Abstract
Objective
To study bidirectional change and predictors of change in estimated glomerular filtration rate (GFR) and proteinuria in lupus nephritis (LN) using a multistate modeling approach.
Methods
Patients in the Systemic Lupus International Collaborating Clinics inception cohort were classified annually into estimated GFR state 1 (>60 ml/minute), state 2 (30–60 ml/minute), or state 3 (<30 ml/minute) and estimated proteinuria state 1 (<0.25 gm/day), state 2 (0.25–3.0 gm/day), or state 3 (>3.0 gm/day), or end-stage renal disease (ESRD) or death. Using multistate modeling, relative transition rates between states indicated improvement and deterioration.
Results
Of 1,826 lupus patients, 700 (38.3%) developed LN. During a mean ± SD follow-up of 5.2 ± 3.5 years, the likelihood of improvement in estimated GFR and estimated proteinuria was greater than the likelihood of deterioration. After 5 years, 62% of patients initially in estimated GFR state 3 and 11% of patients initially in estimated proteinuria state 3 transitioned to ESRD. The probability of remaining in the initial states 1, 2, and 3 was 85%, 11%, and 3%, respectively, for estimated GFR and 62%, 29%, and 4%, respectively, for estimated proteinuria. Male sex predicted improvement in estimated GFR states; older age, race/ethnicity, higher estimated proteinuria state, and higher renal biopsy chronicity scores predicted deterioration. For estimated proteinuria, race/ethnicity, earlier calendar years, damage scores without renal variables, and higher renal biopsy chronicity scores predicted deterioration; male sex, presence of lupus anticoagulant, class V nephritis, and mycophenolic acid use predicted less improvement.
Conclusion
In LN, the expected improvement or deterioration in renal outcomes can be estimated by multistate modeling and is preceded by identifiable risk factors. New therapeutic interventions for LN should meet or exceed these expectations.
Lupus nephritis (LN) is a cardinal feature of systemic lupus erythematosus (SLE). The overall frequency is ~40%, and it is most frequent in patients of younger age, male sex, and non-Caucasian ancestry (1). The majority of patients present with LN within 5 years after the diagnosis of SLE. Initially associated with a 50% 4-year survival rate in the 1950s (2), both life expectancy and renal outcome have steadily improved (3–5). This is attributed to advances in diagnosis, access to care, and treatment protocols. However, some studies suggest that improvements in the most serious outcomes of LN, end-stage renal disease (ESRD) and associated mortality, have reached a plateau over the past 2 decades (6,7).
The prognosis of LN has been studied in clinical trials of therapeutic interventions, cross-sectional studies, and longitudinal observational cohort studies. Clinical outcomes have included overall survival, renal survival, and remission, variously defined. Potential bidirectional change in LN, reflecting improvement or deterioration in renal status, which sometimes occurs several times in an individual patient, has not been optimally captured.
Our aim was 2-fold: to characterize changes in kidney function and proteinuria states over time, and to identify potential predictors of these changes, in an international, observational disease inception cohort of SLE patients. In order to capture the range of dynamic change in renal status over time, we adopted a reversible multistate model that allows for transitions between renal disease states defined by estimated glomerular filtration rate (GFR) and proteinuria.
Multistate models (8) offer a convenient and flexible framework to characterize disease progression in LN, reflecting the clinical reality that both improvement and deterioration are observed over time. Unlike the usual regression approaches which focus on “snapshots” of disease states at various time points, multistate models provide a dynamic representation of the disease in continuous time. They also estimate the duration spent in different states and provide predictive probabilities of being in particular states at the end of specified periods of time. Such summary inferences are more informative than models that focus on single, often dichotomous, outcomes such as the time to a specific clinical event (e.g., ESRD). In addition, estimated probabilities are based on all follow-up data, not simply on data from subsets of patients with a specific followup time. This provides a similar advantage to that of estimated survival curves over proportions of patients with a specific follow-up time experiencing an event.
Patients and Methods
Research study network
The study was conducted by the Systemic Lupus International Collaborating Clinics (SLICC), which comprises 32 academic medical centers in 11 countries (9). Between October 1999 and October 2011, SLICC established an inception cohort for the long-term study of clinical outcomes in SLE. The initial focus was on the study of atherosclerosis and neuropsychiatric disease, but other outcomes were subsequently added, including nephritis (10). Data were collected per protocol at enrollment and annually (±6 months), and entered into a centralized database. Each of the participating centers’ institutional research ethics boards approved the study.
Patients
Patients fulfilled the American College of Rheumatology (ACR) revised classification criteria for SLE (11) (the date the criteria were met was taken as the date of diagnosis) and provided written informed consent. Enrollment occurred up to 15 months following the diagnosis of SLE. Demographic variables included age, sex, race/ethnicity, and education. Lupus-related medications included oral and intravenous corticosteroids, antimalarials, and immunosuppressants (azathioprine, cyclophosphamide, methotrexate, and mycophenolic acid), taken at the time of or between assessments. Other medications included antihypertensives and, separately, angiotensin-converting enzyme (ACE) inhibitors and ACE receptor blockers. Lupus-related variables, such as the SLE Disease Activity Index 2000 (SLEDAI-2K) (12) and SLICC/ACR Damage Index (SDI) (13), were also recorded. With the exception of the SLEDAI-2K scores, which were temporarily linked to an interval of 10 days prior to the assessment, all other clinical, diagnostic, and medication data from any time since the preceding assessment were recorded.
Lupus nephritis
Patients with nephritis, the focus of the study, were identified by the “renal disorder” variable of the ACR classification criteria (11,14) and/or biopsy evidence of nephritis as per the International Society of Nephrology (ISN)/Renal Pathology Society (RPS) criteria (15).
Renal variables and data collection
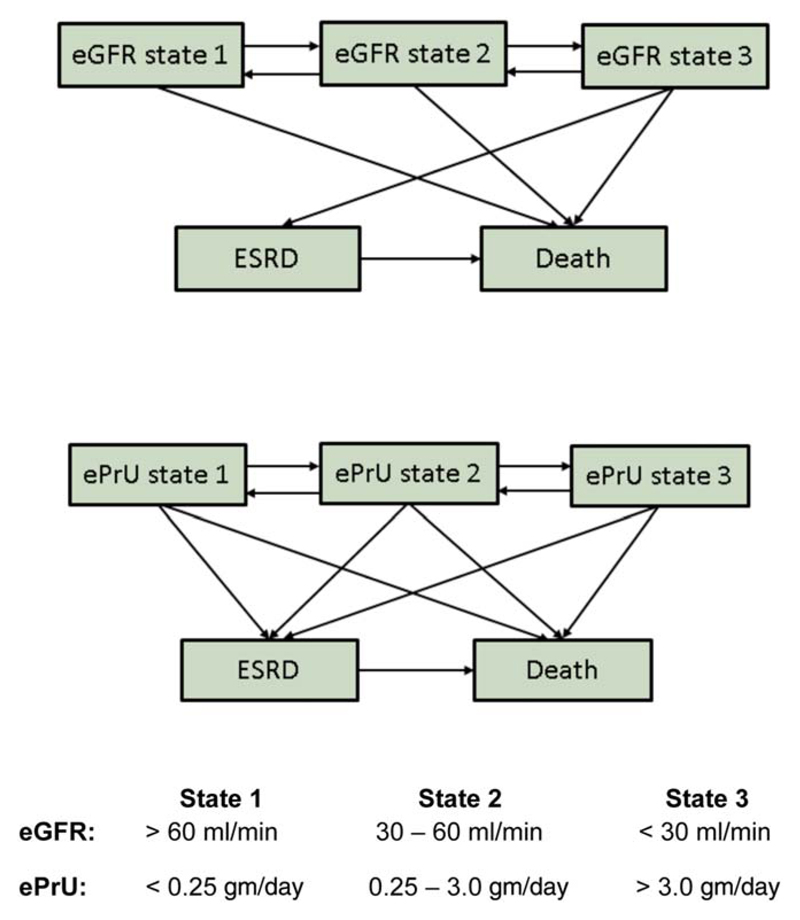
The ISN/RPS classification (15) and activity/chronicity scores (16) were derived from renal biopsy reports. The estimated GFR was determined using the Modification of Diet in Renal Disease equation (17). Proteinuria was estimated using either 24-hour urine collection or spot urine total protein-to-creatinine ratio (18,19). ESRD was identified by the SDI (13). At each assessment, patients were assigned to 1 of 3 GFR states and 1 of 3 proteinuria states or were classified as having ESRD or as having died (Figure 1).
Figure 1.
Reversible multistate Markov model for observed transitions in estimated glomerular filtration rate (eGFR) states and estimated proteinuria (ePrU) states in patients with lupus nephritis. ESRD = end-stage renal disease.
Autoantibodies
Lupus anticoagulant, IgG anticardiolipin (aCL), anti–β2-glycoprotein I, and anti–ribosomal P (anti-P) antibodies, which have been linked to LN (20,21), were measured in baseline sera in a central laboratory (22–25).
Statistical analysis
Two multistate Markov models examined transitions between estimated GFR and estimated proteinuria states and into the ESRD and death states (Figure 1). It was assumed that death could occur when a patient was in any estimated GFR and estimated proteinuria state. Transition to ESRD could occur from estimated GFR state 3 and from any estimated proteinuria state. The modeling was done in continuous time, and therefore an individual observed to move from estimated GFR states 1 or 2 at one assessment to ESRD at the next assessment was assumed to have passed through all states up to state 3 before developing ESRD. This assumption is not clinically defensible for estimated proteinuria states, and hence that model included more possible transitions to ESRD. Similarly, direct transition from an estimated GFR or estimated proteinuria state to any nonadjacent state except for ESRD or death (e.g., transition from estimated GFR or estimated proteinuria state 1 to 3) was not allowed in continuous time. When such changes did occur between assessments, the model assumed that the transition occurred through adjacent states (e.g., transition from estimated GFR or estimated proteinuria state 1 to state 2 to state 3).
In the absence of ESRD or death, transitions could occur into higher and lower estimated GFR and estimated proteinuria states, and each rate of transition was assumed to be constant over time. For a transition between state i and j, the rate of transition at any time t, denoted rij(t | x1, x2, …, xk) was modeled with a relative rate regression model as follows:
Thus, the logarithm of the i-to-j transition rate had a baseline value of aij, which was modified by the product of the value of explanatory, or what we will term predictor, variables (that were coded as x1, x2, …xk) and associated regression coefficients bij1, bij2, … bijk that were estimated. Each regression coefficient represents a log relative rate comparing patients differing by 1 unit in the associated predictor variable. A value of 0 for a regression coefficient represents no effect of the variable on the transition rate and corresponds to a relative rate (RR) value of 1. Explanatory variables, or predictors, were examined for all transitions between estimated GFR and estimated proteinuria states, but, due to limited events, predictors were not examined for transitions to ESRD and death.
Individual predictors potentially associated with transitions to either a worse or better estimated GFR or estimated proteinuria state were examined in the multistate model. Relative rates, but not baseline rates, associated with all forward transitions (i.e., from state 1 to state 2 and from state 2 to state 3) were initially assumed to be identical, as were those for backward transitions (i.e., from state 2 to state 1 and from state 3 to state 2). “Forward” and “backward” transitions are synonymous with deterioration and improvement, respectively, in renal status. If the null hypothesis of this common relative rate assumption was rejected at a 0.05 level through a likelihood ratio test, we allowed the relative rates to differ for all transitions in the model. Predictor variables examined were sex, age at SLE diagnosis, geographic site, race/ethnicity, calendar year, alcohol consumption, smoking, medications (antimalarials, steroids, any immunosuppressant, ACE inhibitors or receptor blockers separately), hypertension, SLEDAI-2K and SDI scores without renal variables, estimated proteinuria/estimated GFR states, diabetes, baseline autoantibodies, ISN/RPS classification, and activity and chronicity scores from renal biopsies. Where appropriate, predictor variables, such as medications, hypertension, SLEDAI-2K, SDI, diabetes, biopsy information, and renal states, were allowed to vary over time.
The analysis used maximum likelihood estimation of the regression coefficients. It can accommodate intermittent missing data (assuming the data are missing at random), right censoring as well as different time between any 2 study assessments (irregular measurement times), assuming that these are unrelated to the estimated GFR and estimated proteinuria states given the observed data.
Results
Characteristics of the LN patients
Between October 1999 and December 2012, LN occurred in 700 (38.3%) of 1,826 patients (Table 1) from SLICC centers in the US (22.9%; n = 160), Europe (22.6%; n = 158), Canada (21.0%; n = 147), Mexico (22.7%; n = 159), and Korea (10.9%; n = 76). Of the 700 patients, 566 (80.9%) were identified at enrollment and 134 (19.1%) were identified during follow-up. Renal biopsies were performed on 395 (56.4%) of the patients, the majority (86.6%) having been performed when nephritis was first suspected, and 377 (95.4%) of the 395 biopsy specimens were of sufficient quality to assign an ISN/RPS class. According to the ISN/RPS criteria, 9 patients (2.4%) had class I, 36 (9.5%) had class II, 101 (26.8%) had class III, 163 (43.2%) had class IV, 120 (31.8%) had class V, and 3 (0.8%) had class VI nephritis. Twenty-one biopsy specimens were classified as class III/V, and 34 were classified as class IV/V. For all 377 biopsy specimens, the mean ± SD activity index was 4.3 ± 3.3, and the mean ± SD chronicity index was 2.7 ± 2.6. In addition to patients with LN, a total of 302 patients had an abnormal estimated GFR (102 had an estimated GFR state of 2 and 11 had an estimated GFR state of 3) and/or abnormal estimated proteinuria (209 had an estimated proteinuria state of 2 and 8 had an estimated proteinuria state of 3) at some time during the study for reasons other than LN (e.g., hypertension, diabetes mellitus, or renal vascular disease). None of these patients progressed to ESRD.
Table 1.
Characteristics of SLE patients at diagnosis of LN*
| No. of patients | 700 |
| Age, mean ± SD years | 31.8 ± 12.0 |
| Sex, no. (%) female/male | 599 (85.6)/101(14.4) |
| Race/ethnicity, no. (%) | |
| Caucasian | 232 (33.1) |
| Hispanic | 172 (24.6) |
| Asian | 123 (17.6) |
| African | 147 (21.0) |
| Other | 26 (3.7) |
| Disease duration, mean ± SD years | 1 ± 1.5 |
| ACR classification criteria, no. % | |
| Malar rash | 223 (31.9) |
| Discoid rash | 61 (8.7) |
| Photosensitivity | 156 (22.3) |
| Oral/nasopharyngeal ulcers | 194 (27.7) |
| Serositis | 189 (27.0) |
| Arthritis | 416 (59.4) |
| Renal disorder | 675 (96.4) |
| Neurologic disorder | 43 (6.1) |
| Hematologic disorder | 413 (59.0) |
| Immunologic disorder | 532 (76.0) |
| Antinuclear antibody | 654 (93.4) |
| SLEDAI-2K, mean ± SD† | 8.2 ± 6.5 |
| SLEDAI-2K without renal variables, mean ± SD | 3.5 ± 3.7 |
| SDI score, mean ± SD‡ | 0.5 ± 0.9 |
| SDI score without renal variables, mean ± SD | 0.4 ± 0.7 |
| Medications, no. (%) | |
| Steroids | 619 (89.2) |
| Antimalarials | 376 (54.0) |
| Immunosuppressants | 487 (70.1) |
| Comorbidities/lifestyle factors | |
| Diabetes, no. (%) | 29 (4.2) |
| Hypertension, no. (%) | 370 (52.9) |
| Current smoker, no. (%) | 83 (11.9) |
| Alcohol consumption, mean ± SD units/week | 0.7 ± 2.7 |
| BMI, mean ± SD kg/m2 | 24.9 ± 5.7 |
| Duration of follow-up, mean ± SD years | 5.2 ± 3.5 |
| Calendar years of follow-up | 1999–2012 |
Percentages are of the number of patients for whom information was available. SLE = systemic lupus erythematosus; LN = lupus nephritis; BMI = body mass index.
The range of possible scores for the SLE Disease Activity Index 2000 (SLEDAI-2K) is 0–105, with higher scores indicating more disease activity.
The range of possible scores for the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (ACR) Damage Index (SDI) is 0–49, with higher scores indicating more organ damage.
Transition rates for estimated GFR and estimated proteinuria states in LN
For patients with LN, there were 3,407 assessments available for analysis, of which 3,254 (95.5%) included both estimated GFR and estimated proteinuria measurements and only 4 (0.1%) were missing both. For patients without LN, there were 5,183 assessments, of which 4,999 (96.4%) included both estimated GFR and estimated proteinuria measurements and only 14 (0.3%) were missing both. For patients with LN, transitions in estimated GFR and estimated proteinuria states, and transitions to ESRD or death, are summarized in Table 2. Some patients remained in the same state over time, while others changed states between 1 or more visits. There was no change in estimated GFR state for 94.8%, 53.3%, and 47.3% of consecutive visit pairs when the initial estimated GFR state was 1, 2, and 3, respectively. The comparable percentages for estimated proteinuria states were 79.9%, 55.6%, and 28.8%. At the patient rather than the visit level, for LN patients who had 2 or more visits there was no observed change in estimated GFR state, over their entire follow-up period, for 71%, 2%, and 0.3% of patients with an initial estimated GFR state at diagnosis of LN of 1, 2, and 3, respectively. For estimated proteinuria states, 22%, 6%, and 0.8% of patients remained in their initial state of estimated proteinuria of 1, 2, or 3 throughout the duration of the study.
Table 2.
Observed transitions in estimated GFR and estimated proteinuria states between assessments*
| Transition to state | ||||||||
|---|---|---|---|---|---|---|---|---|
| Transition from state | Estimated GFR state 1 | Estimated GFR state 2 | Estimated GFR state 3 | Estimated proteinuria state 1 | Estimated proteinuria state 2 | Estimated proteinuria state 3 | ESRD | Death |
| Estimated GFR state 1 | 2,303 | 95 | 5 | – | – | – | 13 | 14 |
| Estimated GFR state 2 | 86 | 136 | 21 | – | – | – | 8 | 4 |
| Estimated GFR state 3 | 1 | 10 | 26 | – | – | - | 15 | 3 |
| Estimated proteinuria state 1 | – | – | – | 1,167 | 257 | 20 | 6 | 10 |
| Estimated proteinuria state 2 | – | – | – | 355 | 547 | 56 | 18 | 7 |
| Estimated proteinuria state 3 | – | – | – | 45 | 85 | 59 | 12 | 4 |
| ESRD | 0 | 0 | 0 | 0 | 0 | 0 | 87 | 1 |
Proteinuria was evaluated by estimated 24-hour urine protein excretion. Values are the number of transitions. GFR = glomerular filtration rate; ESRD = end-stage renal disease.
Predictive probabilities for estimated GFR and estimated proteinuria states in LN
The same multistate models without explanatory variables provided estimates of the probability of transitioning from states 1, 2, or 3 to other estimated GFR and estimated proteinuria states, and to ESRD and death, after 1, 2, and 5 years (Tables 3 and 4). At year 1, the outcome with the highest probability was to remain in the initial estimated GFR or estimated proteinuria state. Following 2 and 5 years, the estimated probabilities indicated that improvement in either estimated GFR or estimated proteinuria was more likely than deterioration. For example, patients in estimated GFR state 1 at diagnosis of LN have a 95% probability of remaining in state 1 after 1 year and an 85% probability of remaining in state 1 after 5 years. As another example, patients in estimated GFR state 2 at diagnosis of LN have a 61% chance of improving to state 1 at 5 years and a 3.8% chance of deteriorating to state 3 at 5 years. The expected time that patients remain in these states over 5 years is 2.257 years for state 1, 1.743 years for state 2, 0.349 years for state 3, and 0.497 years for ESRD (Table 3).
Table 3.
Predictive probabilities of transitioning between states and the time spent in each state for estimated GFR*
| Estimated probability (95% CI) of being in estimated GFR state | Expected time spent in estimated GFR state, years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial state | Estimated GFR state 1 | Estimated GFR state 2 | Estimated GFR state 3 | ESRD | Death | Estimated GFR state 1 | Estimated GFR state 2 | Estimated GFR state 3 | ESRD | Death |
| 1 year | ||||||||||
| Estimated GFR state 1 | 0.95 (0.94–0.96) | 0.039 (0.033–0.047) | 0.003 (0.002–0.005) | 0.0007 (0.0004–0.001) | 0.005 (0.003–0.009) | 0.974 | 0.022 | 0.001 | 0 | 0.003 |
| Estimated GFR state 2 | 0.31 (0.26–0.37) | 0.55 (0.49–0.6) | 0.095 (0.071–0.13) | 0.033 (0.022–0.047) | 0.013 (0.007–0.043) | 0.172 | 0.747 | 0.062 | 0.012 | 0.007 |
| Estimated GFR state 3 | 0.038 (0.021–0.064) | 0.14 (0.079–0.23) | 0.42 (0.3–0.52) | 0.36 (0.26–0.45) | 0.041 (0.018–0.12) | 0.014 | 0.091 | 0.668 | 0.204 | 0.023 |
| ESRD | 0 | 0 | 0 | 0.99 (0.94–1) | 0.01 (0.002–0.064) | 0 | 0 | 0 | 0.995 | 0.005 |
| 2 years | ||||||||||
| Estimated GFR state 1 | 0.92 (0.9–0.93) | 0.06 (0.049–0.07) | 0.008 (0.006–0.011) | 0.004 (0.003–0.006) | 0.011 (0.007–0.018) | 1.908 | 0.073 | 0.007 | 0.002 | 0.011 |
| Estimated GFR state 2 | 0.47 (0.4–0.54) | 0.33 (0.27–0.39) | 0.093 (0.064–0.12) | 0.085 (0.058–0.12) | 0.027 (0.015–0.062) | 0.569 | 1.174 | 0.159 | 0.072 | 0.027 |
| Estimated GFR state 3 | 0.095 (0.056–0.17) | 0.14 (0.082–0.22) | 0.19 (0.11–0.29) | 0.51 (0.38–0.62) | 0.065 (0.031–0.17) | 0.082 | 0.235 | 0.959 | 0.648 | 0.077 |
| ESRD | 0 | 0 | 0 | 0.98 (0.87–1) | 0.02 (0.003–0.13) | 0 | 0 | 0 | 1.98 | 0.02 |
| 5 years | ||||||||||
| Estimated GFR state 1 | 0.85 (0.83–0.87) | 0.078 (0.063–0.094) | 0.016 (0.011–0.023) | 0.025 (0.016–0.033) | 0.029 (0.02–0.048) | 4.554 | 0.288 | 0.046 | 0.042 | 0.071 |
| Estimated GFR state 2 | 0.61 (0.53–0.67) | 0.11 (0.081–0.15) | 0.038 (0.022–0.058) | 0.18 (0.12–0.24) | 0.057 (0.038–0.13) | 2.257 | 1.743 | 0.349 | 0.497 | 0.155 |
| Estimated GFR state 3 | 0.19 (0.11–0.29) | 0.056 (0.03–0.089) | 0.029 (0.012–0.058) | 0.62 (0.42–0.73) | 0.1 (0.052–0.3) | 0.538 | 0.515 | 1.206 | 2.408 | 0.334 |
| ESRD | 0 | 0 | 0 | 0.95 (0.68–0.99) | 0.05 (0.008–0.32) | 0 | 0 | 0 | 4.875 | 0.125 |
GFR = glomerular filtration rate; 95% CI = 95% confidence interval; ESRD = end-stage renal disease.
Table 4.
Predictive probabilities of transitioning between states and the time spent in each state for estimated proteinuria*
| Estimated probability (95% CI) of being in estimated proteinuria state | Expected time spent in estimated proteinuria state, years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial state | Estimated proteinuria state 1 | Estimated proteinuria state 2 | Estimated proteinuria state 3 | ESRD | Death | Estimated proteinuria state 1 | Estimated proteinuria state 2 | Estimated proteinuria state 3 | ESRD | Death |
| 1 year | ||||||||||
| Estimated proteinuria state 1 | 0.81 (0.78–0.83) | 0.17 (0.15–0.19) | 0.011 (0.008–0.013) | 0.004 (0.002–0.016) | 0.006 (0.003–0.011) | 0.892 | 0.1 | 0.004 | 0.001 | 0.003 |
| Estimated proteinuria state 2 | 0.35 (0.33–0.38) | 0.56 (0.53–0.59) | 0.062 (0.05–0.077) | 0.016 (0.011–0.027) | 0.007 (0.004–0.013) | 0.205 | 0.74 | 0.043 | 0.008 | 0.003 |
| Estimated proteinuria state 3 | 0.16 (0.13–0.19) | 0.46 (0.41–0.51) | 0.31 (0.24–0.38) | 0.056 (0.035–0.099) | 0.016 (0.008–0.044) | 0.064 | 0.32 | 0.575 | 0.032 | 0.009 |
| ESRD | 0 | 0 | 0 | 0.99 (0.94–1) | 0.010 (0.001–0.063) | 0 | 0 | 0 | 0.995 | 0.005 |
| 2 years | ||||||||||
| Estimated proteinuria state 1 | 0.72 (0.69–0.74) | 0.24 (0.22–0.26) | 0.022 (0.018–0.028) | 0.010 (0.007–0.027) | 0.012 (0.007–0.02) | 1.649 | 0.311 | 0.021 | 0.008 | 0.012 |
| Estimated proteinuria state 2 | 0.49 (0.46–0.52) | 0.4 (0.37–0.43) | 0.058 (0.046–0.07) | 0.029 (0.021–0.047) | 0.014 (0.009–0.025) | 0.64 | 1.21 | 0.105 | 0.031 | 0.014 |
| Estimated proteinuria state 3 | 0.34 (0.3–0.38) | 0.43 (0.39–0.46) | 0.12 (0.091–0.17) | 0.08 (0.05–0.14) | 0.026 (0.014–0.058) | 0.321 | 0.775 | 0.772 | 0.101 | 0.03 |
| ESRD | 0 | 0 | 0 | 0.98 (0.88–1) | 0.019 (0.002–0.12) | 0 | 0 | 0 | 1.981 | 0.019 |
| 5 years | ||||||||||
| Estimated proteinuria state 1 | 0.62 (0.57–0.64) | 0.28 (0.25–0.3) | 0.034 (0.027–0.043) | 0.033 (0.025–0.078) | 0.031 (0.021–0.053) | 3.618 | 1.122 | 0.112 | 0.071 | 0.076 |
| Estimated proteinuria state 2 | 0.58 (0.54–0.6) | 0.29 (0.26–0.31) | 0.038 (0.029–0.048) | 0.059 (0.043–0.096) | 0.034 (0.024–0.06) | 2.31 | 2.195 | 0.242 | 0.166 | 0.087 |
| Estimated proteinuria state 3 | 0.52 (0.47–0.55) | 0.28 (0.25–0.31) | 0.039 (0.029–0.05) | 0.11 (0.078–0.17) | 0.047 (0.031–0.097) | 1.709 | 1.789 | 0.964 | 0.397 | 0.141 |
| ESRD | 0 | 0 | 0 | 0.95 (0.71–0.99) | 0.047 (0.006–0.29) | 0 | 0 | 0 | 4.882 | 0.118 |
Estimated proteinuria was defined as estimated 24-hour urine protein excretion. 95% CI = 95% confidence interval; ESRD = end-stage renal disease.
Regression analysis for changes in estimated GFR states
Complete results for univariate analyses are available from the corresponding author upon request. The significant predictors from univariate analyses, including age at SLE diagnosis, geographic site, race/ethnicity, calendar year, and immunosuppressant use, in addition to sex and antimalarial use (for adjustment purposes), were included in a primary model for multivariate regression analysis (Table 5). Other important predictors, such as baseline lupus anticoagulant and aCL antibody status, ISN/RPS classes III–VI, and renal biopsy chronicity scores, were initially excluded since they substantially reduced the sample size. Estimated proteinuria states were excluded, since including another measure of kidney function as an explanatory variable is a potential confounder.
Table 5.
Results from the multivariate analysis that identified predictors of transitions between estimated GFR and estimated proteinuria states*
| Estimated GFR analysis |
Estimated proteinuria analysis |
|||
|---|---|---|---|---|
| Transition | RR (95% CI) | P | RR (95% CI) | P |
| Sex | 0.043 | 0.016 | ||
| Female | 1 | 1 | ||
| Male | ||||
| 1→2, 2→3 | 1.168 (0.684–2.00) | 0.858 (0.603–1.22) | ||
| 2→1, 3→2 | 2.159 (1.183–3.94) | 0.594 (0.417–0.85) | ||
| Age at diagnosis† | <0.001‡ | 0.268 | ||
| 1→2 | 1.259 (0.929–1.71) | 0.845 (0.714–1.00) | ||
| 2→1 | 1.545 (1.071–2.23) | 0.962 (0.826–1.12) | ||
| 2→3 | 1.206 (0.526–2.76) | 0.806 (0.554–1.17) | ||
| 3→2 | 2.741 (0.653–11.51) | 0.922 (0.650–1.31) | ||
| Age at diagnosis | ||||
| 1→2 | 1.061 (0.920–1.22) | – | ||
| 2→1 | 0.743 (0.631–0.87) | – | ||
| 2→3 | 0.976 (0.696–1.37) | – | ||
| 3→2 | 0.64 (0.348–1.18) | – | ||
| Geographic site | 0.289 | 0.122 | ||
| Canada | 1 | 1 | ||
| US | ||||
| 1→2, 2→3 | 1.105 (0.579–2.11) | 1.283 (0.800–2.06) | ||
| 2→1, 3→2 | 2.098 (0.970–4.54) | 1.191 (0.768–1.85) | ||
| Mexico | ||||
| 1→2, 2→3 | 0.794 (0.142–4.45) | 1.112 (0.408–3.03) | ||
| 2→1, 3→2 | 0.475 (0.060–3.78) | 0.764 (0.250–2.33) | ||
| Europe | ||||
| 1→2, 2→3 | 0.68 (0.349–1.33) | 1.116 (0.691–1.80) | ||
| 2→1, 3→2 | 1.623 (0.769–3.42) | 1.433 (0.941–2.18) | ||
| Korea | ||||
| 1→2, 2→3 | 0.479 (0.160–1.43) | 1.304 (0.624–2.73) | ||
| 2→1, 3→2 | 1.038 (0.335–3.21) | 0.566 (0.312–1.03) | ||
| Race/ethnicity | <0.001 | 0.013 | ||
| Caucasian | 1 | 1 | ||
| Hispanic | ||||
| 1→2, 2→3 | 2.888 (0.514–16.23) | 1.317 (0.483–3.59) | ||
| 2→1, 3→2 | 4.521 (0.584–34.99) | 0.637 (0.215–1.89) | ||
| Asian | ||||
| 1→2, 2→3 | 4.117 (1.726–9.82) | 1.011 (0.510–2.00) | ||
| 2→1, 3→2 | 7.393 (2.786–19.61) | 1.036 (0.610–1.76) | ||
| African | ||||
| 1→2, 2→3 | 2.818 (1.573–5.05) | 1.142 (0.752–1.73) | ||
| 2→1, 3→2 | 1.528 (0.769–3.04) | 0.506 (0.340–0.75) | ||
| Other | ||||
| 1→2, 2→3 | 0.839 (0.165–4.28) | 1.097 (0.499–2.41) | ||
| 2→1, 3→2 | 2.327 (0.451–12.02) | 0.607 (0.285–1.29) | ||
| Calendar year§ | 0.168 | 0.017 | ||
| 1→2, 2→3 | 0.55 (0.251–1.21) | 0.831 (0.449–1.54) | ||
| 2→1, 3→2 | 1.554 (0.566–4.27) | 2.122 (1.163–3.87) | ||
| Antimalarial use | 0.387 | 0.145 | ||
| No | 1 | |||
| Yes | ||||
| 1→2 | 0.764 (0.487–1.20) | 0.824 (0.605–1.12) | ||
| 2→1 | 1.202 (0.712–2.03) | 0.854 (0.635–1.15) | ||
| 2→3 | 1.954 (0.608–6.28) | 0.735 (0.372–1.46) | ||
| 3→2 | 2.678 (0.465–15.41) | 1.467 (0.773–2.78) | ||
| Immunosuppressant use | 0.113 | 0.046 | ||
| No | 1 | 1 | ||
| Yes | ||||
| 1→2 | 1.448 (0.818–2.57) | 1.538 (1.076–2.20) | ||
| 2→1 | 0.895 (0.490–1.63) | 0.905 (0.635–1.29) | ||
| 2→3 | 11.684 (1.218–112.11) | 0.635 (0.261–1.55) | ||
| 3→2 | 3.962 (0.564–27.83) | 0.809 (0.315–2.08) | ||
| Steroid use | 0.016 | |||
| No | – | 1 | ||
| Yes | – | |||
| 1→2 | – | 1.264 (0.893–1.79) | ||
| 2→1 | – | 1.243 (0.885–1.75) | ||
| 2→3 | – | 2.718 (0.858–8.61) | ||
| 3→2 | – | 0.53 (0.201–1.39) | ||
| SLEDAI-2K (without renal variables) | 0.90 | |||
| 1→2, 2→3 | – | 1.009 (0.963–1.06) | ||
| 2→1, 3→2 | – | 0.998 (0.955–1.04) | ||
| SDI (without renal variables) | 0.045 | |||
| 0 | – | 1 | ||
| 1 | – | |||
| 1→2, 2→3 | – | 1.494 (1.103–2.02) | ||
| 2→1, 3→2 | – | 0.794 (0.580–1.09) | ||
| 2 | – | |||
| 1→2, 2→3 | – | 1.269 (0.879–1.83) | ||
| 2→1, 3→2 | – | 0.792 (0.555–1.13) | ||
| 3 | – | |||
| 1→2, 2→3 | – | 1.626 (0.965–2.74) | ||
| 2→1, 3→2 | – | 1.004 (0.603–1.67) | ||
| ≥4 | – | |||
| 1→2, 2→3 | – | 1.193 (0.596–2.39) | ||
| 2→1, 3→2 | – | 0.878 (0.496–1.56) | ||
GFR = glomerular filtration rate; RR = relative rate; 95% CI = 95% confidence interval; SLEDAI-2K = Systemic Lupus Erythematosus Disease Activity Index 2000; SDI = Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
Standardized as (age – 30)/12.
Global test including quadratic terms for age at diagnosis.
Standardized as (year – 1999)/(2012 – 1999).
In the multivariate analysis, older age at SLE diagnosis (P < 0.001), and Hispanic, Asian, and/or African ancestry (P < 0.001 versus Caucasian) were associated with higher rates of both deterioration and improvement of estimated GFR states. Men had higher rates of improvement than women (P = 0.04).
During the study period, there were changes in standard of care in the treatment of LN, which we anticipated would be reflected in our data. In order to incorporate these changes in our model, apart from including calendar years as a predictor, we further examined whether the outcome varied with the type of immunosuppressant used (azathioprine, oral or intravenous cyclophosphamide, mycophenolic acid, and other immunosuppressants). Overall, there was no evidence for differential effects of the various immunosuppressants on deterioration and improvement rates between estimated GFR states (global P = 0.113). The only suggestive association was between mycophenolic acid (versus azathioprine as the baseline category) and a lower rate of improvement from estimated GFR state 2 to state 1 (RR 0.36 [95% confidence interval (95% CI) 0.18–0.73]).
To examine other potentially important predictors, estimated proteinuria states, baseline aCL antibody status, ISN/RPS classes III–VI, renal biopsy chronicity scores, and the adjustment variables sex, age at SLE diagnosis, geographic site, ethnicity, calendar year, antimalarial use, and immunosuppressant use were included in further multivariate regression models. Higher estimated proteinuria states had a positive association with deterioration as well as a negative association with improvement with regard to estimated GFR states (estimated proteinuria state 2 versus estimated proteinuria state 1 for estimated GFR transition from state 1 to state 2 or from state 2 to state 3: RR 2.28 [95% CI 1.402–3.71]; estimated proteinuria state 2 versus estimated proteinuria state 1 for estimated GFR transition from state 2 to state 1 or from state 3 to state 2: RR 0.53 [95% CI 0.31–0.91]; estimated proteinuria state 3 versus estimated proteinuria state 1 for estimated GFR transition from state 1 to state 2 or from state 2 to state 3: RR 4.88 [95% CI 2.75–8.66]; estimated proteinuria state 3 versus estimated proteinuria state 1 for estimated GFR transition from state 2 to state 1 or from state 3 to state 2: RR 0.42 [95% CI 0.18–0.95]; global P < 0.001).
Further analyses revealed higher rates in the following specific transitions: estimated proteinuria state 2 versus estimated proteinuria state 1 for estimated GFR transition from state 1 to state 2 (RR 1.71 [95% CI 1.02–2.86]), estimated proteinuria state 3 versus estimated proteinuria state 1 for estimated GFR transition from state 1 to state 2 (RR 3.58 [95% CI 1.81–7.11]), and estimated proteinuria state 3 versus estimated proteinuria state 1 for estimated GFR transition from state 2 to state 3 (RR 4.16 [95% CI 1.04–16.70]). In addition, there was a lower rate for estimated GFR transition from state 2 to state 1 for estimated proteinuria state 2 versus estimated proteinuria state 1 (RR 0.56 [95% CI 0.31–0.99]).
The presence of aCL antibodies at baseline was associated with transition rates between estimated GFR states in the multivariate analysis with a borderline global P value of 0.083, and the most notable effect was lower rates of improvement (aCL present versus absent for transitions from state 2 to state 1 and state 3 to state 2: RR 0.41 [95% CI 0.17–0.96]). Higher renal biopsy chronicity scores were associated with a higher deterioration rate from estimated GFR state 1 to state 2 (RR 1.22 [95% CI 1.026–1.45]; global P = 0.002).
Regression analysis for changes in estimated proteinuria states
The methodology used and predictor variables examined for the univariate analyses of estimated proteinuria were identical to those used in the analyses of estimated GFR. Detailed results are available from the corresponding author upon request. Similar to the estimated GFR analyses, important predictors from univariate analyses, such as age at SLE diagnosis, geographic site, race/ethnicity, calendar year, use of antimalarials, use of immunosuppressants and steroids, SLEDAI-2K score (without renal variables), and SDI score (without renal variables), as well as sex (for adjustment purposes) were initially included in a primary model for multivariate regression analysis of estimated proteinuria states (Table 5).
In the multivariate analysis, Hispanics and patients with African ancestry (P = 0.013), earlier calendar years (P = 0.017), and higher SDI scores (without renal variables) (P = 0.045) were associated with higher deterioration and lower improvement in estimated proteinuria states, while men had lower rates of improvement than women (P = 0.016). Immunosuppressant use was linked to a higher deterioration rate from state 1 to state 2 (P = 0.046), and steroid use was linked to higher deterioration rates (P = 0.016).
Age at SLE diagnosis, geographic site, antimalarial use, and SLEDAI-2K score (without renal variables) were no longer significant in the multivariate analysis. Removing geographic site from the model revealed a highly significant effect of race/ethnicity (P < 0.001), which suggests that race/ethnicity predominated when both were included. As was done for estimated GFR states, we also examined whether the outcome in the model for estimated proteinuria states varied with the type of immunosuppressant used. The most notable effect was that patients taking mycophenolic acid (versus azathioprine) had lower estimated improvement rates between estimated proteinuria states (common RR 0.58 [95% CI 0.42–0.79]), and the global test for any differential effects generated a P value of 0.003, primarily on the basis of this effect.
The association with steroid use was studied further. After categorizing the average daily oral prednisone dosage between diagnosis and enrollment or between assessments as low (≤7.5 mg/day), moderate (7.5–30 mg/day), or high (>30 mg/day) and the average daily intravenous pulse methylprednisolone dosage as low (0–100 mg), moderate (101–500 mg), or high (>500 mg), the highest of oral or pulse steroid dosage was assigned to each transition in estimated proteinuria. There were 740 assessment visits with no steroid use and 977, 1,357, and 333 visits with low, moderate, and high dosages, respectively. Compared with no steroid use, there was no association with low and moderate steroid dosage. There was a positive association of high dosages of steroids with improvement from state 2 to state 1 (RR 2.07 [95% CI 1.13–3.78]), but no associations were found for other transitions.
To examine other predictors, such as estimated GFR states, baseline autoantibody status, ISN/RPS classes III–VI, and renal biopsy chronicity scores, we included sex, age at SLE diagnosis, ethnicity, calendar year, antimalarial use, immunosuppressant use, steroid use, SLEDAI-2K score (without renal variables), and SDI score (without renal variables) as adjustment variables in further multivariate regression analyses. Geographic site was not included, since the effect of geography was primarily manifested through race/ethnicity, as described above.
Higher estimated GFR states demonstrated some association with deterioration from estimated proteinuria state 1 to state 2 (estimated GFR state 2 versus estimated GFR state 1: RR 1.93 [95% CI 1.07–3.50]; estimated GFR state 3 versus estimated GFR state 1: RR 2.43 [95% CI 0.77–7.63]), but the overall effect of estimated GFR states on the transition between estimated proteinuria states was not significant (global P = 0.17). The presence of lupus anticoagulant at baseline remained significantly associated (P = 0.007) with lower improvement rates in estimated proteinuria states (lupus anticoagulant present versus absent for estimated proteinuria transitions from state 2 to state 1 and from state 3 to state 2: RR 0.61 [95% CI 0.44–0.87]). The presence of anti-P antibodies at baseline was no longer significant in the multivariate analyses (P = 0.35). Higher renal biopsy chronicity scores remained associated with higher deterioration rates from estimated proteinuria state 1 to state 2 (RR 1.15 [95% CI 1.04–1.27], P = 0.014). ISN/RPS class V remained associated with lower improvement rates for estimated proteinuria state 2 to state 1 and state 3 to state 2 (RR 0.65 [95% CI 0.48–0.90], global P = 0.012). ISN/RPS class III also had a suggestive association with lower improvement rates for estimated proteinuria transitions from state 2 to state 1 and state 3 to state 2 (RR 0.67 [95% CI 0.45– 1.00]) after adjusting for other factors.
Lost to follow-up
Since estimated GFR and estimated proteinuria data were available from a high percentage of assessment visits, there is little intermittent missingness in the data. Thirteen LN patients had an interval between their last assessment and the study cutoff date of >1.5 years, did not have a subsequent study assessment, and were not classified into the ESRD or death state at their last study visit. When a variable identifying these 13 patients was introduced into the multistate models, based on a 2 degrees of freedom test of common forward and backward relative rate estimates, it was not significant in the estimated GFR (P = 0.34) and estimated proteinuria (P = 0.37) multivariate models presented in Table 5. Thus, for transitions between estimated GFR and estimated proteinuria states, there is no evidence that these patients had a different disease course after adjustment for other variables. There was also no significant effect when the variable was added to unadjusted estimated GFR and estimated proteinuria models (P = 0.93 for each). Thus, assumptions that data for patients who were lost to follow-up is missing at random or missing completely at random are plausible, although only the former is needed for the validity of our findings.
Discussion
Nephritis is a pivotal manifestation of SLE and accounts for significant morbidity and mortality. Large observational cohort studies with long-term follow-up provide real-world experience of LN outcomes. This is the first study of LN to examine a reversible multistate model of transitions between disease states. Changes in clinically relevant renal outcomes were evaluated in 700 patients with LN receiving standard of care over a mean of 5 years and a total span of 13 years in an international disease inception cohort. The results provide estimates for improvement or deterioration in estimated GFR and estimated proteinuria and identify predictors of change in renal status. Documenting the probability of change in renal outcomes in patients receiving standard of care provides a target for projected outcomes in clinical trials of new therapeutic interventions.
The SLICC inception cohort has been well characterized previously (26–29). It represents a general lupus population without major selection bias and is well suited to address the objectives of our study. The cumulative frequency of nephritis of 38.3% is almost identical to the overall incidence of 37.8% in 2,290 SLE patients from studies in North America, Europe, and the Middle East (1). The predilection for nephritis to present early in the disease course (30), the higher frequency of nephritis at a younger age (31,32) and in patients of non-Caucasian race/ethnicity (30–33), and the higher frequency of comorbidities such as hypertension (34,35) indicate the validity of the cohort and generalizability of the findings.
In the present study, ESRD and estimates of GFR and proteinuria were selected as the primary renal outcomes. The estimated GFR and estimated proteinuria states reflect clinically meaningful normal and abnormal renal function. Transition rates were derived from the actual change between states over time. Potential applications of multistate modeling include using transition rates as a primary outcome in clinical trials and projecting the cost of care for LN according to the status of the disease. For example, the estimated probability of improving from estimated GFR state 3 to state 1 at 5 years is 0.19. In order to detect a doubling of the odds of achieving this improvement in a clinical trial of LN, the sample size required (with an alpha level of 0.05 and 80% power) would be 362 patients (181 in each group). With only 2 years of follow-up, the corresponding probability is 0.095, and the required sample size would be almost double at 608. Furthermore, by determining the actual costs for each estimated GFR state and knowing the projected proportion of patients and the duration of time in each state, one can predict the costs of care for patients in each estimated GFR state.
Overall, there was more improvement than deterioration in estimated GFR and estimated proteinuria states, as would be expected for patients receiving standard of care. The longer the duration of observation, the greater the likelihood of transitions reflecting improvement (e.g., estimated GFR state 3 to state 1) or deterioration (e.g., estimated GFR state 3 to ESRD), which emphasizes the need for long-term studies in LN. Analyses based on multistate models provide a larger number of effects linked to predictor variables than other regression models. Thus, multivariate analyses are probably the most informative for the identification of the primary associations, and borderline significant effects should be regarded with caution and interpreted in light of the estimated RRs and 95% confidence intervals.
In multivariate analysis the significant predictors of deterioration in estimated GFR state included older age, race/ethnicity (Hispanic, Asian, or African ancestry), higher estimated proteinuria state, higher renal biopsy chronicity scores, and the presence of aCL antibodies at enrollment. Curiously, male sex was the only predictor of improvement in estimated GFR. Multivariate analyses also identified predictors of deterioration in estimated proteinuria state, which included race/ethnicity (Hispanic, Asian, or African ancestry) and higher renal biopsy chronicity score. Lower rates of improvement were associated with the presence of lupus anticoagulant at enrollment and ISN/RPS class V nephritis. The counterintuitive association between any steroid use and deterioration in estimated proteinuria states was no longer apparent when the dosage of medication was included in the analysis, which revealed an association between high-dose steroids and improvement in estimated proteinuria state. Other studies (21,36–41) have reported similar predictors for improvement and deterioration in the outcome of LN over time.
There are a number of limitations to the present study. First, the selection of specific therapies was the result of the treating physician’s recommendation and patient preference rather than study protocol. While this reflects what occurs in routine clinical practice, the apparent impact of medication in an observational study may be confounded by the rationale (e.g., disease severity) for selecting the treatment. When drug allocation is related to the outcome, confounding can also occur. Second, the investigators within the SLICC network practice in academic medical centers and have a special interest in lupus. Thus, our data may not fully reflect community clinical practice. Third, our multistate model approach can handle both intermittent missing data and right censoring under the assumption that they are unrelated to the missing estimated GFR and estimated proteinuria states given the observed data. However, this assumption cannot be verified. Finally, it is inevitable that patients in a disease inception cohort will have a shorter disease duration and younger age at enrollment than prevalent lupus cases. Both factors are associated with chronic kidney disease, and further follow-up is necessary to determine the long-term outcome of LN in this cohort.
Despite these limitations, our study provides very useful information on the change in clinically relevant renal outcomes in patients with LN receiving current standard of care. The multistate model approach is novel and generates probability estimates of transitions between disease states that reflect improvement or deterioration in renal outcomes. This approach can identify predictors of change in renal status and can inform clinical trial design by providing minimum expectations for benefit from new therapeutic interventions for LN.
Role of the Study Sponsor
The Systemic Lupus International Collaborating Clinics (SLICC) and Bristol-Myers Squibb were jointly involved in the study design. SLICC was solely responsible for collection, analysis, and interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication. Publication of this article was jointly approved by SLICC and Bristol-Myers Squibb.
The views expressed herein are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
The Systemic Lupus International Collaborating Clinics (SLICC) research network received funding for this study from Bristol-Myers Squibb. The Hopkins Lupus Cohort is supported by the NIH (grant AR43727). The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research. Dr. Hanly’s work was supported by the Canadian Institutes of Health (research grant MOP-86526). Dr. Bae’s work was supported by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (grant A120404). Dr. Gordon’s work was supported by Lupus UK, Sandwell and West Birmingham Hospitals NHS Trust, and the NIHR/Wellcome Trust Clinical Research Facility in Birmingham. Dr. Clarke holds The Arthritis Society Chair in Rheumatic Diseases at the University of Calgary. Drs. Isenberg and Rahman’s work was supported by the NIHR University College London Hospitals Biomedical Research Centre. Dr. Fortin holds a Tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases at Université Laval, and part of this work was done while he was a Distinguished Senior Investigator of The Arthritis Society. Dr. Bruce is an NIHR Senior Investigator and his work was supported by Arthritis Research UK, the National Institute for Health Research Manchester Biomedical Research Unit, and the NIHR/Wellcome Trust Manchester Clinical Research Facility. Dr. Jacobsen’s work was supported by the Danish Rheumatism Association (A1028) and the Novo Nordisk Foundation (A05990). Dr. Ramsey-Goldman’s work was supported by the NIH (grants 8UL1TR000150 [formerly UL-1RR-025741], K24-AR-02318, and P60AR064464 [formerly P60-AR-48098]). Dr. Dooley’s work was supported by the NIH (grant RR00046). Dr. Ruiz-Irastorza’s work was supported by the Department of Education, Universities and Research of the Basque Government and the Basque Biobank. Drs. Su and Farewell’s work was supported by the MRC (UK) (U105261167).
Dr. Clarke has received consulting fees, speaking fees, and/or honoraria from Eli Lilly and MedImmune/AstraZeneca (less than $10,000 each). Dr. Bruce has received consulting fees, speaking fees, and/or honoraria from Eli Lilly, UCB, Roche, Merck Serono, MedImmune (less than $10,000 each) and grants from UCB, Genzyme Sanofi, and GlaxoSmithKline. Dr. Fortin has received consulting fees, speaking fees, and/or honoraria from Eli Lilly, AbbVie, and Glaxo-SmithKline (less than $10,000 each). Dr. Manzi has received grants from UCB and Human Genome Sciences/GlaxoSmithKline and has received consulting fees from Exagen Diagnostics, GlaxoSmithKline, Eli Lilly, and UBC (less than $10,000 each). Dr. Kalunian has received grants from UCB, Human Genome Sciences/GlaxoSmithKline, Takeda, Ablynx, Bristol-Myers Squibb, Pfizer, and Kyowa Hakko Kirin, and has received consulting fees from Exagen Diagnostics, Genentech, Eli Lilly, Bristol-Myers Squibb, and Anthera (less than $10,000 each).
Footnotes
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Hanly had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Hanly, Urowitz, Romero-Diaz, Gordon, Clarke, Wallace, Isenberg, Ginzler, Petri, Bruce, Fortin, Gladman, Sanchez-Guerrero, Steinsson, Khamashta, Alarcón, Manzi, Nived, Sturfelt, van Vollenhoven, Kalunian, Inanc, Askanase, Farewell.
Acquisition of data. Hanly, Urowitz, Romero-Diaz, Gordon, Bae, Bernatsky, Clarke, Wallace, Merrill, Isenberg, Rahman, Ginzler, Petri, Bruce, Dooley, Fortin, Gladman, Sanchez-Guerrero, Steinsson, Ramsey-Goldman, Khamashta, Aranow, Alarcón, Fessler, Manzi, Nived, Sturfelt, van Vollenhoven, Ramos-Casals, Ruiz-Irastorza, Lim, Kalunian, Inanc, Kamen, Peschken, Jacobsen, Askanase, Theriault.
Analysis and interpretation of data. Hanly, Su, Gordon, Bernatsky, Clarke, Wallace, Petri, Bruce, Dooley, Sanchez-Guerrero, Khamashta, Alarcón, Nived, Sturfelt, Zoma, Kalunian, Askanase, Theriault, Farewell.
References
- 1.Rovin BH, Stillman IE. Kidney. In: Lahita RG, Tsokos G, Buyon J, Koike T, editors. Systemic Lupus Erythematosus. 5th ed. London: Elsevier; 2011. pp. 769–814. [Google Scholar]
- 2.Merrell M, Shulman LE. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis. 1955;1:12–32. doi: 10.1016/0021-9681(55)90018-7. [DOI] [PubMed] [Google Scholar]
- 3.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. Progressive improvement of patient and renal survival and reduction of morbidity over time in patients with lupus nephritis (LN) followed for 20 years. Lupus. 2013;22:810–8. doi: 10.1177/0961203313492576. [DOI] [PubMed] [Google Scholar]
- 4.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35:2152–8. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
- 5.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 6.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63:1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 2011;50:1424–30. doi: 10.1093/rheumatology/ker101. [DOI] [PubMed] [Google Scholar]
- 8.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 9.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group—onwards and upwards? Lupus. 2006;15:606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 10.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. doi: 10.1093/rheumatology/kev311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, the Committee on Prognosis Studies in SLE Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 13.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Weening J, D’Agati V, Schwartz M, Seshan S, Alpers C, Appel G, et al. The classification of glomerulonephritis in systemic lupus nephritis erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 16.Austin HA, III, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–95. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 17.Patel SB, Korbet SM, Lewis EJ. The prognosis of severe lupus nephritis based on the Modification of Diet in Renal Disease (MDRD) study estimated glomerular filtration rate. Lupus. 2011;20:256–64. doi: 10.1177/0961203310385267. [DOI] [PubMed] [Google Scholar]
- 18.Christopher-Stine L, Petri M, Astor BC, Fine D. Urine protein-to-creatinine ratio is a reliable measure of proteinuria in lupus nephritis. J Rheumatol. 2004;31:1557–9. [PubMed] [Google Scholar]
- 19.Matar HE, Peterson P, Sangle S, D’Cruz DP. Correlation of 24-hour urinary protein quantification with spot urine protein: creatinine ratio in lupus nephritis. Lupus. 2012;21:836–9. doi: 10.1177/0961203312437438. [DOI] [PubMed] [Google Scholar]
- 20.De Macedo PA, Borba EF, Viana Vdos S, Leon EP, Testagrossa Lde A, Barros RT, et al. Antibodies to ribosomal P proteins in lupus nephritis: a surrogate marker for a better renal survival? Autoimmun Rev. 2011;10:126–30. doi: 10.1016/j.autrev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Moroni G, Ventura D, Riva P, Panzeri P, Quaglini S, Banfi G, et al. Antiphospholipid antibodies are associated with an increased risk for chronic renal insufficiency in patients with lupus nephritis. Am J Kidney Dis. 2004;43:28–36. doi: 10.1053/j.ajkd.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Merrill JT, Zhang HW, Shen C, Butman BT, Jeffries EP, Lahita RG, et al. Enhancement of protein S anticoagulant function by β2-glycoprotein I, a major target antigen of antiphospholipid antibodies: β2-glycoprotein I interferes with binding of protein S to its plasma inhibitor, C4b-binding protein. Thromb Haemost. 1999;81:748–57. [PubMed] [Google Scholar]
- 23.Merrill JT, Shen C, Gugnani M, Lahita RG, Mongey AB. High prevalence of antiphospholipid antibodies in patients taking procainamide. J Rheumatol. 1997;24:1083–8. [PubMed] [Google Scholar]
- 24.Erkan D, Zhang HW, Shriky RC, Merrill JT. Dual antibody reactivity to β2-glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus. 2002;11:215–20. doi: 10.1191/0961203302lu178oa. [DOI] [PubMed] [Google Scholar]
- 25.Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, et al. for the Systemic Lupus International Collaborating Clinics Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58:843–53. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74:1706–13. doi: 10.1136/annrheumdis-2013-205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanly JG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, et al. Mood disorders in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol. 2015;67:1837–47. doi: 10.1002/art.39111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. for the Systemic Lupus International Collaborating Clinics Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56:265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 29.Urowitz MB, Gladman D, Ibanez D, Fortin P, Sanchez-Guerrero J, Bae S, et al. the Systemic Lupus International Collaborating Clinics Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Rheum. 2008;59:176–80. doi: 10.1002/art.23353. [DOI] [PubMed] [Google Scholar]
- 30.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11:161–7. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 31.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol. 2014;66:357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66:369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alarcon GS, McGwin G Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP, et al. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11:95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 34.Naiker IP, Chrystal V, Randeree IG, Seedat YK. The significance of arterial hypertension at the onset of clinical lupus nephritis. Postgrad Med J. 1997;73:230–3. doi: 10.1136/pgmj.73.858.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis: the importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–8. [PubMed] [Google Scholar]
- 36.Franco C, Yoo W, Franco D, Xu Z. Predictors of end stage renal disease in African Americans with lupus nephritis. Bull NYU Hosp Jt Dis. 2010;68:251–6. [PubMed] [Google Scholar]
- 37.Frankovich JD, Hsu JJ, Sandborg CI. European ancestry decreases the risk of early onset, severe lupus nephritis in a single center, multiethnic pediatric lupus inception cohort. Lupus. 2012;21:421–9. doi: 10.1177/0961203312437805. [DOI] [PubMed] [Google Scholar]
- 38.Kanno A, Hotta O, Yusa N, Taguma Y. Predictive factors of clinical outcome in patients with diffuse proliferative lupus nephritis treated early by intravenous methylprednisolone pulse therapy. Ren Fail. 2007;29:41–7. doi: 10.1080/08860220601038850. [DOI] [PubMed] [Google Scholar]
- 39.Reich HN, Gladman DD, Urowitz MB, Bargman JM, Hladunewich MA, Lou W, et al. Persistent proteinuria and dyslipidemia increase the risk of progressive chronic kidney disease in lupus erythematosus. Kidney Int. 2011;79:914–20. doi: 10.1038/ki.2010.525. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Zhou XJ, Ahn C, Saxena R. A retrospective analysis of clinical presentation of lupus nephritis. Am J Med Sci. 2011;342:467–73. doi: 10.1097/MAJ.0b013e3182199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vozmediano C, Rivera F, Lopez-Gomez JM, Hernandez D. Risk factors for renal failure in patients with lupus nephritis: data from the Spanish Registry of Glomerulonephritis. Nephron Extra. 2012;2:269–77. doi: 10.1159/000342719. [DOI] [PMC free article] [PubMed] [Google Scholar]