis, which showed that the concentrations of formaldehyde, glyoxal, and methylglyoxal were twice as high in SA-O3 than in SA-PM.33 The greater toxicity of SA-O3 relative to SA-PM was also confirmed in parallel studies with rodents done in conjunction with the present study that also found that SA-O3 produced more adverse metabolic, immunologic, and cardiovascular effects than did SA-PM.39–41
Four out of five comparisons showed that the mutagenic potency of SA-PM was not affected by the presence or absence of S9 or heat-inactivated S9, indicating that the mutagenic activity of SA-PM was generally direct-acting (Table 2). Further support for this is shown by the finding that two out of three comparisons showed no significant difference in the mutagenic potency of SA-PM in the presence of either S9 or HI S9 (Table 2). HI S9 had no metabolic capability, as shown by its inability to convert 2-aminoanthracene (2AA) into a mutagen (Tables S5–S7). SA-O3 exhibited both direct- and indirect-acting mutagenicity; however, this was based on only one comparison. Both SAs were cytotoxic when delivered to the cells at 3.5 L/min (Tables S5 and S6). However, SA-O3 was still highly cytotoxic even at 1 L/min, preventing exposures past 3 h, whereas SA-PM had little toxicity at 14 h of exposure at 1 L/min (Tables S7 and S8).
Although the two atmospheres produced similar mutation spectra in terms of the proportional induction of the two classes of mutations, they induced those mutations at different sites in the DNA target of strain TA100, suggesting that although the chemical compositions of the gas phase of each atmosphere may have been similar, they were not identical. Thus, the resulting mutagenicity was driven by a slightly varied mix of atmospheric transformation products.
Organics, not particles per se, can be taken into the bacterial cells, either by diffusion or activate transport, if the organics are physically on the surface of the cell. If a particle is on a cell, it is possible that some of the organics on the particle could be taken up into the cell—but not the particle itself. However, as we have shown (Table S10), neither an aqueous extract nor several different types of organic extracts of the particles were mutagenic. Thus, our data indicate that whatever organics were condensed on the particulate fraction and that were extractable by any of the various solvents we used, none were of sufficient concentration or composition to be mutagenic. Based on this, we have concluded that all of the mutagenicity we observed was due to organics in the gas phase.
Comparison to Other Atmospheres and Agents
Using the method described elsewhere33 to measure the concentration of the gas-phase air pollutant PAN, we found that PAN was present in SA-PM at 233 ± 34 ppbv ± SD and in SA-O3 at 267 ± 17 ppbv ± SD ppb. We have shown previously that exposures of strain TA100 to PAN at ~200–300 ppb for 10–20 h were mutagenic23, 24, 30 However, PAN produces a unique mutation spectrum, consisting of 10% tandem-base mutations (CC → AA),23 which neither SA-PM nor SA-O3 induced. Thus, we conclude that in the complex mixture of these atmospheres, PAN may have played little role in the mutagenicity of these two atmospheres. The absence of PM mutagenicity from SA-PM was unique given that all PM from polluted air tested to date has been mutagenic.3 Thus, the PM we generated was not reflective of that from typical polluted air, whose mutagenicity is highly associated with PAHs and nitroarenes.3 The likely due to the absence of combustion emissions in our SAs.
We have generated atmospheres previously from single, primary reactants and evaluated their mutagenicity in TA100.25, 27, 30 A comparison shows that SA-PM and SA-O3 had mutagenic potencies similar to that of an atmosphere initiated by propylene, whereas atmospheres initiated by either toluene or acetaldehyde had greater mutagenic potencies (Table S16). Although the reaction conditions were somewhat different among these studies, the results indicate that atmospheres initiated by single compounds can be as mutagenic as those initiated by a complex mixture such as gasoline, which is what we used to initiate SA-PM and SA-O3.
There are ~12 studies on the mutagenicity of the gas phase of polluted air, and all were done using complex mixtures consisting of organic extracts of XAD-2 or polyurethane foam in the Salmonella mutagenicity assay.3 Most found that these real-world gas-phase extracts exhibited both direct and indirect mutagenic activity, whereas most of the SAs exhibited only direct-acting activity. In addition, the mutagenic activity of the gas-phase real-world extracts was less than or equal to that of the PM, whereas the PM2.5 from SA-PM was not mutagenic.
These studies on extracts of gas-phase organics from real-world air samples indicate that the gas phase plays a substantial role in the mutagenicity of air overall, perhaps contributing as much as that of PM.3 The finding of S9-dependent mutagenicity in most of the real-world gas-phase samples suggests the possibility that PAHs and other components may have broken through the filter and onto the polyurethane foam or XAD resin in those studies. However, our atmospheres did not contain combustion emissions, which are present in real-world air and can require S9.3 Nonetheless, both these studies of real-world extracts of the gas phase of air, as well as our studies of simulated atmospheres42 (Table S16) show that much, if not most, of the mutagenicity of the gas phase of air is direct-acting. Because the results of these published studies of extracts of real-world air samples were expressed as rev/m3, we cannot compare those results to our gas-phase mutagenicity data, which are expressed as (rev/h)/(mgC/m3).
Studies have shown that the gas-phase mutagenicity of polluted air is likely due to the formation of mutagenic aliphatic and aromatic nitrogen-containing compounds (nitro-PAH lactones) that are formed when organic or non-organic compounds are exposed to NO and UV.12 For example, nitrous acid is readily photo-oxidized to hydroxyl radicals, which can then initiate the atmospheric transformation process.13 Studies also have found associations between the mutagenicity of the gas phase of polluted air and the PAH concentrations in the gas phase43 as well as the likely nitrogen fixation of arenes produced by photo-oxidation.44
Mutation Spectra
The mutation spectra of SA-PM and SA-O3 were significantly different from that of the gas-phase air pollutant PAN23 and organic extracts of PM2.5 from polluted air in TA10045 (P < 0.001). The only known agent that produces a mutation spectrum similar to SA-PM and SA-O3 is the plant-derived dietary supplement, angelicin46 (P = 0.17). Thus, our SAs were otherwise unique in terms of the proportions of the classes of mutations they induced. How reflective this is of the gas phase of real-world polluted air is unknown because such data have not been reported.3
On average the SAs produced a greater proportion of C → A than C → T mutations, and this proportionality is also found in the P53 gene in lung tumors from non-smokers, whose cancers are possibly linked to air pollution47 (Table S17); in contrast, C → T mutations are found predominantly in the P53 gene of lung tumors from smokers and are induced by cigarette smoke48 or PM2.545 from polluted air in TA100 (Table S17). Thus, to the extent that the gas phase of the atmospheres we have generated reflects that of typical polluted air, our data suggest that the gas phase of polluted air is directly mutagenic and that a predominant class of mutation induced by the gas phase is the predominant class found in lung tumors of non-smokers, where air pollution may have played a role in the tumor induction. This implicates a potential role of the gas phase of polluted air in lung cancer associated with air pollution. However, due to a paucity of data, the gas phase has not been used to evaluate the carcinogenicity of polluted air; only PM has been used for such an evaluation.3
An Air Index for Mutagenicity
The air index we used (AQHI) to design this study was not developed to assess air with concentrations of pollutants as high as we had in our atmospheres or to assess air mutagenicity. Nonetheless the two atmospheres, which had similar AQHI values, induced similar mutation spectra in terms of the proportion of mutational classes, and they had mutagenic potencies that were associated with the partitioning of the organics between the particulate and gas phase. Although not perfect, such results suggest that an AQHI as used here might be predictive of the mutagenicity of the gas phase of various atmospheres. Perhaps with further refinement, an index could be developed that would be predictive of a variety of health effects of polluted air and would distinguish subtle differences in health effects among a variety of air sheds.
Among thousands of samples of ambient PM from around the world that have been studied for mutagenicity, the mutagenic potency per mass of PM varied by only ~1 order of magnitude,3 suggesting that the general mix of organics in PM is somewhat similar worldwide. In contrast, the mutagenicity of air when expressed per cubic meter of air varied 5 orders of magnitude.3 This indicates that it is largely the concentration of PM per cubic meter of air, not so much the composition of the PM, that accounts for the variability in air mutagenicity around the world.
Although there are limited data on the mutagenicity of the gas phase of polluted air,3, 12, 13 previous (Table S16) and new42 work from our laboratory suggest that a somewhat similar mix of secondary reaction products may result from the photo-oxidation of a range of primary reactants. Thus, the mutagenic potencies of simulated atmospheres vary a little over 1 order of magnitude42 (Table S16).
Perhaps like PM, the mutagenicity of the gas phase is due largely to the variable concentration of the same several hundred atmospheric transformation products worldwide.42 Considering studies on the mutagenicity of extracts of the gas phase of ambient air3 and of simulated atmospheres initiated either by single compounds25, 27, 31 or a complex mixture (gasoline) in this study, it appears that secondary reaction products, which are typically not monitored or regulated, may account for much of the direct-acting mutagenicity of the gas phase of ambient air.
Supplementary Material
Acknowledgments
This study was funded in part by an Oak Ridge Institute for Science and Education (RISE) postdoctoral fellowship to JZ. This research was funded by the Intramural Research Program of the Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC. We thank Drs. Jeffrey Ross and Brian Chorley (U.S. EPA) for their helpful comments on the manuscript. This article was reviewed by the National Health and Environmental Effects Research Laboratory, U.S. EPA, and approved for publication. Approval does not signify that the contents reflect the views of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.Gaffney JS, Marley NA. Atmospheric chemistry and air pollution. TheScientificWorldJournal. 2003;3:199–234. doi: 10.1100/tsw.2003.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sexton KG, Jeffries HE, Jang M, Kamens RM, Doyle M, Voicu I, Jaspers I. Photochemical products in urban mixtures enhance inflammatory responses in lung cells. Inhalation toxicology. 2004;16(Suppl 1):107–114. doi: 10.1080/08958370490443196. [DOI] [PubMed] [Google Scholar]
- 3.IARC. IARC monographs. France: World Health Organization International Agency for Research on Cancer; 2016. Outdoor air pollution; pp. 1–449. [Google Scholar]
- 4.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 5.Brook RD. Cardiovascular effects of air pollution. Clinical science (London, England: 1979) 2008;115(6):175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 6.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environmental research. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Quality, Atmosphere & Health. 2013;6(1):47–56. [Google Scholar]
- 8.Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. American journal of respiratory and critical care medicine. 2013;187(7):721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council. Toxicity testing in the 21st century: A vision and a strategy. National Academies Press; 2007. [Google Scholar]
- 10.Tokiwa H, Morita K, Takeyoshi H, Takahashi K, Ohnishi Y. Detection of mutagenic activity in particulate air pollutants. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1977;48(2):237–248. doi: 10.1016/0027-5107(77)90165-8. [DOI] [PubMed] [Google Scholar]
- 11.Talcott R, Wei E. Airborne mutagens bioassayed in Salmonella typhimurium. Journal of the National Cancer Institute. 1977;58(2):449–451. doi: 10.1093/jnci/58.2.449. [DOI] [PubMed] [Google Scholar]
- 12.Claxton LD, Matthews PP, Warren SH. The genotoxicity of ambient outdoor air, a review: Salmonella mutagenicity. Mutation Research/Reviews in Mutation Research. 2004;567(2):347–399. doi: 10.1016/j.mrrev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutation Research/Reviews in Mutation Research. 2007;636(1):95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.de Bruijne K, Ebersviller S, Sexton KG, Lake S, Leith D, Goodman R, Jetters J, Walters GW, Doyle-Eisele M, Woodside R, Jeffries HE, Jaspers I. Design and testing of electrostatic aerosol in vitro exposure system (EAVES): an alternative exposure system for particles. Inhal Toxicol. 2009;21(2):91–101. doi: 10.1080/08958370802166035. [DOI] [PubMed] [Google Scholar]
- 15.Lichtveld K, Ebersviller SM, Sexton KG, Vizuete W, Jaspers I, Jeffries H. In vitro exposures in diesel exhaust atmospheres: Resuspension of PM from filters versus direct deposition of PM from air. Environmental Science & Technology. 2012;46(16):9062–9070. doi: 10.1021/es301431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakand S, Hayes A. Troubleshooting methods for toxicity testing of airborne chemicals in vitro. Journal of pharmacological and toxicological methods. 2010;61(2):76–85. doi: 10.1016/j.vascn.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Zavala J. UNC Electronic Theses and Dissertations. University of North Carolina; Chapel Hill: 2014. Development of an electrostatic air sampler as an alternative method for aerosol in vitro exposure studies. [Google Scholar]
- 18.Aufderheide M, Gressmann H. A modified Ames assay reveals the mutagenicity of native cigarette mainstream smoke and its gas vapour phase. Experimental and Toxicologic Pathology. 2007;58(6):383–392. doi: 10.1016/j.etp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Aufderheide M, Gressmann H. Mutagenicity of native cigarette mainstream smoke and its gas/vapour phase by use of different tester strains and cigarettes in a modified Ames assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2008;656(1):82–87. doi: 10.1016/j.mrgentox.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Aufderheide M, Scheffler S, Mohle N, Halter B, Hochrainer D. Analytical in vitro approach for studying cyto- and genotoxic effects of particulate airborne material. Analytical and bioanalytical chemistry. 2011;401(10):3213–3220. doi: 10.1007/s00216-011-5163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilford J, Thorne D, Payne R, Dalrymple A, Clements J, Meredith C, Dillon D. A method for assessment of the genotoxicity of mainstream cigarette-smoke by use of the bacterial reverse-mutation assay and an aerosol-based exposure system. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2014;769:20–28. doi: 10.1016/j.mrgentox.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Claxton LD, de Umbuzeiro AG, DeMarini DM. The Salmonella mutagenicity assay: the stethoscope of genetic toxicology for the 21st century. Environmental health perspectives. 2010;118(11):1515. doi: 10.1289/ehp.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeMarini DM, Shelton ML, Kohan MJ, Hudgens EE, Kleindienst TE, Ball LM, Walsh D, de Boer JG, Lewis-Bevan L, Rabinowitz JR, Claxton LD, Lewtas J. Mutagenicity in lung of big Blue((R)) mice and induction of tandem-base substitutions in Salmonella by the air pollutant peroxyacetyl nitrate (PAN): predicted formation of intrastrand cross-links. Mutation research. 2000;457(1–2):41–55. doi: 10.1016/s0027-5107(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 24.Kleindienst TE, Shepson PB, Smith DF, Hudgens EE, Nero CM, Cupitt LT, Bufalini JJ, Claxton LD. Comparison of mutagenic activities of several peroxyacyl nitrates. Environ Mol Mutagen. 1990;16(2):70–80. doi: 10.1002/em.2850160204. [DOI] [PubMed] [Google Scholar]
- 25.Dumdei BE, Kenny DV, Shepson PB, Kleindienst TE, Nero CM, Cupitt LT, Claxton LD. MS/MS analysis of the products of toluene photooxidation and measurement of their mutagenic activity. Environ Sci Technol. 1988;22(12):1493–8. doi: 10.1021/es00177a017. [DOI] [PubMed] [Google Scholar]
- 26.Shepson PB, Kleindienst TE, Nero CM, Hodges DN, Cupitt LT, Claxton LD. Allyl chloride: the mutagenic activity of its photooxidation products. Environ Sci Technol. 1987;21(6):568–73. doi: 10.1021/es00160a007. [DOI] [PubMed] [Google Scholar]
- 27.Shepson PB, Kleindienst TE, Edney EO, Nero CM, Cupitt LT, Claxton LD. Acetaldehyde: the mutagenic activity of its photooxidation products. Environ Sci Technol. 1986;20(10):1008–13. doi: 10.1021/es00152a007. [DOI] [PubMed] [Google Scholar]
- 28.Kleindienst TE, Shepson PB, Edney EO, Claxton LD, Cupitt LT. Wood smoke: measurement of the mutagenic activities of its gas- and particulate-phase photooxidation products. Environ Sci Technol. 1986;20(5):493–501. doi: 10.1021/es00147a009. [DOI] [PubMed] [Google Scholar]
- 29.Shepson PB, Kleindienst TE, Edney EO, Cupitt LT, Claxton LD. The mutagenic activity of the products of ozone reaction with propylene in the presence and absence of nitrogen dioxide. Environ Sci Technol. 1985;19(11):1094–8. doi: 10.1021/es00141a013. [DOI] [PubMed] [Google Scholar]
- 30.Kleindienst TE, Shepson PB, Edney EO, Claxton LD. Peroxyacetyl nitrate: measurement of its mutagenic activity using the Salmonella/mammalian microsome reversion assay. Mutat Res. 1985;157(2–3):123–8. doi: 10.1016/0165-1218(85)90106-5. [DOI] [PubMed] [Google Scholar]
- 31.Kleindienst TE, Shepson PB, Edney EO, Cupitt LT, Claxton LD. Mutagenic activity of the products of propylene photooxidation. Environ Sci Technol. 1985;19(7):620–7. doi: 10.1021/es00137a007. [DOI] [PubMed] [Google Scholar]
- 32.Stieb DM, Burnett RT, Smith-Doiron M, Brion O, Shin HH, Economou V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. Journal of the Air & Waste Management Association. 2008;58(3):435–450. doi: 10.3155/1047-3289.58.3.435. [DOI] [PubMed] [Google Scholar]
- 33.Krug JD, Lewandowski M, Offenberg JH, Turlington JM, Lonneman WA, Modak N, Krantz QT, King C, Gavett SH, Gilmour MI, DeMarini DM, Kleindienst TE. The Photochemical conversion of surrogate emissions for use in toxicological studies: role of particulate- and gas-phase products. Environmental Science & Technology. 2018 doi: 10.1021/acs.est.7b04879. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutlu E, Warren SH, Ebersviller SM, Kooter IM, Schmid JE, Dye JA, Linak WP, Gilmour MI, Jetter JJ, Higuchi M. Mutagenicity and pollutant emission factors of solid-fuel cookstoves: comparison with other combustion sources. Environmental health perspectives. 2016;124(7):974. doi: 10.1289/ehp.1509852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YH, Warren SH, Krantz QT, King C, Jaskot R, Preston WT, Hays MD, Landis MS, Higuchi M, DeMarini DM, Gilmour MI. Mutagenicity and lung toxicity of smoldering versus flaming emission from various biomass fuels: implications for health effects from wildland fire. Environmental Health Perspectives. 2018 doi: 10.1289/EHP2200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutation Research/Environmental Mutagenesis and Related Subjects. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 37.Ebersviller S, Lichtveld K, Sexton K, Zavala J, Lin YH, Jaspers I, Jeffries H. Gaseous VOCs rapidly modify particulate matter and its biological effects–Part 1: Simple VOCs and model PM. Atmospheric Chemistry and Physics. 2012;12(24):12277–12292. doi: 10.5194/acpd-12-5065-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMarini DM. Influence of DNA repair on mutation spectra in Salmonella. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000;450(1):5–17. doi: 10.1016/s0027-5107(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 39.Hargrove MM, Snow SJ, Luebke RW, Wood CE, Krug JD, Krantz QT, King C, Copeland CB, McCullough SD, Gowdy KM, Kodavanti UP, Gilmour MI, Gavett SH. Effects of simulated smog atmospheres in rodent models of metabolic and immunologic dysfunction. Environmental Science & Technology. 2018 doi: 10.1021/acs.est.7b06534. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazari MS, Stratford KM, Krantz QT, King C, Krug JD, Farraj AK, Gilmour MI. Comparative cardiopulmonary effects of particulate matter- and ozone-enhanced smog atmospheres in mice. Environmental Science & Technology. 2018 doi: 10.1021/acs.est.7b04880. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratford KM, Haykal-Coates N, Thompson L, Krantz QT, King C, Krug JD, Gilmour MI, Farraj AK, Hazari MS. Early-life persistent vitamin D deficiency alters cardiopulmonary responses to particulate matter-enhanced atmospheric smog in adult mice. Environmental Science & Technology. 2018 doi: 10.1021/acs.est.7b04882. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riedel TP, DeMarini DM, Zavala J, Warren SH, Corse EW, Offenberg JH, Kleindienst TE, Lewandowski M. Mutagenic atmospheres resulting from the photooxidation of aromatic hydrocarbon and NO2 mixtures. Atmospheric Environment. 2018 doi: 10.1016/j.atmosenv.2018.01.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai JH, Being-Hwa P, Ding-Zang L, Lee CC. PAH characteristics and genotoxicity in the ambient air of a petrochemical industry complex. Environment international. 1995;21(1):47–56. [Google Scholar]
- 44.de Pollok FS, Aneja VP, Hughes TJ, Claxton LD. Chemical and mutagenic analysis of volatile organic compounds in Raleigh air samples at three different elevations before, during, and after hurricane gordon. Chemosphere. 1997;35(4):879–893. doi: 10.1016/s0045-6535(97)00130-6. [DOI] [PubMed] [Google Scholar]
- 45.DeMarini DM, Shelton ML, Bell DA. Mutation spectra in Salmonella of complex mixtures: comparison of urban air to benzo [a] pyrene. Environmental and molecular mutagenesis. 1994;24(4):262–275. doi: 10.1002/em.2850240403. [DOI] [PubMed] [Google Scholar]
- 46.Koch WH, Henrikson EN, Kupchella E, Cebula TA. Salmonella typhimurium strain TA100 differentiates several classes of carcinogens and mutagens by base substitution specificity. Carcinogenesis. 1994;15(1):79–88. doi: 10.1093/carcin/15.1.79. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian J, Govindan R. Molecular profile of lung cancer in never smokers. European Journal of Cancer Supplements. 2013;11(2):248–253. doi: 10.1016/j.ejcsup.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeMarini DM, Shelton ML, Levine JG. Mutation spectrum of cigarette smoke condensate in Salmonella: comparison to mutations in smoking-associated tumors. Carcinogenesis. 1995;16(10):2535–2542. doi: 10.1093/carcin/16.10.2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


