Summary
We analysed the spatiotemporal variations of bovine tuberculosis (bTB) incidence between 1965 and 2000 in France at the department level (95 areas). Using a Bayesian space–time model, we studied the association between the evolution of bTB incidence and changes of cattle population structure and of herd management practices. Several spatiotemporal hierarchical Bayesian models were compared, and the deviance information criterion was used to select the best of them. Southern France remained a high-risk area over the analysed period, whereas central and western regions were low-risk areas. Besides the frequency of tuberculin skin testing (fixed according to bTB incidence in the preceding years), four factors were associated with an increased risk of bTB: the average herd density and size, the percentage of dairy cows in the cattle population, and the percentage of permanent grassland in cultivated surfaces area. These four factors are linked to the progressive professionalization and specialization of cattle farming, with the disappearance of family farms and of the intensification of breeding systems (especially in dairy farms after the application of the milk quota system in the 1980s). Both trends probably played a significant role in reducing the risk of bTB in France between 1965 and 2000, besides mandatory detection and control procedures.
Keywords: bovine tuberculosis, spatiotemporal, professionalization, specialization
Introduction
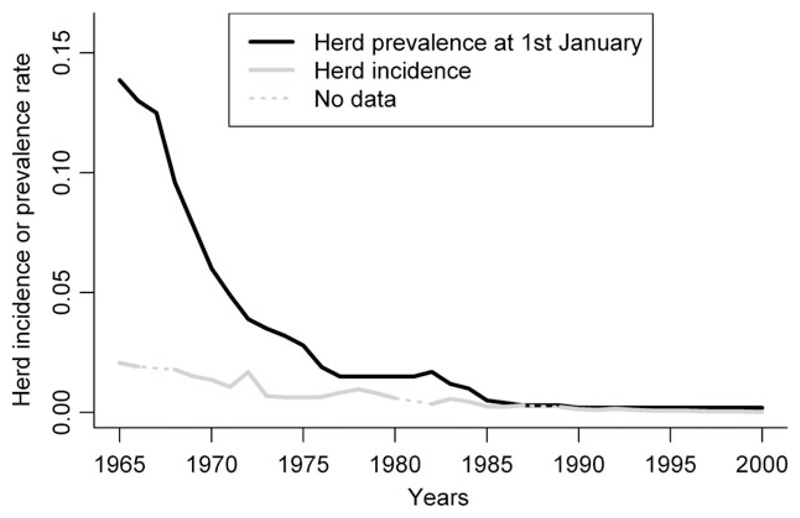
In France, the bovine tuberculosis (bTB) eradication programme started in 1954 and became compulsory throughout France by 1965. In 2000, France was recognized officially free of bTB (herd prevalence rate <0.1% for at least 6 years) by the European Union (E.U). The instantaneous prevalence of bTB in French herds has been decreased from about 13% in 1965 to 1.5% in 1980 and then to <0.1% in 2000 (Fig. 1); the prevalence thus decreased rapidly between 1965 and 1980 and more slowly thereafter (Fig. 1). This evolution, however, has not been even across the country, and the decrease was faster in some departments than in others (Figure S1). For example, on the 1st of January of the years 1965, 1980 and 2000, the herd prevalence in the Cantal department was, respectively, 21.3%, 4.06% and 0.06%, whereas in the Vienne department, the rates were 21.2%, 0.8% and 0.02%, respectively.
Fig. 1.
Evolution of the incidence and prevalence rates of bovine tuberculosis in France, from 1965 to 2000.
Since 1965, the national control programme has been based on detection of infected herds by: (i) screening for infected animals using the intradermal tuberculin test (IDTT) and (ii) inspection of carcasses at slaughterhouse. Intradermal tuberculin test reactors are culled to prevent spread. In 1990, the control programme was reinforced by an important measure to protect free herds: introduced animals can only come from bTB-free farms and are submitted to an IDTT on arrival (movement restrictions are imposed if this measure is not applied by the farmer). From 1999, the low bTB prevalence allowed introducing in the control programme the slaughter of all cattle in infected herds. Although the same control programme has been implemented in all departments, the frequency of tuberculin skin testing has been adapted to the herd prevalence rate in each department, according to the EU Council Directive (64/432/EEC). Thus, tuberculin skin testing could be annual in some departments, biennial, triennial or quadrennial in others, and this frequency could change according to the year. In some cases, IDTT screening was stopped. Similarly, although infected herds are in theory subjected to a total slaughter since 1999, an exception to this rule was applied in the Camargue region from 2003 and in the Côte d’Or and Dordogne departments since 2009.
The population of cattle in France has profoundly changed since the 1950s. Between 1950 and 1980, the dominant production was milk in traditional structures of family type, and herd sizes were generally small. From 1980, the dairy production declined, whereas meat production increased. This was the consequence of European regulations that aimed at limiting milk production (via the introduction of milk quotas in 1984) and that supported beef production. In parallel, the number of cattle herds decreased by three quarters between 1965 and 2000, although the total number of cattle has remained roughly unchanged: the average herd size thus significantly increased. Cattle herd bTB status is strongly dependent on herd management practices: Munyeme et al. (2008) reported that the risk of bTB infection in Zambia was greater for transhumant herds than for sedentary herds [odds ratio (OR) = 3 (1.1–8.6)] (Munyeme et al., 2008). In the UK, Reilly and Courtenay (2007) analysed bTB breakdowns using case–control studies: they reported that the storage of manure for ≥6 months increased the odds ratio of transient (i.e. under breakdown restrictions for ≤6 months) bTB breakdown [OR = 4.4 (1.3–15.4)] (Reilly and Courtenay, 2007). Similarly, in a study conducted in the southwest of England between 2001 and 2004 by Ramirez-Villaescusa et al. (2010), spreading slurry or manure all year round was found to be associated with an increased risk of bTB herd breakdown [OR = 2.23 (1.10–4.51)] (Ramirez-Villaescusa et al., 2010). In Belgium, Humblet et al. (2010) analysed bTB outbreaks identified between 1995 and 2006 (n = 415) and reported that cattle density was a risk factor for bTB (Humblet et al., 2010). In several studies, the risk of bTB was higher for dairy herds than for other herd types (beef/mixed) (Ramirez-Villaescusa et al., 2010; Karolemeas et al., 2011; Vial et al., 2011; Alvarez et al., 2012). Herd size has also been identified as a risk factor in several studies conducted at the herd level (Griffin et al., 1996; Munroe et al., 1999; Green and Cornell, 2005; Reilly and Courtenay, 2007; Carrique-Mas et al., 2008; Brooks-Pollock and Keeling, 2009; Ramirez-Villaescusa et al., 2010; Wolfe et al., 2010). Other authors highlighted the association between cattle herd bTB status and the number of cattle movements in the infected area (Gilbert et al., 2005). A study conducted in Belgium identified the proportion of cattle movements from an infected area during the current year as the main risk factor for bTB (Humblet et al., 2010). History of tuberculosis, in the herd or in the region, has also been identified as a risk factor in several studies (Olea-Popelka et al., 2004; Carrique-Mas et al., 2008; Humblet et al., 2010; Karolemeas et al., 2011). Finally, the proximity to an outbreak was a risk factor for between-herd transmission of bTB (Griffin et al., 1996; Gilbert et al., 2005; Karolemeas et al., 2011).
In France, between 1950 and 2000, there have been simultaneous changes in farming practices (with the disappearance of family farms and professionalization of breeder activity), herd structures (changes in both size and type of herd), in the pattern of disease (the incidence of tuberculosis decreasing more or less rapidly depending on the department) and in the control programme (gradually adapted, according to the evolution of the disease, in each department). The declining prevalence and incidence of bTB in France since the 1950s is presumably due to the effectiveness of the bTB control programme, but the profound changes to herd structures and management practices during this period are also likely to have contributed.
The aim of this work was to analyse the spatiotemporal variations of the incidence of bTB in France between the implementation of mandatory prophylaxis (in 1965) and obtaining the bTB-free status (in 2000). In particular, this work (i) highlights areas of high and low relative risks that remained so throughout the 35-year period and (ii) analyses the association between the evolution of the incidence of bTB and the evolution of cattle population structure and of herd management practices.
Materials and Methods
Data
Bovine tuberculosis incidence
The number of newly reported infected herds (herds that became positive for bTB during a given year after having been negative the previous year) was obtained from the French Ministry of Agriculture, Food and Forestry (MAFF). The annual incidence has been recorded only since 1981. The herd incidence (n) before that date was calculated from the number of infected herds on the 1st of January for each year (N1), the number of herds cleaned during the year (A) and the remaining number of infected herds on the 31st of December (N2): n = N2 + A – N1. Herd incidence data were thus obtained for each year between 1965 and 2000 included, with the exception of 3 years (1967, 1981, 1988) for which the MAFF annual detailed report on bTB did not include these data, for unknown reasons.
Frequency of tuberculin skin testing
During the study period, French cattle herds were tested every 1, 2, 3 or 4 years using the simple intradermal tuberculin test (IDTT) for evidence of infection with M. bovis. Moreover, screening was stopped in some areas for part of the study period. For each department and for the period 1989–2000, the frequency of tuberculin skin testing was obtained from MAFF. For the period before 1988, we used the frequency of tuberculin skin testing recommended by the EU Council Directive (64/432/EEC), implemented by the MAFF. This directive sets the rules for changing the frequency of testing according to the observed herd prevalence in the area:
-
1
Screening should be biennial if the herd prevalence rate in the area has been <1% for 2 years.
-
2
Screening should be triennial if the herd prevalence rate in the area has been <0.2% for 4 years.
-
3
Screening should be quadrennial (or can be stopped) if herd prevalence rate in the area has been <0.1% for 6 years.
Breeding structures and farming practices
Annual data for permanent grassland (ha), cultivated surface (ha), number of cattle, number of dairy cows and number of cattle herds for each department were provided by the MAFF.
Model
Model structure
We considered a spatiotemporal hierarchical Bayesian approach to model the incidence of bTB by department, taking into account a set of covariates: the proportion of dairy cows in the cattle population, the proportion of permanent grassland in the total cultivated area, the average herd size (number of animals) and the cattle herds density (herds/km2). Note that the proposed model is an extension of the hierarchical Bayesian spatial model proposed by Besag, York and Mollie (Besag et al., 1991). This spatial model has been widely used in epidemiology because they improve the estimation of the relative risk by taking into account the spatial dependence between the epidemiological units.
At the first level of the hierarchy of our model, we assumed that the number of reported infected cases (denoted Yit) in department i (1 ≤ i ≤ N), during period t (1 ≤ t ≤ T), follows a Poisson distribution:
Where
-
1
Eit is the number of expected cases (i.e. the product of the national incidence rate during period t by the number of cattle herds in the department i during the same period) and
-
2
λit is the unknown relative risk (RR) in department i and period t, which corresponds to the standardized incidence ratio (SIR) in that department for that period.
At the second level, the log-RRs are decomposed into:
Where:
α is the intercept of the model,
Xitk is a vector of K covariates, and βk are the associated coefficients,
μi is the main spatial random effect for department i,
νi is the spatial unstructured heterogeneity random effect for department i,
γt is the main temporal random effect of the period t.
ζit is the space–time interaction random effect for department i and period t.
The two spatial random effects μi and νi represent latent variables capturing the effects of risk factors that may be unknown or unmeasured, spatially structured μi or not νi. Similarly, the temporal random effect γt represents a latent variable capturing temporal changes due to unknown or unmeasured risk factors in the whole study region. The space–time interaction term ζit captures any departure from the main spatial and temporal patterns.
The random unstructured heterogeneity νi was specified as a Normal distribution:
where σν is the standard deviation parameter for the unstructured heterogeneity effect.
The spatially correlated heterogeneity component μi had been specified by a Gaussian conditional autoregressive (CAR) model (Kunsch, 1987), which allows to express the spatial dependence between observations in adjacent locations. It is defined as follows:
With W = (wij) is an adjacency matrix, where wij = 1 if we consider departments i and j adjacent and 0 otherwise. σμ is the standard deviation parameter that controls the conditional variability of relative risk in the spatially structured component.
Similarly, for the temporal effect, a (CAR) model had been specified as:
with Z = (ztd) is the temporal adjacency matrix, where ztd = 1 if t and d are neighbours and 0 otherwise. Here, we consider the first-order neighbourhood system which considers periods t–1 and t + 1 as the neighbours for period t. σγ is the standard deviation parameter.
Knorr-Held (2000) distinguished four different types of space–time interactions, namely space–time exchangeable, spatial independent but linked in time, time-independent linked in space and space–time inseparable (Knorr-Held, 2000). For the sake of generality, we considered a space–time inseparable interaction, using the approach proposed by Abellan et al. (2008). The space–time interaction was assumed to be generally low, departures from this rule being, however, possible. This led to use a mixture model with two components for the distribution of the ζit (Abellan et al., 2008):
The two components have Gaussian distributions with mean 0 and variances and , respectively, with assumed to be small. To insure identification of both components, we used the re-parametrization proposed by Robert (2007), according to which = + k (Robert, 2007).
Model parameterization
In some departments, few or even no infected herds were reported during some years (especially after 1990). Thus, data sets were aggregated over seven 5-year periods: 1965–1970, 1971–1974, 1975–1978, 1979–1984, 1985–1990, 1991–1995 and 1996–2000 (Table 1). As data were missing for 3 years (1967, 1981, 1988), periods 1 (1965–1970), 4 (1979–1984) and 5 (1985–1990) are periods of 6 years corresponding to 5 years with data and 1 year for which data were missing. To define five periods of average duration 5 years, periods 2 (1971–1974) and 3 (1975–1978) had to be shortened to 4 years. These two ‘shortened’ periods were chosen in a plateau of the national incidence curve to minimize effects on the estimation of incidence ratios by the model (Fig. 1).
Table 1.
Evolution at the national level of the herd incidence rate, of the frequency of tuberculin skin testing and of indicators of cattle population structures and of farming practices
| Variables | 1965–1970 | 1971–1974 | 1975–1978 | 1979–1984 | 1985–1990 | 1991–1995 | 1996–2000 |
|---|---|---|---|---|---|---|---|
| Permanent grassland (%) | 41.21% | 41.88% | 40.54% | 39.89% | 38.54% | 37.07% | 35.78% |
| Dairy cows (% of cattle population) | 38.96% | 37.64% | 36.91% | 30.23% | 26.97% | 23.01% | 21.55% |
| Herd density (herds/km2) | 2.28 | 1.81 | 1.55 | 1.31 | 1.04 | 0.78 | 0.62 |
| Herd size | 17.37 | 23.43 | 28.53 | 31.71 | 39.58 | 48.80 | 60.21 |
| Frequency of tuberculin skin testing (number of departments) | |||||||
| Stopped or quadrennial | 1 | 1 | 2 | 2 | 0 | 1 | 8 |
| Annual | 94 | 86 | 74 | 69 | 35 | 23 | 13 |
| Biennial | 0 | 4 | 13 | 24 | 52 | 48 | 38 |
| Triennial | 0 | 0 | 0 | 0 | 7 | 23 | 36 |
| Mixed | 1 | 5 | 7 | 1 | 2 | 0 | 0 |
| Herd incidence rate | 0.0175 | 0.01035 | 0.00765 | 0.00519 | 0.00233 | 0.00109 | 0.000473 |
| Herd prevalence rate (1st January) | 0.0718 | 0.0236 | 0.0149 | 0.0108 | 0.0043 | 0.0016 | 0.00053 |
All quantitative covariates were standardized. To assess the effect of these covariates on the herd incidence of bTB, we used a difference of one standard deviation.
The frequency of tuberculin skin testing obtained by IDTT was grouped into three categories: annual, biennial (reference) and ‘lightened’: triennial, quadrennial, mixed (several different rhythms during the period) or stopped.
Following a Bayesian approach, the parameters are treated as random variables and therefore prior knowledge is incorporated via prior distributions. Specifically we used an improper uniform distribution for the fixed effects α, β. The prior for π was uniform on [0,1]. We chose an inverse gamma with parameters 0.5 and 0.0005 for the standard deviation parameters (σμ, σν, σγ), following (Kelsall and Wakefield, 1999).
Half-normal distributions were used for σ1 and k as in Abellan et al. (2008):
Bayesian estimation is carried out using the WinBUGS software (version 1.4) (Lunn et al., 2000, 2009), generating with Markov chain Monte Carlo (MCMC) multiple samples of the parameters of the statistical model. We generated a total of 50 000 iterations (with a thin = 50) discarding the first 20 000 iterations as a burn-in period of MCMC. To check the convergence of the simulated sequences, we used the convergence diagnostic (Gelman and Rubin, 1992), which was nearer to 1.0 for all parameters as well as visual inspection of the plots of the sampled parameters. A sensitivity analysis on prior distributions of hyper parameters was performed to measure the robustness of the results. The posterior distributions showed the consistency of results.
Model exploitation
To detect collinearity between dependent variables, Spearman’s rank correlation coefficients were computed for each pair of covariates. Different models have been compared to choose the best one describing our data. This was achieved by first fitting the model just with fixed effects which allowed us, using a forward stepwise approach, to choose the best combination of covariates. Then we included the different random effects and selected the best model including both fixed and random effects. Models were compared by considering the deviance information criterion (DIC) (Spiegelhalter et al., 2002), which is computed routinely by WinBUGS. The model with smaller DIC value was chosen.
Residuals, that can be used to assess how well the data fits the selected model, were computed as the difference between the predicted standardized incidence ratios (λit) and the estimated SIRs: the ratio of the number of herds reported infected in a given department for a given period over the expected number of herds reported infected (i.e. the product of the national incidence rate during the period by the number of herds in the department) (Figure S1).
For each department, we computed a 0–7 score corresponding to the number of periods for which the department was at risk (λit > 1). To map SIRs and covariates, we used the zoning proposed by Pluvinage (1971), with a small modification to take into account the evolution of the agricultural situation in some departments since 1970. This zoning groups the French departments into six major areas (Fig. 2a), each presenting relatively homogeneous characteristics for cattle population:
-
1
North area (N): cattle farms in this area are mostly dairy herds in lowlands.
-
2
East area (E): a mountainous dairy production area. Meat production is limited to lightweight calves for slaughter and culled cows.
-
3
Centre area (C): the ‘Massif Central’ region and the bordering regions in the North; it is specialized in the production of meat, and most cattle are beef cows.
-
4
Southeast area (SE): cattle farming is poorly developed, due to the Mediterranean climate which is poorly conducive to forage production. This area, however, contains the Camargue region where a specific type of extensive breeding exists for fighting bulls.
-
5
Southwest area (SW): cattle farming is not of primary importance here, but the herd density is not negligible, with similar proportions of dairy and beef herds.
-
6
West area (W): this is the leading dairy region and also the leading region for meat production (calves for slaughter and culled cows) although the cows are mainly dairy cows.
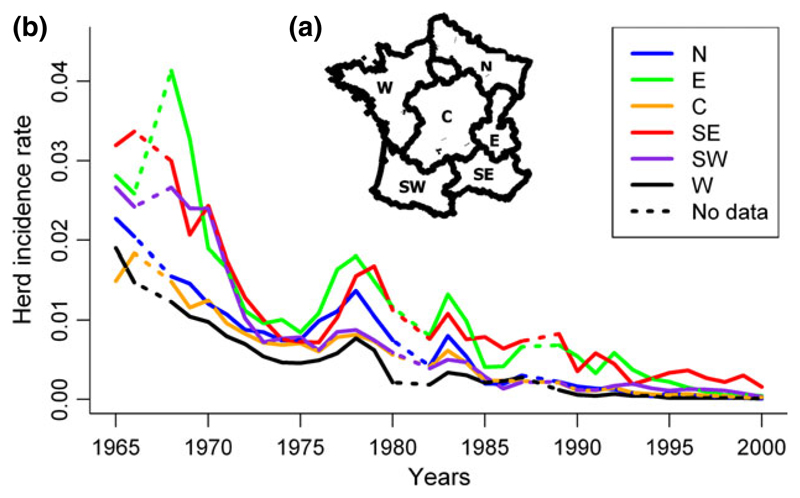
Fig. 2.
Evolution of the incidence rate for each region in France between 1965 and 2000; (a) definition of six homogeneous zones for the characteristics of the cattle (adapted from Pluvinage, 1971); (b) evolution of incidence rate.
Choropleth maps were constructed using the Quantum Gis software (QGIS Development Team, 2012).
Results
Descriptive analysis
National-level herd incidence rate quickly declined between 1965 and 1983 and more slowly afterwards. It became roughly stable from 1990 (Fig. 1). This decrease occurred in parallel in the six areas, starting from different incidence levels in 1965: at that time, the most severely affected area was E, and the least affected was W (Fig. 2b). Since 1990, the incidence rate has been low and similar in all areas, except in the SE area where it remained higher than elsewhere (Fig. 2b).
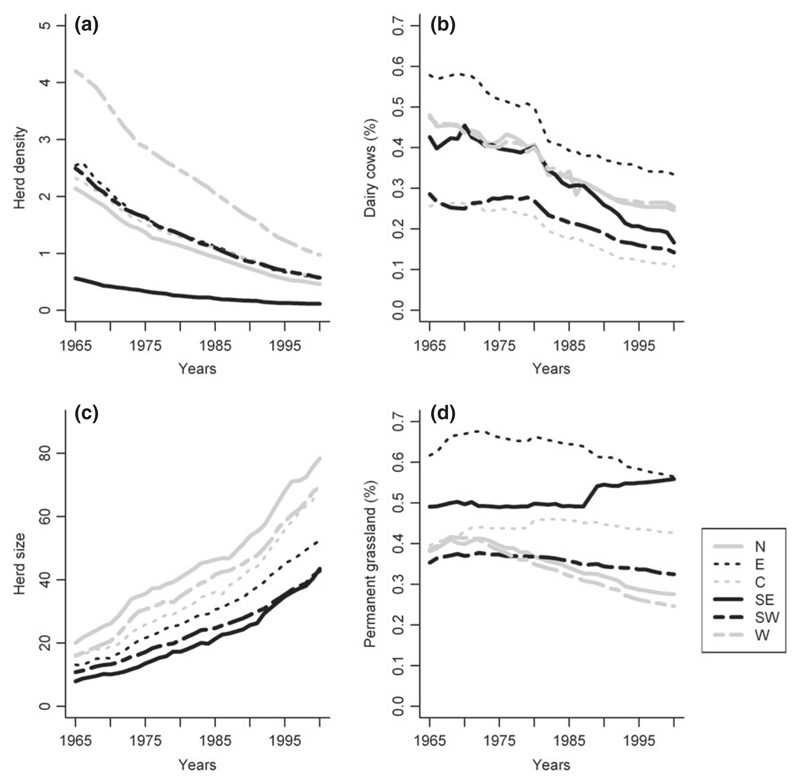
At the national level, the proportion of dairy cows decreased over time, as well as the herd density and the proportion of permanent grassland in the cultivated surface area (that decreased only slowly) (Table 1). The average herd size increased significantly: it was 3.5 times larger during period 7 (1996–2000) than during period 1 (1965–1970) (Table 1). The W area had the highest herd density throughout the study period and the SE area the lowest (Figs 3a and S2). Average herd size did increase continuously and generally homogeneously in the six areas (Figs 3c and S3). The largest average herd sizes were observed in the N area and the lowest in the SE area (Figs 3c and S3). Between periods 1 (1965–1970) and 3 (1975–1978), the percentage of dairy cows was high (≥41%) in four areas: W, N, E and SE (Figs 3b and S4). From period 4 (1979–1984) and because of the application of the milk quota system, the percentage of dairy cows decreased in all areas (Figs 3b and S4). The proportion of permanent grassland in the cultivated surface area remained stable between periods 1 (1965–1970) and 3 (1975–1978) (Figs 3d and S5). It decreased afterwards in all areas, except in the SE area where it continuously increased (Figs 3d and S5).
Fig. 3.
Evolution, from 1965 to 2000, of (a) herd density per km2; (b) the percentage dairy cows in the total cattle population; (c) the average herd size; (d) the percentage of permanent grassland in the cultivated area.
The percentage of departments with yearly tuberculin skin testing was above 70% from 1965 until 1984 and then decreased gradually to 13.5% during the most recent period (1996–2000) (Table 1).
Spatiotemporal hierarchical Bayesian model
None of the covariate pairs were significantly correlated (maximal value of the Spearman’s rank correlation coefficient: 0.62), and all of the covariates were thus kept for multivariate analysis.
Using our model selection procedure, as described in section Model exploitation, the DIC suggested for the fixed effects selection that all the covariates need to be kept in the model. As for the inclusion of the random effects, the lower DIC value was associated with the model that includes the spatial, temporal and space–time effects, in addition to the five covariates (Table 2).
Table 2.
Model comparison criterion (DIC) for all fitted models
| Model | DIC |
|---|---|
| PGC + SH + HDN + PDC + FREQ + μi + γt + νi + ζit | 5189.1 |
| PGC + SH + HDN + PDC + FREQ + μi + γt + νi | 34490.0 |
| PGC + SH + HDN + PDC + FREQ + γt + νi | 34463.8 |
| PGC + SH + HDN + PDC + FREQ + μt + νi | 35437.0 |
PGC: percentage of the permanent grassland area in the total cultivated area; SH: average herd size; HDN: herd density per km2; PDC: percentage of dairy cows in cattle population; FREQ: frequency of tuberculin skin testing; γt: temporal autocorrelation; μi: spatial autocorrelation; ζit: space-time interaction; νi: unstructured spatial heterogeneity.
In the selected model, as expected, a significantly higher SIR was associated with an annual rather than biennial rhythm of prophylaxis [incidence ratio (IR) = 1.13, 95% credible interval (CI): (1.02–1.26)] (Table 3). No significant difference in incidence was associated with the ‘lightened’ rhythm (Table 3). The increase in average herd size was associated with a significantly higher SIR, with an IR of 1.15 for an increase of one standard deviation (19 animals) [95% CI: (1.01–1.40)] (Table 3). Similarly, an increase of the proportion of permanent grassland in the total cultivated area was a risk factor for bTB incidence, with an IR of 1.03 for an increase of one standard deviation (24%) [95% CI: (1.001–1.13)] (Table 3). An increase in herd density in the department was associated with a significantly higher SIR, with an IR of 1.02 for an increase of one standard deviation (1.3 herds/km2) [95% CI: (1.0005–1.08)] (Table 3). Likewise an aggravating effect was associated with the percentage of dairy cows in the cattle population, with an IR of 1.02 for an increase of one standard deviation (16%) [95% CI: (1.001–1.09)] (Table 3).
Table 3.
Posterior medians and 95% CIs for the different parameters of the selected model
| Variable | Median | Credible interval at 95% |
|---|---|---|
| Permanent grassland (%) | 1.03a | 1.001–1.13 |
| Herd size | 1.15b | 1.01–1.40 |
| Herd density (Herds/km2) | 1.02c | 1.0005–1.08 |
| Dairy cows (% of cattle population) | 1.02d | 1.001–1.09 |
| Frequency of tuberculin testing: | ||
| Every 2 years | Ref | – |
| Every year | 1.13e | 1.02–1.26 |
| Other | 0.98e | 0.96–1.05 |
| Temporal autocorrelation: | ||
| 1965–1970 | 0.09 | −0.08; 0.46 |
| 1971–1974 | −0.15 | −0.27; −0.04 |
| 1975–1978 | −0.004 | −0.09; 0.10 |
| 1979–1984 | 0.10 | 0.02; 0.18 |
| 1985–1990 | 0.09 | 0.0002; 0.18 |
| 1991–1995 | −0.02 | −0.15; 0.11 |
| 1996–2000 | −0.10 | −0.32; 0.085 |
| 0.029 | 0.005; 0.10 | |
| 0.53 | 0.27; 0.91 | |
| 0.10 | 0.021; 0.227 | |
| 0.409 | 0.36; 0.45 | |
| 3.32 | 1.47; 7.63 |
Incidence ratio computed for a difference of 24% (standard deviation of the variable).
Incidence ratio computed for a difference of 19.2 animals (standard deviation of the variable).
Incidence ratio computed for a difference of 1.3 herds/km2 (standard deviation of the variable).
Incidence ratio computed for a difference of 16% (standard deviation of the variable).
Incidence ratios.
The temporal autocorrelation γt did not show any clear trend, with positive or negative values according to the period (Table 3). The spatial autocorrelation μi was negatively linked to bTB risk in the W and C areas and in some departments of the N, E and SW areas. On the contrary, it was positively linked to bTB risk in the SE area and in some departments of the N, E and SW areas (Figure S6).
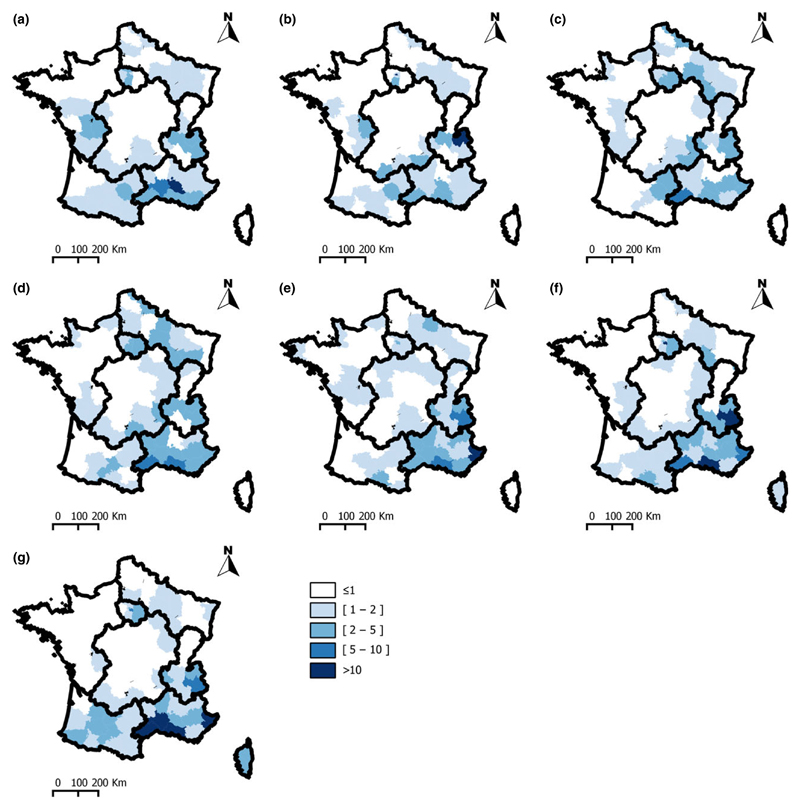
The distribution of departments according to the number of times the department was predicted to be at risk (λit > 1) is shown in Fig. 4 (see also Table S1). The areas E (southeastern part), SE, SW and C (for departments bordering the SE and SW areas) were high-risk areas (λit > 1 for 6 or 7 of the seven periods). Conversely, the areas C (northern and central parts) and W (northern part) were low-risk areas (λit > 1 for 0 or 1 of the seven periods).
Fig. 4.
Number of periods for which departments-specific predicted standardized incidence ratios were >1.
Three patterns were observed for the evolution of the predicted SIR over time (Fig. 5):
-
1
An increase and followed by a decrease, in the N area and in the C area,
-
2
A decrease in the W area and
-
3
A stability in the south of E area or an increase in the south of France (SE and SW areas, the western part of the SW area being, however, predicted to be at low risk until period 6).
Fig. 5.
Posterior means of relative risks between 1965 and 2000. (a)1965–1970; (b) 1971–1974; (c) 1975–1978; (d) 1979–1984; (e) 1985–1990; (f) 1991–1995; (g) 1996–2000.
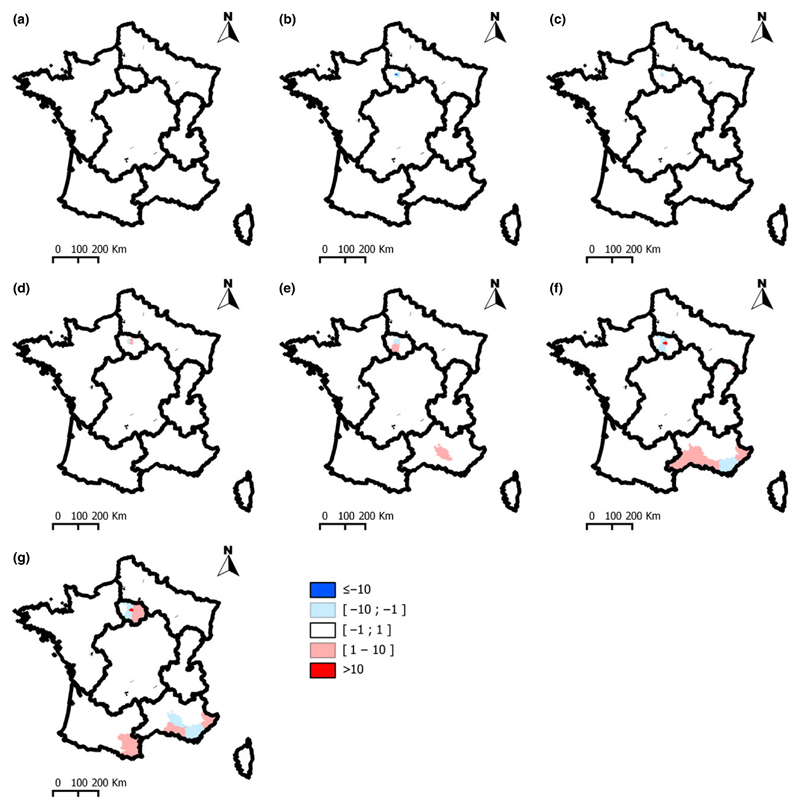
Residuals analysis showed that the model predictions for periods 1, 2, 3, 4 and 5 (1965–1990) were good, with a single department for which the predicted SIR was slightly over-estimated (Fig. 6). For periods 6 and 7 (1991–2000), the model slightly under- or over-estimated the SIR for some departments of the SE area. However, the absence of spatial and/or temporal clustering of the departments where the SIR was either overestimated or underestimated suggests a good quality of predictions.
Fig. 6.
Spatiotemporal distribution of model residuals. (a)1965–1970; (b) 1971–1974; (c) 1975–1978; (d) 1979–1984; (e) 1985–1990; (f) 1991–1995; (g) 1996–2000.
Discussion
Bovine tuberculosis (bTB) is a slowly progressing disease, and long data series are necessary for studying bTB risk factors or for analysing the efficacy of control programmes at the country level. Only few data sets are available in European countries to document the evolution of bTB herd incidence or prevalence over long periods (>30 years). However, some descriptive studies have been published about the evolution of the epidemiological situation of bTB and about disease control strategies since the beginning of bTB control. In the Irish Republic, bTB incidence decreased from 2.99% in 1960 to 0.33% in 2004 (Good, 2006). In Great Britain, the control programme launched in 1950 reduced the number and incidence of IDTT reactors from nearly 15 000 (16.2 reactors per 10 000 cattle tests) in 1961 to 569 (2.3 reactors per 10 000 tests) in 1982 (de la Rua-Domenech, 2006). This progress was stopped in the mid-1980s, when the situation began to gradually worsen to a point where in 2004 Great Britain has one of the highest incidences of bTB in the EU (number and incidence of IDTT reactors: more than 25 000, more than 50 reactors per 10 000 cattle tested) (de la Rua-Domenech, 2006). In West Germany, the percentage of IDTT reactors decreased from 1.52% in 1961 to 0.20% in 1980 (Schliesser, 1982).
Our study is the first that describes both the evolution of the incidence and prevalence of bTB from the start of the compulsory control programme (1965) to the free status (2000), and analyses the association between bTB control progress and changes to cattle population structures and herd management practices. This study was performed on aggregated data (in time and space) and is thus subjected to ecologic bias (ecological fallacy) when making causal inferences at the herd level (Morgenstern, 1982; Richardson et al., 1987). Aggregated data capture only partly the heterogeneity of the exposure level and of the values of covariates between herds of the same department, during the same period (Morgenstern, 1995). Therefore, the effect of covariates, estimated at department level, should be interpreted with caution when inferring results at the herd level or when comparing with others studies conducted at the farm level. The predicted SIRs allowed us to describe the evolution of the distribution of bTB in France. Most at-risk departments were those of the southern half of France, where SIRs remained stable or increased during the seven periods. Conversely, the northern half of France (especially the C and W areas) was a low-risk area, with a rapid decrease in SIR over time.
This geographical variation in the distribution of bTB between the south (SE area) and the north (W and C areas) of France may be linked to development of cattle breeding and to the dominant herd type in these areas. Indeed, the type and the economic importance of livestock cattle vary greatly between these three areas. Cattle farming is strongly developed in the W area: it is the leading region for both dairy and meat production. The C area mainly produces sucklers, a sector that has undergone a significant professionalization since the implementation of the European regulations that aimed at limiting milk production (via the introduction of milk quotas, in the early 1980s). Cattle farming remains poorly developed in the SE area and is of little weight in the local economy: the Mediterranean climate is not suitable for the production of fodder needed to feed cattle. However, this area contains the Camargue region where fighting bulls are bred. The difficulty in applying screening tests by IDTT and the low sensitivity of these tests due to the stress of animals when tested may explain why this region remained at risk.
According to the DIC criterion, the best model incorporated all the covariates, the spatial autocorrelation, the temporal autocorrelation and the space–time interaction random effect. Several factors are known to influence the epidemiology of bTB, including herd size, herd (or animal) density (Humblet et al., 2010), herd management practices (Allepuz et al., 2011) and environmental variables such as the annual amplitude of mean middle-infrared temperature (Humblet et al., 2010). Due to the absence of data for the whole study period (1965–2000), it was not possible to incorporate in the model covariates describing cattle movements or environmental conditions.
In our study, the average herd size was a risk factor for bTB outbreaks, [IR = 1.15 (1.01–1.40)]. This result is consistent with previous findings (Griffin et al., 1996; Munroe et al., 1999; Green and Cornell, 2005; Reilly and Courtenay, 2007; Carrique-Mas et al., 2008; Brooks-Pollock and Keeling, 2009; Ramirez-Villaescusa et al., 2010; Wolfe et al., 2010). A study of 151 dairy and 477 beef herds between 1985 and 1994 in Canada showed that increasing herd size was associated with an increased risk of being positive for bTB, herds of >80 animals having an odds ratio of 9.3 [3.2– 27.6] compared to herds of <16 animals (Munroe et al., 1999). In the United Kingdom, Brooks-Pollock and Keeling (2009) showed that herd size was a risk factor for bTB persistence: after a breakdown, nearly 90% of herds with fewer than 20 animals successfully passed two IDTT, against 55% for herds containing 400 animals (Brooks-Pollock and Keeling, 2009). In the Republic of Ireland, Wolf et al., (2010) found in a retrospective study that herds of 30–79 animals were more at risk than herds <30 cattle [hazard ratio: 1.8 (1.5–2.1)] (Wolfe et al., 2010). Several studies in the UK have shown that an increase of one log unit in herd size was associated with an OR significantly higher than 1 (Griffin et al., 1996; Green and Cornell, 2005; Reilly and Courtenay, 2007; Carrique-Mas et al., 2008; Ramirez-Villaescusa et al., 2010). British scientists explain the effect of size on bTB by an increased probability of contact between cattle, and because large farms buy more cattle, thus increasing the likelihood of introducing infected animals. Furthermore, the likelihood of detecting at least one infected animal during IDTT screening increases with herd size, as herd sensitivity of screening tests increases with the number of tested animals.
Like herd size, we found increasing herd density to be a risk factor for bTB [IR = 1.02 (1.0005–1.08) IC 95%]. This result was expected: as the number of herds in a region increases, the probability for a herd to be exposed to infection increases, due to contacts on pastures between animals of different farms. During the 35 years of the study period, the cattle herd density decreased in all French regions. This decrease is linked to the professionalization of farming, with the disappearance of family farms and of small herds, which were no more economically viable. In Belgium, cattle density was identified as a risk factor for bTB (Humblet et al., 2010).
The extent of permanent grassland (as a percentage of cultivated surface area) was identified as a risk factor for bTB [IR = 1.03 (1.001–1.13)]. This covariate is a proxy for the area dedicated to pastures. A higher pasture density increases the number of neighbouring herds, thus the risk of bTB transmission, as suggested by an English questionnaire-based study performed in 1999 (Humblet et al., 2009). Furthermore, a higher pasture density increases the probability of contact with wildlife and the corresponding bTB transmission risk (Johnston et al., 2005).
The model identified the percentage of dairy cows as a risk factor [IR = 1.02 (1.001–1.09)]. Today dairy farms are intensively managed, the animals being frequently or continuously kept in stables. Intensively managed herds are at higher risk of bTB breakdowns than other herds (Griffin et al., 1993; O’Connor et al., 1993): in stables, cattle tend to have closer contacts than when at pasture, potentially increasing both cattle-to-cattle transmission and the interactions with wildlife visiting the buildings (Johnston et al., 2011). Whatever the management system (intensive or extensive, as it was the case in France until the 1970s), close contact between cows during milking increases the risk of bTB transmission (Barlow et al., 1997), and production stresses experienced by dairy cattle may increase their susceptibility or lessen their immune resistance to infection (Griffin et al., 1993). All these elements suggest that the within-herd transmission of M. bovis is more intense in dairy herds than in suckling herds. Several studies in the UK showed an association between the risk of bTB and dairy production: Ramirez-Villaescusa et al. (2010), in a retrospective cohort study in the southwest of England, found that the presence of dairy cows on a farm was a risk factor [OR = 2.18 (1.12–4.24)] (Ramirez-Villaescusa et al., 2010). In the case–control study (including 1150 case-herds and 2852 control farms) by Vial et al. (2011), dairy herds showed a significantly higher risk of bTB than non-dairy herds [OR = 1.30 (1.12–1.58)] (Vial et al., 2011). The case–control study (including 84 case-herds and 213 control farms) by Karolemeas et al. (2011) found that the risk of bTB breakdown recurrence was higher in dairy herds than beef/other herds [OR = 2.5 (1.3–5.1)] (Karolemeas et al., 2011). In a study in New Zealand (Porphyre et al., 2008) between 1984 and 2004, the risk was higher for dairy herds than for beef herds [RR = 3.43 (1.70–6.92)]. In Spain, Alvarez et al. (2012) showed that within-herd transmission dynamics (β) of bTB was higher in dairy herds (median 4.7) than in beef or bullfighting herds (2.3 and 2.2 respectively) (Alvarez et al., 2012). The authors explain these results as a consequence of dairy cattle reaching an older age than beef cattle and therefore being exposed to the risk of bTB infection for longer, there being more time to incubate infection (Ramirez-Villaescusa et al., 2010). This argument is valid for France until the 1980s, but after the implementation of milk quotas, the age at culling of dairy cows decreased: 90% of dairy cows are now culled after 2.5 lactations (Derville et al., 2009). Indeed, the application of the milk quota system from 1984 induced changes in the demographic structure of dairy herds, with a decrease in the age at culling, an increase in the culling rate and a wider use of heifers born on the farm to restock cow herds. These changes reduced the bTB risk in dairy herds during the last three periods. Nevertheless, the percentage of dairy cows was a risk factor when considering the seven time periods, from 1965 to 2000.
A significant association was observed between the frequency of tuberculin skin testing and the risk of bTB in the department. This result was expected because the frequency of tuberculin testing was determined by the annual herd prevalence rate. The absence of association between SIR and ‘lightened’ frequencies of tuberculin testing suggests that having stopped IDTT screening does not explain the resurgence of the disease, observed in some departments from 2004.
The predicted spatial autocorrelation values were negatively linked to bTB risk in the northern half of the country (except the centre of the N area) and positively linked to bTB risk in the southern half of France. This contrast was also observed for the predicted SIRs. It may reflect disparities in the application of the control programme, linked to lower financial resources allocated by local authorities in southern France where the importance of cattle breeding in the local economy is weak. It may also reflect a higher proportion of professional farms in the northern half of France than in the southern half.
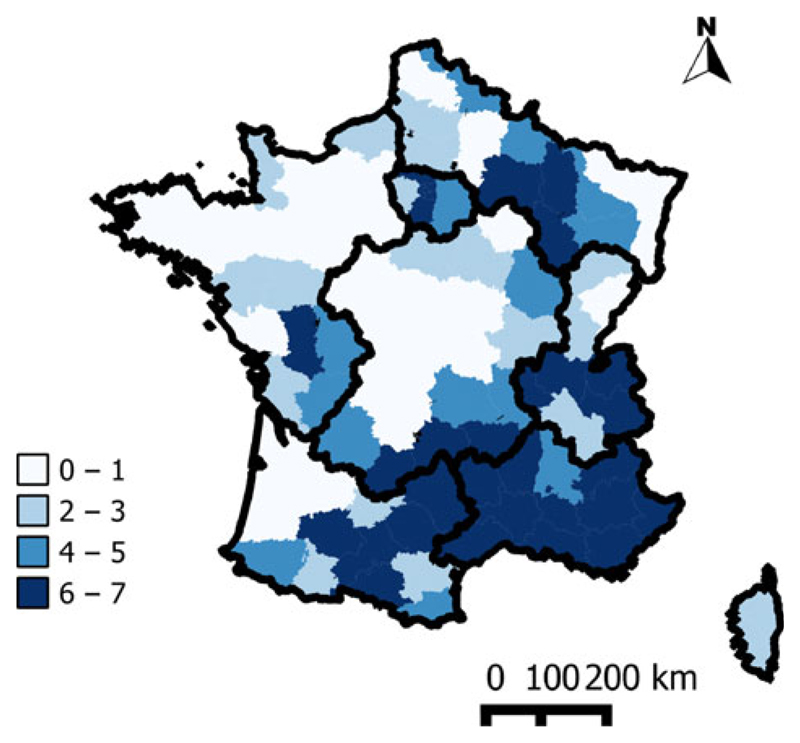
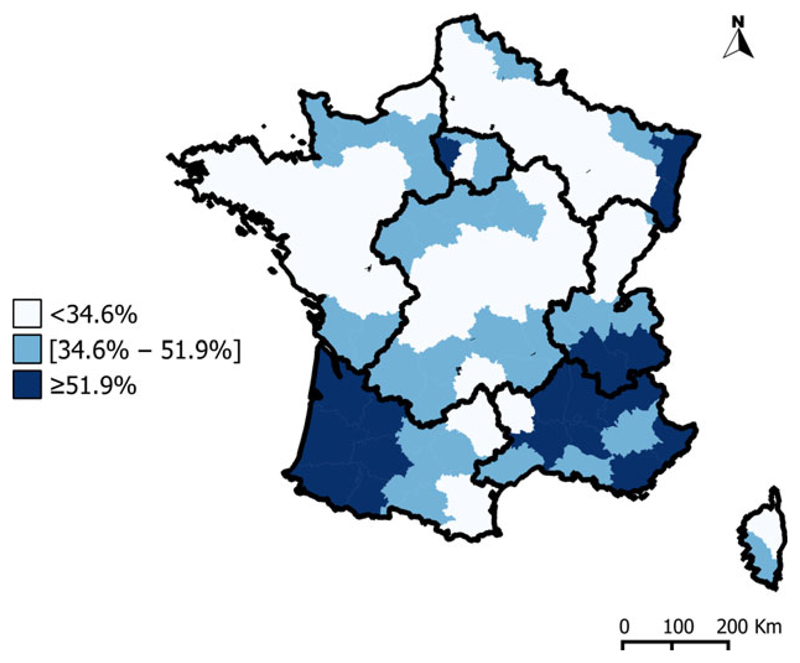
Indeed, the professionalization of cattle farming had a significant effect on the evolution of bTB. The transition from family structures to professional farming was accompanied by improvements of biosafety conditions, regular veterinary monitoring, compliance with good breeding practices (quarantine, control of introduced animals, husbandry) and the separation of animals in distinct groups (according to their age). The professionalization of farming thus improved the effectiveness of control programme both in dairy and in beef herds and allowed implementing key measures for cattle traceability, such as the individual identification of animals. In our study, the predicted SIR values rapidly decreased in areas where professionalization began earlier (the W, N and C areas). Cattle farming has a weak economic weight in the southern half of France, where professionalization was less marked, and SIR values remained stable or even increased over time in that part of the country. These north–south differences in the professionalization of cattle farming persisted to the end of study period, as illustrated by the higher percentage of non-professional farms (defined by the MAFF as farms with <20 cattle) in the southern half of France, in 2000 (Fig. 7). The specialization over time of professionally farmed herds has been associated with the decline of dairy herds and the increase in beef herds. These developments are direct consequences of European regulations limiting milk production, via the introduction of milk quotas (decreasing the percentage of dairy cows and inducing changes in demographic structure) and the financial incentives that favoured beef production from 1980. This specialization may partly explain the reduction of SIR values from period 5 (1979–1984) in the northern part of France.
Fig. 7.
Geographical variations of the non-professional cattle herds (<20 animals) in 2000 (34.6% is the national-level percentage).
In conclusion, our study shows that the evolution of the herd incidence of bTB in France between the beginning of the compulsory control programme (1965) and the bTB-free status (2000) was partly attributable to changes in herd management practices and in cattle population structures. Professionalization has also been a factor reducing the risk of bTB, especially in northern France. In the southern part of France where the professionalization of herds is lower, the risk of the disease remained stable over time. The effects of European regulations limiting milk production and favouring beef production may also have contributed to reducing the disease risk.
Supplementary Material
Acknowledgements
The authors would like to thank the Ministry of Agriculture, Food and Forestry (MAFF) and especially Mrs. Colombani from AGRESTE service, for providing of historical data.
References
- Abellan JJ, Richardson S, Best N. Use of space-time models to investigate the stability of patterns of disease. Environ Health Perspect. 2008;116:1111–1119. doi: 10.1289/ehp.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allepuz A, Casal J, Napp S, Saez M, Alba A, Vilar M, Domingo M, Gonzalez MA, Duran-Ferrer M, Vicente J, Alvarez J, et al. Analysis of the spatial variation of Bovine tuberculosis disease risk in Spain (2006–2009) Prev Vet Med. 2011;100:44–52. doi: 10.1016/j.prevetmed.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Perez A, Bezos J, Marques S, Grau A, Sarez JL, Minguez O, de Juan L, Dominguez L. Eradication of bovine tuberculosis at herd-level in Madrid, Spain: study of within-herd transmission dynamics over a 12 year period. BMC Vet Res. 2012;8:100. doi: 10.1186/1746-6148-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow ND, Kean JM, Hickling G, Livingstone PG, Robson AB. A simulation model for the spread of bovine tuberculosis within New Zealand cattle herds. Prev Vet Med. 1997;32:57–75. doi: 10.1016/s0167-5877(97)00002-0. [DOI] [PubMed] [Google Scholar]
- Besag J, York J, Mollie A. Bayesian image restoration with applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–20. [Google Scholar]
- Brooks-Pollock E, Keeling M. Herd size and bovine tuberculosis persistence in cattle farms in Great Britain. Prev Vet Med. 2009;92:360–365. doi: 10.1016/j.prevetmed.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Carrique-Mas JJ, Medley GF, Green LE. Risks for bovine tuberculosis in British cattle farms restocked after the foot and mouth disease epidemic of 2001. Prev Vet Med. 2008;84:85–93. doi: 10.1016/j.prevetmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Derville M, Patin S, Avon L. Races bovines de France: Origine, Standard, Sélection. 1st edn. Edition France Agricole; Paris: 2009. [Google Scholar]
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–511. [Google Scholar]
- Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R, Wint W. Cattle movements and bovine tuberculosis in Great Britain. Nature. 2005;435:491–496. doi: 10.1038/nature03548. [DOI] [PubMed] [Google Scholar]
- Good M. Bovine tuberculosis eradication in Ireland. Ir Vet J. 2006;59:154–162. [Google Scholar]
- Green LE, Cornell SJ. Investigations of cattle herd breakdowns with bovine tuberculosis in four counties of England and Wales using VETNET data. Prev Vet Med. 2005;70:293–311. doi: 10.1016/j.prevetmed.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Hahesy T, Lynch K, Salman MD, McCarthy J, Hurley T. The association of cattle husbandry practices, environmental factors and farmer characteristics with the occurrence of chronic bovine tuberculosis in dairy herds in the Republic of Ireland. Prev Vet Med. 1993;17:145–160. [Google Scholar]
- Griffin JM, Wayne Martin S, Thorburn MA, Eves JA, Hammond RF. A case-control study on the association of selected risk factors with the occurrence of bovine tuberculosis in the Republic of Ireland. Prev Vet Med. 1996;27:217–229. [Google Scholar]
- Humblet MF, Boschiroli ML, Saegerman C. Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet Res. 2009;40:50. doi: 10.1051/vetres/2009033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblet MF, Gilbert M, Govaerts M, Fauville-Dufaux M, Walravens K, Saegerman C. New assessment of bovine tuberculosis risk factors in Belgium based on nationwide molecular epidemiology. J Clin Microbiol. 2010;48:2802–2808. doi: 10.1128/JCM.00293-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WT, Gettinby G, Cox DR, Donnelly CA, Bourne J, Clifton-Hadley R, Le Fevre AM, McInerney JP, Mitchell A, Morrison WI, Woodroffe R. Herd-level risk factors associated with tuberculosis breakdowns among cattle herds in England before the 2001 foot-and-mouth disease epidemic. Biol Lett. 2005;1:53–56. doi: 10.1098/rsbl.2004.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WT, Vial F, Gettinby G, Bourne FJ, Clifton-Hadley RS, Cox DR, Crea P, Donnelly CA, McInerney JP, Mitchell AP, Morrison WI, et al. Herd-level risk factors of bovine tuberculosis in England and Wales after the 2001 foot-and-mouth disease epidemic. Int J Infect Dis. 2011;15:e833–e840. doi: 10.1016/j.ijid.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Karolemeas K, McKinley TJ, Clifton-Hadley RS, Goodchild AV, Mitchell A, Johnston WT, Conlan AJ, Donnelly CA, Wood JL. Recurrence of bovine tuberculosis breakdowns in Great Britain: risk factors and prediction. Prev Vet Med. 2011;102:22–29. doi: 10.1016/j.prevetmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Kelsall JE, Wakefield JC. Discussion of “Bayesian models for spatially correlated disease and exposure data”, by Best et al. In: Bernardo JM, Berger JO, Dawid AP, Smith AFM, editors. Bayesian Statistics. Vol. 6. University Press; Oxford: 1999. p. 151. [Google Scholar]
- Knorr-Held L. Bayesian modelling of inseparable spacetime variation in disease risk. Stat Med. 2000;19:2555–2567. doi: 10.1002/1097-0258(20000915/30)19:17/18<2555::aid-sim587>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kunsch HR. Intrinsic autoregressions and related models on the two dimensional lattice. Biometrika. 1987;74:517–524. [Google Scholar]
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med. 2009;28:3049–3067. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- Morgenstern H. Uses of ecologic analysis in epidemiologic research. Am J Public Health. 1982;72:1336–1344. doi: 10.2105/ajph.72.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]
- Munroe FA, Dohoo IR, McNab WB, Spangler L. Risk factors for the between-herd spread of Mycobacterium bovis in Canadian cattle and cervids between 1985 and 1994. Prev Vet Med. 1999;41:119–133. doi: 10.1016/s0167-5877(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Munyeme M, Muma JB, Skjerve E, Nambota AM, Phiri IG, Samui KL, Dorny P, Tryland M. Risk factors associated with bovine tuberculosis in traditional cattle of the livestock/wildlife interface areas in the Kafue basin of Zambia. Prev Vet Med. 2008;85:317–328. doi: 10.1016/j.prevetmed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- O’Connor R, Conway A, Murphy M. Study of Socio-Economic Impediments to Bovine Tuberculosis Eradication. A Report by The Economic and Social Research Institute for the Eradication of Animal Disease Board. Dublin: 1993. p. 215. [Google Scholar]
- Olea-Popelka FJ, White PW, Collins JD, O’Keeffe J, Kelton DF, Martin SW. Breakdown severity during a bovine tuberculosis episode as a predictor of future herd breakdowns in Ireland. Prev Vet Med. 2004;63:163–172. doi: 10.1016/j.prevetmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Pluvinage J. Regard sur l’élevage Bovin Franjçais et Son Avenir. 1st edn. Vol. 1. Editions Genin-Librairies Techniques; Paris: 1971. p. 971. [Google Scholar]
- Porphyre T, Stevenson MA, McKenzie J. Risk factors for bovine tuberculosis in New Zealand cattle farms and their relationship with possum control strategies. Prev Vet Med. 2008;86:93–106. doi: 10.1016/j.prevetmed.2008.03.008. [DOI] [PubMed] [Google Scholar]
- QGIS Development Team. version 1.7.4. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2012 Available at http://qgis.osgeo.org.
- Ramirez-Villaescusa AM, Medley GF, Mason S, Green LE. Risk factors for herd breakdown with bovine tuberculosis in 148 cattle herds in the south west of England. Prev Vet Med. 2010;95:224–230. doi: 10.1016/j.prevetmed.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Reilly LA, Courtenay O. Husbandry practices, badger sett density and habitat composition as risk factors for transient and persistent bovine tuberculosis on UK cattle farms. Prev Vet Med. 2007;80:129–142. doi: 10.1016/j.prevetmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Richardson S, Stucker I, Hemon D. Comparison of relative risks obtained in ecological and individual studies: some methodological considerations. Int J Epidemiol. 1987;16:111–120. doi: 10.1093/ije/16.1.111. [DOI] [PubMed] [Google Scholar]
- Robert C. The Bayesian Choice. From Decision-Theoretic Foundations to Computational Implementation. 2nd edn. Springer; New York: 2007. [Google Scholar]
- de la Rua-Domenech R. Bovine Tuberculosis in the European Union and other countries: current status, control programmes and constrains to eradication. Gov Vet J. 2006;16:19–45. [Google Scholar]
- Schliesser T. History and development of the fight against bovine tuberculosis (author’s transl) Zbl Bakt-Int J Med M. 1982;251:326–340. [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J Roy Stat Soc B. 2002;64:583–639. [Google Scholar]
- Vial F, Johnston WT, Donnelly CA. Local cattle and badger populations affect the risk of confirmed tuberculosis in British cattle herds. PLoS ONE. 2011;6:e18058. doi: 10.1371/journal.pone.0018058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe DM, Berke O, Kelton DF, White PW, More SJ, O’Keeffe J, Martin SW. From explanation to prediction: a model for recurrent bovine tuberculosis in Irish cattle herds. Prev Vet Med. 2010;94:170–177. doi: 10.1016/j.prevetmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.