Abstract
Radical cyclizations have a rich history in organic chemistry and have been particularly generous to the field of natural product synthesis. Owing to their ability to operate in highly congested molecular quarters, and with significant functional group compatibility, these transformations have enabled the synthesis of numerous polycyclic terpenoid natural products over the past several decades. Moreover, when programmed accordingly into a synthetic plan, radical cascade processes can be used to rapidly assemble molecular complexity, much in the same way nature rapidly constructs terpene frameworks through cationic cyclization pathways. This review highlights recent total syntheses of complex terpenoids (from 2011–2017) employing C–C bond-forming radical cascade sequences.
Introduction
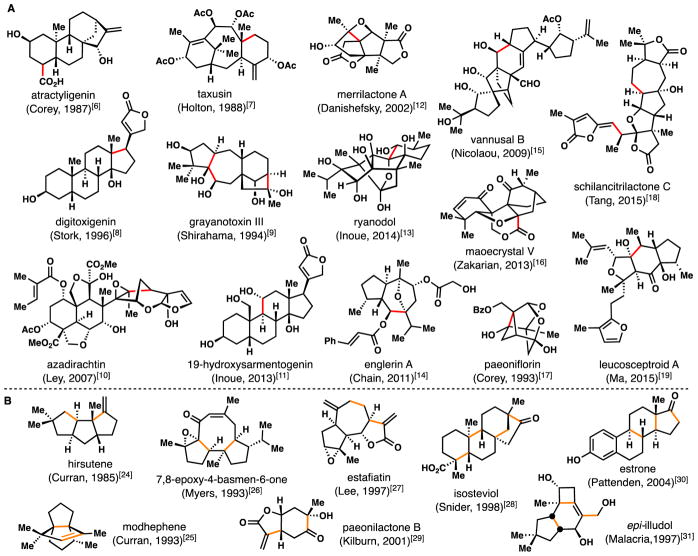
Although once overlooked synthetically–or more likely feared–organic reactions proceeding via radical intermediates are now embraced and incorporated into nearly every facet of modern organic synthesis.1–4 While the evolution from physical organic chemistry peculiarity to synthetic method required decades, by the 1980s the radical’s time had come for inclusion into the ultimate synthetic methodology testing ground–natural product synthesis.5 Accordingly, the last three decades have witnessed a profusion of total syntheses employing radical cyclization reactions. Owing to complex and stereochemically-rich polycyclic ring systems, terpene natural products in particular have proven to be ideal candidates for radical cyclization reactions. Figure 1A highlights an assortment of architecturally diverse terpene natural products that have employed radical cyclizations as key steps over the past thirty years.6–19 Many of these natural products are some of the most synthetically challenging terpenoids ever assembled by synthetic chemists and in many cases, one could argue that radical chemistry enabled their chemical syntheses. In many instances, this was due to the ability of radicals to form highly congested C–C bonds with functional group tolerance toward polar functionality–conditions which cationic, anionic, and many metal-mediated methodologies often struggle with.
Figure 1.
Terpenoid natural products prepared using key radical C–C bond forming reactions. A) Selected total syntheses of highly complex targets which have employed a radical cyclization as a key step (C–C bond formed shown in red). B) Selected syntheses which have utilized radical cascade processes to form multiple carbon-carbon bonds in a single step (C–C bonds formed shown in orange.
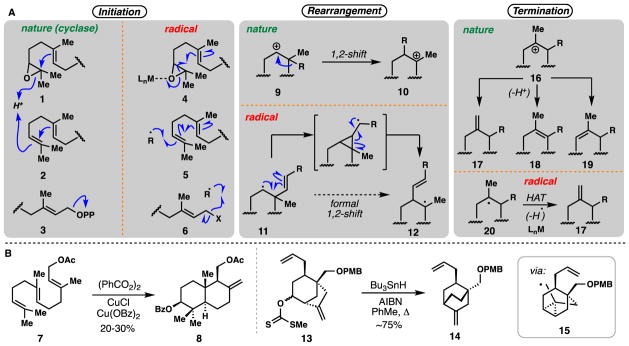
In addition to their potential in forming recalcitrant carbon-carbon bonds, one of the true powers of radicals lies in their ability to take part in cascade processes wherein multiple bonds are formed or broken.20–23 Embodied by Curran’s classic 1985 synthesis of hirsutene,24 radical cascade sequences have featured prominently in terpene syntheses and notable examples are shown (Figure 1B).24–31 Although not reviewed explicitly herein, modes of radical cyclization are diverse1–5,32,33 and often complementary to nature’s cyclase-mediated cationic processes.34 Nevertheless, analogies exist between stages of abiotic radical cyclizations of polyprenyl-derived chains and those of terpene biosynthesis. Salient comparisons are shown in Figure 2 and an excellent 2011 review by Gansäuer and Cuerva discusses this topic in detail.35 Nature’s enzymes frequently initiate cationic cyclizations by protonation of an epoxide or alkene (see 1 and 2) or by ionization of a pyrophosphate leaving group (see 3). These processes can be mimicked by transition metal-mediated homolytic epoxide opening (see 4), addition of an existing radical to an alkene (see 5), or homolytic cleavage (C-X scission) to generate an allylic radical (see 6). Breslow’s early report on the benzoylative radical cyclization of geranyl acetate (7) to polycycle 8 serves as a historic example illustrating these aspects of radical terpene biomimicry (Figure 2B).36
Figure 2.
Biosynthesis vs. radical synthesis. A) Key features of terpene biosynthesis that can be mimicked with radical chemistry. B) Selected examples in synthesis.
In a second process, cyclase enzymes often promote the rearrangement of initially formed carbocyclic rings through series of hydride and alkyl migrations (see 9→10).34 While these processes do not have an exact radical equivalent, homoallylic rearrangements serve to formally migrate vinyl units in a 1,2-fashion (see 11→12). A recent example in terpene synthesis comes from Nicolaou’s synthesis of platencin wherein xanthate 13 was converted into [2.2.2] bicycle 14, via the intermediacy of cyclopropane-containing radical 15 (Figure 2B);37 an additional example can be found in the syntheses of andrastin D and terretonin L (Section 3.8).
Finally, cyclase-mediated cationic cascades often terminate with regiospecific proton loss (see 16→17–19), a process which is often difficult to emulate in biomimetic synthesis wherein thermodynamic product ratios are often observed (i.e. 18 and 19 are formed in preference to 17). Radical cyclization involving transitions metals, however, can terminate via a hydrogen atom transfer (HAT) process from less hindered methyl groups, thus affording 1,1-disubstituted alkene products (see 20→17).35,36,38 Breslow’s conversion of 7 to 8 also highlights this process.
While biomimicry can often lead to expedient total syntheses,39–40 radical transformations also open up a wealth of C–C bond disconnections nature does not have in its arsenal–as far as we are aware–and effort has been made to highlight such processes. The syntheses discussed have been selected from the period of 2011-present, a time range complementary to Gansäuer and Cuerva’s insightful report.35 In order to expand the breadth of chemistry discussed, we have also chosen to highlight select total syntheses of meroterpenes (polyketide/terpene hybrids) as well as terpene-derived alkaloids.
2. Ketyl Radical Initiated Cascade Processes
While not utilized by nature in terpene biosynthesis, ketyl radicals, typically formed via single-electron reduction of carbonyl groups, have proven to be highly valuable reactive intermediates in initiating radical cascades in natural product synthesis.41–43 Moreover, since secondary and tertiary alcohols are directly produced during these transformations, such reactions have been particularly useful to the synthesis of hydroxylated terpene natural products. Three representative syntheses employing ketyl radical cascades are discussed below.
2.1. Procter’s synthesis of (+)-pleuromutilin (2013)
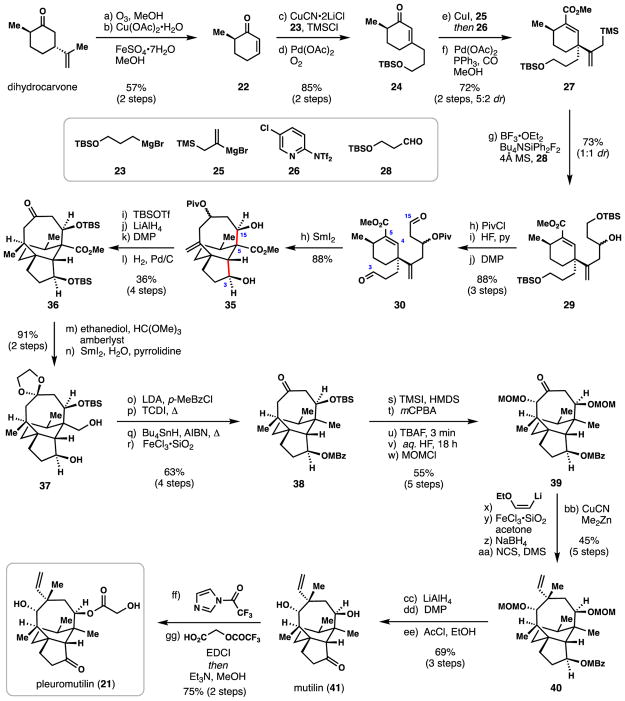
In 1951, Kavanagh and co-workers isolated the potent antibacterial fungal secondary metabolite (+)-pleuromutilin (21) from Pleurotus mutilus (Scheme 1).44,45 Over a time-span of decades, analogues of this unique ribosome-targeting antibiotic entered the clinic, ultimately leading to marketed products for the treatment of veterinary diseases (Denegard® and Econor®) and more recently human skin infections (Altabax®). Two racemic total syntheses of 21 have been reported, the first by Gibbons in 1982,46 and another by Boeckman in 1989.47 Asymmetric routes to pleuromutilin have been slower to transpire, the first being Procter’s 2013 synthesis,48 and very recently, Herzon’s 2017 report.49,50 In addition, a number of approaches to the nucleus of 21 have been documented.51–54 Herein we discuss Procter’s 2013 synthesis which utilized an impressive SmI2-mediated radical cascade to construct two of the three rings found in the target in a single step (Scheme 1).
Scheme 1.
Procter’s total synthesis of (+)-pleuromutilin (21).
Procter’s synthesis commenced with known, two-step conversion of dihydrocarvone to (R)-α-methyl cyclohexenone (22).55 Enone 22 underwent copper-mediated conjugate addition of Grignard reagent 23, and following Saegusa-Ito oxidation, enone 24 was obtained in excellent yield. A second conjugate addition (CuI, 25) followed by enolate trapping with Comin’s reagent (26) produced a vinyl triflate that underwent Pd-catalyzed methoxycarbonylation (Pd(OAc)2, PPh3, CO, MeOH) to afford unsaturated methyl ester 27. Subsequent Lewis acid-mediated allylation of this material with aldehyde 28 afforded 29, and after a protection/deprotection/oxidation sequence, key cyclization precursor 30 was obtained.
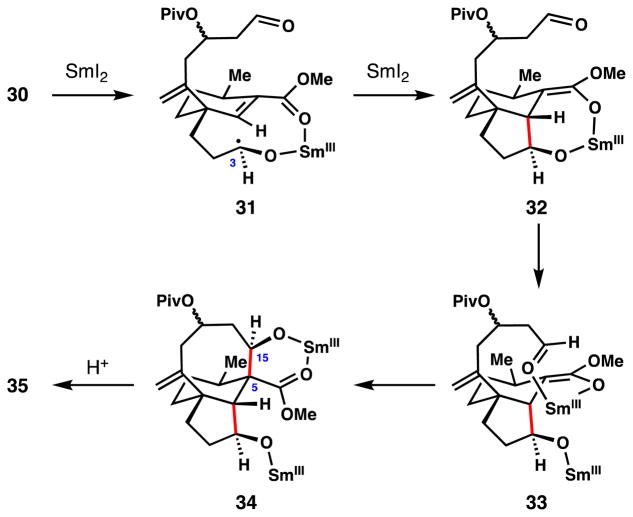
Procter’s key radical cascade sequence was initiated by treating dialdehyde 30 with 2.5 equivalents of SmI2 in THF/t-BuOH. It was proposed that ketyl radical formation begins at C3 and that this species adds, in a conjugate sense, to the neighbouring unsaturated ester, possibly via the samarium chelate shown (see 30→31→32, Figure 3). The resulting radical is then presumably reduced with another equivalent of SmI2 to form samarium enolate 32. Coordination of the samarium (III) center to the proximal aldehyde (see 33) enforces a diastereoselective aldol cyclization furnishing 34 (C-5/C-15 bond formation), and ultimately 35, after protonation. In addition to facile construction of a challenging 8-membered carbocycle in high yield (88%),56 four new stereogenic centers were generated during this powerful cascade.
Figure 3.
Analysis of Procter’s key cascade sequence.
With the core structure of 21 in hand in only eleven steps, the remainder of the synthesis involved adding the final three carbon units as well as adjusting various oxidation states. Diol 35 was first silylated (TBSOTf, Et3N), and then treated with LiAlH4 to remove the pivalate protecting group (Scheme 1). A subsequent Dess-Martin periodinane oxidation and palladium-catalyzed hydrogenation afforded ketone 36 in 36% yield over 4 steps. Conversion of the methyl ester moiety to a requisite methyl group was then achieved in a six-step process. First, the ketone was protected as its ethylene glycol ketal (1,2-ethanediol, HC(OMe)3, amberlyst), a process which also removed the C-3 hydroxyl protecting group. The authors then found that the powerful reductant SmI2/pyrrolidine/H2O could reduce the sterically hindered methyl ester to a primary hydroxyl group in high yield (95% yield). Diol 37 could be mono para-methylbenzoylated at the C-3 hydroxyl position with LDA/p-MeBzCl; the small amount of material protected at the primary hydroxyl position (20%) could be recycled by basic hydrolysis (NaOMe, MeOH). The free primary alcohol was then converted to its corresponding thiocarbamate (1,1-thiocarbonyldiimidazole (TCDI), Δ) thus allowing for a subsequent Barton-McCombie deoxygenation (n-Bu3SnH, AIBN, Δ). After removal of the ketal protecting group, ketone 38 was unveiled in 63% yield over 4 steps from diol 37. The next task in the synthesis was to install the final secondary hydroxyl group found in 21. Regioselective silyl enol ether formation (TMSI, HMDS) allowed for a Rubottom-type oxidation, and following protecting group manipulations, ketone 39 was obtained in 55% yield over 5 steps.
The remaining challenge in the synthesis of pleuromutilin was installation of the key all-carbon quaternary center bearing a vinyl and methyl group. (Z)-2-Ethoxyvinyl lithium was first added to ketone 39 and the resulting tertiary alcohol was dehydrated (FeCl3•SiO2) to reveal a conjugate aldehyde intermediate. 1,2-Reduction of this material with sodium borohydride followed by chlorination (NCS, Me2S) afforded an allylic chloride intermediate. Inspired by Gibbons’ and Boeckman’s prior work,46,47 a stereoselective, Cu-mediated SN2′ displacement with dimethyl zinc furnished the hallmark quaternary center as a single diastereomer. From this point, three more steps were needed to arrive at mutilin (41), which lacks the critical ester side chain found in 21. Lithium aluminium hydride removed the para-methyl benzoate protecting group, allowing for a Dess-Martin oxidation of the resulting secondary hydroxyl group. Finally, the MOM protecting groups were cleaved with in situ generated hydrogen chloride (AcCl, EtOH). With 41 in hand, a two-pot process developed by SmithKline
Beecham researchers was utilized to convert 41 into 21.48 While approximately 35 steps were needed to ultimately furnish the full natural product, the Procter synthesis is a striking example of the power of radical cascades in forging synthetically challenging polycycles in rapid, high-yielding fashion. Not surprisingly this cascade has already been employed in the synthesis of diverse carbocyclic analogs of 21.57
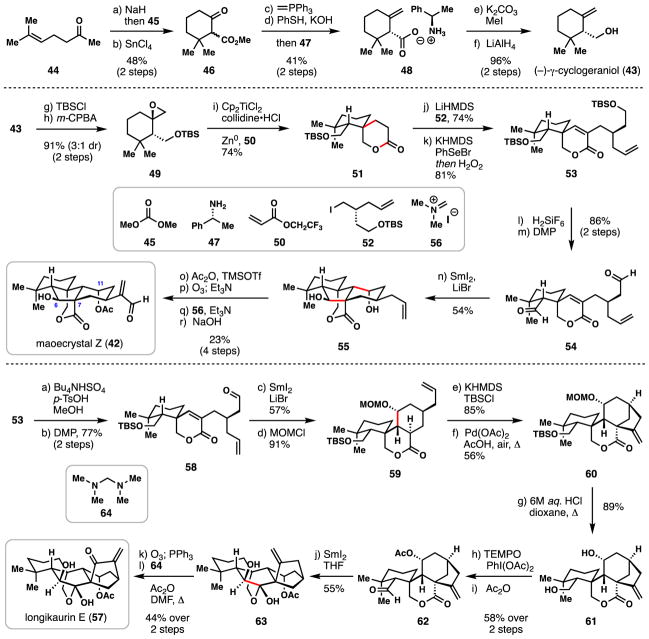
2.2. Reisman’s synthesis of (−)-maoecrystal Z (2011) and (−)-longikaurin E (2013)
In 2006, Xu and coworkers isolated the diterpene natural product maoecrystal Z (42) from the flowering plant Isodon eriocalyx.58 Owing to their longstanding use in traditional Chinese medicine, plants of the Isodon genus have been subject to intense natural product isolation efforts.59 Maoecrystal Z in particular displayed moderate cytotoxicity against several human tumor cell lines (IC50 = 1–3 μg/mL). Moreover, the compact and densely functionalized 6,7-seco-ent-kauranoid skeleton of 42 makes it an attractive target for synthetic chemistry exploration.
In 2011, Reisman and coworkers reported the first total synthesis of 42,60 employing a powerful ketyl radical cascade transformation to assemble the 5,6-fused bicyclic ring system (Scheme 2). (−)-γ-cyclogeraniol (43) was identified as a key building block en route to 42 and was assembled by a previously described route from ketone 44.61 First, treatment of ketone 44 with base (NaH) and dimethylcarbonate (45) furnished an intermediate β-ketoester which underwent smooth SnCl4-mediated cyclization generating ester 46. Methyl Wittig olefination and hydrolysis gave a carboxylic acid product, which could be resolved with chiral amine 47 thus furnishing enantioenriched carboxylate 48. Esterification (MeI, K2CO3) and reduction with lithium aluminum hydride then afforded (−)-γ-cyclogeraniol (43). This material was subsequently silylated (TBSCl) and oxidized (m-CPBA), resulting in epoxide 49 (91%, 3:1 dr) and setting up one of two key radical couplings in the synthesis.
Scheme 2.
Reisman’s syntheses of (−)-maoecrystal Z (42) and (−)-longikaurin E (57).
Treatment of epoxide 49 with Cp2TiCl2, Zn(0), and 2,4,6-collidine•HCl promoted homolytic epoxide opening forming a tertiary radical intermediate.62,63 In analogy to RajanBabu’s findings,64 this species then underwent radical conjugate addition to 2,2,2-trifluoroethylacrylate (50) subsequently forming lactone 51 in excellent yield (74%) and as a single diastereomer. Notably, electron-deficient acrylate 50 proved to be an optimal radical acceptor for this transformation. Concurrently, iodide 52 was prepared in 4 steps by way of Myers’ asymmetric alkylation and then reacted with the lithium enolate of 51.65 An α–selenation/oxidation sequence (KHMDS, PhSeBr; then H2O2) dehydrogenated the lactone moiety giving 53, and following removal of TBS groups and DMP oxidation, dialdehyde 54 was obtained, setting the stage for the key radical cascade cyclization.
Reminiscent of Procter’s radical cascade (Scheme 1),48 treatment of 54 with SmI2/LiBr triggered ketyl radical formation and subsequent addition to the α,β-unsaturated lactone, correctly setting the C-11 stereocenter in the process. Following radical reduction, a stereoselective aldol reaction ensued, also constructing both the C-7 and C-6 centers with the proper configurations. Overall, an impressive 54% yield of a single diastereomer was obtained in this cascade sequence; mono-cyclized products proved to be the major side products identified.
Diol 55 was then globally acetylated (Ac2O, cat. TMSOTf) and the allyl fragment cleaved via ozonolysis. The resulting aldehyde (not shown) was α-methylenated using Eschenmoser’s salt (56), and following deacetylation of the C-6 hydroxyl group with sodium hydroxide, maeocrystal Z (42) was obtained in 38% yield, along with fully deacetylated product (24%) and mono C-11 deacetylated product (24%). Overall, the synthesis of maeocrystal Z required only 18 steps from known ketone 44. Notably, the Ti(III)-mediated spirolactonization and the ketyl radical cascade transformation swiftly built the carbon network of 42 in highly stereoselective and concise manner.66
In 2013, Reisman and coworkers also accomplished the total synthesis of longikaurin E (57),67 a biosynthetically-related kaurane-type natural product (Scheme 2). Utilizing common intermediate 53 as a starting point, selective desilylation of the more accessible TBS ether (p-TsOH, n-Bu4NHSO4, MeOH) followed by DMP oxidation afforded cyclization precursor 58. When aldehyde 58 was treated with the aformentioned SmI2/LiBr system, smooth monocyclization ensued (57%) forging tricyclic lactone 59 after hydroxyl group protection. Diverging from their previous work, the authors developed a two-step sequence to construct the hallmark bicyclo[3.2.1]-octane motif characteristic of many ent-kaurenoids. The potassium enolate of ester 59 was first silylated (TBSCl) setting up an oxidative palladium(II)-mediated, 5-exo-trig cyclization. The combination of one equivalent of Pd(OAc)2 and 0.5 equivalents of acetic acid in DMSO at 45 °C under air was found to be optimal in promoting this challenging transformation; under these conditions alkenylated tetracycle 60 was obtained in 48% yield over two steps. Treating 60 with strong protic acid cleaved both protecting groups and delivered diol 61 in good yield. Selective oxidation of the primary hydroxy group with TEMPO/PIDA and acetylation of secondary alcohol (Ac2O) arrived at aldehyde 62, setting the stage for a second essential Sm(II)-mediated reaction. Treating 62 with SmI2 promoted a pinacol-type coupling furnishing the final ring of the target molecule in 55% yield, and importantly, with the correct stereochemistry at the newly-formed hydroxyl group. With the full ent-kaurenoid skeleton in hand, a final ozonolysis and α-methylenation using 64 smoothly produced longikaurin E (57) in only 12 steps from common building block 53.
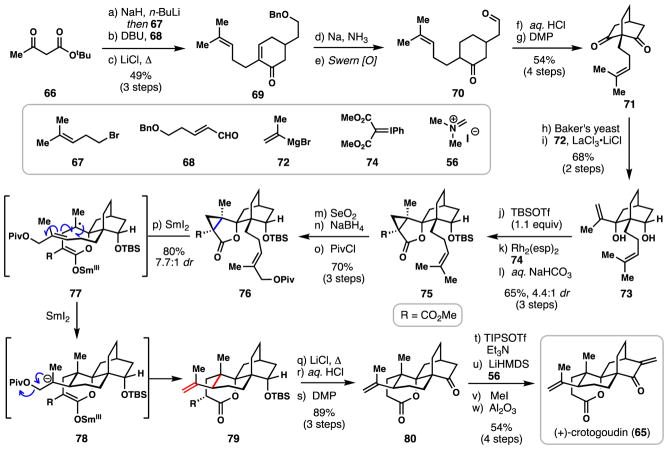
2.3. Carreira’s synthesis of (+)-crotogoudin (2013)
In 2010, crotogoudin (65), a cytotoxic 3,4-seco-atisane diterpenoid, was isolated by Rasoanaivo and coworkers from the extraction of Croton barorum and Croton goudotii.68 This structurally intriguing natural product quickly received attention from the synthetic community, and has already seen elegant total syntheses reported by the groups of Carreira (2013),69 Liu (2015),70 and recently Sarpong (2017).71 Herein we highlight Carreira’s 2013 pathway featuring a creative ketyl radical induced fragmentation/cyclization cascade.
The Carreira synthesis commenced with alkylation of the dianion derived from acetoacetate 66 with homoprenyl bromide (67), a subsequent Michael addition/aldol condensation with enal 68, and a final Krapcho decarboxylation to generate cyclic enone 69 in 49% yield over 3 steps. Dissolving metal reduction of this material (Na, NH3) simultaneously reduced the enone moiety as well as removed the benzyl protecting group giving aldehyde 70 after Swern oxidation. An acid-mediated aldol reaction then efficiently constructed the bicylo[2.2.2]octane core and following treatment with Dess-Martin Periodinane, meso dione 71 was generated in 54% yield over 4 steps. Employing a Baker’s yeast-catalyzed asymmetric ketone reduction,72 this material could be efficiently desymmetrized, and the remaining ketone subjected to a LaCl3-promoted nucleophilic addition reaction with Grignard reagent 72. Reagent approach occurred from the exo face in this transformation, resulting in the formation of 73 as both a single enantiomer and diastereomer. From this point, cyclopropane 75 was strategically assembled, via TBS protection of the less hindered secondary alcohol, rhodium-catalyzed cyclopropanation of the isopropenyl employing 74 (4.4:1 dr), and lactone formation under mildly basic conditions (aqueous NaHCO3, methanol). Position selective allylic oxidation (SeO2), aldehyde reduction, and pivaloylation afforded lactone 76 for use in the key radical cascade.
Treating cyclopropane 76 with 2.5 equivalents of SmI2 in a THF/N,N′-dimethylpropyleneurea (DMPU) mixture generated a ketyl radical species via single-electron reduction of the lactone. This process then initiated radical cyclopropane cleavage (presumably generating 77) and the newly-formed alkyl radical underwent clean 6-exo-trig radical cyclization onto the pendant alkene chain. The final tertiary radical intermediate (not shown) is assumed to be reduced by additional Sm(II) to anion 78 which triggers elimination of the pivalate leaving group and formation of tetracycle 79. Notably this cascade occurred in high yield (80%), displayed good diastereocontrol, and completed construction of the entire carbocyclic skeleton of the natural product.
From polycycle 79, a Krapcho decarboxylation, hydroxyl group deprotection, and DMP oxidation smoothly produced 80 (89% yield over 3 steps). To complete the total synthesis, the authors first protected the lactone as its silyl ketene acetal (TIPSOTf, Et3N) thus allowing for three-step α-methylenation of the cyclic ketone which was found to be less reactive than the lactone otherwise. Ultimately crotogoudin (65) was realized in 54% yield over 4 steps. The Carreira synthesis nicely showcases the strategic incorporation and orchestration of a strained ring cleavage reaction within a radical cascade process.
3 Alkyl Radical Initiated Cascade Processes
Alkyl radicals have historically been the most heavily investigated radical species for use in natural product synthesis. While early studies typically generated these reactive intermediates from alkyl halide and xanthate ester precursors, a variety of more recent metal-mediated processes have greatly expanded the ability of other functional groups to serve as radical generators. Herein we further highlight processes exploiting olefins, epoxides, ketones, and carboxylic acid derivatives as initiators for radical cascade processes.
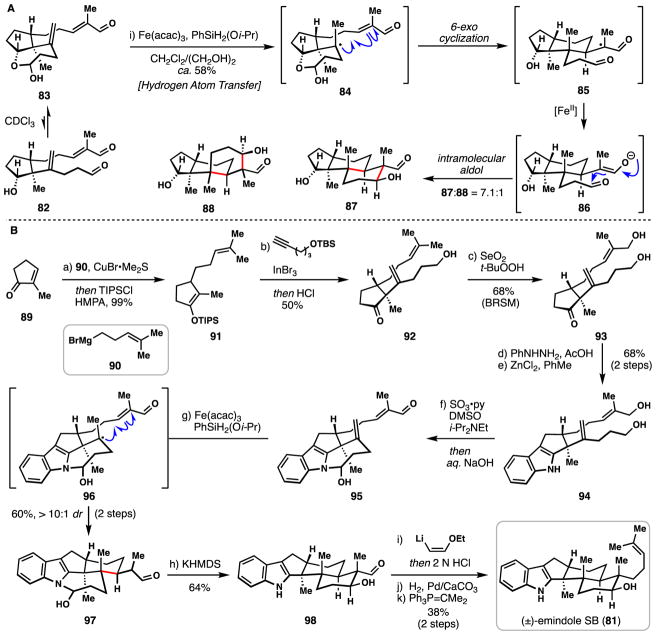
3.1 Pronin’s synthesis of (±)-emindole SB (2015)
(±)-Emindole SB (81) belongs to the fungal-derived family of Paxilline indole diterpene alkaloids,73 a class of natural products to which significant synthetic resources have been devoted.74 Herein, we focus on the inaugural total synthesis of 81 by Pronin and co-workers which explored a radical-polar crossover polycyclization for rapid core assembly.75
Pronin’s overarching strategy towards the construction of the key trans-hexahydroindene core relied upon a radical-polar cyclization cascade, which was initially investigated with model dialdehyde 82 (Scheme 4A). Easily accessible from 2-methylcyclopentenone, this dialdehyde (82) was found to exist as a circa 2:1 mixture favouring its conformationally rigid hemiacetal 83. Under the action of iron(III) acetylacetonate and (isopropoxy)phenylsilane,76 a tertiary radical was generated chemoselectively at the more electron-rich olefin via a hydrogen atom transfer (HAT) process and this species cyclized onto the pendant enal π-system via a 6-exo-trig radical cyclization.38 These events presumably formed radical intermediate 85 which underwent single-electron reduction by the Fe(II) formed after the initial HAT. The resulting enolate (86) then participated in an intramolecular aldol reaction generating products 87 and 88 (~7:1 mixture favouring 87). The rigidifying element provided by the hemiacetal linkage in 83 was important in biasing the product ratio toward 87; in its absence, near equal product ratios were observed.
Scheme 4.
Pronins’s synthesis of (±)-emindole SB (81)
With a blueprint identified for formation of the core structure in hand, the synthesis of (±)-emindole SB was completed in a concise fashion (Scheme 4B). Copper-mediated conjugate addition of Grignard 90 to 2-methylcyclopentenone (89) followed by silylative trapping generated silyl enol ether 91. An indium (III)-mediated alkenylation process coupled 91 with the alkyne shown, resulting in the formation of one of the three challenging all-carbon quaternary centers found in the target. Notably these researchers have further developed this chemistry into an efficient catalytic methodology.77 Following in situ deprotection with hydrochloric acid, alcohol 92 was obtained and then converted to diol 93 by a selenium dioxide-mediated allylic oxidation. A Fischer indole synthesis then coupled phenylhydrazine with 93, resulting in formation of indole 94 in good yield (68%). Parikh-Doering-type oxidation of this material generated an aldehyde intermediate which cyclized to hemiaminal 95, setting the stage for the pivotal radical cyclization. Results from the model study (Scheme 4A) suggested that the hemiaminal locking element would be crucial in enforcing substrate rigidity and enhancing diastereoselectivity. Pleasingly, subjecting 95 to Fe(acac)3/PhSiH2Oi-Pr promoted the formation of intermediate 96 which underwent an analogous cyclization to that of 83. In this system, the stability of the cyclic template prevented final aldol cyclization from occurring in the same pot. Treating isolated product 97 with KHMDS, however, cleanly elicited this step in a stereoselective manner.
To finish the synthesis of (±)-emindole SB (81), a three-step procedure was developed to convert the aldehyde group into the requisite homoprenyl appendage. The addition of 2-ethoxyvinyllithium to 98 followed by acidic quench led to an enal intermediate which was subsequently hydrogenated (Pd/CaCO3, H2). Finally, Wittig olefination (Ph3P=CMe2) gave (±)-emindole SB (81), thus completing an efficient 11-step synthesis. As this synthesis clearly demonstrates, the ability to utilize simple olefins as radical precursors via HAT chemistry opens up a plethora of powerful bond-disconnections in natural product total synthesis.
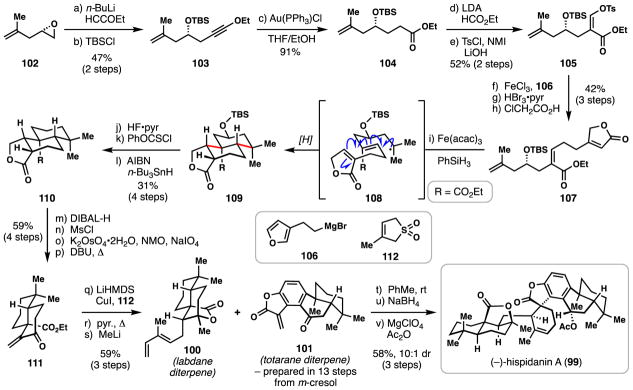
3.2 Liu’s synthesis of (−)-hispidanin A (2017)
Isolated in 2014 from the rhizomes of Isodon hispida, a plant native to southwest China and used in traditional herbal medicine, hispidanin A (99) is one of four dimeric diterpenoids comprised of a labdane (see 100) and totarane (see 101) diterpene scaffold.79 Hispidanins exhibit significant cytotoxicity towards a number of cancer cell lines, and coupled with their obvious structural complexity, such molecules are compelling synthetic targets. Indeed, 99 has already seen two distinct total syntheses by the groups of Liu and the combined efforts of Lan, Gong, and Yang.80,81 Of these, Liu’s synthesis features a radical cascade for the assembly of the labdane-type diene (100) needed for downstream, biomimetic union with the totarane-derived fragment.
Forays into construction of diene 100 began with the nucleophilic opening of epoxide 102 with (ethoxyethynyl)lithium followed by hydroxyl group silylation (TBSCl) to yield 103. Mild hydration of the alkyne moiety with Au(PPh3)Cl allowed for the preparation of ester 104 without formation of a γ-lactone, a side reaction observed when numerous other acids were employed.82 Direct formylation of the lithium enolate of ester 104 with ethyl formate followed by stereoselective (Z)-enol tosylation (TsCl, N-methylimidazole) generated vinyl tosylate 105.83 An iron(III)-catalyzed cross-coupling smoothly merged 105 with Grignard reagent 106, maintaining the olefin geometry in the process. Oxidation of the furan ring (HBr3•pyr) followed by acid-mediated rearrangement afforded butenolide 107, a requisite precursor for the key radical cascade cyclization.
Treating 107 with a similar reagent combination to those utilized by Pronin in the synthesis of 81 (Fe(acac)3/PhSiH3) generated a tertiary radical from the electron-rich alkene, and this species (see 108) underwent a radical polycyclization event (6-endo-trig/6-endo-trig cyclization) leading to tricycle 109. Notably manganese and cobalt-based catalysts proved ineffective for this transformation.78 Four contiguous stereocenters were set during this process and a chair-like transition state was invoked to account for the stereochemistry of the major diastereomer.
The chiral secondary alcohol, which helped to orchestrate this cascade, was then removed via a three-step sequence involving desilylation (HF•pyr) and Barton-McCombie deoxygenation. Reduction of the lactone with diisobutylaluminum hydride and mesylation of the resulting lactol afforded a dihydrofuran intermediate (not shown) which was then oxidatively cleaved (K2OsO4•2H2O/NMO, NaIO4) and subjected to base-promoted elimination, ultimately affording enone 111. Copper-mediated 1,4-addition of the anion derived from sulfone 112 to 111 followed by thermal, chelotropic extrusion of sulfur dioxide forged the requisite diene unit. Finally, addition of methyl lithium triggered a 1,2-addition/lactonization sequence thus completing the synthesis of the key labdane fragment (100). Reacting this material with exocyclic methylene lactone-containing totarane fragment 101 (prepared in 13 steps) led to a smooth [4+2] cycloaddition; this Diels-Alder reaction proceeded at room temperature and with good diastereocontrol (10:1), suggesting a similar process may be involved in the biogenesis of hispidanin A (99). A final reduction of the benzylic ketone and acetylation furnished 99 in 22 steps (longest linear sequence).
The HAT-induced radical cyclization of 107 again clearly showcased the power of this chemistry in stereocontrolled polycycle synthesis, in this case forging a ring system likely made in nature through a cationic cyclization process.34
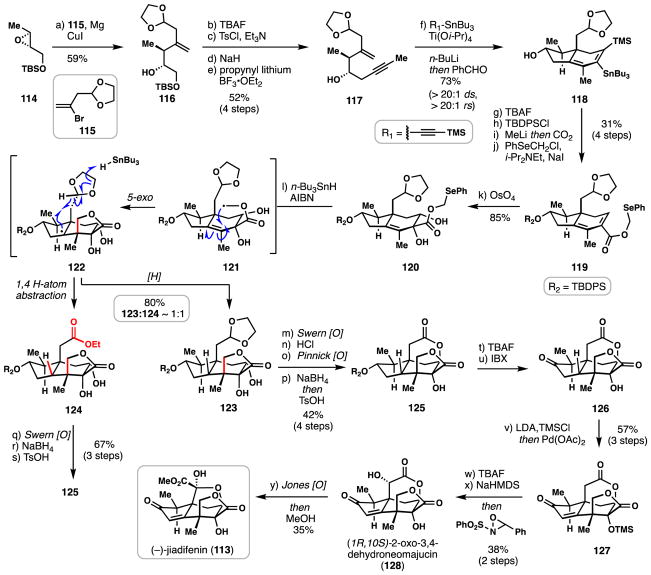
3.3 Micalizio’s synthesis of (−)-jiadifenin (2016)
Illicium sesquiterpenes are a moderately sized family of natural products exclusive to the Illicium genus of plants which includes the well-known “star anise” fruits and spices commonly found in Asian cuisine.84 In recent years, newly isolated members bearing the seco-prezizaane core ring system, including (−)-jiadifenin (113) among others,85,86 have displayed significant in vitro neurotrophic activity–a process thought to stem from modulation of the γ-aminobutyric acid (GABA) receptors.87 Owing to their potential for treating neurodegenerative disease and their compact and highly oxygenated molecular frameworks, various Illicium sesquiterpenes have proven to be highly attractive synthetic targets. (−)-Jiadifenin in particular has already seen multiple syntheses reported to date;88–93 herein we will examine Micalizio’s radical-based route to 113 (Scheme 6).94
Scheme 6.
Micalizio’s synthesis of (−)-jiadifenin (113).
The pathway to 113 commenced with nucleophilic opening of chiral epoxide 114 with vinyl bromide 115 under the mediation of magnesium and copper. The resulting alcohol formed (see 116) was then elaborated to enyne 117 via a four-step sequence consisting of: i) deprotection and mesylation of the primary hydroxyl group, ii) epoxide formation under basic conditions, and iii) subsequent epoxide opening with propynyl lithium. At this stage, assembly of the seco-prezizaane skeleton could be impressively accomplished via a low valent, titanium-mediated [2+2+2] annulation of enyne 117 with the alkynyl stannane shown, demonstrating methodology previously developed by the group.95 Impressively, 118 was formed as nearly a single regio- and diastereomer during this process which also forged a challenging all-carbon quaternary stereocenter. With bicycle 118 in hand, protecting group manipulations followed by lithium-tin exchange and quenching of the resulting lithiate with carbon dioxide accomplished formation of the full sesquiterpene carbon framework. Alkylation of the newly formed carboxylic acid with PhSeCH2Cl provided radical precursor 119 and a subsequent osmium-mediated dihydroxylation established compound 120, setting the stage for the pivotal cyclization.
Subjecting 120 to standard tin-based radical initiation conditions (n-Bu3SnH, AIBN, Δ) lead to formation of α-oxygen stabilized primary radical 121 which underwent a facile 5-exo-trig cyclization onto the neighboring tetrasubstituted alkene; this process generated the hallmark γ-lactone system of jiadifenin. Typically, in many similar reactions the final radical is quenched via a hydrogen atom transfer (HAT) reaction with n-Bu3SnH and indeed this occurs in this system as well, thus forming acetal 123. Quite unexpectedly, however, a similar amount of ethyl ester 124 was also formed presumably by a process wherein the tertiary radical abstracts the weak C-H bond on the neighboring dioxolane ring (via 1,4-hydrogen atom abstraction) initiating ring fragmentation. A final HAT process with n-Bu3SnH then forms the ethyl ester moiety. This process is notable in that the oxidation state of the dioxolane carbon is changed from formally an aldehyde to that of an ester–a process which is beneficial for the ensuing synthetic plan. While the 1:1 ratio of 124:123 could be altered to ~4.7:1 via switching to (TMS)3SiH as the H-atom donor, the efficiency of this process was lower, and for preparative purposes, routes were developed to process both 123 and 124. Acetal 123 could be converted into δ-lactone 125 via a four-step sequence consisting of: i) Swern oxidation of the secondary alcohol, ii) acetonide deprotection and subsequent Pinnick oxidation to a carboxylic acid, iii) stereoselective reduction of the cyclic ketone, and iv) translactonization under acidic conditions. Gratifyingly, the route to 125 from ethyl ester 124 did not require the Pinnick step owing to the aforementioned oxidation state change at the dioxolane carbon.
With 125 in hand, the TBDPS protecting group was removed and the resulting secondary alcohol oxidized (IBX) generating cyclopentanone 126. Saegusa-Ito oxidation of this material constructed the requisite enone system of the target, and following fluoride-mediated desilylation and stereoselective enolate oxidation with Davis’ oxaziridine, the synthesis of the natural product (1S,10R)-2-oxo-3,4-dehydroneomajucin (128) was completed. Known Jones oxidation of this material followed by treatment with methanol rearranged the δ-lactone structure into the hallmark hemiketal motif, thereby completing a synthesis of jiadifenin (113).88–92 While not shown, this work also allowed access to the related sesquiterpene natural product (2S)-hydroxy-3,4-dehydroneomajucin. The Micalizio route to 113 not only showcases the power of radical cyclizations in the construction of hindered, all-carbon quaternary stereocenters, but also nicely highlights the cascade capabilities of these reactive intermediates when weak C-H bonds are present in close spatial proximity.
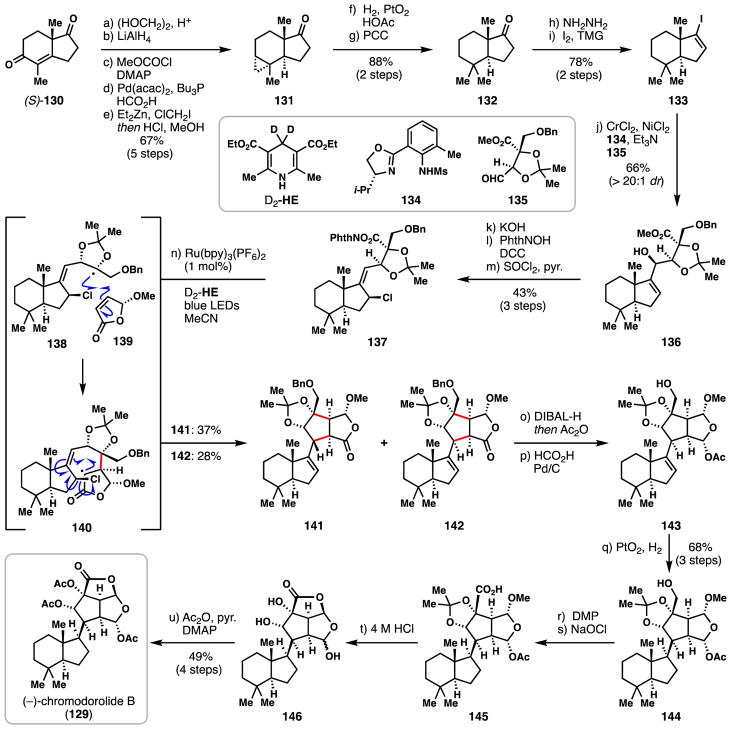
3.4 Overman’s synthesis of (−)-chromodorolide B (2016)
Marine nudibranches of the Chromodoris genus produce a collection of rearranged spongian diterpenes including the synthetically challenging metabolite (−)-chromodorolide B (129) which possesses ten contiguous stereogenic centers.96 While the biological activities of constituents of this family are diverse, special interest has been placed on the Golgi modifying properties of related members and simplified analogues.97 Herein we discuss the only synthetic route to a chromodorolide member, that of (−)-chromodorolide B by Overman and coworkers which employed a striking intermolecular radical addition/cyclization/fragmentation cascade as the key step.98
Beginning with enantioenriched enedione 130, a five-step sequence was used to construct trans-hydrindanone cyclopropane 131 (Scheme 7). First, the cyclopentanone carbonyl was converted to its corresponding dioxolane thus allowing for 1,2-reduction of the nearby cyclohexenone group with LiAlH4 and subsequent carbonate formation. This material then participated in a stereospecific, palladium-catalyzed reductive transposition (Pd(acac)2, Bu3P, HCO2H) setting the key trans-fused 5,6-ring junction.99 The resulting trisubstituted alkene formed was then cyclopropanated under Simmons-Smith conditions arriving at 131 after acidic removal of the dioxolane protecting group. Reductive cleavage of the strained cyclopropane was accomplished via hydrogenation with Adam’s catalyst, and following PCC oxidation ketone 132 was formed. Conversion of this material to vinyl iodide 133 was conveniently accomplished using Barton’s two-step hydrazone iodination protocol. In the first key C-C bond forming step in the synthesis, vinyl iodide 133 was coupled to aldehyde 135 using the venerable Nozaki-Hiyama-Kishi coupling, thus resulting in complex fragment 136. The use of Kishi’s chiral sulfonamide ligand (134) was crucial in obtaining exquisite levels of diastereocontrol during this process (>20:1 dr).100 Ester 136 was hydrolyzed under basic conditions, revealing a hindered carboxylic acid which could be coupled to N-hydroxyphthalimide with N,N′-dicyclohexylcarbodiimide (DCC). Chlorination of the secondary allylic alcohol (SOCl2) with concomitant allylic rearrangement gave activated ester 137 and set the stage for the key radical cascade.
Scheme 7.
Overman’s synthesis of (−)-chromodorolide B (129).
Photoinduced fragmentation of the N-acyloxyphthalimide moiety mediated by the catalyst Ru(bpy)3(PF6)2 generated hindered tertiary radical 138, which added diastereoselectively to chiral butenolide fragment 139.101,102 The resulting α-carbonyl-stabilized radical 140 then underwent non-diastereoselective, intramolecular 5-exo-trig radical cyclization onto the pendant alkene followed by β-fragmentation of the C–Cl bond to deliver pentacyclic products 141 and 142 (in 37% and 28% yields, respectively). Several other coupled, but not fully cyclized, products were also formed; their quantities, however, could be minimized by use of a deuterated Hantzsch ester (D2-HE) additive, which presumably suppresses premature radical reduction. Moving forward, the minor product (lactone 142) was converted to its corresponding lactol with DIBAL, acetylated in situ, and hydrogenated to afford alcohol 143. The key stereocenter at the carbon linking the two complex halves of the rearranged spongian skeleton was then conveniently set by a simple heterogeneous hydrogenation (PtO2/H2). Two-step oxidation of the primary alcohol to a carboxylic acid gave compound 145, and following treatment with acid, lactol 146 was readily formed. Exhaustive acetylation then furnished (−)-chromodorolide B (129) in 21 total steps. The ability to couple complex, stereochemically-rich fragments with high efficiency via decarboxylative radical coupling has recently enabled the synthesis of many complex terpenes.101 When combined with tandem radical cyclization processes, as demonstrated herein, such methodology is nearly unmatched in its power to rapidly generate molecular complexity.
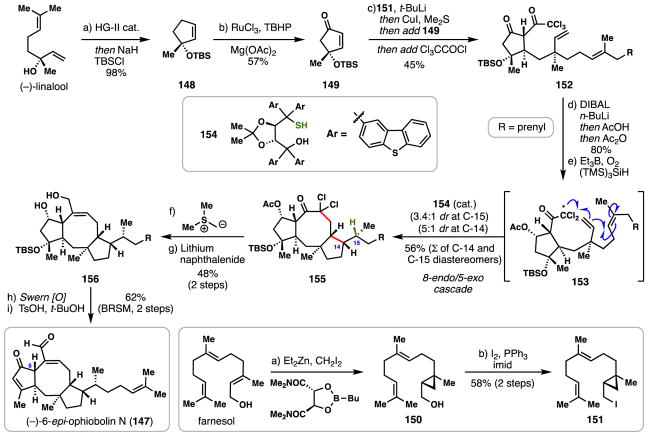
3.5 Maimone’s synthesis of (−)-6-epi-ophiobolin N (2016)
The ophiobolin sesterterpenes are a moderately sized family of natural products possessing complex, stereochemically-rich 5,8,5-fused tricyclic skeletons. While originally isolated and investigated for their phytotoxic effects which negatively impacted several agricultural crops,103 ophiobolins have recently exhibited interesting anti-cancer effects against a number of cancer cell lines.104 To date, only three ophiobolins have succumbed to total synthesis efforts: ophiobolin C by Kishi by 1989,105 ophiobolin A by Nakada in 2011,106 and 6-epi-ophiobolin N (147) by Maimone in 2016 which employed a radical cascade cyclization and is discussed below.107
Utilizing inexpensive (−)-linalool as a chiral pool building block,108 ring-closing metathesis employing Hoveyda-Grubbs second generation catalyst (HG-II) provided alkene 148 after in situ silylation (Scheme 8). Ruthenium-catalyzed allylic oxidation then gave enone 149. In a separate sequence (see lower insert, Scheme 8), trans, trans farnesol was subjected to Charette’s asymmetric cyclopropanation protocol,109 forging enantioenriched alcohol 150. An Appel-type reaction then converted this material into iodide 151. In the first key C–C bond-forming step of the synthesis, iodide 151 was treated with tert-butyllithium inducing anionic cyclopropane ring opening.110 Treating this presumed lithiate intermediate with copper iodide, followed by enone 149 and finally trichloroacetyl chloride led to the formation of 152 via a 1,4-addition/trapping sequence (60%, dr = 3:1). Cyclopentanone carbonyl reduction of 152 (DIBAL/n-BuLi “ate” complex) followed by acetylation of the resulting secondary alcohol set the stage for the key radical cyclization.
Scheme 8.
Maimone’s synthesis of (−)-6-epi-ophiobolin N (147).
Subjecting this material to conditions which promote reductive radical cyclizations ((TMS)3SiH, Et3B/O2 as initiator) led to a tandem 8-endo-trig/5-exo-trig radical cyclization cascade (see 153), thus forging the entire ophiobolin skeleton. Key to the success of this process was the inclusion of bulky thiol 154 as a polarity reversal catalyst,111 which influenced the final radical termination step (HAT process) at the C-15 position (shown in green) to favor the correct isomer. When simple, achiral thiols were utilized, the incorrect C-15 diastereomer predominated (dr ~ 3:2).
With compound 155 in hand, a Corey-Chaykovsky epoxidation completed the synthesis of the full C-25 carbon framework and following reductive, dehalogenative epoxide opening (promoted by lithium naphthalenide) diol 156 was obtained. Double Swern oxidation followed by acid-promoted E1cB elimination of the tertiary silyl ether group then completed a nine-step synthesis of (−)-6-epi-ophiobolin N. The Maimone synthesis of 147 is noteworthy in that it showcases not only the power of radical cascades to rapidly assemble complex terpene ring systems, but also the stereochemical challenges–and potential solutions–that can be encountered during their use.
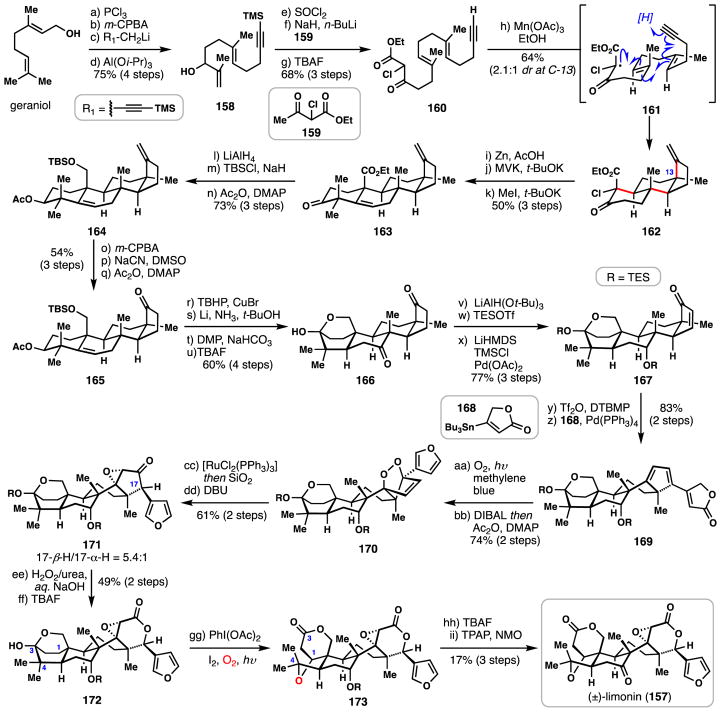
3.6 Yamashita’s synthesis of (±)-limonin (2015)
Limonoids are a series of triterpene derivatives commonly found in citrus fruit. Hundreds of congeners have been cataloged, including limonin (157), which was identified as early as 1841.112 Due to their unique architectures and broad spectrum biological activities, various synthetic approaches have been detailed in the literature.113 The first synthesis of limonin by Yamashita and co-workers featured a powerful tandem radical cascade polycyclization which will be examined below.114
The Yamashita synthesis commenced with conversion of geraniol into polyene 158 via a four-step sequence (Scheme 9). First, chlorination of the allylic alcohol and epoxidation of the terminal alkene afforded epoxygeranyl chloride. Next, a lithium acetylide displacement reaction and aluminum-mediated epoxide elimination gave rise to 158 in excellent yield. A second alcohol chlorination (SOCl2) generated an allylic chloride which could be displaced by the dianion of β-ketoester 159 and following fluoride-mediated deprotection, polyene 160 was formed. Taking inspiration from the work of Snider,115 treatment of 160 with Mn(OAc)3 generated an α-keto radical (see 161) which engaged the polyene system in a radical polycyclization. This cascade presumably terminated by reduction of the final vinyl radical with a hydrogen atom provided by ethanol.115 It is worth noting that while the key all-carbon quaternary center at C-13 was set with 2.1:1 diasteroselectivity during this process, a related all alkene-based oxidative polycyclization employing a Mn(III)/Cu(II) system provided the undesired isomer almost exclusively.
Scheme 9.
Yamashita’s synthesis of (±)-limonin (157).
Moving forward, reductive dechlorination of 162 and subsequent Robinson annulation established the androstane framework, and following a γ-deprotonation/methylation sequence, diene 163 was constructed. Global carbonyl reduction, selective silylation, and a final acetylation afforded acetate 164. The exocyclic methylene carbon was expunged by epoxidation following by treatment with cyanide, a process presumably involving oxirane opening and subsequent ejection of acetonitrile. Reacetylation of this material furnished ketone 165. A copper-catalyzed allylic oxidation then produced a B-ring enone intermediate which could be stereoselectively reduced via dissolving metal reduction, thus establishing the trans-fused A/B ring junction. These conditions also cleaved the acetate protecting group which allowed for Dess-Martin oxidation of the resulting secondary alcohol, and upon treatment with TBAF, cyclic hemiketal 166 was revealed. The hemiketal cage conveniently allowed for chemo- and stereoselective reduction of the B-ring ketone (LiAlH(Ot-Bu)3) and the resulting alcohol product was immediately silylated. A Saegusa-Ito oxidation then provided enone 167. Formation of a vinylogous enol triflate was accomplished by treating 167 with triflic anhydride and 2,6-di-tert-butyl-4-methylpyridine (DTBMP) which permitted a subsequent Pd-catalyzed Stille coupling to proceed with stannane 168. The butenolide-containing product formed (169) then participated in a diastereoselective [4+2] cycloaddition reaction with singlet oxygen (O2, methylene blue) and following conversion of the butenolide to a furan (DIBAL then Ac2O) endoperoxide 170 was obtained. Isomerization of 170 under ruthenium(II) catalysis produced a bis(epoxide) intermediate (not shown), which rearranged to epoxyketone 171 by way of an acid-promoted 1,2-hydride shift. The diasteromeric ratio of 171 to its C-17 epimer could be enhanced by treatment with DBU whereby a 5.4:1 ratio favoring 171 could be established. Advanced intermediate 171 was then converted into lactone 172 by way of a Baeyer-Villiger oxidation and selective deprotection.
In a second key radical-based step in the synthesis, 172 was subjected to Suárez’s oxygen radical generating conditions (PhI(OAc)2, I2, hν) which cleaved the C-3/C-4 bond, resulting in a lactone ring and a carbon-centered radical on C-4. This radical was intercepted with molecular oxygen and postulated to ultimately form an alkoxy radical on C-4 which underwent intramolecular C-H abstraction from the C-1 position (i.e. 1,5 abstraction), iodination, and C–O bond formation leading to 173. Finally, (±)-limonin (157) was obtained after a hydroxyl group deprotection and subsequent oxidation, thus completing a 35-step synthesis. As mentioned in the introduction, radical chemistry can provide orthogonal solutions to complex polycyclizations typically performed in nature using cationic chemistry;34 Yamashita’s route to limonin powerfully demonstrates this reaction dichotomy.
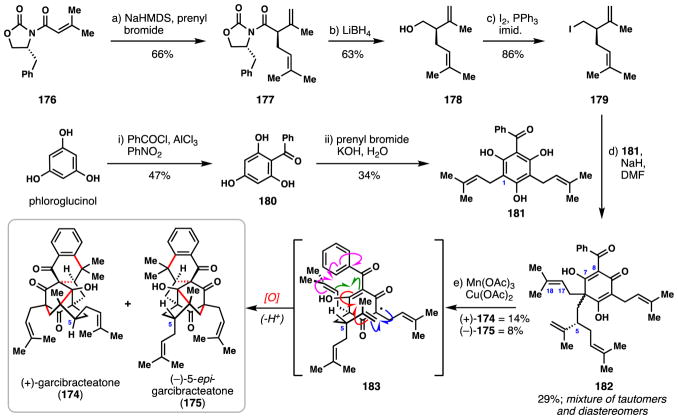
3.7 George’s syntheses of (+)-garcibracteatone (2014)
Embodying a large array of intricate bicyclo[3.3.1]nonane-containing natural products from the plants of the Clusiaceae and Hypericaceae families,116 polycyclic polyprenylated acylphloroglucinols (PPAPs) have garnered intense scrutiny from synthetic chemists over the past two decades, culminating in numerous completed syntheses.117 Garcibracteatone (174) represents a particularly complex problem in PPAP synthesis owing to its compact caged architecture containing seven stereocenters, four of which are all-carbon quaternary centers (Scheme 10). In 2012, George and co-workers reported a shockingly short route to racemic 174 using an elegant, bio-inspired radical cascade.118 Herein we will highlight the group’s 2014 route to (+)-174 which also secured the absolute configuration of the natural product.119
Scheme 10.
George’s total synthesis of (+)-garcibracteatone (174) and (−)-5-epi-garcibracteatone (175).
In order to access enantiopure lavandulyl iodide (179), a key fragment needed for their approach, an asymmetric synthesis was devised using chiral auxiliary methodology (Scheme 10).120 The enolate of Evans oxazolidinone 176 was cleanly alkylated with prenyl bromide affording 177 and upon reduction and iodination, 179 was generated. In a separate sequence, Friedel-Crafts reaction of phloroglucinol with benzoyl chloride gave benzophenone 180 which could be diprenylated (prenylbromide, KOH) to give 181. In the first key step in the synthesis, 179 and 181 were coupled by way of a dearomative phenolic para-alkylation reaction which produced 182 as a mixture of four compounds–two diastereomers each of which were vinylogous acid tautomers. In a single incredible step, (+)-garcibracteatone (174) and (−)-5-epi-garcibracteatone (175) were obtained in 22% combined yield via a Mn(III)-mediated oxidative cyclization cascade. In this process, the reaction of 182 with Mn(III) is believed to first form α-keto radical intermediate 183, which then undergoes 7-endo-trig cyclization onto the pendant lavandulyl chain (see blue arrows). The resulting tertiary radical then participated in two successive 5-exo-trig cyclizations, first with the Δ7,8 enol double bond and then the Δ17,18 olefin (see red and green arrows, respectively). The cascade terminates with coupling of the C-18 carbon to the aromatic ring (see magenta arrows), a process which could involve radical addition to the arene and subsequent oxidation (as depicted) or possibly radical oxidation and a subsequent Friedel-Crafts alkylation. Incredibly, this total synthesis was only five linear steps owing to the ability of the radical cascade to construct four C–C bonds in a single step. Moreover, George’s work also clearly established the absolute configuration of natural garcibracteatone.
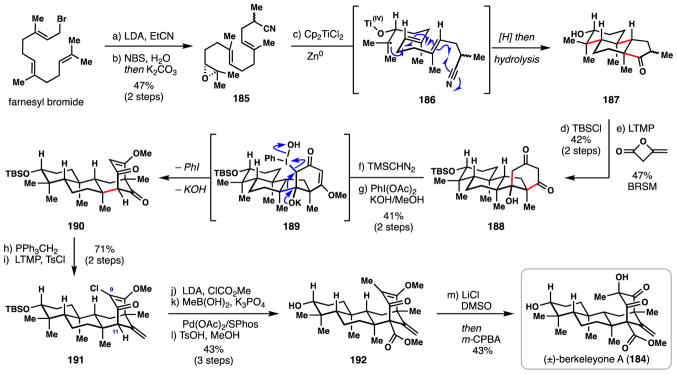
3.8 Maimone’s synthesis of (±)-berkeleyone A (2016) and related meroterpenes (2017)
Extremophiles often possess unique metabolic capabilities and the ability to synthesize structurally and functionally interesting secondary metabolites. The Berkeley Pit (Butte, MT, USA), a former open copper mine turned toxic waste lake is home to the fungal strain Penicillium rubrum which has been found to produce a variety of meroterpene natural products derived from 3,5-dimethylorsellinic acid (DMOA) and farnesyl pyrophosphate, many of which possess anti-inflammatory effects.121,122 Among these is berkeleyone A (184) which features a bicyclo[3.3.1]nonane-containing core skeleton and is likely a biosynthetic precursor to even more complex metabolites within this class (Scheme 11).123 Berkeleyone A has recently succumbed to two unique syntheses by the groups of Maimone and Newhouse, works which also represent the first routes to any complex DMOA-derived meroterpenes.124,125 Maimone’s efforts feature a radical cascade for assembly of a key portion of the polycyclic scaffold and will be showcased below.
Scheme 11.
Maimone’s total synthesis of (±)-berkeleyone A (184).
The synthesis of 184 began with the alkylation of the lithium anion derived from propionitrile with farnesyl bromide, and following regioselective epoxidation–via the intermediacy of a bromohydrin–terminal epoxide 185 was obtained (Scheme 11). Inspired by the work of Fernández-Mateos,126 a titanocene-mediated reductive epoxide cyclization was envisioned to offer rapid access to three of the rings of 184 in a stereocontrolled manner.35,127,128 Towards this end, treating 185 with in situ generated Ti(III) (formed from Cp2TiCl2, Zn0) induced homolytic epoxide opening and cyclization onto the polyene system (see 186). The radical cascade terminated with a 5-exo-dig cyclization onto the nitrile followed by further reduction; following aqueous work-up, the intermediate imine was then hydrolyzed to a ketone resulting in polycycle 187. Protection of 187 (TBSCl) then set the stage for a second key C–C bond-forming step wherein diketene underwent a formal [4+2] annulation reaction with the lithium enolate of protected-187. Methylation of the 1,3-diketone moiety with trimethylsilyldiazomethane afforded a stable vinylogous ester which underwent clean oxidative ring expansion to the bicyclo[3.3.1]nonane framework (see 189) when treated with PhI(OAc)2 under basic conditions.129,130 An ensuing Wittig olefination and chlorination of the C-9 position yielded vinyl chloride 191 from 190. Bridgehead (i.e. C-11) deprotonation of 191 was accomplished with lithium tetramethylpiperidide allowing for a remarkably hindered acylation reaction to occur with methylchloroformate. A Suzuki coupling installed the final carbon atom of the target and acidic hydrolysis of the tert-butyldimethylsilyl ether revealed advanced intermediate 192. A final Krapcho demethylation and stereoselective hydroxylation of the vinylogous acid moiety completed a 13-step synthesis of racemic berkeleyone A.
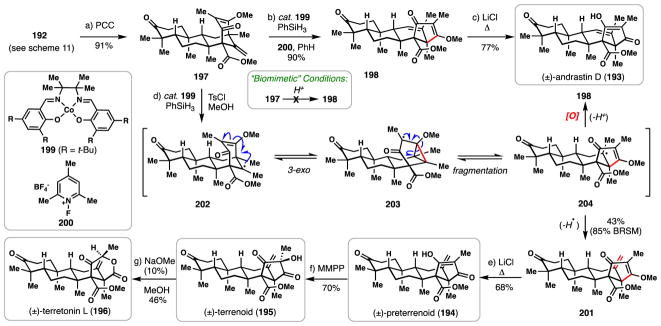
Following disclosure of the synthesis of (±)-berkeleyone A (184), conditions were developed to convert advanced intermediate 192 into the related fungal meroterpenes andrastin D (193), preterrenoid (194), terrenoid (195), and terretonin L (196) (Scheme 12).131 While the biosynthesis of 193–196 presumably involve similar initial steps to berkeleyone A, these metabolites possess a 5,6-fused diketone motif in place of the bicyclo[3.3.1]nonane architecture. It was hypothesized that 197, prepared via PCC oxidation of 192, could be converted into this motif (i.e. 198) via an acid-promoted quasi-biomimetic 1,2-ring shift. While the authors found no success realizing this rearrangement with acid, conditions reported by Shigehisa for cobalt-catalyzed oxidative hydrofunctionalization (cat. 199, PhSiH3, F+ oxidant 200) smoothly delivered 198 in high yield (90%) and as a single alkene isomer.132 A simple demethylation then afforded andrastin D (193). Natural products 194–196 were envisioned to be more challenging to synthesize than 193 owing to the exocyclic olefin present. It was discovered that simply switching oxidant 200 for TsCl afforded 201 in moderate yield and with good selectivity for the less substituted alkene isomer (<5% of 198 was observed).133 The authors hypothesized that these cobalt-catalyzed rearrangements may in fact be examples of the classic homoallyl rearrangement discussed earlier (Figure 2). In this scenario, radical intermediate 202 is initially generated via HAT and undergoes 3-exo-trig cyclization onto the alkene of the vinylogous acid generating cyclopropane 203. Fragmentation of 203 into radical 204 followed by formal loss of a hydrogen atom (HAT) from the less hindered methyl group then delivers 201, the less stable alkene isomer. It is proposed that the more strongly oxidizing conditions containing 200 promote radical oxidation of 204 to a tertiary carbocation, and following proton loss, the more stable isomer (i.e 198) is generated. With 201 in hand, Krapcho demethylation afforded preterrenoid (194) which could be oxidized stereoselectively to terrenoid (195) using magnesium monoperoxyphthalate (MMPP). Terrenoid is believed to be the biosynthetic precursor to the terretonin family and indeed it was found that simple treatment of 195 with NaOMe/MeOH afforded terretonin L (196), possibly by way of a retro-Claisen/intramolecular esterification cascade process.134
Scheme 12.
Radical-based conversion of berkeleyone congeners into andrastin and terretonin frameworks.
The route to berkeleyone A is yet further testament to the ability of radical cyclizations to mimic biosynthetic processes, in this case the opening of an epoxide followed by a polyene cyclization. Moreover, the pathways to 193–196 also highlight an instance where a mechanistically distinct radical reaction was capable of producing a product which biomimetic, cationic chemistry failed to prepare.
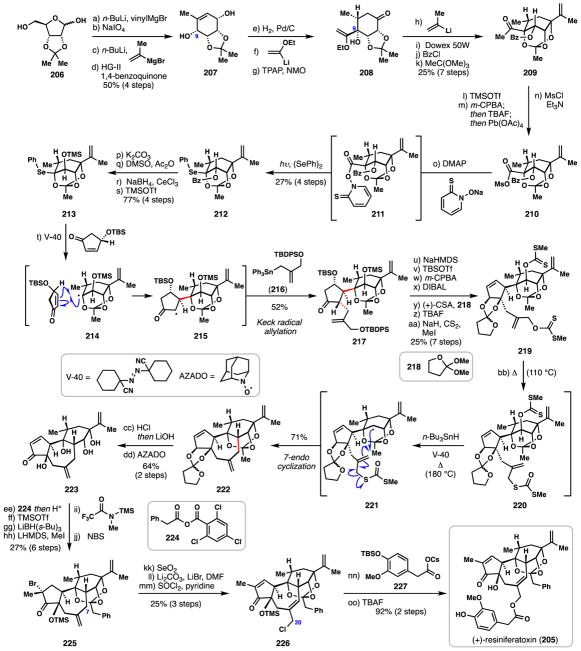
3.9 Inoue’s synthesis of (+)-resiniferatoxin (2017)
The plant family Euphorbiaceae produces an extensive collection of biologically active natural products, most notably the complex tigliane,135,136 and closely related daphnane diterpenes.137 (+)-Resiniferatoxin (205), a potent irritant isolated from Euphorbia resinifera in 1975 is perhaps the flagship daphnane diterpene (Scheme 13).138 Its synthetically challenging tricyclo[9.3.3.0]tetradecane skeleton coupled with potent modulatory properties toward the transient receptor potential vanilloid 1 (TRPV1) receptors have made 205 both a high profile synthetic target and chemical tool for studying pain.139 Although intense effort has been devoted to the chemical synthesis of diterpenes from Euphorbiaceae,136 resiniferatoxin remains the only daphnane natural product prepared by total synthesis; furthermore, Wender’s historic 1997 synthesis140 and Inoue’s very recent 2017 report remain the only fully synthetic routes reported to 205.141 Herein, we explore Inoue’s de novo synthesis of resiniferatoxin utilizing several powerful radical cascades to construct the stereochemically-rich and synthetically challenging polycyclic daphnane ring system.
Scheme 13.
Inoue’s synthesis of (+)-resiniferatoxin (205)
The synthetic pathway to 205 utilized commercially available D-ribose derivative 206 as starting material (Scheme 13). Addition of n-BuLi (to deprotonate acidic functionality) allowed for addition of vinylmagnesium bromide to the unmasked aldehyde to take place. Oxidative diol cleavage (NaIO4) then furnished a hemiacetal intermediate which was again treated with n-BuLi and a Grignard reagent, in this instance isopropenylmagnesium bromide. Finally, the cyclohexene ring was constructed via ring closing metathesis with Hoveyda-Grubbs second-generation catalyst in presence of 1,4-benzoquinone to prevent alkene migration thus forming 207. Interestingly, subjecting 207 to hydrogenation conditions (H2, Pd/C) led to stereoselective alkene isomerization giving a ketone at the C-9 position. Diastereoselective addition of 1-ethoxyvinyl lithium to this material followed by Ley oxidation of the remaining hydroxyl group generated ketone 208. A fourth organometallic addition, this time utilizing 1-methylvinyl lithium, was employed to construct the isopropenyl group of the target. The acetonide group was then removed with acidic resin (Dowex 50W) which also conveniently furnished a methyl ketone from the preexisting vinyl ether group. The intermediate tetraol then underwent site-selective benzoylation, and following treatment with trimethylorthoformate, the three-remaining hydroxyl groups engaged in orthoester formation delivering 209. Reacting methyl ketone 209 with TMSOTf/Et3N afforded an enol ether which could be chemoselectively epoxidized to give an α-hydroxy ketone. Oxidative cleavage of this material with Pb(OAc)4 generated an intermediate carboxylic which upon treatment with MsCl/pyridine formed acyl mesylate 210. Conversion of 210 to Barton ester 211 was conveniently achieved using 2-mercaptopyridine N-oxide sodium salt and DMAP and the somewhat unstable intermediate formed was immediately irradiated with light in the presence of diphenyl diselenide inducing decarboxylative phenylselenation.142 Selenide 212 was then taken through a four-step sequence consisting of desilylation, alcohol oxidation, stereoselective reduction, and protection to generate 213, setting the stage for the key radical cascade.
When 213 was heated in the presence of the radical initiator V-40, deselenation occurred forming oxygen-stabilized bridgehead radical 214. In the presence of the enone shown, a diastereoselective radical conjugate addition took place forging α-keto radical 215. Finally, this radical species participated in a Keck-type radical allylation with stannane 216 delivering ketone 217.143 An impressive 52% overall yield was achieved in this remarkable 3-component radical coupling which forges several sterically hindered C–C linkages.
Several further synthetic manipulations were required in preparation for the 7-membered ring-forming event. From cyclopentanone 217, E1cB elimination of the silyl ether group employing NaHMDS fashioned an enone which was subjected to two-step Rubottom oxidation (TBSOTf then m-CPBA). Stereoselective 1,2-reduction with DIBAL generated a cis-fused diol which could be masked as an oxacyclopentylidene orthoester when reacted with 218 under acidic conditions. Fluoride-mediated global desilylation followed by global xanthate formation (NaH, CS2, MeI) afforded key radical precursor 219.
In a single masterful stroke, simple heating of bisxanthate 219 at 110 °C promoted a clean [3,3]-sigmatropic rearrangement of the allylic xanthate into dithiocarbonate 220. This maneuver was followed by further heating (180°C) in the presence of the radical initiator V-40 and Bu3SnH which generated radical intermediate 221. A diastereoselective 7-endo-trig cyclization subsequently ensued leading to 222 after ejection of the dithiocarbonate group. Given the plethora of intricate chemistry which occurs during this cascade, the 71% yield obtained is quite impressive.
With the daphnane 5,7,6-fused ring system in hand, the authors needed only to append one remaining carbon atom to the terpene framework and interconvert several functional groups to arrive at resiniferatoxin. To this end, global deprotection of 222 and chemoselective oxidation of the allylic alcohol provided enone 223. A six-step sequence involving: i) formation of the hallmark orthoester group via Yamaguchi esterification with 224, ii) TMS protection of the tertiary hydroxyl group, iii) enone 1,4-reduction using LiBH(s-Bu)3, iv) enolate formation and alkylation with MeI, v) silyl enol ether formation using N-methyl-N-(trimethylsilyl)trifluoroacetamide, and vi) NBS-mediated bromination then afforded 225 in 27% over six steps. Next, the authors leveraged SeO2 to install a hydroxyl group at C-7 via regioselective allylic oxidation. Heating this material in the presence of lithium carbonate and lithium bromide induced elimination of the tertiary bromine atom, thereby restoring the cyclopentenone system. Finally, chlorination (SOCl2) of the allylic alcohol with concomitant transposition afforded C-20 allylic chloride 226. To complete the synthesis, the allylic chloride was displaced with cesium carboxylate 227 and the silyl protecting group removed with TBAF. In the end, (+)-resiniferatoxin (205) was constructed in 41 steps. The two radical cascades employed in Inoue’s work are particularly impressive when one considers both the complexity of the substrates utilized, as well as the high diastereoselectivities and yields obtained. It would be difficult to envision a significant number of polar bond-forming events which can craft such complexity so efficiently.
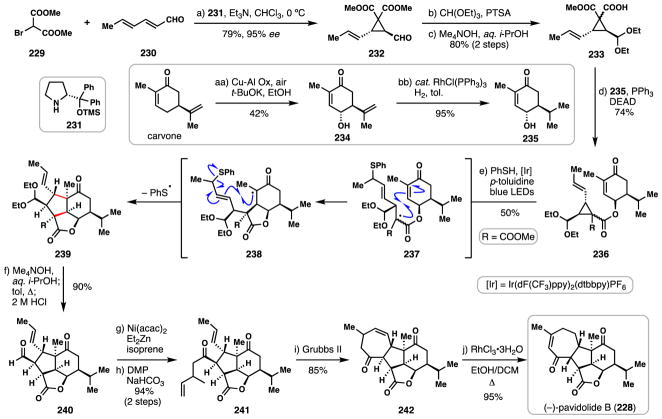
3.10 Gong and Yang’s synthesis of (−)-pavidolide B (2017)
Marine cembranoids are a diverse group of macrocycle-derived diterpenes often produced by various species of coral.144 The soft coral Sinularia pavidai in particular produces the tetracyclic 7,5,5,6-fused cembranoid (−)-pavidolide B (228) which possesses inhibitory activity against various human promyelocytic leukemia cell lines (Scheme 14).145 Gong and Yang’s inaugural 2017 route to this complex natural product featured an impressive radical cascade employing a vinyl cyclopropane cleavage/formal annulation process to forge an advanced tricyclic intermediate and ultimately delivering a short 10-step total synthesis of 228.146
Scheme 14.
Gong and Yang’s synthesis of (−)-pavidolide B (228).
In order to assemble a key cyclopropane fragment, dimethyl bromomalonate (229) and dieneal 230 were merged via a domino Michael addition/alkylation using proline-derived catalyst 231 (Scheme 14).147 The enantioenriched cyclopropane formed (232) was then subjected to acetal formation followed by mild ester monohydrolysis to arrive at carboxylic acid 233 as an inconsequential pair of diastereomers. Concurrently, (+)-carvone was regio- and stereoselectively oxidized to allylic alcohol 234 using copper-aluminum mixed oxide (Cu-Al Ox) in the presence of air and sodium tert-butoxide.148 Hydrogenation of the isopropenyl group in 234 with Wilkinson’s catalyst furnished alcohol 235. The union of acid 233 and alcohol 235 was achieved under Mitsunobu conditions giving intermediate 236 in high yield and setting the stage for the critical radical cascade.
Visible light irradiation of the Ir(III) complex shown in the presence of p-toluidine and thiophenol resulted in the generation of the phenylthiyl radical which added to the alkene of 236 giving radical 237 after cyclopropane ring opening.149–151 This species then underwent diastereoselective addition to the neighboring enone system generating α-keto radical (see 238) which then cyclized back onto the alkene (5-exo-trig process) initially formed during the cyclopropane fragmentation. Finally, expulsion of the phenylthiyl radical delivered the annulated product (239) in 50% yield and impressively as a single diastereomer. With tricycle 239 in hand, the extraneous ester group was removed by hydrolysis to a carboxylic acid (Me4NOH, i-PrOH/H2O) and thermal decarboxylation, and following treatment with HCl to remove the acetal group, aldehyde 240 was generated. Next, a Nickel-catalyzed hydroacylation of 240 with isoprene followed by DMP-oxidation gave rise to olefin metathesis precursor 241 which was cyclized to 242 under the mediation of Grubbs’ second generation catalyst. Finally, RhCl3/i-PrOH isomerized the γ,δ-unsaturated enone into conjugation, thus completing the synthesis of (−)-pavidolide B (228). The Gong and Yang 10-step route to 228 is both elegant and efficient; without question its efficiency stems from both the judicious choice of chiral pool building blocks and the ability to form two rings and four contiguous stereocenters in a single operation via a diastereoselective radical cascade.
4. Alkenyl Radical Initiated Cascade Processes
Radical cyclizations originating at a sp2-hybridized carbon center are less frequently encountered in natural product total synthesis perhaps owing to the rise of modern transition metal-catalyzed Heck and cross-coupling methodology. Nevertheless, notable examples of diverse aryl, alkenyl, and acyl radical cyclization permeate the literature.152–164 As discussed previously with alkyl radicals, tin and silicon-based methods using AIBN decomposition have historically been employed to initiate radical generation, yet modern photoredox methods are increasingly offering new solutions to this problem.149–151 In addition, radical additions to alkynes offer convenient entry into vinyl radicals, an example of which is discussed below.
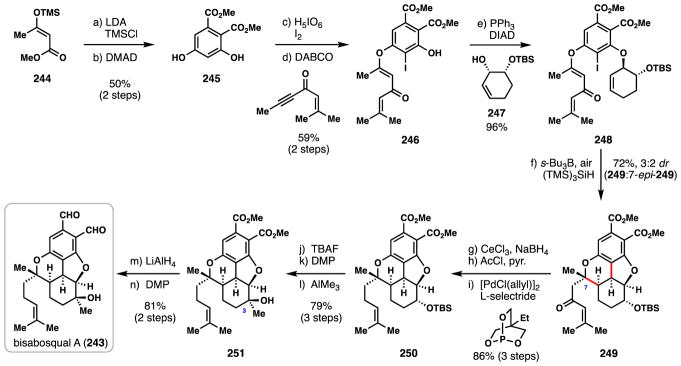
4.1. Parker’s synthesis of (±)-bisabosqual A (2013)
During efforts to discover new microsomal squalene synthase inhibitors, Minagawa and co-workers isolated the polycyclic meroterpene bisabosqual A (243) from a strain of Stachybotrys fungi.165 This natural product features a compact, cis, cis-fused tetracyclic ring system with five contiguous stereogenic centers. The group of Parker reported an efficient 14-step total synthesis of 243 in 2013, in which an aryl radical-induced cascade transformation rapidly forged the core structure.166
Parker’s synthesis began with a 2-step procedure to prepare known phenolic ester 245 which entailed silylation of 244 to produce a diene and a subsequent [4+2] cycloaddition with dimethyl acetylenedicarboxylate (DMAD) (Scheme 15).167 Iodination of 245 followed by a DABCO-catalyzed 1,4-addition to the ynone shown afforded vinylogous ester 246 with high geometric purity. Coupling of 246 with allylic alcohol 247 was achieved under Mitsunobu conditions wherein 248 was produced in outstanding yield.
Scheme 15.
Parker’s synthesis of (±)-bisabosqual A (243).
In the key step of the synthesis–which was inspired by previous synthetic work on morphine–aryl radical generation was initiated with s-Bu3B/air producing an intermediate that underwent a tandem 5-exo-trig cyclization onto the cyclohexene ring, followed by a 6-exo-trig cyclization onto the vinylogous ester.152 The final α-keto radical produced was then terminated via a hydrogen atom transfer reaction with supersilane ((TMS)3SiH). Overall, the tetracyclic product (249) was isolated in 72% yield combined with its C-7 epimer (~3:2 mixture favouring 249). Nevertheless, the creation of two rings and three stereogenic centers in a single step is quite impressive and the minor, unwanted diastereomer (7-epi-249) could be epimerized under basic conditions (1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), then NaHCO3) to give 94% of a 2:1 mixture favouring 249.
From this point, a three-step procedure was developed to remove the extraneous ketone group. Luche reduction and formation of an allylic acetate provided a suitable substrate for a palladium-catalyzed π-allyl reduction giving 250 in 86% overall yield. Subsequent removal of TBS protecting group (TBAF), DMP oxidation, and diastereoselective addition of trimethyl aluminium to the resulting ketone forged tertiary alcohol 251. Finally, the dialdehyde motif was unveiled by global reduction and reoxidation, thus completing the first total synthesis of (±)-243 in only 14 steps and further showcasing the power of radical cascade chemistry in a complex milieu.
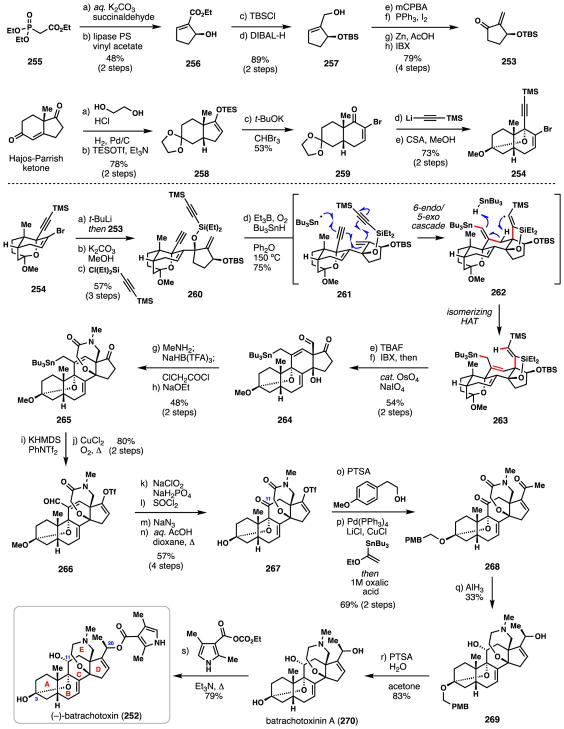
4.2. Du Bois’ synthesis of (−)-batrachotoxin (2016)
The steroidal alkaloid (−)-batrachotoxin (252) is the primary toxic constituent of Colombian poison dart frogs and acts as a potent agonist of voltage-gated sodium channels (NaVs). This acute toxin was first isolated by Witkop and co-workers from the skin extracts of Phyllobates bicolor in 1963,168 and its structure was secured later in 1969 by X-Ray crystallographic analysis of a derivative.169 Driven by its potent biological activity and intriguing chemical structure, Du Bois and co-workers completed a total synthesis of both 252 and its enantiomer in 2016,170 work which represents only the second ever synthesis of this highly challenging target.171 A remarkable tin radical-induced cyclization cascade serves as the cornerstone of this synthetic work, details of which are provided below.
Du Bois’ and co-workers began by assembling key fragments 253 and 254 which would be later merged in convergent fashion (Scheme 16). First, ethyl diethylphosphonoacetate (255) was converted to enantioenriched allylic alcohol 256 by way of a Horner Emmons-type coupling with succinaldehyde and enzymatic resolution (lipase PS).172 Protection of this material (TBSCl) and reduction with DIBAL afforded 257 which was taken through a four-step sequence to arrive at enone 253. These steps entailed epoxidation of the allylic alcohol and Appel reaction, zinc-mediated reductive epoxide opening, and a final IBX oxidation of the resulting secondary alcohol. Using chemistry inspired by previous work from Parson,173 the second key fragment, vinyl bromide 245, was assembled from the Hajos-Parrish ketone. Ketalization and enol ether formation gave 258, which when exposed to in situ generated dibromocarbene, underwent cyclopropanation and ring expansion producing vinyl bromide 259. Addition of lithium (trimethylsilyl)acetylide to 259 followed by treatment with camphor sulfonic acid and methanol led to key bicycle 254.
Scheme 16.
Total synthesis of (−)-batrachotoxin (252) by Du Bois and co-workers.
In a key coupling event, treatment of 254 with t-Buli produced a lithiate which underwent diastereoselective 1,2-addition to chiral cyclopentenone 253. The TMS group was then removed with basic methanol and the tertiary alcohol coupled with the chlorosilane shown resulting in radical cascade precursor 260. Under tributyltin radical generating condition (Et3B, O2, n-Bu3SnH, 150 °C), the unprotected alkyne first reacted with Bu3Sn• leading to formation of a vinyl radical intermediate which participated in a 6-endo-trig/5-exo-dig cyclization cascade (see 261→262). Elevated reaction temperatures favored the desired initial 6-endo process over the competing 5-exo pathway. Finally, allylic hydrogen atom abstraction prior to the final HAT reaction with Bu3SnH afforded isomerized product 263 from vinyl radical intermediate 262.
With the batrachotoxin C-ring assembled, the authors turned toward construction of the homomorpholine E-ring. Global desilylation (TBAF), oxidation of secondary alcohol (IBX), and in situ oxidative cleavage of the mono-substituted alkene afforded aldehyde 264 in 54% yield from 263. One-pot reductive amination of 264 with methylamine, acylation with chloroacetyl chloride, and intramolecular etherification then afforded lactam 265. The D-ring ketone was converted to its corresponding vinyl triflate and in a somewhat unexpected finding, treating this compound with CuCl2 under aerobic conditions afforded aldehyde 266 as opposed to the expected allylic chloride product. A sequence consisting of Pinnick oxidation, acyl azide formation, Curtius rearrangement, and acid-mediated hydrolysis then afforded 267. It should be noted that the somewhat circuitous route to install the C-11 ketone (i.e 265→267) was a result of difficulties encountered in trying to achieve the more direct protodestannylation/oxidative alkene cleavage pathway to this moiety. For purification purposes, the C-3 hemiketal was reprotected by condensing 267 with p-methoxyphenethyl alcohol, and following palladium-catalyzed Stille coupling and vinyl ether hydrolysis, methyl ketone 268 was generated. Treatment of 268 with freshly prepared alane (AlH3) accomplished a remarkable, diasteroselective global carbonyl reduction which set two stereocenters and also forged the crucial cyclic amine. Finally, acid-mediated removal of ketal protecting group furnished batrachotoxinin A (270) which could be esterified at the C-20 position to afford (−)-batrachotoxin (252). Moroever, the authors also prepared (+)-batrachotoxin leading to the identification of this enantiomer as a fairly potent channel antagonist which likely shares some overlapping binding regions to the inner pore cavity as the natural antipode.
The Du Bois route to batrachotoxin is a testament to the power of chemical synthesis in addressing biological questions not answerable using naturally occurring substances alone. Moreover, the power of vinyl radical cyclization cascades to enable the rapid assembly of complex molecular complexity was on full display during this exercise in complex molecule total synthesis.
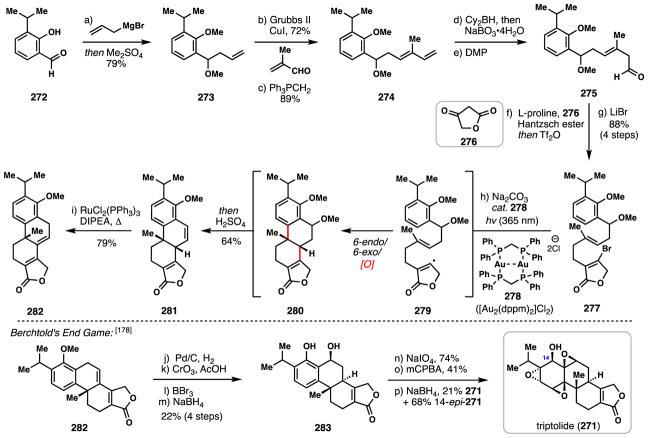
4.3. Barriault’s formal synthesis of (±)-triptolide (2016)
Triptolide (271), a diterpene triepoxide, was isolated in 1972 by Kupchan and coworkers from the Chinese thunder god vine (Tripterygium wilfordii).174 Due to its potent antitumor, anti-inflammatory, and immunosuppressive properties, this natural product has been extensively studied from both a chemical and biological perspective; derivatives of 271 have even entered clinical trials for the treatment of pancreatic cancer.175–177 In 2016, Barriault and co-workers reported a formal synthesis of racemic triptolide using a vinyl radical polycyclization initiated by gold photoredox catalysis.178
Barriault’s synthesis began with allyl magnesium bromide addition to commercially available benzaldehyde derivative 272 and following in situ global methylation, anisole 273 was formed in 79% yield. A four-carbon extension of the homoallyl side chain was achieved via cross metathesis of 273 and methacrolein using Grubbs’ second-generation catalyst followed by Wittig methylenation. The resulting diene (274) was then converted to aldehyde 275 by a hydroboration-oxidation sequence. Proline-catalyzed coupling of 275 with tetronic acid (276), and in situ reduction and triflation delivered a vinylogous acid triflate which could be converted into 277 with LiBr.
The key radical cyclization was initiated by irradiating a solution of 277 and the dimeric gold catalyst [Au2(dppm)2]Cl2 (278) with ultraviolet light (365 nm). Under these conditions, vinyl radical 279 is presumably generated via reduction of the C–Br bond through a single electron transfer (SET) pathway involving the excited state gold complex. Vinyl radical 279 then undergoes a tandem radical cyclization cascade to give 280 after an oxidation event, presumably also mediated by the Au complex. The reaction mixture was then directly treated with sulfuric acid which furnished elimination product 281 in 64% yield overall yield from 277. Finally, isomerization of the newly formed olefin (cat. RuCl2(PPh3)3, DIPEA) into conjugation completed the synthesis of 282, an intermediate which Berchtold had previously converted into triptolide by the seven-step synthetic sequence shown.179
At nine steps, Barriault’s synthesis of 282 is quite concise owing to strategic incorporation of a redox neutral radical polycyclization cascade. Moreover, the use of photoredox catalysis to initiate such transformations offers both environmental and safety advantages over classic tin-based reagents and potentially dangerous radical initiators such as Et3B/O2 and AIBN.
Conclusions
The described works above give strong testament to the ability of radical cascades to rapidly assemble complex molecular frameworks. In particular, their ability to avoid falling under the influence of steric effects, which can plague many alternative reaction types, makes them particularly useful tools in the synthesis of complex, polycyclic molecules such as terpenes. Synthetic radical research of the past has established many of the “rules” governing the basic reactivity and cyclization modes of free radicals.1–3,32,33 Modern day efforts seek to exploit more ubiquitous functional groups as radical progenitors, develop more environmentally benign radical reactions, and to merge radical chemistry with that of transition metal catalysis and organocatalysis.180–192 So what is left to explore? While many of the aforementioned areas still offer many exciting avenues of inquiry, one could argue that stereocontrol over HAT processes which form stereogenic centers (both diastereocontrol and especially enantiocontrol) is very much an unsolved problem–especially in the realm of small molecule catalysis.193,194 The synthesis of 6-epi-ophiobolin N (Section 3.5) highlights such challenges as the authors had to take a largely empirical approach to improving the reaction outcome, the results of which were far from ideal. Thus, there is much reason to expect that the future of synthetic radical chemistry will feature many exciting areas of research, with the possibility of elevating the field of complex molecule synthesis to greater heights than is currently imaginable.
Scheme 3.
Carreira’s synthesis of (+)-crotogoudin (65).
Scheme 5.
Liu’s synthesis of (−)-hispidanin A (99).
Scheme 17.
Barriault’s formal synthesis of (±)-triptolide (271)
Acknowledgments
Financial support is provided by the NIH (GM116952) and the NSF (CAREER Award# 1554544). T.J.M. is a Research Corporation Cottrell Scholar and acknowledges unrestricted support from Novartis, Bristol-Myers Squibb, Amgen, and Eli Lilly. X.H. and K.H. thank Bristol-Myers Squibb and Eli Lilly for graduate research fellowships respectively.
References
- 1.Renaud P, Sibi MP, editors. Radicals in Organic Synthesis. Wiley-VCH; 2001. [Google Scholar]
- 2.Togo H. Advanced Free Radical Reactions for Organic Synthesis. Elsevier; 2004. [Google Scholar]
- 3.Zard S. Radical Reactions in Organic Synthesis. Oxford; USA: 2003. [Google Scholar]
- 4.Yan M, Lo JC, Edwards JT, Baran PS. J Am Chem Soc. 2016;138:12692–12714. doi: 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Jasperse CP, Curran DP, Fevig TL. Chem Rev. 1991;91:1237–1286. [Google Scholar]; b) Plesniak MP, Huang H-M, Procter DJ. Nat Rev Chem. 2017;1 Article Number 0077. [Google Scholar]
- 6.Singh AK, Bakshi RK, Corey EJ. J Am Chem Soc. 1987;109:6187–6189. [Google Scholar]
- 7.Holton RA, Juo RR, Kim HB, Williams AD, Harusawa S, Lowenthal RE, Yogai S. J Am Chem Soc. 1988;110:6558–6560. [Google Scholar]
- 8.Stork G, West F, Lee HY, Isaacs RCA, Manabe S. J Am Chem Soc. 1996;118:10660–10661. [Google Scholar]
- 9.Kan T, Hosokawa S, Nara S, Oikawa M, Ito S, Matsuda F, Shirahama H. J Org Chem. 1994;59:5532–5534. [Google Scholar]
- 10.Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV. Angew Chem Int Ed. 2007;46:7629–7632. doi: 10.1002/anie.200703027. [DOI] [PubMed] [Google Scholar]
- 11.Mukai K, Urabe D, Kasuya S, Aoki N, Inoue M. Angew Chem Int Ed. 2013;52:5300–5304. doi: 10.1002/anie.201302067. [DOI] [PubMed] [Google Scholar]
- 12.Birman VB, Danishefsky SJ. J Am Chem Soc. 2002;124:2080–2081. doi: 10.1021/ja012495d. [DOI] [PubMed] [Google Scholar]
- 13.Nagatomo M, Koshimizu M, Masuda K, Tabuchi T, Urabe D, Inoue M. J Am Chem Soc. 2014;136:5916–5919. doi: 10.1021/ja502770n. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Nakashige M, Chain WJ. J Am Chem Soc. 2011;133:6553–6556. doi: 10.1021/ja201921j. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou KC, Ortiz A, Zhang H. Angew Chem Int Ed. 2009;48:5648–5652. doi: 10.1002/anie.200902029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu P, Gu Z, Zakarian A. J Am Chem Soc. 2013;135:14552–14555. doi: 10.1021/ja408231t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey EJ, Wu YJ. J Am Chem Soc. 1993;115:8871–8872. [Google Scholar]
- 18.Wang L, Wang H, Li Y, Tang P. Angew Chem Int Ed. 2015;54:5732–5735. doi: 10.1002/anie.201501169. [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Liu J, Ma D. Angew Chem Int Ed. 2015;54:1298–1301. doi: 10.1002/anie.201410134. [DOI] [PubMed] [Google Scholar]
- 20.Ardkhean R, Caputo DFJ, Morrow SM, Shi H, Xiong Y, Anderson EA. Chem Soc Rev. 2016;45:1557–1569. doi: 10.1039/c5cs00105f. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaou KC, Chen JS. Chem Soc Rev. 2009;38:2993–3009. doi: 10.1039/b903290h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 23.Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; 2006. [Google Scholar]
- 24.Curran DP, Rakiewicz DM. J Am Chem Soc. 1985;107:1448–1449. [Google Scholar]
- 25.Curran DP, Shen W. Tetrahedron. 1993;49:755–770. [Google Scholar]
- 26.Myers AG, Condroski KR. J Am Chem Soc. 1995;117:3057–3083. [Google Scholar]
- 27.Lee E, Lim JW, Yoon CH, Sung Y-S, Kim YK, Yun M, Kim S. J Am Chem Soc. 1997;119:8391–8392. [Google Scholar]
- 28.Snider BB, Kiselgof JY, Foxman BM. J Org Chem. 1998;63:7945–7952. [Google Scholar]
- 29.Boffey RJ, Whittingham WG, Kilburn JD. J Chem Soc, Perkin Trans. 2001;1:487–496. [Google Scholar]
- 30.Pattenden G, Gonzalez MA, McCulloch S, Walter A, Woodhead SJ. Proc Natl Acad Sci. 2004;101:12024–12029. doi: 10.1073/pnas.0401925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott MR, Dhimane A-L, Malacria M. J Am Chem Soc. 1997;119:3427–3428. [Google Scholar]
- 32.Curran DP, Porter NA, Giese B. Stereochemistry of Radical Reactions. Wiley-VCH; 1996. [Google Scholar]
- 33.McCarroll AJ, Walton JC. Angew Chem Int Ed. 2001;40:2224–2248. doi: 10.1002/1521-3773(20010618)40:12<2224::aid-anie2224>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Yoder RA, Johnston JN. Chem Rev. 2005;105:4730–4756. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justicia J, Álvarez de Cienfuegos L, Campaña AG, Miguel D, Jakoby V, Gansäuer A, Cuerva JM. Chem Soc Rev. 2011;40:3525–3537. doi: 10.1039/c0cs00220h. [DOI] [PubMed] [Google Scholar]
- 36.Breslow R, Barrett E, Mohacsi E. Tetrahedron Lett. 1962;3:1207–1211. [Google Scholar]
- 37.Nicolaou KC, Tria GS, Edmonds DJ, Kar M. J Am Chem Soc. 2009;131:15909–15917. doi: 10.1021/ja906801g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosley SWM, Barabé F, Shenvi RA. J Am Chem Soc. 2014;136:16788–16791. doi: 10.1021/ja5105602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poupon E, Nay B. Biomimetic Organic Synthesis. 1–2 Wiley-VCH; Weinheim: 2011. [Google Scholar]
- 40.Razzak M, De Brabander JK. Nat Chem Biol. 2011;7:865–875. doi: 10.1038/nchembio.709. [DOI] [PubMed] [Google Scholar]
- 41.Nicolaou KC, Ellery SP, Chen JS. Angew Chem Int Ed. 2009;48:7140–7165. doi: 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmonds DJ, Johnston D, Procter DJ. Chem Rev. 2004;104:3371–3404. doi: 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]
- 43.Molander GA, Harris CR. Chem Rev. 1996;96:307–338. doi: 10.1021/cr950019y. [DOI] [PubMed] [Google Scholar]
- 44.Kavanagh F, Hervey A, Robbins WJ. Proc Natl Acad Sci U S A. 1951;37:570–574. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh F, Hervey A, Robbins WJ. Proc Natl Acad Sci U S A. 1952;38:555–560. doi: 10.1073/pnas.38.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibbons EG. J Am Chem Soc. 1982;104:1767–1769. [Google Scholar]
- 47.Boeckman RK, Jr, Springer DM, Alessi TR. J Am Chem Soc. 1989;111:8284–8286. [Google Scholar]
- 48.Fazakerley NJ, Helm MD, Procter DJ. Chemistry - A European Journal. 2013;19:6718–6723. doi: 10.1002/chem.201300968. [DOI] [PubMed] [Google Scholar]
- 49.Murphy SK, Zeng M, Herzon SB. Science. 2017;356:956–959. doi: 10.1126/science.aan0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.While this manuscript was under review, a 2018 radical-based synthesis of 21 by Reisman appeared, see: Farney EP, Feng SS, Schafers F, Reisman SE. J Am Chem Soc. 2018 doi: 10.1021/jacs.7b13260. Article ASAP.
- 51.Kahn M. Tetrahedron Lett. 1980;21:4547–4548. [Google Scholar]
- 52.Paquette LA, Wiedeman PE, Bulman-Page PC. J Org Chem. 1988;53:1441–1450. [Google Scholar]
- 53.Bacque E, Pautrat F, Zard SZ. Org Lett. 2003;5:325–328. doi: 10.1021/ol027312m. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Lotesta SD, Sorensen EJ. Chem Commun. 2011;47:1500–1502. doi: 10.1039/c0cc04077k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreiber SL. J Am Chem Soc. 1980;102:6163–6165. [Google Scholar]
- 56.Petasis NA, Patane MA. Tetrahedron. 1992;48:5757–5821. [Google Scholar]
- 57.Ruscoe RE, Fazakerley NJ, Huang H, Flitsch S, Procter DJ. Chemistry. 2016;22:116–119. doi: 10.1002/chem.201504343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han QB, Cheung S, Tai J, Qiao CF, Song JZ, Tso TF, Sun HD, Xu HX. Org Lett. 2006;8:4727–4730. doi: 10.1021/ol061757j. [DOI] [PubMed] [Google Scholar]
- 59.Liu M, Wang W-G, Sun H-D, Pu J-X. Nat Prod Rep. 2017;34:1090–1140. doi: 10.1039/c7np00027h. [DOI] [PubMed] [Google Scholar]
- 60.Cha JY, Yeoman JT, Reisman SE. J Am Chem Soc. 2011;133:14964–14967. doi: 10.1021/ja2073356. [DOI] [PubMed] [Google Scholar]
- 61.a) Tanimoto H, Oritani T. Tetrahedron. 1997;53:3527–3536. [Google Scholar]; b) Sum FW, Weiler L. Tetrahedron Lett. 1979;20:707–708. [Google Scholar]
- 62.Gansäuer A, Fleckhaus A. Encyclopedia of Radicals in Chemistry, Biology and Materials, Online. Wiley; 2012. Epoxides in Titanocene-Mediated and -Catalyzed Radical Reactions. [Google Scholar]
- 63.Gansäuer A, Pierobon M, Bluhm H. Angew Chem Int Ed. 1998;37:101–103. [Google Scholar]
- 64.RajanBabu TV, Nugent WA. J Am Chem Soc. 1989;111:4525–4527. [Google Scholar]
- 65.Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J Am Chem Soc. 1997;119:6496–6511. [Google Scholar]
- 66.Morcillo SP, Miguel D, Campaña AG, Álvarez de Cienfuegos L, Justicia J, Cuerva JM. Org Chem Front. 2014;1:15–33. [Google Scholar]
- 67.Yeoman JTS, Mak VW, Reisman SE. J Am Chem Soc. 2013;135:11764–11767. doi: 10.1021/ja406599a. [DOI] [PubMed] [Google Scholar]
- 68.Rakotonandrasana OL, Raharinjato FH, Rajaonarivelo M, Dumontet V, Martin M-T, Bignon J, Rasoanaivo P. J Nat Prod. 2010;73:1730–1733. doi: 10.1021/np1005086. [DOI] [PubMed] [Google Scholar]
- 69.Breitler S, Carreira EM. Angew Chem Int Ed. 2013;52:11168–11171. doi: 10.1002/anie.201305822. [DOI] [PubMed] [Google Scholar]
- 70.Song L, Zhu G, Liu Y, Liu B, Qin S. J Am Chem Soc. 2015;137:13706–13714. doi: 10.1021/jacs.5b08958. [DOI] [PubMed] [Google Scholar]
- 71.Finkbeiner P, Murai K, Ropke M, Sarpong R. J Am Chem Soc. 2017;139:11349–11352. doi: 10.1021/jacs.7b06823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Servi S. Synthesis. 1990:1–25. [Google Scholar]
- 73.Nozawa K, Nakajima S, Kawai K-i, Udagawa S-i. J Chem Soc, Perkin Trans I. 1988:2607–2610. [Google Scholar]
- 74.George DT, Kuenstner EJ, Pronin SV. Synlett. 2017;28:12–18. [Google Scholar]
- 75.George DT, Kuenstner EJ, Pronin SV. J Am Chem Soc. 2015;137:15410–15413. doi: 10.1021/jacs.5b11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.a) Lo JC, Gui J, Yabe Y, Pan CM, Baran PS. Nature. 2014;516:343–348. doi: 10.1038/nature14006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lo JC, Yabe Y, Baran PS. J Am Chem Soc. 2014;136:1304–1307. doi: 10.1021/ja4117632. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Obradors C, Martinez RM, Shenvi RA. J Am Chem Soc. 2016;138:4962–4971. doi: 10.1021/jacs.6b02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmbo SD, Godfrey NA, Hirner JJ, Pronin SV. J Am Chem Soc. 2016;38:12316–12319. doi: 10.1021/jacs.6b06847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crossley SWM, Obradors C, Martinez RM, Shenvi RA. Chem Rev. 2016;116:8912–9000. doi: 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang B, Xiao C-J, Huang Z-Y, Tian X-Y, Cheng X, Dong X, Jiang B. Org Lett. 2014;16:3552–3555. doi: 10.1021/ol501417k. [DOI] [PubMed] [Google Scholar]
- 80.Li F, Tu Q, Chen S, Zhu L, Lan Y, Gong J, Yang Z. Angew Chem Int Ed. 2017;56:5844–5848. doi: 10.1002/anie.201700838. [DOI] [PubMed] [Google Scholar]
- 81.Deng H, Cao W, Liu R, Zhang Y, Liu B. Angew Chem Int Ed. 2017;56:5849–5852. doi: 10.1002/anie.201700958. [DOI] [PubMed] [Google Scholar]
- 82.García-García P, Fernández-Rodríguez MA, Aguilar E. Angew Chem Int Ed. 2009;48:5534–5537. doi: 10.1002/anie.200901269. [DOI] [PubMed] [Google Scholar]
- 83.Nakatsuji H, Nishikado H, Ueno K, Tanabe Y. Org Lett. 2009;11:4258–4261. doi: 10.1021/ol9013359. [DOI] [PubMed] [Google Scholar]
- 84.Fukuyama Y, Huang J-M. In: Stud Nat Prod Chem. Part L. Atta ur R, editor. Vol. 32. Elsevier; 2005. pp. 395–427. [Google Scholar]
- 85.Yokoyama R, Huang J-M, Yang C-S, Fukuyama Y. J Nat Prod. 2002;65:527–531. doi: 10.1021/np010571k. [DOI] [PubMed] [Google Scholar]
- 86.Kubo M, Okada C, Huang J-M, Harada K, Hioki H, Fukuyama Y. Org Lett. 2009;11:5190–5193. doi: 10.1021/ol9021029. [DOI] [PubMed] [Google Scholar]
- 87.(a) Shenvi RA. Nat Prod Rep. 2016;33:535–539. doi: 10.1039/c5np00160a. [DOI] [PubMed] [Google Scholar]; (b) Ohtawa M, Krambis MJ, Cerne R, Schkeryantz JM, Witkin JM, Shenvi RA. J Am Chem Soc. 2017;139:9637–9644. doi: 10.1021/jacs.7b04206. [DOI] [PubMed] [Google Scholar]
- 88.Cho YS, Carcache DA, Tian Y, Li Y-M, Danishefsky SJ. J Am Chem Soc. 2004;126:14358–14359. doi: 10.1021/ja045939p. [DOI] [PubMed] [Google Scholar]
- 89.Carcache DA, Cho YS, Hua Z, Tian Y, Li Y-M, Danishefsky SJ. J Am Chem Soc. 2006;128:1016–1022. doi: 10.1021/ja056980a. [DOI] [PubMed] [Google Scholar]
- 90.Yang Y, Fu X, Chen J, Zhai H. Angew Chem Int Ed. 2012;51:9825–9828. doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]
- 91.Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Chem Eur J. 2013;19:6398–6408. doi: 10.1002/chem.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Org Lett. 2011;13:4554–4557. doi: 10.1021/ol201742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Condakes ML, Hung K, Harwood SJ, Maimone TJ. J Am Chem Soc. 2017;139:17783–17786. doi: 10.1021/jacs.7b11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng X, Micalizio GC. J Am Chem Soc. 2016;138:1150–1153. doi: 10.1021/jacs.5b12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizoguchi H, Micalizio GC. J Am Chem Soc. 2015;137:6624–6628. doi: 10.1021/jacs.5b02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris SA, Silva EDd, Andersen RJ. Can J Chem. 1991;69:768–771. [Google Scholar]
- 97.Schnermann MJ, Beaudry CM, Egorova AV, Polishchuk RS, Sütterlin C, Overman LE. Proc Natl Acad Sci U S A. 2010;107:6158–6163. doi: 10.1073/pnas.1001421107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tao DJ, Slutskyy Y, Overman LE. J Am Chem Soc. 2016;138:2186–2189. doi: 10.1021/jacs.6b00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandai T, Matsumoto T, Kawada M, Tsuji J. J Org Chem. 1992;57:1326–1327. [Google Scholar]
- 100.Wan Z-K, Choi H-w, Kang F-A, Nakajima K, Demeke D, Kishi Y. Org Lett. 2002;4:4431–4434. doi: 10.1021/ol0269805. [DOI] [PubMed] [Google Scholar]
- 101.Jamison CR, Overman LE. Acc Chem Res. 2016;49:1578–1586. doi: 10.1021/acs.accounts.6b00284. [DOI] [PubMed] [Google Scholar]
- 102.Pratsch G, Lackner GL, Overman LE. J Org Chem. 2015;80:6025–6036. doi: 10.1021/acs.joc.5b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Au TK, Chick WSH, Leung PC. Life Sci. 2000;67:733–742. doi: 10.1016/s0024-3205(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 104.Tian W, Deng Z, Hong K. Marine drugs. 2017;15:229. doi: 10.3390/md15070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rowley M, Tsukamoto M, Kishi Y. J Am Chem Soc. 1989;111:2735–2737. [Google Scholar]
- 106.Tsuna K, Noguchi N, Nakada M. Angew Chem Int Ed. 2011;50:9452–9455. doi: 10.1002/anie.201104447. [DOI] [PubMed] [Google Scholar]
- 107.Brill ZG, Grover HK, Maimone TJ. Science. 2016;352:1078–1082. doi: 10.1126/science.aaf6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brill ZG, Condakes ML, Ting CP, Maimone TJ. Chem Rev. 2017;117:11753–11795. doi: 10.1021/acs.chemrev.6b00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Charette AB, Juteau H, Lebel H, Molinaro C. J Am Chem Soc. 1998;120:11943–11952. [Google Scholar]
- 110.Charette AB, Naud J. Tetrahedron lett. 1998;39:7259–7262. [Google Scholar]
- 111.Roberts BP. Chem Soc Rev. 1999;28:25–35. [Google Scholar]
- 112.Bernays Justus Liebigs Annalen der Chemie. 1841;40:317–317. [Google Scholar]
- 113.Heasley B. Eur J Org Chem. 2011;2011:19–46. [Google Scholar]
- 114.Yamashita S, Naruko A, Nakazawa Y, Zhao L, Hayashi Y, Hirama M. Angew Chem Int Ed. 2015;54:8538–8541. doi: 10.1002/anie.201503794. [DOI] [PubMed] [Google Scholar]
- 115.Snider BB. Chem Rev. 1996;96:339–364. doi: 10.1021/cr950026m. [DOI] [PubMed] [Google Scholar]
- 116.Ciochina R, Grossman RB. Chem Rev. 2006;106:3963–3986. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 117.Richard J-A, Pouwer RH, Chen DYK. Angew Chem Int Ed. 2012;51:4536–4561. doi: 10.1002/anie.201103873. [DOI] [PubMed] [Google Scholar]
- 118.Pepper HP, Lam HC, Bloch WM, George JH. Org Lett. 2012;14:5162–5164. doi: 10.1021/ol302524q. [DOI] [PubMed] [Google Scholar]
- 119.Pepper HP, Tulip SJ, Nakano Y, George JH. J Org Chem. 2014;79:2564–2573. doi: 10.1021/jo500027k. [DOI] [PubMed] [Google Scholar]
- 120.Cardillo G, D’Amico A, Orena M, Sandri S. J Org Chem. 1988;53:2354–2356. [Google Scholar]
- 121.Stierle AA, Stierle DB. Stud Nat Prod Chem. 2005;32:1123–1175. [Google Scholar]
- 122.Stierle DB, Stierle AA, Patacini B, McIntyre K, Girtsman T, Bolstad E. J Nat Prod. 2011;74:2273–2277. doi: 10.1021/np2003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsuda Y, Abe I. Nat Prod Rep. 2016;33:26–53. doi: 10.1039/c5np00090d. [DOI] [PubMed] [Google Scholar]
- 124.Ting CP, Xu G, Zeng X, Maimone TJ. J Am Chem Soc. 2016;138:14868–14871. doi: 10.1021/jacs.6b10397. [DOI] [PubMed] [Google Scholar]
- 125.Elkin M, Szewczyk SM, Scruse AC, Newhouse TR. J Am Chem Soc. 2017;139:1790–1793. doi: 10.1021/jacs.6b12914. [DOI] [PubMed] [Google Scholar]
- 126.Fernández-Mateos A, Teijón PH, Clemente RR, González RR, González FS. Synlett. 2007:2718–2722. [Google Scholar]
- 127.Gansäuer A, Fleckhaus A. In Encyclopedia of Radicals in Chemistry, Biology and Materials, Online. Wiley; 2012. Epoxides in Titanocene-Mediated and –Catalyzed Radical Reactions. [Google Scholar]
- 128.Barrero AF, Quilez del Moral JF, Sanchez EM, Arteaga JF. Eur J Org Chem. 2006:1627–1641. [Google Scholar]
- 129.Ting CP, Maimone TJ. J Am Chem Soc. 2015;137:10516–10519. doi: 10.1021/jacs.5b06939. [DOI] [PubMed] [Google Scholar]
- 130.Ting CP, Maimone TJ. Synlett. 2016;27:1443–1449. [Google Scholar]
- 131.Xu G, Elkin M, Tantillo DJ, Newhouse TR, Maimone TJ. Angew Chem Int Ed. 2017;56:12498–12502. doi: 10.1002/anie.201705654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shigehisa H, Aoki T, Yamaguchi S, Shimizu N, Hiroya K. J Am Chem Soc. 2013;135:10306–10309. doi: 10.1021/ja405219f. [DOI] [PubMed] [Google Scholar]
- 133.Gaspar B, Carreira EM. Angew Chem Int Ed. 2008;47:5758–5760. doi: 10.1002/anie.200801760. [DOI] [PubMed] [Google Scholar]
- 134.Matsuda Y, Iwabuchi T, Wakimoto T, Awakawa T, Abe I. J Am Chem Soc. 2015;137:3393–3401. doi: 10.1021/jacs.5b00570. [DOI] [PubMed] [Google Scholar]
- 135.Shi Q-W, Su X-H, Kiyota H. Chem Rev. 2008;108:4295–4327. doi: 10.1021/cr078350s. [DOI] [PubMed] [Google Scholar]
- 136.Vasas A, Hohmann J. Chem Rev. 2014;114:8579–8612. doi: 10.1021/cr400541j. [DOI] [PubMed] [Google Scholar]
- 137.Liao S-G, Chen H-D, Yue J-M. Chem Rev. 2009;109:1092–1140. doi: 10.1021/cr0782832. [DOI] [PubMed] [Google Scholar]
- 138.Hergenhahn M, Adolf W, Hecker E. Tetrahedron Lett. 1975;16:1595–1598. [Google Scholar]
- 139.Raisinghani M, Pabbidi RM, Premkumar LS. J Physiol. 2005;567:771–786. doi: 10.1113/jphysiol.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wender PA, Jesudason CD, Nakahira H, Tamura N, Tebbe AL, Ueno Y. J Am Chem Soc. 1997;119:12976–12977. [Google Scholar]
- 141.Hashimoto S, Katoh S-i, Kato T, Urabe D, Inoue M. J Am Chem Soc. 2017;139:16420–16429. doi: 10.1021/jacs.7b10177. [DOI] [PubMed] [Google Scholar]
- 142.Crich D, Quintero L. Chem Rev. 1989;89:1413–1432. [Google Scholar]
- 143.(a) Keck GE, Yates JB. J Am Chem Soc. 1982;104:5829. [Google Scholar]; (b) Grignon J, Pereyre M. J Organomet Chem. 1973;61:C33–C35. [Google Scholar]
- 144.Coll JC. Chem Rev. 1992;92:613–631. [Google Scholar]
- 145.Shen S, Zhu H, Chen D, Liu D, Ofwegen LV, Proksch P, Lin W. Tetrahedron Lett. 2012;53:5759–5762. [Google Scholar]
- 146.Zhang P-P, Yan Z-M, Li Y-H, Gong J-X, Yang Z. J Am Chem Soc. 2017;139:13989–13992. doi: 10.1021/jacs.7b07388. [DOI] [PubMed] [Google Scholar]
- 147.Hayashi Y, Gotoh H, Hayashi T, Shoji M. Angew Chem Int Ed. 2005;44:4212–4215. doi: 10.1002/anie.200500599. [DOI] [PubMed] [Google Scholar]
- 148.García-Cabeza AL, Marín-Barrios R, Azarken R, Moreno-Dorado FJ, Ortega MJ, Vidal H, Gatica JM, Massanet GM, Guerra FM. Eur J Org Chem. 2013;2013:8307–8314. [Google Scholar]
- 149.Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 150.(a) Yoon TP. Acc Chem Res. 2016;49:2307–2315. doi: 10.1021/acs.accounts.6b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ravelli D, Protti S, Fagnoni M. Chem Rev. 2016;116:9850–9913. doi: 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]
- 152.Parker KA, Fokas D. J Am Chem Soc. 1992;114:9688–9689. [Google Scholar]
- 153.Smith MW, Snyder SA. J Am Chem Soc. 2013;135:12964–12967. doi: 10.1021/ja406546k. [DOI] [PubMed] [Google Scholar]
- 154.Patro B, Murphy JA. Org Lett. 2000;2:3599–3601. doi: 10.1021/ol006477x. [DOI] [PubMed] [Google Scholar]
- 155.Li F, Tartakoff SS, Castle SL. J Am Chem Soc. 2009;131:6674–6675. doi: 10.1021/ja9024403. [DOI] [PubMed] [Google Scholar]
- 156.Stork G, Baine NH. Tetrahedron Lett. 1985;26:5927–5930. [Google Scholar]
- 157.Yun SY, Zheng JC, Lee D. Angew Chem Int Ed. 2008;47:6201–6203. doi: 10.1002/anie.200801587. [DOI] [PubMed] [Google Scholar]
- 158.Harrington-Frost NM, Pattenden G. Tetrahedron Lett. 2000;41:403–405. [Google Scholar]
- 159.Enquist JA, Jr, Stoltz BM. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boger DL, Hong J. J Am Chem Soc. 2001;123:8515–819. doi: 10.1021/ja011271s. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L, Koreeda M. Org Lett. 2004;6:537–540. doi: 10.1021/ol0363063. [DOI] [PubMed] [Google Scholar]
- 162.Pattenden G, Roberts L. Tetrahedron Lett. 1996;37:4191–4194. [Google Scholar]
- 163.Herzon SB, Myers AG. J Am Chem Soc. 2005;127:5342–5344. doi: 10.1021/ja0510616. [DOI] [PubMed] [Google Scholar]
- 164.Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chem Rev. 1999;99:1991–2070. doi: 10.1021/cr9601425. [DOI] [PubMed] [Google Scholar]
- 165.Minagawa K, Kouzuki S, Nomura K, Yamaguchi T, Kawamura Y, Matsushima K, Tani H, Ishii K, Tanimoto T, Kamaguchi T. J Antibiot. 2001;54:890–895. doi: 10.7164/antibiotics.54.890. [DOI] [PubMed] [Google Scholar]
- 166.am Ende CW, Zhou Z, Parker KA. J Am Chem Soc. 2013;135:582–585. doi: 10.1021/ja3108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamamoto K, Suzuki S, Tsuji J. Chem Lett. 1978:649–652. [Google Scholar]
- 168.Marki F, Witkop B. Experientia. 1963;19:329–338. doi: 10.1007/BF02152303. [DOI] [PubMed] [Google Scholar]
- 169.Tokuyama T, Daly J, Witkop B, Karle IL, Karle J. J Am Chem Soc. 1968;90:1917–1918. doi: 10.1021/ja01009a052. [DOI] [PubMed] [Google Scholar]
- 170.Logan MM, Toma T, Thomas-Tran R, Du Bois J. Science. 2016;354:865–869. doi: 10.1126/science.aag2981. [DOI] [PubMed] [Google Scholar]
- 171.Kurosu M, Marcin LR, Grinsteiner TJ, Kishi Y. J Am Chem Soc. 1998;120:6627–6628. [Google Scholar]
- 172.Takano S, Yamane T, Takahashi M, Ogasawara K. Synlett. 1992;1992:410–412. [Google Scholar]
- 173.Lacrouts P, Parsons PJ, Penkett CS, Rauf Raza A. Synlett. 2005:27670–2768. [Google Scholar]
- 174.Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. J Am Chem Soc. 1972;94:7194–7195. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 175.Ziaei S, Halaby R. Avicenna J Phytommed. 2016;6:149–164. [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Q. Int Immunopharmacol. 2011;11:377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 177.Zhou Z-L, Yang Y-X, Ding J, Li Y-C, Miao Z-H. Nat Prod Rep. 2012;29:457–475. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
- 178.Cannillo A, Schwantje TR, Begin M, Barabe F, Barriault L. Org Lett. 2016;18:2592–2595. doi: 10.1021/acs.orglett.6b00968. [DOI] [PubMed] [Google Scholar]
- 179.Lai CK, Buckanin RS, Chen SJ, Zimmerman DF, Sher FT, Berchtold GA. J Org Chem. 1982;47:2364–2369. [Google Scholar]
- 180.Zhou J, Fu GC. J Am Chem Soc. 2003;125:14726–14727. doi: 10.1021/ja0389366. [DOI] [PubMed] [Google Scholar]
- 181.Zhao Y, Weix DJ. J Am Chem Soc. 2014;136:48–51. doi: 10.1021/ja410704d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tellis JC, Primer DN, Molander GA. Science. 2014;345:433–436. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ackerman LKG, Anka-Lufford LL, Naodovic M, Weix DJ. Chem Sci. 2015;6:1115–1119. doi: 10.1039/c4sc03106g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao Y, Weix DJ. J Am Chem Soc. 2015;137:3237–3240. doi: 10.1021/jacs.5b01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huihui KMM, Caputo JA, Melchor Z, Olivares AM, Spiewak AM, Johnson KA, DiBenedetto TA, Kim S, Ackerman LKG, Weix DJ. J Am Chem Soc. 2016;138:5016–5019. doi: 10.1021/jacs.6b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.(a) Lu X, Xiao B, Zhang Z, Gong T, Su W, Yi J, Fu Y, Liu L. Nat Commun. 2016;7(11129) doi: 10.1038/ncomms11129. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang G, Shang R, Cheng W, Fu Y. J Am Chem Soc. 2017;139:18307–18312. doi: 10.1021/jacs.7b10009. [DOI] [PubMed] [Google Scholar]
- 190.Green SA, Matos JLM, Yagi A, Shenvi RA. J Am Chem Soc. 2016;138:12779–12782. doi: 10.1021/jacs.6b08507. [DOI] [PubMed] [Google Scholar]
- 191.Zweig JE, Kim DE, Newhouse TR. Chem Rev. 2017;117:11680–11752. doi: 10.1021/acs.chemrev.6b00833. [DOI] [PubMed] [Google Scholar]
- 192.Twilton J, Le C, Zhang P, Shaw MH, Evans RW, Macmillan DWC. Nat Rev Chem. 2017;1 Article Number 0052. [Google Scholar]
- 193.Cai Y, Roberts BP, Tocher DA. J Chem Soc Perkin Trans 1. 2002:1376–1386. [Google Scholar]
- 194.For recent examples using enzymes, see: Emmanuel MA, Greenberg NR, Oblinsky DG, Hyster TK. Nature. 2016;540:414–417. doi: 10.1038/nature20569.Sandoval BA, Meichan AJ, Hyster TK. J Am Chem Soc. 2017;139:11313–11316. doi: 10.1021/jacs.7b05468.