Abstract
Antioxidants have been reported to have anti-inflammatory effects but there is a lack of research comparing food to supplement antioxidant sources. The aim of this study was to determine if increases in intake of foods naturally rich in antioxidants would lower blood levels of inflammatory markers more than consuming antioxidant supplements among adults with cardiovascular disease (CVD) risk factors. Eighty-eight generally healthy adults with ≥1 elevated risk factors for CVD were randomized in a single-blind (diets)/double blind (supplements), parallel group study for eight weeks. Participants consumed 1) usual diet and placebo pills (n=29), 2) usual diet and antioxidant supplements (n=29), or 3) antioxidant-rich foods closely matched to antioxidant content of supplements and placebo (n=30). Usual diet combined with antioxidant supplements or increased antioxidant-rich food intake were designed to approximately double daily habitual antioxidant intake. Antioxidant pills included carotenoids, mixed tocopherols, vitamin C and selenium. Fasting blood samples were analyzed for inflammatory marker concentrations of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1) and soluble intercellular adhesion molecule-1 (sICAM-1). Participants in the intervention groups successfully doubled most antioxidants as verified by diet records and elevated blood concentrations in treatment groups. Baseline levels of inflammatory markers for the entire study group were 110 ± 65 pg/mL for MCP-1, 0.9 ± 0.7 pg/mL for IL-6, and 217 ± 56 ng/mL for sICAM-1 [means ± standard deviation (SD)] and did not differ by treatment arm. After eight weeks, there were no significant within-group changes or between-group 8-week change differences in inflammatory marker concentrations. In conclusion, no beneficial effects were detected on the inflammatory markers investigated in response to antioxidants from foods or supplements.
Keywords: Adults, antioxidants, inflammatory markers, diet, supplements
1. Introduction
Chronic inflammation plays a major role in the development of atherosclerosis and cardiovascular disease (CVD)[1]. In recent years, there has been a great interest in the potential anti-inflammatory effects of dietary patterns and food components, especially antioxidants[2]. Dietary antioxidants have been recognized as playing an important role in the prevention of CVD by quenching reactive-oxygen species, and thereby interfering with the oxidative processes that contribute to atherogenesis[3,4]. Antioxidants as defined by the Federal Drug Administration (FDA), - are “substances that, following absorption from the gastrointestinal tract, participate in physiological, biochemical, or cellular processes that inactivate free radicals or prevent free radical-initiated chemical reactions”[5].
In the past ~15 years several epidemiological studies and clinical trials have investigated the potential role of dietary antioxidants in modulating inflammation as evidenced by changes in circulating concentrations of inflammatory markers. Findings from observational studies have uniformly supported the hypothesis that dietary intake and/or plasma concentrations of antioxidants – vitamin C, carotenoids, and alpha-tocopherol – are inversely correlated with biomarkers of inflammation[6–9]. In addition, consuming foods rich in antioxidants such as nuts, olive oil, fruits[10], and soy[11], or closely following dietary patterns high in antioxidant-rich foods, such as the Mediterranean Diet[12], has been associated with lower concentrations of several inflammatory markers.
Despite these findings, clinical trials have been less consistent in their results. The majority of studies have investigated the effects of either dietary supplements[13–18] or single foods[19–28] on various biomarkers of inflammation, in generally healthy but high-risk individuals (e.g. with one or more CVD risk factor, the metabolic syndrome, or mildly elevated C-reactive protein (CRP)). Trials using dietary supplements – vitamin C, tocopherols, quercetin, resveratrol, green tea extract, selenium, coenzyme Q10 – have largely failed to observe a beneficial effect[13–18]. In contrast, the majority of trials examining foods high in antioxidants – soy, almonds, cherries, bilberries, and wine – have shown a decrease in selected inflammatory markers[19–28]. Possible explanations for these inconsistent findings may include the wide range of doses and combinations of the antioxidants examined, the differential effects of isolated antioxidants compared to antioxidant combinations, or the different mechanisms of actions of dietary as opposed to supplemental sources of antioxidants.
To date, very few studies have investigated the effect of dietary patterns high in antioxidants on inflammatory markers[29–33]. In general, these studies have concluded that dietary patterns high in vegetables, fruits, almonds, soy and other antioxidant-rich foods may lower biomarkers of inflammation. To our knowledge, the effects of an antioxidant-rich diet versus supplements have not been directly compared in the same trial. Therefore, our objective was to determine the short-term efficacy of foods naturally rich in antioxidants vs. supplemental pill antioxidants on markers of inflammation in generally healthy subjects with at least one elevated risk factor for CVD. The central hypothesis was that blood concentrations of inflammatory markers would be more favorably decreased by increases in intake of foods naturally rich in antioxidants vs. antioxidant supplements, among adults with cardiovascular disease (CVD) risk factors
2. Methods and Materials
2.1 Trial Design
This was a randomized, single-blind (diets)/double blind (supplements), parallel group study with three different arms including: dietary antioxidants, antioxidant supplements, and placebo pills only. The primary outcome for this study was to investigate the short-term effect of foods naturally rich in antioxidants versus antioxidant supplements on selected markers of inflammation.
2.2 Subjects
Eighty-eight adults (32 men and 56 women) were recruited from the local community through radio and newspaper advertisements and completed an on-line questionnaire and a screening clinic visit. Inclusion and exclusion criteria were chosen to maximize generalizability and achieve relative homogeneity to avoid outliers with confounding metabolic or health disorders. The study participants included generally healthy subjects with at least one elevated risk factor for CVD as a proxy for elevated oxidative stress. Participants were included if they had one of the following characteristics: 1) BMI ≥ 27, 2) pre-hypertension (Systolic BP ≥120 but <140, or Diastolic BP ≥ 80 but <90), 3) moderately elevated LDL-cholesterol (≥130 mg/dL but <160 mg/dL) or low HDL-cholesterol (<40 mg/dL). Participants were excluded if they had a BMI ≥ 40, diabetes, renal disease, significant liver enzyme abnormality, were pregnant or lactating, were currently smoking, had a history of CVD, inflammatory disease, malignant neoplasm, clotting disorder, or were taking anti-inflammatory, lipid lowering or anti-hypertensive drugs. Participants who already consumed a diet high in fruits and vegetables (≥ 5 servings per day), as assessed by a 3-day food record at screening, were also excluded. Informed consent was obtained from all participants. The Stanford University Human Subjects Committee approved the study annually.
2.3 Intervention
Before randomization, participants were instructed to abstain from any antioxidant supplements, any supplement containing omega-3 fatty acids, and foods rich in or supplemented with antioxidants for the duration of the trial. Participants were then randomly assigned to one of three treatment arms for a total of eight weeks to continue their habitual diet (hereafter referred to as “usual”) and receive placebo pills (n=29, Usual/Plac), continue their usual diet and receive antioxidant supplement pills (n=29, Usual/Supp), or increase their daily intake of certain dietary antioxidants and take a placebo pill (n=30, Antiox-Food/Plac). Participants, data collectors and those assessing outcomes were blinded to pill assignment; all but participants were blinded to diet assignment. Participants assigned to the Antiox-Food group were educated about increasing their dietary antioxidant intake during a one-hour group class, followed by one one-hour individual sessions provided by the study’s registered dietitian. Participants were instructed to eat more vegetables, fruits, nuts/seeds/oils, and whole grains to achieve their daily antioxidant intake goal. In order to maximize adherence to the dietary modifications, the study dietitian instructed participants to fill out daily antioxidant intake log sheets, and gave them sample daily menus and handouts listing foods high in antioxidants and their content. In addition, she gave participants a supply of Brazil nuts to ensure adequate intake of selenium, as these nuts have a high selenium content. A second one-hour individual session was provided to those participants who were struggling to increase their dietary antioxidant intake. Study pills, containing the active supplements or placebo, were dispensed twice, beginning and mid-study, in bottles containing sufficient supplements for 4 weeks. The pills were provided by Nutrilite, Inc., and contained approximately 100 mg selenium, 100 mg vitamin C, 10 mg vitamin E (as mixed tocopherols), and 10 mg beta carotene (as mixed carotenoids). Antioxidant supplement dose and increased antioxidant-food intake was intentionally designed to be similar to habitual intake, so that in combination with habitual intake would double usual daily intake. Participants were instructed to follow the national guidelines for physical activity during the study.
2.4 Data Collection
Data were collected from questionnaires, blood samples, blood pressure assessment and anthropometric variables. Participants completed three on-study clinic visits: at baseline, 4 and 8 wk after randomization. Data were collected at the Stanford Prevention Research Center (SPRC) and the Stanford Clinical and Translations Research Unit (CTRU). At baseline and 4 wk, participants received a 4-wk supply of study capsules, and at 4 and 8 wk they returned used bottles for leftover capsule counting. At each visit, anthropometric variables, blood pressure, and a fasting blood sample were collected. Blood pressure was measured three times at two-minute intervals after resting for five minutes. The first measurement was discarded and the last two averaged. Blood samples were analyzed for three inflammatory markers– monocyte chemotactic protein-1 (MCP-1), interleukin-6 (IL-6), and soluble intercellular adhesion molecule-1 (sICAM-1). Participants completed three unannounced 24-hour dietary recalls at baseline, 4, and 8 weeks after randomization to assess adherence to dietary guidelines of the intervention. Participants also completed Stanford 7-day Physical Activity Recalls (PAR) prior to randomization and at the end of the study.
2.5 Biochemical analyses
Plasma concentrations of MCP-1, IL-6, and sICAM-1 were measured with high-sensitivity ELISA kits from R&D Systems in duplicate, with a minimal detectable concentration of 5.0 ng/L, 0.04 ng/L, and 96 fg/L, respectively. All samples from each person were analyzed on the same day and in the same assay. Inter- and intra-assay variation (expressed as CV%) were 7.4/7.8, 5.8/5.7, and 4.6/5.5, for IL-6, MCP-1, and sICAM-1, respectively. Carotenoids and tocopherols were measured by normal-phase high performance liquid chromatography[34] (ARUP, Salt Lake City). Blood concentrations of selenium and vitamin C were not analyzed due to the limited sensitivity of those assessments to changes in dietary intake [35–36].
2.6 Dietary data
Dietary intake data were collected by telephone-administered, 3-day, unannounced, 24-hour dietary recalls using Nutrition Data System for Research software, versions 4.05.33, 4.06.34, and 5.0.35 (Nutrition Coordinating Center, University of Minnesota, Minneapolis). The study registered dietitian who collected dietary intake data was trained and certified by the Nutrition Coordination Center. The recalls occurred on two weekdays and one weekend day per time point (baseline, 4 and 8 wk), on nonconsecutive days whenever possible. Local foods not found in the comprehensive database were added to the database manually. A “food amounts booklet” was used to assist participants with portion size estimation.
2.7 Randomization
Treatment randomization was done in blocks of 30 assignments (i.e., 10/arm for the three arms) drawing participant ID numbers from an envelope and matching to a predetermined random sequence of the assignments prepared for an n=90. Throughout the study period, the study coordinator was responsible for maintaining the master code list and for dispensing the sets of pills to the participants.
2.8 Statistical analyses
The primary aim of this study was to test whether blood changes in selected inflammatory markers (MCP-1, IL-6, and sICAM-1) from baseline to 8 weeks were different in the supplement or diet group relative to placebo. Sample size determination of n=30/arm was based on power calculations using a power of 80% and a 2-sided test with an α of 0.05, and a modest to large effect size of d=0.75. The objective of randomizing 90 participants included an anticipated 15% attrition rate, allowing for a sample size of > 25 participants/arm. Linear mixed models[37] with a random effect for individual and fixed effects for time, treatment group, and time by treatment group interaction were employed to assess these changes over time. Linear mixed models can account for the correlated nature of within person changes while using all available data. For each outcome, the null hypothesis of no time by treatment interaction term in the model was tested by an F-test with two degrees of freedom in the numerator and Kenward-Roger denominator degrees of freedom[38] using the R package lmerTest [39]. When the F-test rejected this null hypothesis, differences in baseline to 8 week changes between groups were tested via the Westfall test [40] to account for multiple comparisons, as implemented in the R package multcomp [41]. All statistical tests were two-tailed with type 1 error assumed to be 0.05. All analyses were conducted using R version 3.3.2[42].
3. RESULTS
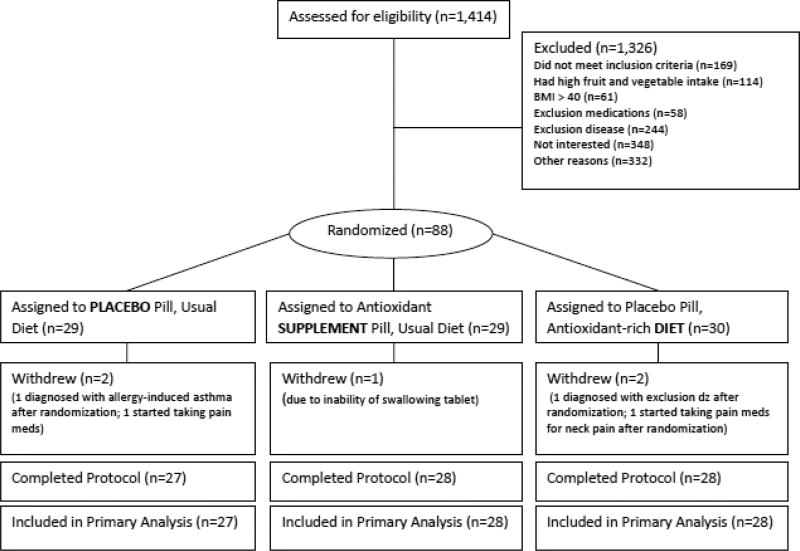
The participant flow is shown in Figure 1. Eighty-eight participants were randomized to 1 of the 3 treatment groups, and 5 withdrew from the study (94% retention). Two participants started taking pain medications after randomization, 2 participants were diagnosed with exclusion diseases after randomization, and 1 participant dropped out due to inability to swallow study tablets.
Figure 1.
Diagram illustrates the selection and participation of subjects
3.1 Baseline characteristics
The participants’ baseline characteristics are shown in Table 1. Baseline characteristics were similar among all three arms of the study with no significant differences in age, race, body mass index (BMI) or cardiovascular risk factors. Participants were middle aged adults, age 51 ± 10 years, [means ± standard deviation (SD)], with an average BMI of 27 ± 4 kg/ m2, predominantly women (64%), and Caucasian (69%).
Table 1.
Baseline Participant Characteristics
| Usual/Plac | Usual/Supp | Antiox- Food/Plac |
|
|---|---|---|---|
| Characteristics | |||
| Demographics | |||
| Sex, Women, [n (%)] | 19 (66%) | 19 (66%) | 18 (60%) |
| Age (y) | 52 ± 9 | 52 ± 11 | 49 ± 10 |
| Education (y) | 18 ± 2 | 17 ± 4 | 18 ± 2 |
| Race/Ethnicity [n (%)] | |||
|
| |||
| White | 22 (76%) | 21 (72%) | 18 (60%) |
| Asian | 6 (21%) | 5 (17%) | 9 (30%) |
| Hispanic | 0 | 1 (3%) | 2 (7%) |
| Black | 1 (3%) | 2 (7%) | 1 (3%) |
| Anthropometrics | |||
| Body mass index (kg/m2) | 27 ± 3 | 27 ± 5 | 27 ± 4 |
| Cardiovascular disease risk factors | |||
| LDL-C (mg/dL) | 134 ± 30 | 136 ± 30 | 127 ± 26 |
| HDL-C (mg/dL) | 60 ± 15 | 63 ± 12 | 56 ± 11 |
| Triglycerides (mg/dL) | 116 ± 48 | 109 ± 53 | 130 ± 68 |
| Blood pressure (mm HG) | |||
|
| |||
| Systolic | 116 ± 15 | 118 ± 20 | 116 ± 17 |
| Diastolic | 70 ± 10 | 72 ± 12 | 67 ± 8 |
All values are means ± SD, unless otherwise indicated.
Usual/Plac, placebo group (usual diet and placebo pills), n=29; Usual/Supp, supplement group (usual diet and antioxidant supplement), n=29; Antiox-Food/Plac, diet group (antioxidant-rich diet and placebo pills), n=30.
3.2 Capsule adherence and antioxidant pill content
Study capsule adherence was high in all groups with a mean across all three arms of approximately 74% (1 pill/day): Usual/Plac 75 ± 12%, Usual/Supp 74% ± 12%, and Antiox-Food/Plac 72% ± 13% (means ± SD). Twenty-five percent of the participants took 80% or more pills, 63% took between 61 and 79%, and the rest took between 43 and 60%. An independent external laboratory confirmed antioxidant supplement pill content. The antioxidant content and change over an 18-month period are shown in Supplemental Table S1. With the exception of an unexplained and perhaps artifactual increase in selenium, and a modest decrease in alpha-carotene, the pill antioxidant content remained fairly stable over 18 months.
3.3 Diet and physical activity data
Completion of dietary recalls was high; 98% of diet recalls (747 recalls) were completed by the 83 participants who finished the study. Energy intake between baseline and 8 wk did not differ among groups. Dietary intake of antioxidants (e.g. non-supplement intake) at baseline and 8 wk are shown in Table 2. As expected, and by design, the Usual/Plac and Usual/Supp groups show non-significant and similar changes between baseline and 8 wk. In contrast, the findings demonstrate expected significant differences 8-week increases for the Antiox-Food/Plac group relative to the other two diet arms in most but not all cases. Dietary intake data show participants in this group achieved a 73% increase in carotenoids, a 20% increase in tocopherols, a 135% increase in selenium, and a 161% increase in vitamin C. Analyses of 7-day PAR data showed no within or between group differences in energy expenditure throughout the study (data not presented). The dietary intake for individual carotenoids and tocopherols at baseline and 8 weeks is presented in Supplemental Table S2.
Table 2.
Dietary intake of antioxidants at baseline and 8 weeks1
| Antioxidant | Usual/Plac | Usual/Supp | Antiox-Food/Plac | P value* |
|---|---|---|---|---|
| Total Carotenoids (µg/d) | ||||
| Baseline | 12664 ± 1223 | 8178 ± 746 | 14955 ± 2054 | |
| 8 weeks | 12632 ± 1233 | 13561 ± 3803 | 25849 ± 2850 | .061 |
| Alpha carotene (µg/d) | ||||
| Baseline | 640 ± 106 | 491 ± 105 | 598 ± 142 | |
| 8 weeks | 659 ± 131 | 1680 ± 1062 | 1236 ± 304 | .462 |
| Beta carotene (µg/d) | ||||
| Baseline | 3633 ± 325 | 3066 ± 408 | 3891 ± 531 | |
| 8 weeks | 4356 ± 782 | 5290 ± 2284 | 9478 ± 1531 | .130 |
| Lutein (µg/d) | ||||
| Baseline | 2450 ± 289 | 2096 ± 258 | 2826 ± 494 | |
| 8 weeks | 2558 ± 416a | 2005 ± 286a | 6157 ± 1268b | .010 |
| Lycopene (µg/d) | ||||
| Baseline | 5706 ± 1000 | 2355 ± 391 | 7144 ± 1579 | |
| 8 weeks | 4836 ± 675 | 4344 ± 556 | 8170 ± 1349 | .190 |
| Cryptoxanthin (µg/d) | ||||
| Baseline | 234 ± 46 | 170 ± 35 | 496 ± 252 | |
| 8 weeks | 223 ± 44 | 242 ± 108 | 809 ± 145 | .444 |
| Total Tocopherols (mg/d) | ||||
| Baseline | 24.5 ± 1.9 | 24.7 ± 1.7 | 28.8 ± 2.1 | |
| 8 weeks | 24.7 ± 2.8 | 24.0 ± 1.5 | 34.9 ± 2.1 | .053 |
| Alpha Tocopherol (mg/d) | ||||
| Baseline | 9.1 ± 0.6 | 8.0 ± 0.6 | 10.7 ± 1.1 | |
| 8 weeks | 9.8 ± 1.1a | 8.7 ± 0.9a | 17.3 ± 1.1b | .0004 |
| Beta Tocopherol (mg/d) | ||||
| Baseline | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | <.0001 |
| 8 weeks | 0.4 ± 0.0a | 0.4 ± 0.0a | 1.0 ± 0.1b | |
| Gamma Tocopherol (mg/d) | ||||
| Baseline | 13.3 ± 1.4 | 14.7 ± 1.3 | 15.5 ± 1.3 | .865 |
| 8 weeks | 12.7 ± 1.9 | 13.2 ± 1.2 | 14.9 ± 1.2 | |
| Delta Tocopherol (mg/d) | ||||
| Baseline | 1.8 ± 0.3 | 1.6 ± 0.2 | 2.0 ± 0.3 | .334 |
| 8 weeks | 1.7 ± 0.4 | 1.7 ± 0.2 | 1.6 ± 0.2 | |
| Selenium (µg/d) | ||||
| Baseline | 129 ± 9 | 117 ± 7 | 122 ± 7 | |
| 8 weeks | 129 ± 14a | 120 ± 7a | 287 ± 24b | <.0001 |
| Vitamin C (mg/d) | ||||
| Baseline | 95 ± 9 | 84 ± 7 | 107 ± 13 | |
| 8 weeks | 98 ± 9a | 79 ± 7a | 280 ± 23b | <.0001 |
All values are means ± SEM.
Dietary intake reflects intake from foods only, and excludes intake from supplements Usual/Plac, placebo group (usual diet and placebo pills), n=29; Usual/Supp, supplement group (usual diet and antioxidant supplement), n=29; Antiox-Food/Plac, diet group (antioxidant-rich diet and placebo pills), n=30.
Obtained using a test for treatment by time interaction in a linear mixed model. When this effect was significant (p<.05), shared superscripts indicate no difference in baseline to 8 week changes between groups by Westfall’s test for multiple comparisons; no shared superscripts indicate significant difference.
3.4 Blood concentrations
of antioxidants For ease of presentation, we report baseline and 8-wk data only (i.e., data are available for mid-study blood sample analyses but were consistent with 8-wk results). Table 3 shows blood measures total carotenoids and tocopherols at baseline and 8 wk. Carotenoids more than doubled in the Usual/Supp group, almost doubled in the Antiox-Food/Plac group, and both were significantly increased relative to the Usual/Plac group. Alpha- and beta-carotene also doubled or tripled in the treatment groups, with no change in the Usual/Plac group. Alpha-tocopherol increased in the Supp/Plac group, but not in the other two groups; neither gamma-tocopherol nor total tocopherols were observed to increase significantly in either of the active treatment groups.
Table 3.
Blood measures of carotenoids and tocopherols at baseline and 8 weeks
| Usual/Plac | Usual/Supp | Antiox- Food/Plac |
P value* | |
|---|---|---|---|---|
| Total carotenoids (µg/L) | ||||
| Baseline | 713 ± 85 | 632 ± 62 | 474 ± 39 | |
| 8 weeks | 615 ± 74a | 1439 ± 153b | 765 ± 108c | <.0001 |
| Alpha carotene (µg/L) | ||||
| Baseline | 141 ± 32 | 115 ± 18 | 80 ± 9 | |
| 8 weeks | 117 ± 21a | 215 ± 47b | 164 ± 32b | .007 |
| Beta carotene (µg/L) | ||||
| Baseline | 384 ± 57 | 318 ± 43 | 220 ± 24 | |
| 8 weeks | 328 ± 52a | 1040 ± 110b | 407 ± 67c | <.0001 |
| Lutein (µg/L) | ||||
| Baseline | 158 ± 10 | 162 ± 13 | 145 ± 14 | |
| 8 weeks | 142 ± 11 | 150 ± 10 | 160 ± 17 | .080 |
| Zeaxanthin (µg/L) | ||||
| Baseline | 30 ± 3 | 29 ± 3 | 28 ± 2 | |
| 8 weeks | 27 ± 2 | 35 ± 3 | 35 ± 4 | .051 |
| Total tocopherols (mg/L) | ||||
| Baseline | 16.1 ± 0.5 | 15.7 ± 0.5 | 15.8 ± 0.5 | |
| 8 weeks | 15.9 ± 0.5 | 16.8 ± 0.5 | 15.8 ± 0.6 | .092 |
| Alpha-tocopherol (mg/L) | ||||
| Baseline | 14.6 ± 0.5 | 13.9 ± 0.4 | 14.2 ± 0.5 | |
| 8 weeks | 14.4 ± 0.5a | 15.2 ± 0.5b | 14.3 ± 0.6ab | .028 |
| Gamma-tocopherol (mg/L) | ||||
| Baseline | 1.5 ± 0.2 | 1.8 ± 0.1 | 1.6 ± 0.1 | |
| 8 weeks | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | .065 |
All values are means ± SEM.
Usual/Plac, placebo group (usual diet and placebo pills), n=29; Usual/Supp, supplement group (usual diet and antioxidant supplement), n=29; Antiox-Food/Plac, diet group (antioxidant-rich diet and placebo pills), n=30.
Obtained using a test for treatment by time interaction in a linear mixed model. When this effect was significant (p<.05), shared superscripts indicate no difference in baseline to 8 week changes between groups by Westfall’s test for multiple comparisons; no shared superscripts indicate significant difference.
3.5 Inflammatory markers
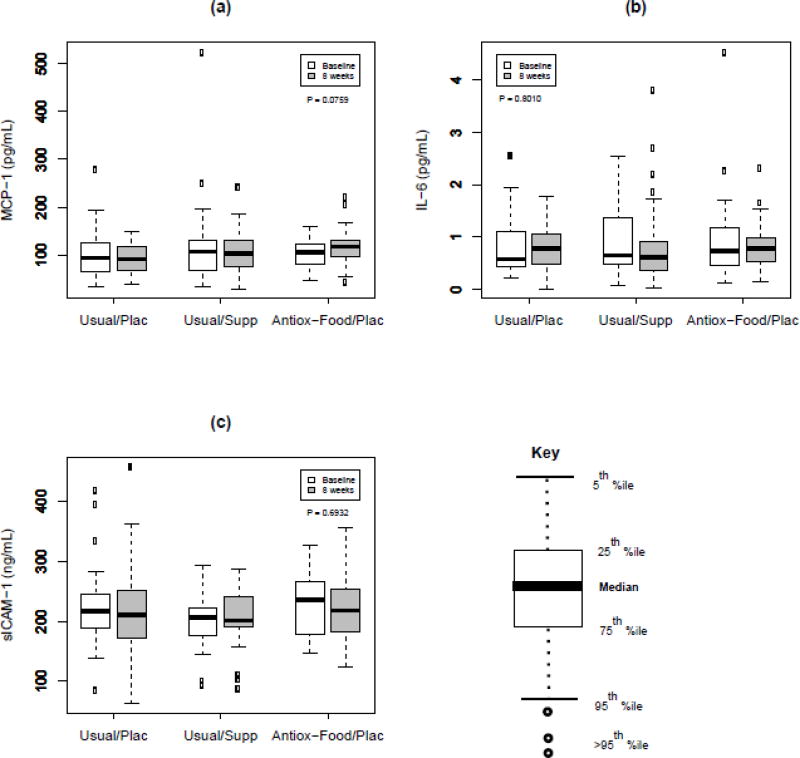
Baseline values of inflammatory markers across all three groups combined were 110 ± 65 pg/mL for MCP-1, 0.9 ± 0.7 pg/mL for IL-6, and 217 ± 56 ng/mL for sICAM-1 (means ± SD). The post-intervention inflammatory marker results are illustrated in Figure 2. After eight weeks of treatment, there were no significant changes in MCP-1, IL-6, or sICAM-1 among the three arms of the study. Changes in concentrations from baseline to 8 wk (mean and 95% confidence interval) for MCP-1 were: −10 (−31, 10), −12 (−32, 7), 12 (−8, 33) for the Usual/Plac, Usual/Supp, and Antiox-Food/Plac groups, respectively; for IL-6: −0.1 (−0.3, 0.2), 0.0 (−0.3, 0.2), −0.1 (−0.3, 0.1), respectively; for sICAM-1: −2.5 (−17, 12), 4 (−10.5, 18), −2 (−17, 12), respectively.
Figure 2.
Box plots of MCP-1 (a), IL-6 (b), and sICAM-1 (c) by time point and treatment. P-value is test of time by treatment interaction in linear mixed model. Usual/Plac, placebo group (usual diet and placebo pills); Usual/Supp, supplement group (usual diet and antioxidant supplement); Antiox-Food/Plac, diet group (antioxidant-rich diet and placebo pills).
4. Discussion
In this study, we sought to determine whether antioxidants from either food or supplement sources that essentially doubled the daily habitual intake, would lower blood concentrations of selected markers of inflammation (IL-6, MCP-1, and sICAM-1) in adults with at least one elevated risk factor for CVD after eight weeks of treatment. Our results do not support this hypothesis. There were no significant changes in any of the three inflammatory markers investigated, regardless of the source of antioxidants from diet or supplements, despite evidence from blood sampling and dietary records that overall antioxidant intake was substantially increased.
Our study compared an antioxidant-rich diet to supplements, whereas most previous studies in this field have focused on one or the other delivery strategies. Clinical trials investigating the effect of different antioxidant supplements on markers of inflammation have mostly reported null findings[13–18]. For example, Shargorodsky et al.[18] used a combination of antioxidant supplements similar to that used in our study (vitamin C, vitamin E, selenium, and coenzyme Q-10) in a population with at least two risk factors for CVD, but failed to show a significant change in high-sensitivity CRP after six months of treatment. Egert et al. also reported no significant changes in CRP or TNF-α in adults with the metabolic syndrome who took 150 mg quercetin daily for 6 weeks[14]. Similarly, there were no significant changes in CRP, IL-6 or tumor necrosis factor (TNF)-α in men with high cholesterol taking alpha-tocopherol and vitamin C for 3 years[17]. In contrast, Devaraj et al.[15] observed a significant decrease in CRP and tumor necrosis factor (TNF) -α in subjects with the metabolic syndrome who took a combination of alpha-tocopherol (800 mg/d) and gamma-tocopherol (800 mg/d) for six weeks. No change was detected in the groups taking only one of the two supplements, suggesting a high dosage may be needed to observe a significant effect on inflammatory markers.
Clinical trials investigating antioxidant-rich foods have had mixed but largely positive results. In a cross-over feeding study, Rajaram et al.[19] observed a reduction in CRP and E-selectin, but not IL-6, in healthy participants consuming a low or high intake of almonds compared to a heart-healthy control diet without nuts. Similarly, postmenopausal women consuming a diet rich in soy protein or soy nuts (30 g/day) for eight weeks experienced significant reductions in CRP, TNF-α, E-selectin, and interleukin-18 (IL-18), but not in other markers analyzed[22]. Jenkins et al. failed to show a significant effect of soy foods providing ~50 g protein per day on CRP, TNF-α, IL-6, and serum amyloid A (SAA) in hypercholesterolemic adults[27]. Clinical trials investigating wine, on the other hand, have shown positive effects of moderate daily wine consumption on several inflammatory markers in healthy men and women[24,25].
More interestingly, studies of diet patterns, such as the Mediterranean diet or other diets rich in antioxidants, have been consistent in showing significant reductions in CRP and interleukins[29–33]. The effects have been attributed to either a very high intake of fruits and vegetables (8 servings/day compared to 5 or 2 servings per day)[31], or a diet with high antioxidant capacity, compared to one with similar quantities of fruits and vegetables but with lower antioxidant capacity[30]. It is possible that our study, in spite of being successful in doubling the participants’ baseline level of antioxidant intake, did not reach a high enough level of antioxidants to exert significant changes in the inflammatory markers investigated. Among the studies in our literature review, only one reports blood antioxidant levels[31]. After consuming a diet with either two, five, or eight daily servings of carotenoid-rich fruits and vegetables, participants had mean blood levels of total carotenoids between 1.6 and 3.0 µmol/L, compared to 1.4 µmol/L (765 mg/L) in the Antiox-Food/Plac group. The Usual/Supp reached concentrations in the range of this study (2.7 µmol/L/1,439 mg/L), but failed to elicit a decrease in selected inflammatory markers.
A potentially important difference between studies in this field is a differential in starting, baseline levels of inflammatory markers, and the room for improvement. Participants in the current study were not screened for concentrations of inflammatory markers in order to determine eligibility. Rather, they were enrolled on the basis of meeting the criterion of at least one elevated risk factor for CVD, a condition that has been associated with chronic inflammation[1]. This resulted in enrolling some participants that had relatively low inflammatory marker concentrations to begin with, with little room for improvement. In the soy protein and soy nuts study cited previously[22], mean IL-6 concentrations after the 8-week intervention (during which they decreased from baseline values) were between 1.7 and 1.8 pg/ml, or about double the baseline value in our study (0.9 ± 0.7 pg/mL). Eight-week concentrations of sICAM-1 were also higher in that study (range: 280–286 ng/mL), compared to baseline values in our study (217 ± 56 ng/mL). Similarly, in an investigation of a Mediterranean diet pattern and omega-3 acid supplementation on inflammatory markers, Trøseid et al.[29] enrolled subjects with mean baseline IL-6 concentrations between 1.5 and 1.6 pg/ml, and mean baseline MCP-1 values approximately 4-fold those in our study, 429–442 and 110 ± 65 pg/mL, respectively.
There were several strengths in the design and conduct of the current study: enrolling participants with elevated risk for CVD and therefore room for health improvement; comparing food versus supplement sources of antioxidants; and achieving double daily habitual dose of antioxidant intake for most of the antioxidants in both diet and supplement groups. In addition, the retention rate and adherence to the study protocol were excellent. Finally, the assessment of diet and supplement intake of antioxidants indicated that these potentially confounding factors remained stable from baseline to the end of the trial.
The trial also included several limitations. An important limitation was the observed finding that not all four antioxidants were similarly doubled, on average, in the supplement arm (as assessed by blood concentrations), or the diet arm (as assessed by diet assessment and blood assessment). In some cases the increases were in the right direction but not statistically significant. In other cases the lack of observed increases were for some of the minor carotenoids. Blood concentrations of overall tocopherols were observed to show negligible changes, despite indications of significant changes by dietary assessments. However, by design the study targeted increasing four dietary antioxidants simultaneously, and by doing this the intervention did achieve an substantial increase in overall dietary antioxidant intake. Another limitation is that the inflammatory markers investigated in this trial may not have been the most suitable to capture the effects of antioxidants, as numerous inflammatory markers have been documented in the literature [43]. Also, we did not try to account for other potential nutrient or phytochemical changes in diet, aside from antioxidant intake, that may have affected inflammatory markers in the Antiox-Food/Plac group, as this was likely different for different individuals within that group; the level of complexity involved with accounting for all other potential dietary factors was beyond the scope of this analysis. Finally, these results can only be generalized to the specific study population enrolled in this study and may not be extended to other individuals that may have different underlying levels of chronic inflammation, other race/ethnic or groups, etc.
Although this study did not show any significant effect of antioxidant supplementation or dietary intake of antioxidant-rich foods on the inflammatory markers studied, there may be other mechanisms involved that are not well understood and should be the focus of future study. As found in this trial, antioxidant supplementation or consumption of antioxidant-rich foods did not lower concentrations of the specific markers of inflammation investigated. The reason for this finding may be due to multiple factors including the population studied, the dosage used, or the specific inflammatory markers chosen. It is possible that antioxidants may have a significant anti-inflammatory effect in populations with higher baseline levels of inflammation, or that higher doses may be required in order to observe any effect. Growing awareness of the important role of inflammation in the development of atherosclerosis and CVD has prompted the investigation of a plethora of biomarkers of inflammation as predictors of CVD risk. However, more research is needed to clarify which markers are most relevant to CVD risk and most reliably and accurately measured using standardized assays[43,44].
In conclusion, adults with at least one elevated CVD risk factor, doubling their dietary intake of antioxidants from either food or supplement sources for eight weeks, did not show a reduction in the blood concentrations of selected inflammatory markers. Further research is warranted to better elucidate the mechanism of action of antioxidants from dietary or supplement sources.
Supplementary Material
Acknowledgments
Authors would like to thank Gretchen George for assessing dietary composition. Authors report no conflict of interest. This investigation was supported by National Institute of Health [grant number 1R21AT003245-01]; supported in part by the Clinical and Translational Science Award [award number 1UL1 RR025744] for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, National Institutes of Health. The funding sources had no involvement in the design or the study; collection, analysis or interpretation of the data; or decision to submit the article for publication.
Abbreviations
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- FDA
Federal Drug Administration
- IL-6
Interleukin-6
- MCP-1
Monocyte chemotactic protein-1
- SAA
Serum amyloid A
- sICAM-1
Soluble intercellular adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis - An Inflammatory Disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Visioli F, Poli A. Modulation of Inflammation by Nutritional Interventions. Curr Atheroscler Rep. 2008;10:451–3. doi: 10.1007/s11883-008-0069-0. [DOI] [PubMed] [Google Scholar]
- 3.Tribble DL. Antioxidant Consumption and Risk of Coronary Heart Disease: Emphasis on Vitamin C, Vitamin E, and β-Carotene. Circulation. 1999;99:591 LP–595. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 4.Díaz MN, Frei B, Vita JA, Keaney JF. Antioxidants and Atherosclerotic Heart Disease. N Engl J Med. 1997;337:408–16. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food & Drug Administration. Guidance for Industry: Food Labeling; Nutrient Content Claims; Definition for “High Potency” and Definition for “Antioxidant” for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods; Small Entity Compliance Guide. [accessed January 30, 2017]; n.d. https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm063064.htm.
- 6.Helmersson J, Arnlöv J, Larsson A, Basu S. Low dietary intake of beta-carotene, alpha-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Br J Nutr. 2009;101:1775–82. doi: 10.1017/S0007114508147377. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee SG, Lowe GDO, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006;83:567–74. doi: 10.1093/ajcn.83.3.567. doi:83/3/567 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Brighenti F, Valtueña S, Pellegrini N, Ardigò D, Del Rio D, Salvatore S, et al. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. 2005;93:619–25. doi: 10.1079/BJN20051400. [DOI] [PubMed] [Google Scholar]
- 9.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 10.Salas-Salvadó J, Garcia-Arellano a, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62:651–9. doi: 10.1038/sj.ejcn.1602762. [DOI] [PubMed] [Google Scholar]
- 11.Wu SH, Shu XO, Chow W-H, Xiang Y-B, Zhang X, Li H-L, et al. Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet. 2012;112:996–1004. 1004–4. doi: 10.1016/j.jand.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA study. J Am Coll Cardiol. 2004;44:152–8. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Bakker GC, van Erk MJ, Pellis L, Wopereis S, Rubingh CM, Cnubben NH, et al. An antiinflammatory dietary mix modulates inflammation and oxidative\nand metabolic stress in overweight men: a nutrigenomics approach. Am J Clin Nutr. 2010;91:1044–59. doi: 10.3945/ajcn.2009.28822. [DOI] [PubMed] [Google Scholar]
- 14.Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–74. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 15.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008;44:1203–8. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega-López S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with ω3 Polyunsaturated Fatty Acids and all-rac Alpha-Tocopherol Alone and in Combination Failed to Exert an Anti-inflammatory Effect in Human Volunteers. Metabolism. 2004;53:236–40. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Bruunsgaard H, Poulsen HE, Pedersen BK, Nyyssönen K, Kaikkonen J, Salonen JT. Long-term combined supplementations with α-tocopherol and vitamin C have no detectable anti-inflammatory effects in healthy men. J Nutr. 2003;133:1170–3. doi: 10.1093/jn/133.4.1170. [DOI] [PubMed] [Google Scholar]
- 18.Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr {&} Metab. 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutr. 2010;103:907–12. doi: 10.1017/S0007114509992480. [DOI] [PubMed] [Google Scholar]
- 20.Beavers KM, Serra MC, Beavers DP, Cooke MB, Willoughby DS. Soymilk supplementation does not alter plasma markers of inflammation and oxidative stress in postmenopausal women. Nutr Res. 2009;29:616–22. doi: 10.1016/j.nutres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Rankin JW, Andreae MC, Chen CYO, O’Keefe SF. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes, Obes Metab. 2008;10:1086–96. doi: 10.1111/j.1463-1326.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 22.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: A cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30:967–73. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 23.Blum A, Monir M, Peleg A, Blum N. Tomato-rich (Mediterranean) diet does not modify inflammatory markers. Clin Investig Med. 2007;30:70–5. doi: 10.25011/cim.v30i2.982. [DOI] [PubMed] [Google Scholar]
- 24.Vázquez-Agell M, Sacanella E, Tobias E, Monagas M, Antúnez E, Zamora-Ros R, et al. Inflammatory markers of atherosclerosis are decreased after moderate consumption of cava (sparkling wine) in men with low cardiovascular risk. J Nutr. 2007;137:2279–84. doi: 10.1093/jn/137.10.2279. 137/10/2279 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Avellone G, Di Garbo V, Campisi D, De Simone R, Raneli G, Scaglione R, et al. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. Eur J Clin Nutr. 2006;60:41–7. doi: 10.1038/sj.ejcn.1602265. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DS, Rasooly R, Jacob Ra, Kader Aa, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136:981–6. doi: 10.1093/jn/136.4.981. doi:136/4/981 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJA, Kendall CWC, Connelly PW, Jackson CJC, Parker T, Faulkner D, et al. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51:919–24. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
- 28.Karlsen A, Paur I, B??hn SK, Sakhi AK, Borge GI, Serafini M, et al. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49:345–55. doi: 10.1007/s00394-010-0092-0. [DOI] [PubMed] [Google Scholar]
- 29.Trøseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism. 2009;58:1543–9. doi: 10.1016/j.metabol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Valtuena S, Pellegrini N, Franzini L, Bianchi MA, Ardigo D, Del Rio D, et al. Food selection based on total antioxidant capacity can modify antioxidant intake, systemic inflammation, and liver function without altering markers of oxidative stress. Am J Clin Nutr. 2008;87:1290–7. doi: 10.1093/ajcn/87.5.1290. doi:87/5/1290 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Watzl B, Kulling SE, Möseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052–8. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins DJA, Kendall CWC, Marchie A, Faulkner DA, Josse AR, Wong JMW, et al. Direct comparison of dietary portfolio vs statin on C-reactive protein. Eur J Clin Nutr. 2005;59:851–60. doi: 10.1038/sj.ejcn.1602152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome - A randomized trial. JAMA. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong NC, Paganga G, Brunner E, Miller NJ, Shipleyb M, Rice-evans CA, et al. Reference Values for α -Tocopherol and β -Carotene in the Whitehall II Study Reference Values for a-Tocopherol and p-Carotene in the Whitehall I1 Study. 2009:5762. doi: 10.3109/10715769709097853. [DOI] [PubMed] [Google Scholar]
- 35.Loria CM, Whelton PK, Caulfield LE, Szklo M, Klag MJ. Agreement among indicators of vitamin C status. Am J Epidemiol. 1998 Mar 15;147(6):587–96. doi: 10.1093/oxfordjournals.aje.a009491. [DOI] [PubMed] [Google Scholar]
- 36.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr. 2009 Jun;89(6):2025S–2039S. doi: 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- 37.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 38.Kenward MG, Roger JH. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics. 1997;53:983–97. doi: 10.2307/2533558. [DOI] [PubMed] [Google Scholar]
- 39.Kuznetsova Alexandra, Brockhoff Per Bruun, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. [accessed January 1, 2017];R package version 2.0-32. 2016 https://cran.r-project.org/package=lmerTest%0D.
- 40.Westfall PH. Multiple Testing of General Contrasts Using Logical Constraints and Correlations. J Am Stat Assoc. 1997;92:299–306. doi: 10.1080/01621459.1997.10473627. [DOI] [Google Scholar]
- 41.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50:346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 42.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- 43.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. doi:54/1/24 [pii]\r10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 44.Koenig W. Update on integrated biomarkers for assessment of long-term risk of cardiovascular complications in initially healthy subjects and patients with manifest atherosclerosis. Ann Med. 2009;41:332–43. doi: 10.1080/07853890902769675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.