Abstract
Background:
Hemodialysis patients are at an increased risk of polypharmacy as they have the highest pill burden of all chronically ill patient populations, with an estimated average of 12 medications per day.
Objectives:
The aim of this study was to evaluate prescribing patterns of outpatient medications in patients receiving in-center hemodialysis. This was done to identify potential candidate medications for future quality improvement initiations to optimize prescribing.
Design:
We conducted a descriptive retrospective cross-sectional study in the province of Ontario, Canada, using several linked health care databases housed at the Institute for Clinical Evaluative Sciences (ICES).
Setting:
We considered outpatient medications dispensed to patients eligible for the Ontario Drug Benefit program.
Patients:
Patients were receiving chronic in-center hemodialysis at one of the 69 facilities in the province of Ontario, Canada as of October 1, 2013.
Measurements:
We assessed whether any of our 28 study medications of interest were recently dispensed (within the prior 120 days), the type of prescribing physician, and the associated medication costs. The 28 included medications of interest (ie, proton pump inhibitors, benzodiazepines) were selected because they may not have a true indication for dialysis patients and/or there are safety concerns with their use in this population. Results are presented as median (25th, 75th percentile).
Methods:
We conducted this study at ICES according to a prespecified protocol approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Toronto, Ontario).
Results:
A total of 3094 patients on chronic in-center hemodialysis received a study drug of interest (age: 76.5 years [SD: 7.3]), 44% women). Patients were dispensed 11 (8, 14) unique medication products with more than two-thirds of patients dispensed 9 or more different medications. The median number of annual health care visits was 7 (3-15) with more than half the cohort receiving prescriptions from 3 or more specialists. The 10 most commonly dispensed study medications cost more than 3 million dollars in direct costs in 1 year.
Limitations:
Our study was also subjected to some limitations of health care databases.
Conclusions:
Polypharmacy is frequent in in-center hemodialysis patients. To decrease polypharmacy and its associated negative outcomes, health care providers need to implement tools to optimize medication use and deprescribe medications that lack evidence for efficacy and safety in hemodialysis patients. Therefore, strategies to improve prescribing and discontinue ineffective medications warrant testing for better patient outcomes and reduced health care costs.
Keywords: polypharmacy, hemodialysis, deprescribing, demographics
Abrégé
Contexte:
Parmi la population atteinte d’une maladie chronique, le patient hémodialysé présente la charge médicamenteuse la plus lourde, soit une prise moyenne estimée à 12 médicaments distincts chaque jour.
Objectif:
Nous avons analysé les habitudes de prescription externe de médicaments chez les patients hémodialysés en centre hospitalier afin de cibler des médicaments qui pourraient représenter des cibles d’amélioration pour une prescription médicamenteuse optimisée.
Type d’étudeL:
Il s’agit d’une étude transversale, descriptive et rétrospective menée en Ontario, au Canada. L’étude tire ses données de plusieurs bases interreliées, hébergées à l’Institut de recherche en services de santé (IRSS).
Cadre de l’étude:
Notre analyse s’est concentrée sur les médicaments prescrits aux patients ambulatoires admissibles au Programme de médicaments de l’Ontario.
Participants:
Étaient considérés dans l’étude tous les patients hémodialysés à long terme à l’un des 69 établissements prodiguant l’hémodialyse en Ontario depuis le 1er octobre 2013.
Mesures:
Nous avons vérifié si l’un des 28 médicaments d’intérêt avait été administré (au cours des 120 jours précédents), et nous avons noté la spécialité du médecin prescripteur et les coûts associés au traitement. Les 28 médicaments considérés (p. ex. inhibiteurs de la pompe à protons, benzodiazépines) ont été sélectionnés pour au moins l’une des deux raisons suivantes : i) il n’existe pas de réelle indication pour le traitement de patients hémodialysés; ii) l’innocuité du médicament n’est pas claire pour la population étudiée. Les résultats sont présentés sous forme de médiane et de percentile (du 25e au 75e centile).
Méthodologie:
L’étude a été menée à l’IRSS conformément au protocole préalablement approuvé par le comité d’éthique en recherche du Centre des sciences de la santé Sunnybrook (à Toronto).
Résultats:
Un total de 3 094 patients hémodialysés prenaient au moins un médicament d’intérêt; 44 % étaient de sexe féminin, et l’âge moyen était de 76,5 ans (ÉT : 7,3). Les patients prenaient 11 (8 à 14) médicaments distincts, et plus des deux tiers d’entre eux en prenaient au moins neuf. Le nombre de visites médicales annuelles médian était de 7 (3 à 15). Plus de la moitié de la population à l’étude recevait des prescriptions médicamenteuses de trois prescripteurs ou plus. Les 10 médicaments les plus communément prescrits représentaient des coûts directs annuels de plus de 3 millions de dollars.
Limites:
Les bases de données en santé utilisées comportaient certaines contraintes pour notre étude.
Conclusion:
La polypharmacie est fréquente chez les patients hémodialysés en centre. Pour endiguer le phénomène et ses répercussions, les fournisseurs de soins de santé doivent implanter des outils d’optimisation de la médication chez les patients hémodialysés et cesser tout médicament dont l’innocuité ou l’efficacité n’ont pas été suffisamment démontrées chez ces patients. On devra donc tester des stratégies d’amélioration des habitudes de prescription pour atteindre de meilleurs résultats sur les plans de la santé du patient et des coûts de soins de santé.
What was known before
Patients on hemodialysis are exposed to polypharmacy as a consequence of their high pill burden, which results in decreased adherence and higher risk of adverse drug events, hospitalization, and mortality. Types of medications, prescribing habits, and costs have not been evaluated.
What this adds
Our study demonstrated that hemodialysis patients in Ontario were dispensed 11 unique medication products, and the median number of annual health care visits was 7 with more than half the cohort receiving prescriptions from 3 or more specialists. The top 10 medications dispensed were projected to cost more than 3 million dollars per year.
Introduction
The use of multiple concurrent medications, or polypharmacy, is an issue of growing concern in North America. The term polypharmacy refers to the use of multiple medications, typically 5 or more.1 The term is also used to describe the use of inappropriate medications, or more medications than clinically indicated. Consequences of polypharmacy include adverse drug reactions, drug-drug interactions, nonadherence, cognitive impairment, impaired balance and falls, higher health care costs, and a higher risk of morbidity, hospitalization, and mortality.2-8
Up to 10% of hospital admissions may be attributable to adverse drug events, with 30% to 55% deemed preventable.9 The risk of an adverse drug event increases with the number of medications used, ranging from 6% for those using 2 medications to 82% for those using 7 or more medications.9
Most patients receiving hemodialysis treatments for kidney failure also have many concurrent chronic conditions. These conditions often include hypertension, diabetes, and cardiovascular disease, all of which require medications. Patients receiving hemodialysis take an average of 19 pills per day, giving them the greatest pill burden of all chronic conditions.10,11 Patients with kidney failure are rarely included in clinical trials, raising uncertainty about the effectiveness of many therapies. Some medications are not adequately eliminated by dialysis, or may be eliminated too readily, which also increases the risk of poor outcomes. In a cross-sectional study from Japan, more than 50% of hemodialysis patients were taking a potentially inappropriate medication.12 In 2 of our hemodialysis-based studies assessing medication management and adherence, patients took a mean (standard deviation) of 12 (5) medications per day where 70% of these medications were potentially inappropriate.13,14
We conducted this study to improve our understanding of current medication prescribing in patients receiving in-center hemodialysis. Specifically, we were interested in which drugs were dispensed, which types of physicians prescribed the drugs, and the associated medication costs. Most drugs were selected because of potential safety concerns within the hemodialysis population; however, we also included commonly used medications to examine their relative use to the potentially inappropriate medications. This was done as part of a staged program of research to develop and test quality improvement strategies to optimize medication use and deprescribe inappropriate medications in this at-risk population.
Materials and Methods
Design and Setting
We conducted a descriptive retrospective cross-sectional study in the province of Ontario, Canada, using several linked health care databases housed at the Institute for Clinical Evaluative Sciences (ICES). This was done to assess outpatient medications dispensed to outpatients receiving ongoing in-center hemodialysis in 69 facilities in Ontario as of October 1, 2013. Ontario has about 13.7 million residents, 16% of whom are aged 65 years and older. All Ontario residents receive universal access to hospital and physician services, while elderly residents and select populations receive prescription medication coverage through a universal drug program. We conducted this study at ICES according to a prespecified protocol approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Toronto, Ontario). Participant informed consent was not required for this study. The reporting of this study followed guidelines for observational studies (Supplemental Table 1).15
Data Sources
We obtained baseline characteristics, drug use information, and costs using health records from 7 administrative databases. Vital statistics were obtained from the Registered Persons Database which contains demographic information on all Ontario residents with a health card. The Ontario Drug Benefit (ODB) database provided prescription drug data. This database contains highly accurate records of outpatient prescriptions dispensed to patients aged 65 and older, patients residing in long-term care homes, and those enrolled in assistance programs, with an error rate of less than 1%.16 We obtained diagnostic and procedural information on all hospital admissions and emergency department visits from the Canadian Institute for Health Information Discharge Abstract Database and National Ambulatory Care Reporting System database. The Ontario Health Insurance Plan database was used to ascertain baseline characteristics, and includes claims for inpatient and outpatient physician services. The ICES Physician Database contains prescriber and specialist demographic and practice data. The Canadian Organ Replacement Register (CORR) was used to identify individuals on chronic in-center hemodialysis (identified receipt of continuous treatment for a specific period of time following hemodialysis initiation) and information related to this. All datasets were linked using unique, encoded identifiers, and analyzed at ICES. Previous studies have used these databases to study adverse drug events and health outcomes in the hemodialysis population.17-19 With the exception of prescriber specialty (missing in up to 20% of prescriptions), the databases were complete (less than 0.1% missing for rural residence and neighborhood income quintile) for all variables used in this study. Codes from the International Statistical Classification of Diseases and Related Health Problems, Canadian 10th revision (ICD-10-CA) were used to assess baseline comorbidities in the 5 years prior to October 1, 2013.
Cohort Selection
We identified outpatients receiving chronic in-center hemodialysis in Ontario as of October 1, 2013. We excluded patients with evidence of a dialysis modality change in the prior 120 days, and excluded patients not eligible for receiving universal prescription drug benefits. This was defined as patients with no evidence of a recent prescription drug within 120 days of October 1, 2013, in the ODB database.
Drug Use and Costs
Active prescription drug use was defined as evidence of at least 1 medication dispensed in ODB in the prior 120 days (in Ontario, the maximum prescription duration is 100 days, and we allowed an additional 20 day buffer to account for potential noncompliance). We assessed the proportion of patients dispensed each of our study drugs of interest. These study drugs were allopurinol, alpha adrenergic blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, anticonvulsants, antidepressants, typical antipsychotics, prescription aspirin, benzodiazepines/hypnotics, beta blockers, bisphosphonates, bowel prokinetics, calcitriol, calcium channel blockers, digoxin, diuretics, dopamine agonists, fibrates, H2 receptor antagonists, oral hyperglycemics, insulin, levodopa and combinations, prescription nonsteroidal anti-inflammatory drugs (NSAIDs) other than aspirin, opioids, proton pump inhibitors, statins, tamsulosin, and warfarin (Supplemental Table 2). Most of these medications were selected because they may not have a true indication for dialysis patients and/or there are safety concerns with their use in this population.20 For instance, drugs such as alpha blockers and diuretics may not have a true indication for dialysis patients. We also selected proton pump inhibitors, allopurinol, benzodiazepines, and opioids as there is substantial evidence for safety concerns with their use.20 In addition, we included drugs that are commonly used for other chronic conditions prevalent in the hemodialysis population such as hypertension, diabetes, and cardiovascular disease (ACE inhibitors, calcium channel blockers, and hypoglycemics/insulin) to determine their use relative to other medications used in the hemodialysis population. We could not include over-the-counter medications as the database only contains prescription medications.
Statistical Analysis
We used descriptive statistics to summarize the baseline characteristics of in-center hemodialysis patients who were dispensed at least 1 study drug of interest. Baseline characteristics were summarized for the total cohort and separately by study drug group. Continuous variables were reported using mean and standard deviation, or median and interquartile range (IQR), and categorical variables were reported as a percentage.
The percentage of patients prescribed a study drug was calculated by dividing the total number with a prescription for each drug (numerator) by the total number of patients in our ODB-eligible cohort (denominator) during the 120-day period prior to October 1, 2013. We obtained the total annual cost for each study drug by multiplying the number of prescriptions dispensed in the 1 year following October 1, 2013, by the cost of each drug. We conducted all analyses with SAS version 9.4 (SAS Institute, Cary, North Carolina) at the ICES Western facility (London, Ontario).
Results
Demographics
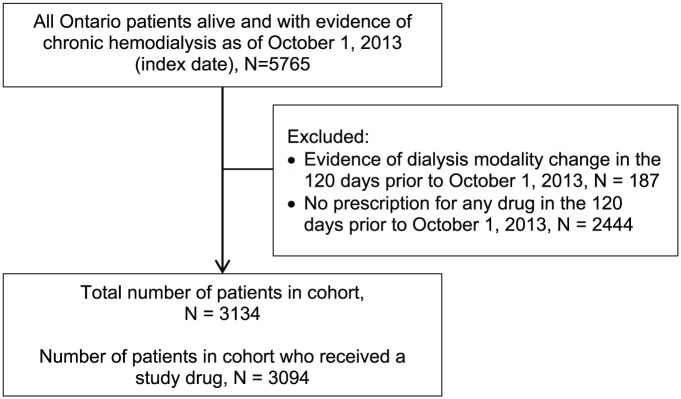
A total of 5765 individuals were receiving in-center hemodialysis in Ontario as of October 1, 2013. After exclusions were applied, the cohort contained 3134 patients who were receiving universal prescription drug coverage from ODB. Of these, 3094 patients received at least 1 study drug of interest (Figure 1). Patient characteristics are summarized in Table 1. The mean (±standard deviation) age was 76.5 ± 7.3 years with 1373 (44%) being women. There were 209 (7%) individuals living in a rural setting (municipal population <10 000). There were 277 (9%) patients living in long-term care facilities. About 48% of patients were in the first or second lowest neighborhood income quintile. The 6 most common comorbidities were coronary artery disease including angina (62%), diabetes mellitus (53%), heart failure (52%), chronic lung disease (42%), arrhythmia (29%), and atrial fibrillation (22%). The median (25th, 75th percentile) Charlson comorbidity index score was 4 (2-5), where 57% had a score between 3 and 6. The median duration of in-center hemodialysis was 3 (1-6) years.
Figure 1.
Cohort selection.
Table 1.
| N | % | |
|---|---|---|
| Characteristic | ||
| Age, years | ||
| Mean (SD) | 76.5 (7.3) | |
| Median (IQR) | 76 (70-82) | |
| ≤65 years | 121 | 3.9 |
| 66-69 years | 536 | 17.3 |
| 70-74 years | 646 | 20.9 |
| 75-79 years | 697 | 22.5 |
| 80-84 years | 622 | 20.1 |
| 85-89 years | 335 | 10.8 |
| 90+ years | 137 | 4.4 |
| Sex | ||
| Women | 1373 | 44.4 |
| Men | 1721 | 55.6 |
| Locationc | ||
| Urban | 2885 | 93.2 |
| Rural | 209 | 6.8 |
| Long-term care residence | 277 | 9.0 |
| Neighborhood income quintiled | ||
| 1 (lowest) | 779 | 25.2 |
| 2 | 690 | 22.4 |
| 3 | 592 | 19.2 |
| 4 | 536 | 17.4 |
| 5 (highest) | 489 | 15.8 |
| Comorbidities | ||
| Coronary artery disease (including angina) | 1911 | 61.8 |
| Diabetes mellitus | 1628 | 52.6 |
| Heart failure | 1592 | 51.5 |
| Chronic lung disease | 1298 | 42.0 |
| Arrhythmia (bradyarrhythmia and tachyarrhythmia) | 907 | 29.3 |
| Atrial fibrillation/flutter | 667 | 21.6 |
| Myocardial infarction | 519 | 16.8 |
| Peripheral vascular disease | 474 | 15.3 |
| Chronic liver disease | 344 | 11.1 |
| Stroke or transient ischemic attack | 226 | 7.3 |
| Coronary revascularization | 152 | 4.9 |
| Aortic aneurysm repair or bypass | 33 | 1.1 |
| Renal transplant | 11 | 0.4 |
| Charlson comorbidity index | ||
| Mean (SD) | 3.9 (2.4) | |
| Median (IQR) | 4 (2-5) | |
| 0 | 366 | 11.8 |
| 1-2 | 568 | 18.4 |
| 3-4 | 990 | 32.0 |
| 5-6 | 775 | 25.0 |
| ≥7 | 395 | 12.8 |
| Dialysis vintage, years | ||
| Mean (SD) | 4.2 (4.6) | |
| Median (IQR) | 3 (1-6) | |
| 0-<1 years | 715 | 23.1 |
| 1-<2 years | 483 | 15.6 |
| 2-<3 years | 403 | 13.0 |
| 3+ years | 1488 | 48.2 |
Note. SD = standard deviation; IQR = interquartile range.
Based on study prescription closest to October 1, 2013.
Some results removed due to privacy regulations (sample size too small).
Location was missing in ≤0.1% of patients; missing values combined with “urban” category due to privacy regulations.
Income was categorized into quintiles of average neighborhood income on the index date. Income was missing in ≤0.1% of patients; missing values combined with the middle category due to privacy regulations.
Medication and Health Care Use
Medication use is summarized in Table 2. Patients were dispensed a median of 11 (8-14) unique medication products in the 120 days prior to October 1, 2013. More than two-thirds of the patients (70%) were dispensed 9 or more different medications. Patients were dispensed a median of 5 (4-7) of the 28 medication classes examined in this study. A comprehensive table summarizing prevalence of comorbidities by study medication class use is shown in Supplemental Table 3.
Table 2.
Medication and Health Care Use of Patients Receiving a Study Medication (N = 3094).a
| Characteristic | N | % |
|---|---|---|
| Medication use (of all medications in ODB) | ||
| Number of unique drug names | ||
| Mean (SD) | 11.3 (5.0) | |
| Median (IQR) | 11 (8-14) | |
| ≤4 drug names | 212 | 6.9 |
| 5-8 drug names | 752 | 24.3 |
| 9-12 drug names | 984 | 31.8 |
| 13-16 drug names | 680 | 22.0 |
| 17+ drug names | 466 | 15.1 |
| Study medication use (of 28 study medications only) | ||
| Number of unique drug names | ||
| Mean (SD) | 5.4 (2.3) | |
| Median (IQR) | 5 (4-7) | |
| Health care use (prior 365 days) | ||
| Primary care visits | ||
| Mean (SD) | 12.0 (14.8) | |
| Median (IQR) | 7 (3-15) | |
| 0 visits | 202 | 6.5 |
| 1-3 visits | 712 | 23.0 |
| 4-6 visits | 541 | 17.5 |
| 7-9 visits | 369 | 11.9 |
| ≥10 visits | 1270 | 41.0 |
| Prescriber information | ||
| Number of prescriber specialtiesb | ||
| Mean (SD) | 2.8 (1.3) | |
| Median (IQR) | 3 (2-4) | |
| 1 specialty | 391 | 12.7 |
| 2 specialties | 1031 | 33.4 |
| 3+ specialties | 1672 | 54.0 |
| Prescribing physician specialtya | ||
| General practitioner | 1312 | 49.40 |
| Nephrologist | 1189 | 44.80 |
| Cardiologist | 60 | 2.30 |
| Internist | 49 | 1.80 |
| Endocrinologist | 45 | 1.70 |
Note. ODB = Ontario Drug Benefit; SD = standard deviation; IQR = interquartile range.
Based on study prescription closest to October 1, 2013.
Based on all prescriptions dispensed from the Ontario Drug Benefit in the 120 days prior to October 1, 2013.
The median number of primary care visits among all patients in the 1 year prior to October 1, 2013, was 7 (3-15). The median number of different prescriber specialties per patient for any drug was 3 (2-4), with 391 (13%) patients receiving prescriptions from only 1 specialty, and 1031 (33%) and 1672 (54%) patients receiving prescriptions from 2, or 3 or more specialties, respectively. When examining prescriptions for study drugs only, general practitioners were the most common prescribers (49% of the study medications) followed by nephrologists (45% of the study medications). Certain drug classes were more commonly prescribed by general practitioners, as opposed to nephrologists, including aspirin (62% vs 36%), antidepressants (69% vs 29%), H2 receptor antagonists (57% vs 37%), insulin (54% vs 23%), opioids (71% vs 27%), proton pump inhibitors (60% vs 37%), statins (56% vs 38%), and tamsulosin (63% vs 34%).
Top Study Medications Used and Associated Costs
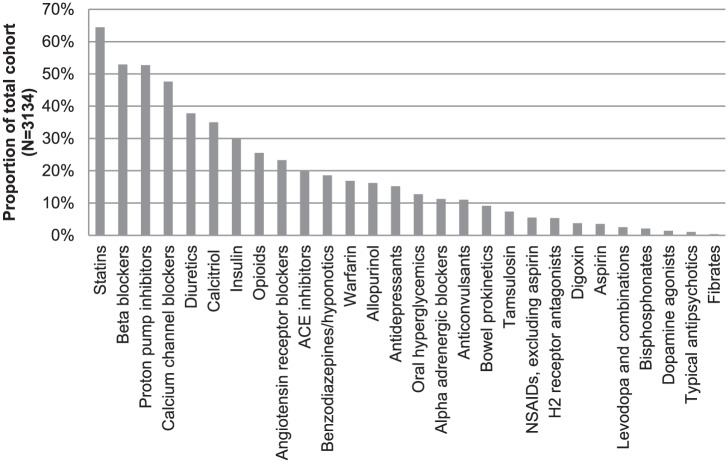
Data on observed drug costs informed our estimates of the 1-year costs of each drug. With the exception of ARBs, being the 9th most common medication prescribed while oral hyperglycemic agents were the 15th most prescribed medication class, the 10 most commonly dispensed medication classes were also the 10 most costly when prescription costs were summed over 1 year, with the data summarized in Figure 2 and Table 3. Taken together, these 10 medications cost more than 3 million dollars annually in direct costs. When antihypertensives and hypoglycemic agents (including insulin) are removed, the top drugs in cost from highest to lowest included proton pump inhibitors, calcitriol, statins, opioids, diuretics, antidepressants, anticonvulsants, warfarin, alpha blockers, and benzodiazepines.
Figure 2.
Percentage of study medication use in chronic in-center hemodialysis patients.
Note. ACE = angiotensin-converting enzyme; NSAIDs = nonsteroidal anti-inflammatory drugs.
Table 3.
Annual Costs for Most Costly Study Medications.
| Study medication | Total prescriptions | Total annual costa |
|---|---|---|
| Insulin | 6320 | $662 112 |
| Proton pump inhibitors | 31 847 | $433 093 |
| Calcitriol | 18 710 | $404 305 |
| Statins | 35 771 | $384 073 |
| Calcium channel blockers | 24 865 | $312 209 |
| Beta blockers | 29 789 | $228 950 |
| Opioids | 8989 | $201 132 |
| Oral hyperglycemics | 7937 | $164 476 |
| Diuretics | 20 230 | $141 529 |
| ACE inhibitors | 10 014 | $131 530 |
Note. ACE = angiotensin-converting enzyme.
Calculated in the 1 year (365 days) following October 1, 2013.
Discussion
The objective of our study was to examine current prescribing practices for medications with efficacy and safety concerns in the Ontario in-center hemodialysis population, and the associated costs of these drugs to the health care system. Our results revealed that polypharmacy is a major concern in hemodialysis patients. Patients were taking a median of 11 different medications; however, this does not account for potential over-the-counter medications such as phosphate binders (ie, calcium carbonate), and other medications for hemodialysis paid by the Ministry of Health and Long-term Care and delivered during hemodialysis treatments such as erythropoietin-stimulating agents and some preparations of vitamin D. Therefore, the daily medication use is likely even higher. Furthermore, the annual cost for the top 10 medications in the in-center hemodialysis population was over 3 million dollars.
Although our study could not evaluate the indication for the medications dispensed to the hemodialysis patients, these medications were chosen to be evaluated in our database because of their potential for misuse, and thus, the focus of this study was to evaluate medications that potentially do not have a true indication and/or may have safety concerns with their use in both the general population and especially patients on chronic hemodialysis. Nonetheless, like many other studies, our study demonstrated that polypharmacy in the in-center hemodialysis population is a major concern.20 Although we did not measure the consequences of polypharmacy, other studies have demonstrated the potential for harmful consequences of polypharmacy; these include adverse drug reactions, drug-drug interactions, nonadherence, increased risk of cognitive impairment, impaired balance and falls, and increased risk of morbidity, hospitalization, and mortality, all which can contribute to increased health care costs.20-25 It is thus essential to develop tools to optimize medication use and prevent the consequences of polypharmacy in hemodialysis patients. Our results provide some insight and understanding of the medication use in the in-center hemodialysis population, which will be used to guide the future implementation of our optimization strategies such as the development of deprescribing algorithms for specific medications in the hemodialysis population.
It is important to understand the potential reasons for polypharmacy in the hemodialysis population to implement methods to optimize medications. For instances, patients with concurrent comorbidities, multiple prescribers, and age-related changes in organ systems are at the greatest risk of polypharmacy.26 In fact, studies have shown that patients with multiple chronic conditions resulted in more medications prescribed.26-30 Our results showed that greater than 50% of our in-center hemodialysis populations had significant comorbidities besides kidney disease such as diabetes, coronary artery disease, and heart failure.
Furthermore, prescribers are often reluctant to change medications other prescribers have started, and may also have difficulty recognizing medication side effects, increasing the risk of prescribing cascades (ie, new medication added to manage side effects).26 Indeed, our results showed 54% of the patients on in-center hemodialysis had 3 or more prescribers, and 78% of the patients had more than 3 health care visits per year, resulting in more than 90% of the hemodialysis patients taking more than 5 medications within our study period.
Finally, elderly patients (and patients on hemodialysis) are underrepresented in clinical trials, yet they are the greatest consumers of medications.27-30 A study examining randomized controlled trials of 4 commonly used medications found that the proportion of older patients (>65 years) enrolled was significantly lower than the proportion in the average of population; out of 155 trials, only 3 included the elderly.31 Therefore, despite the large quantity of guidelines available for chronic disease states (eg, hypertension, diabetes), few specifically address how to approach the elderly and hemodialysis patients.32 For instance, statins were one of the top dispensed medications from our database study, with two-thirds of the patients taking a statin. Although excellent data support the use of statins in the general population for primary prevention, the same data are lacking in the hemodialysis population.33-35 Therefore, prescribers should consider the patient’s life expectancy, goals of care, and time to potential benefit when prescribing medications such as statins for hemodialysis patients.
It is also important to recognize the use of inappropriate medications and their cost to the health care system. In our study, the annual cost for the top 10 medications in the in-center hemodialysis population was over 3 million dollars. Therefore, optimizing the use of medications in this population would not only potentially decrease morbidity and mortality and their associated costs but could also decrease the direct costs of the medications themselves.
Our study was able to demonstrate the state of polypharmacy in the in-center hemodialysis population and provides ideas for potential strategies to guide clinicians in optimizing medication use in this population.
A major strength of our study was the use of Ontario health care databases, which capture a large sample of patients who received the study drugs in routine care and accurately examine patient comorbidities at the time of prescription. However, our study was also subjected to some limitations of health care data. For instance, ODB data are restricted to patients aged 65 years and older, or who are on disability and social works, and thus, our results may not be generalizable to all hemodialysis patients. In addition, we were restricted to outpatient prescription data and could not examine over-the-counter medications. We were also unable to determine the specific indication for which the drugs were prescribed, so ascertaining whether the drug is truly indicated or not is difficult with our data. Finally, we could not assess scheduled compared with “as needed” medications, and thus, we may not have truly accounted for tablet burden. Although direct medications costs were calculated, we did not evaluate the potential costs of treating adverse outcomes associated with polypharmacy. It is also important to note that data obtained from ICES are restricted to Ontario residents only; thus, our findings may not be generalizable to other regions depending on prescribing practices and drug costs in other provinces. The CORR database was used in our study to obtain our cohort of chronic in-center hemodialysis patients residing in Ontario, and the health care system in Ontario contains some aspects unique to the province. Despite this, we feel that our in-center hemodialysis patient population would be similar to those in other regions as patients on maintenance hemodialysis may experience similar health-related issues that are beyond geographic boundaries. Indeed, drug and comorbidity information using health care databases in other provinces is warranted to compare the burden of polypharmacy across Canada.
Polypharmacy is a growing problem for patients on hemodialysis in scope and impact. The key clinical implication is that clinicians need to regularly review and optimize chronic medications, particularly in people with polypharmacy or whose life expectancy is limited. Health care providers must share the responsibility with patients to ensure that potentially inappropriate medications are minimized, appropriate medications and doses are used, drug interactions are monitored, side effects are not treated with more medications without first investigating medication-related causes, and pill burden is minimized. Future studies should be aimed at developing tools to aid clinicians and patients on how to best optimize medication use and deprescribe medications that lack any efficacy or safety data to prevent adverse outcomes associated with polypharmacy.
Supplemental Material
Supplemental material, suppl_file/Polypharm_Supplemental for A Province-wide, Cross-sectional Study of Demographics and Medication Use of Patients in Hemodialysis Units Across Ontario by Marisa Battistella, Racquel Jandoc, Jeremy Y. Ng, Eric McArthur and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors, and not necessarily those of CIHI. The authors thank IMS Brogan Inc for use of their Drug Information Database.
Footnotes
Ethics Approval and Consent to Participate: We conducted this study at the Institute for Clinical Evaluative Sciences (ICES) according to a prespecified protocol approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Toronto, Ontario). Participant informed consent was not required for this study.
Consent for Publication: All authors consent to this manuscript being published.
Availability of Data and Materials: The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the data set publicly available, access can be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS.
Author Contributions: MB made substantial contributions to the design of the study, collection of data as well as the interpretation and analysis of the data. MB extensively revised the manuscript critically, and gave final approval of the version to be published. RJ managed and coordinated project planning and execution, made substantial contributions to the interpretation of the data, extensively revised the manuscript critically, and gave final approval of the version to be published. JYN made substantial contributions to the interpretation and analysis of the data. JYN revised the manuscript critically, and gave final approval of the version to be published. EM performed statistical programming to analyze and synthesize the data, reviewed the manuscript, and gave final approval of the version to be published.
AXG made substantial contributions to the design of the study, provided data resources for conducting the analysis, reviewed the manuscript, and gave final approval of the version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Institute for Clinical Evaluative Sciences (ICES) Western site. The ICES is funded by an annual grant from the Ontario Ministry of Health and Long-term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Western facility, who are supported by a grant from the Canadian Institutes of Health Research (CIHR). The opinions, results, and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHR, or the MOHLTC is intended or should be inferred. Amit X. Garg was supported by the Dr Adam Linton Chair in Kidney Health Analytics and a Canadian Institutes of Health Research Clinician Investigator Salary Award. Research personnel who worked on this project were provided space in the Lilibeth Caberto Kidney Clinical Research Unit.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Reason B, Terner M, McKeag AM, Tipper B, Webster G. The impact of polypharmacy on the health of Canadian seniors. Fam Pract. 2012;29:427-432. [DOI] [PubMed] [Google Scholar]
- 2. Salazar JA, Poon I, Nair M. Clinical consequences of polypharmacy in elderly: expect the unexpected, think the unthinkable. Expert Opin Drug Saf. 2007;6:695-704. [DOI] [PubMed] [Google Scholar]
- 3. Shah B, Hajjar E. Polypharmacy, adverse drug reactions and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186. [DOI] [PubMed] [Google Scholar]
- 4. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayes BD, Klein-Schwartz W, Barrueto F. Polypharmacy and the geriatric patient. Clin Geriatr Med. 2007;23:371-390. [DOI] [PubMed] [Google Scholar]
- 6. Huang A, Mallet L, Rochefort C, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29:359-376. [DOI] [PubMed] [Google Scholar]
- 7. Hajjar ER, Cariero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345-351. [DOI] [PubMed] [Google Scholar]
- 8. Cross C. Introducing deprescribing into culture of medication. CMAJ. 2013;185:E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529-537.e4. [DOI] [PubMed] [Google Scholar]
- 10. Patterson SM, Hughes C, Kerse N, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012;5:CD008165. [DOI] [PubMed] [Google Scholar]
- 11. Lindsay J, Dooley M, Martin J, Fray M, Kearney A, Barras M. Reducing potentially inappropriate medications in palliative cancer patients: evidence to support deprescribing approaches. Support Care Cancer. 2014;22:1113-1119. [DOI] [PubMed] [Google Scholar]
- 12. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START Criteria. Clin Pharmacol Ther. 2011;89(6):845-854. [DOI] [PubMed] [Google Scholar]
- 13. Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18(4):121-124. [DOI] [PubMed] [Google Scholar]
- 14. Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm. 2013;66(3):201-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67-71. [PubMed] [Google Scholar]
- 17. Harel Z, Wald R, McArthur E, et al. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26(12):3141-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weir MA, Dixon SN, Fleet JL, et al. β-blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol. 2015;26(4):987-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dev V, Dixon SN, Fleet JL, et al. Higher anti-depressant dose and major adverse outcomes in moderate chronic kidney disease: a retrospective population-based study. BMC Nephrol. 2014;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kondo N, Nakamura F, Yamazaki S, et al. Prescription of potentially inappropriate medications to elderly hemodialysis patients: prevalence and predictors. Nephrol Dial Transplant. 2015;30:498-505. [DOI] [PubMed] [Google Scholar]
- 21. Reeve E, Wiese MD. Benefits of deprescribing on patients’ adherence to medications. Int J Clin Pharm. 2014;36:26-29. [DOI] [PubMed] [Google Scholar]
- 22. Manley HJ, McClaran ML, Overbay DK, et al. Factors associated with medication-related problems in ambulatory hemodialysis patients. Am J Kidney Dis. 2003;41(2):386-393. [DOI] [PubMed] [Google Scholar]
- 23. Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battistella M, Fleites R, Wong R, Jassal V. Development, validation, and implementation of a medication adherence survey to seek a better understanding of the hemodialysis patient. Clin Nephrol. 2016;85:12-22. [DOI] [PubMed] [Google Scholar]
- 25. Chan WWY, Mahalingam G, Richardson RMA, Fernandes OA, Battistella M. A formal medication reconciliation programme in a haemodialysis unit can identify medication discrepancies and potentially prevent adverse drug events. J Ren Care. 2015;41(2):104-109. [DOI] [PubMed] [Google Scholar]
- 26. Anthierens S, Tansens A, Petrovic M, Christiaens T. Qualitative insights into general practitioners’ views on polypharmacy. BMC Fam Pract. 2010;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Spall H, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233-1140. [DOI] [PubMed] [Google Scholar]
- 28. Dodd K, Saczynski J, Zhao Y, Goldberg RJ, Gurwitz JH. Exclusion of older adults and women from recent trials of acute coronary syndromes. J Am Geriatr Soc. 2011;59:506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zulman D, Sussman J, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011;26:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cherubini A, Oristrell J, Pia X, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550-556. [DOI] [PubMed] [Google Scholar]
- 31. Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7(3):e33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmes H, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609. [DOI] [PubMed] [Google Scholar]
- 33. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248. [DOI] [PubMed] [Google Scholar]
- 34. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009:360:1395-1407 [DOI] [PubMed] [Google Scholar]
- 35. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2182-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, suppl_file/Polypharm_Supplemental for A Province-wide, Cross-sectional Study of Demographics and Medication Use of Patients in Hemodialysis Units Across Ontario by Marisa Battistella, Racquel Jandoc, Jeremy Y. Ng, Eric McArthur and Amit X. Garg in Canadian Journal of Kidney Health and Disease