Figure 10.
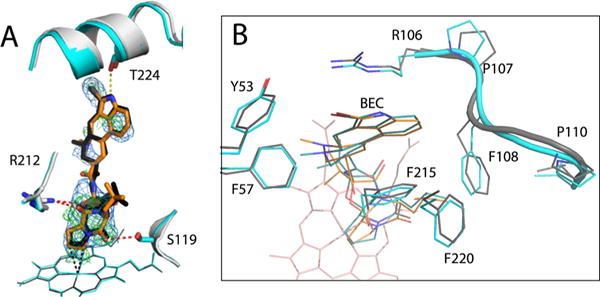
Crystal structure of the BEC-bound Cys-less CYP3A4. (A) Structural superposition of the BEC-bound forms of Cys-less (gray and orange) and WT CYP3A4 (PDB entry 3UA1, cyan and black). In both structures, BEC binds in an extended and productive conformation. However, in Cys-less CYP3A4, the cyclic tripeptide moiety is closer to the heme and H-bonded to Ser119 and Arg212 (red dotted lines), whereas the lysergic group is farther from Thr224 and cannot form the H-bond observed in the 3UA1 structure (yellow dotted line). The 2Fo – Fc and simulated annealing Fo – Fc omit maps around BEC in Cys-less CYP3A4 are contoured at 1σ and 3σ and shown as blue and green mesh, respectively. (B) Top view of the active site showing residues that flank the lysergic moiety of BEC. In Cys-less CYP3A4, the 105–111 fragment is folded differently and Phe108 clashes with BEC’s lysergic group, forcing it to move aside and lose a H-bond connection with Thr224.
