Abstract
Objective
TNF system (TNF and its soluble receptors sTNFR1 and 2) has been investigated as a potential molecular target in bipolar disorder. The aim of the study was to compare plasma levels of these receptors in unmedicated bipolar depressed patients compared with healthy controls, and to evaluate the effects of a 6-week lithium treatment on sTNFR1 and sTNFR2 levels.
Methods
The study enrolled 29 patients with unmedicated bipolar disorder in a major depressive episode and 27 matched controls. Patients had blood collected at baseline and after 6 weeks of lithium treatment. The concentration of sTNFRs was measured by ELISA.
Results
sTNFR1 and sTNFR2 levels were significantly increased in bipolar depression in comparison with healthy subjects. Lithium treatment did not significantly change sTNFR1 and sTNFR2 levels from baseline to endpoint. There was no correlation between improvement in depressive symptoms and the change in sTNFR1 or sTNFR1 levels.
Conclusion
These results reinforce the involvement of an activated immune response system in the pathophysiology of bipolar disorder, with no impact of lithium treatment on the related biomarkers.
Keywords: bipolar disorder, bipolar depression, lithium, sTNFR1, sTNFR2
INTRODUCTION
Several lines of evidence have pointed out the role of immune system dysfunction in the pathophysiology of bipolar disorder (BD) (Teixeira et al., 2013; Barbosa et al., 2014). For instance, post-mortem brain studies in patients with BD have found elevated expression of inflammation related pro-apoptosis and oxidative stress genes (Gigante et al., 2011; Brown et al., 2014). In line with these findings, a recent positron emission tomography (PET) study reported an increase in [11C]-(R)-PK11195 binding potential, which is indicative of activated microglia, in the hippocampus of BD patients (Haarman et al., 2014). In the periphery, increased blood levels of inflammatory mediators are frequently described in subjects with BD across affective states (Munkholm et al., 2013a). Interestingly, TNF levels and its soluble receptor sTNFR1 were found to be more elevated in manic than euthymic patients (Munkholm et al., 2013b). There are much less available data on the immune/inflammatory profile in depression BD.
TNF has been investigated as a potential molecular target in BD (Brietzke and Kapczinski, 2008), and its circulating levels were found to correlate with cognitive impairment in patients (Barbosa et al., 2012; Doganavsargil-Baysal et al., 2013). Indeed TNF seems to play a modulatory role in the brain, influencing glutamate neurotransmission and excitotoxicity (McAfoose and Baune, 2009). TNF exerts its main effects by binding to the receptors TNFR1 and TNFR2. There are soluble forms of these receptors (sTNFR1 and sTNFR2) that, despite not transducing signals, are able to modulate the biological activity of TNF by protecting the molecule against proteolytic degradation and prolonging its half-life (Diez-Ruiz et al., 1995). Moreover, their measurement is useful to determine the overall production of TNF. As TNF is less stable than its soluble receptors, sTNFR1 and sTNFR2 are suitable markers of TNF activity and, as consequence, of inflammatory activity (Coelho et al., 2008; Barbosa et al., 2011).
Lithium is the gold standard agent for the treatment of BD, including bipolar depression (Yatham et al., 2013). Its mechanisms of action are complex, influencing multiple signaling pathways (Machado-Vieira et al., 2009, 2014). A growing body of evidence suggests that lithium may also have an anti-inflammatory role (Nassar and Azab, 2014), being possible that its therapeutic effects might be associated with changes in inflammatory parameters in BD.
Therefore the aim of the current study was to compare the plasma levels of sTNFR1 and sTNFR2 between unmedicated bipolar depression and healthy subjects, and to evaluate the effect of a 6-week lithium treatment on sTNFR1 and sTNFR2 levels. We hypothesized that sTNFR1 and sTNFR2 levels would be elevated in bipolar depression compared with healthy control subjects and that lithium treatment would decrease their levels.
METHODS
Subjects
This study was conducted in the outpatient clinic of the Institute of Psychiatry, University of Sao Paulo, Brazil. To be enrolled, patients were required to have a score ≥18 on the 21-item Hamilton Depression Scale (HAM-D) (Hamilton, 1960). BD patients were excluded if they presented with an unstable medical condition, including infectious or autoimmune diseases, or if they had substance abuse or dependence in the last year.
The study enrolled 29 patients (M/F, 8/21; mean age ± SD, 28.4 ± 5.5) with BD in a major depressive episode; 11 (37.9%) had a diagnosis of type I BD and 18 (62.1%) type II BD. Most patients (n = 25, 86.2%) were drug-free for at least 6 weeks before their enrollment, and 21 (72.4%) were drug-naïve. The mean duration of illness was 36.6 months (±18.9). The diagnosis of BD was performed following the Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID) (First et al., 1997). Besides HAM-D, Young Mania Rating Scale (YMRS) (Young et al., 1978) was applied to assess the severity of symptoms.
For comparison, matched healthy subjects (n = 27) (M/F, 8/19; mean age ± SD, 28.0 ± 7.7) were recruited. Controls did not have lifetime history of any psychiatric disorder (as evaluated with the SCID) or the presence of mood or psychotic disorders in any first-degree relatives.
This study had approval by the local Ethics Committee. All participants provided written informed consent before entering the study.
Study protocol
At baseline, patients received lithium carbonate at 450 mg/day with subsequent adjustments according to the clinical status and serum lithium levels. The use of hypnotics (benzodiazepines or zolpidem) as needed for insomnia was allowed. Most patients were kept on lithium monotherapy; only four patients received lithium with concomitant mood stabilizers. Systematic psychometric assessments were performed at baseline and at endpoint (week 6). Response was defined as a decrease of 50% or more in the HAM-D at endpoint, while remission criteria was defined as a HAM-D <8 and YMRS <8 at endpoint.
Blood sampling and laboratorial analysis
Patients had blood collected at baseline and after 6 weeks of lithium treatment, while controls had blood collected only at one time point. Ten milliliters of blood after 8 h fasting was drawn from each subject by venipuncture into a sodium heparin tube on the same day of the clinical assessment. All procedures were performed between 8 and 10 am to minimize biological differences due to circadian rhythms. The blood was immediately centrifuged at 3000 g for 10 min. The plasma was collected and stored at −80 °C until assayed.
The concentration of sTNFRs was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) according to the procedures supplied by the manufacturer (DuoSet, R&D Systems, Minneapolis, MN), as routinely performed in our laboratory (Coelho et al., 2008; Barbosa et al., 2011, 2012; Rocha et al., 2014). All samples were assayed in duplicate, and the intra- and the inter-assay coefdicients of variability were below 5 and 10%, respectively. The detection limit of these assays was 10 pg/mL. Concentrations are expressed as pg/mL.
Statistical analysis
Kolmogorov–Smirnov test was used to check if the sample presented normal distribution. Between-group comparisons of the demographic variables were done using the Chi square test for categorical variables, and Mann–Whitney test for continuous variables. Wilcoxon Signed Ranks test was used to investigate changes in sTNFRs levels before and after lithium treatment. Correlations between variables were assessed with Spearman’s correlation coefficient. All tests were two-tailed with a significance level set at 0.05.
RESULTS
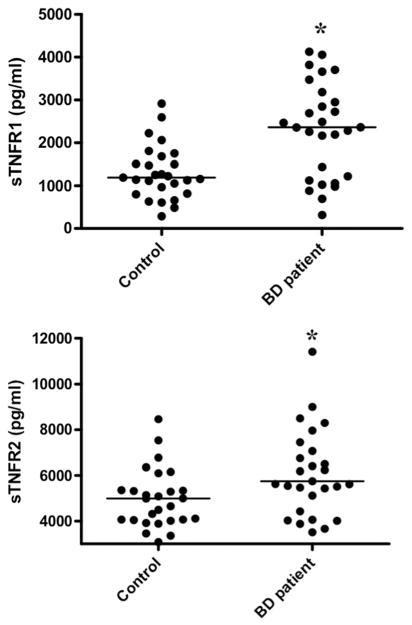
At baseline, sTNFR1 levels were increased in depressed BD patients (mean ± SD, 2584.1 ± 1857.4 pg/mL) in comparison with healthy subjects (1307.8 ± 627.8 pg/mL) (z = −3.38, p <0.001) (Figure 1). Baseline sTNFR2 levels also were elevated in patients (7006.9 ± 4274.1 pg/mL) in comparison with controls (4978.7 ± 1282.0 pg/mL) (z = −2.55, p = 0.007). The severity of depressive symptoms, as assessed by the HAM-D, did not correlate with sTNFR1 (p = 0.35) or sTNFR2 (p = 0.38). Interestingly, the length of the disease correlated with sTNFR1 levels (ρ = 0.37, p = 0.05), but not sTNFR2 (ρ = 0.30, p = 0.12). The levels of sTFNR1 and sTNFR2 were highly correlated (ρ = 0.69, p <0.001).
Figure 1.
Plasma levels of soluble TNF receptor 1 (sTNFR1) and 2 (sTNFR2) in 27 healthy controls and 29 bipolar depression subjects at baseline. When compared with controls, patients had increased levels of sTNFR1 and sTNFR2 levels. Horizontal lines represent median values
After lithium treatment, BD patients exhibited a significant decrease in symptoms severity (baseline mean ± SD HAM-D score: 22.6 ± 3.6; endpoint: 7.2 ± 6.0; z = −4.60, p <0.001). Response was observed in 24 (82.8%) patients, while remission occurred in 18 (62.1%) patients; and the mean (SD) serum lithium at endpoint was 0.49 (0.20) mEq/L.
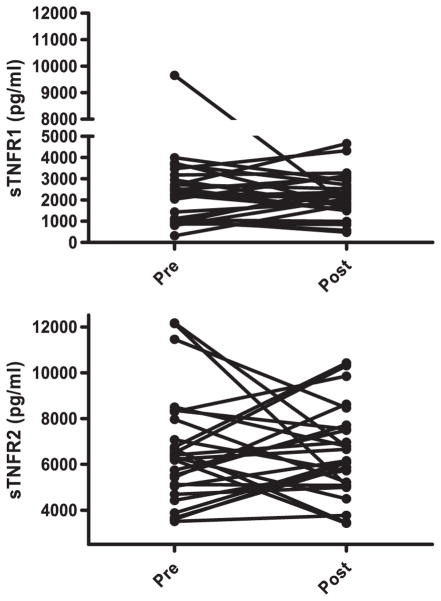
Lithium treatment did not significantly change sTNFR1 plasma levels from baseline (mean±SD, 2584.1 ±1857.4pg/mL) to endpoint (2445.8±1793.2pg/mL) (z=−0.89, p=0.37). Also, sTNFR2 levels were not significantly altered from baseline (7006.9±4274.1pg/mL) to endpoint (7027.3±2534.0pg/mL) (z=−1.09, p=0.27) (Figure 2).
Figure 2.
Plasma levels of soluble TNF receptor 1 (sTNFR1) and 2 (sTNFR2) in 29 bipolar depression patients before (pre) and after (post) lithium treatment for six weeks. Lithium treatment did not significantly change sTNFR1 and sTNFR2 levels
There was no correlation between the change in HAM-D scores and the change in sTNFR1 or sTNFR1 levels. There was no difference in sTNFR1 and sTNFR2 levels between responders/remitters and non-responders/non-remitters. Serum lithium did not correlate with endpoint sTNFR1 or endpoint sTNFR2. The use of hypnotics did not influence clinical or bio-marker measures.
DISCUSSION
This study is the first to assess sTNFR1 and sTNFR2 in unmedicated depressed BD patients. It is also the first study to evaluate the impact of a specific therapeutic strategy, i.e. lithium, on inflammatory biomarkers in BD.
We confirmed previous studies that reported increased circulating levels of sTNFR1 and sTNFR2 in BD patients (Barbosa et al., 2011; Hope et al., 2011; Tsai et al., 2012; Doganavsargil-Baysal et al., 2013). Barbosa et al. (2011) reported elevated plasma levels of sTNFR1 in BD patients in euthymia and in mania in comparison with controls. Manic patients had higher sTNFR1 levels than euthymic patients, and sTNFR1 levels correlated with the length of BD as also reported in the current study. Doganavsargil-Baysal et al. (2013) described increased plasma levels of sTNFR1 and sTNFR2 in BD patients in euthymia compared with controls.
Only a few studies have investigated the immune/inflammatory profile of bipolar depression (Munkholm et al., 2013b). TNF was assessed in three studies with depressed BD patients of which two described significantly elevated levels among patients in comparison with controls (O’Brien et al., 2006; Ortiz-Dominguez et al., 2007; Kapczinski et al., 2011). A single study evaluated sTNFR1 in depressed BD patients, reporting levels similar to healthy subjects (Hope et al., 2011). This latter study enrolled stable patients under treatment what could explain the lack of difference in sTNFR1 levels between depressed patients and controls.
One of the strengths of the current study is indeed the enrollment of a significant percentage of drug-naive patients, making possible the control of potential confounder of drugs on immune or inflammatory parameters. It is worth mentioning that our study also comprised young patients, with short duration of illness, increasing the specificity of findings, but preventing the extrapolation for other BD populations, notably to those older patients and/or with long-term disease. Other limitations of the study include the sample size and the limited assessment of circulating biomarkers.
The levels of these inflammatory markers did not change after lithium treatment and were not associated with clinical improvement. This result has two implications. First, sTNFR1 and sTNFR2, among other inflammatory mediators, may be regarded as BD trait biomarkers as they do not modify along with clinical changes. Second, the therapeutic effect of lithium may be independent of this inflammatory pathway, despite experimental evidence indicating that lithium may present both pro- and anti-inflammatory activities depending on the model studied (Nassar and Azab, 2014). In support of this assumption, previous studies have demonstrated increased sTNFR1 and sTNFR2 levels in euthymic BD patients treated with lithium and other mood stabilizing drugs (Barbosa et al., 2011; Doganavsargil-Baysal et al., 2013). Alternatively the inflammatory ‘signature’ in the blood may take longer time, i.e. over 6 weeks, to return to baseline levels. It is worth mentioning that patients with unipolar depression also exhibited increased circulating levels of sTNFR1 and sTNFR2 which is line with an activated TNF system in this condition (Grassi-Oliveira et al., 2009). Interestingly, treatment with serotonin and serotonin/noradrenaline reuptake inhibitors seems to decrease the circulating levels of TNF in depressed patients (Hannestad et al., 2011; Li et al., 2013). Moreover, one study reported that the responder group had a greater reduction in TNF compared with the non-responder group, indicating that TNF system might be regarded as a ‘target’ for antidepressant treatment (Li et al., 2013). On the other hand, a placebo-controlled study in unipolar depression revealed that the level of inflammatory molecules reduced not only in the active treatment groups, but also in the placebo arm (Brunoni et al., 2014). Whether these contrasting results reflect either distinct pathophysiological mechanisms in unipolar depression and bipolar depression or a specific anti-inflammatory property of antidepressants remains to be determined. Further, placebo-controlled studies assessing the impact of treatment on inflammatory molecules in bipolar depression are necessary.
In conclusion, these results reinforce the role played by an activated immune response system in BD. Further studies are needed to evaluate the effect of different mood stabilizing drugs on cytokines and other immune mediators, confirming or refuting their putative role as biomarkers and/or targets of treatment in BD.
Acknowledgments
This work was funded by CNPq, Minas Gerais Research Foundation (Fapemig), and Sao Paulo Research Foundation (Fapesp, Brazil 2009/14891-9).
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
References
- Barbosa IG, Huguet RB, Mendonna VA, et al. Increased plasma levels of soluble TNF receptor I in patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(2):139–143. doi: 10.1007/s00406-010-0116-z. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Rocha NP, Huguet RB, et al. Executive dysfunction in euthymic bipolar disorder patients and its association with plasma bio-markers. J Affect Disord. 2012;137(1–3):151–155. doi: 10.1016/j.jad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Machado-Vieira R, Soares JC, Teixeira AL. The immunology of bipolar disorder. Neuroimmunomodulation. 2014;21(2–3):117–122. doi: 10.1159/000356539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietzke E, Kapczinski F. TNF-alpha as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1355–1361. doi: 10.1016/j.pnpbp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014 doi: 10.1016/j.psychres.2014.04.005. pii: S0165–1781(14)00282-0. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Machado-Vieira R, Zarate CA, et al. Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): results from a factorial, randomized, controlled trial. Psychopharmacology (Berl) 2014;231(7):1315–1323. doi: 10.1007/s00213-013-3322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FM, Reis HJ, Nicolato R, et al. Increased serum levels of inflammatory markers in chronic institutionalized patients with schizophrenia. Neuroimmunomodulation. 2008;15(2):140–144. doi: 10.1159/000148197. [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54(1):1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Doganavsargil-Baysal O, Cinemre B, Aksoy UM, et al. Levels of TNF-alpha, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol. 2013;28(2):160–167. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Clinical Version (SCID-I/CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Gigante AD, Young LT, Yatham LN, et al. Morphometric postmortem studies in bipolar disorder: possible association with oxidative stress and apoptosis. Int J Neuropsychopharmacol. 2011;14(8):1075–1089. doi: 10.1017/S146114571000146X. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Brietzke E, Pezzi JC, Lopes RP, Teixeira AL, Bauer ME. Increased soluble tumor necrosis factor-alpha receptors in patients with major depressive disorder. Psychiatry Clin Neurosci. 2009;63(2):202–208. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Haarman BC, Riemersma-Van der Lek RF, de Groot JC, et al. Neuroinflammation in bipolar disorder - A [11C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.03.016. pii: S0889–1591(14) 00082-8. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45(12):1608–1616. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dal-Pizzol F, Teixeira AL, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011;45:156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Li Z, Qi D, Chen J, et al. Venlafaxine inhibits upregulation of plasma tumor necrosis factor-alpha (TNF--alpha in the Chinese patients in major depressive disorder: a prospective longitudinal study. Psychoneuroendocrinology. 2013;38(1):107–114. doi: 10.1016/j.psyneuen.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA., Jr The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J Biol Psychiatry. 2014;15(2):84–95. doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive functions. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013a;47(9):1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013b;144(1–2):16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Nassar A, Azab AN. Effects of Lithium on Inflammation. ACS Chem Neurosci. 2014 doi: 10.1021/cn500038f. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Ortiz-Dominguez A, Hernandez ME, Berlanga C, et al. Immune variations in bipolar disorder: phasic differences. Bipolar Disord. 2007;9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Rocha NP, Teixeira AL, Scalzo PL, et al. Palotder: phasic difPlasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov Disord. 2014;29(4):527–531. doi: 10.1002/mds.25752. [DOI] [PubMed] [Google Scholar]
- Teixeira AL, Barbosa IG, Machado-Vieira R, Rizzo LB, Wieck A, Bauer ME. Novel biomarkers for bipolar disorder. Expert Opin Med Diagn. 2013;7(2):147–159. doi: 10.1517/17530059.2013.734807. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Chung KH, Wu JY, Kuo CJ, Lee HC, Huang SH. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J Affect Disord. 2012;136(1–2):110–116. doi: 10.1016/j.jad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. doi: 10.1111/bdi.12025. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. Brit J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]