Abstract
Objective:
To compare the image quality (IQ), radiation dose and diagnostic reliability of standard-dose and low-dose lumbar spine (L-spine) multi-detector CT (SDCT and LDCT, respectively) with iterative reconstruction (IR) in trauma patients.
Methods:
We retrospectively analysed the data of 263 consecutive patients (male:female, 133:130; mean age, 44.3 years) who underwent SDCT (200–300 mAs; 120 kVp) with IR (n = 126) or LDCT (80–150 mAs; 120 kVp) with IR (n = 137) for suspected L-spine fractures between November 2015 and September 2016. Patients were categorized according to their body mass index, as follows: Group 1, ~22.9 kg m–2; Group 2, 23–24.9 kg m–2 or Group 3, ≥25 kg m–2. We compared the quantitative IQ (signal-to-noise ratio), qualitative IQ (subjective image noise and diagnostic acceptability [4-point rating scale; score 1–4], image sharpness [5-point rating scale; score 1–5]) and diagnostic accuracy between the two scan types. Interobserver agreement was also calculated.
Results:
Overall, SDCT exhibited slightly better diagnostic performance than did LDCT (sensitivity, 96.7–100% vs 94–98.5%; specificity, 95.6–97.0% vs both 95.7%; accuracy, 96.0–98.4%vs94.9–97.1%). However, none of these parameters was significantly different between SDCT and LDCT, either in the whole cohort (p ≥ 0.50) or among the three body mass index groups (p ≥ 0.49). All interobserver agreements were excellent or good (range, 0.776–0.985).
Conclusions:
The diagnostic performance of LDCT with IR for L-spine fractures was comparable to that of SDCT with IR, with a 47–69% reduction in the radiation dose.
Advances in knowledge:
LDCT scan can be used as a diagnostic imaging tool for evaluating trauma patients with suspected L-spine fractures.
INTRODUCTION
Since the early 2000s, the number of CT examinations has significantly increased; therefore, optimizing the radiation exposure during CT examinations to ensure that it is in line with the “as low as reasonably achievable (ALARA)” concept is critical.1 However, it is just as essential to have the optimal image quality (IQ) for visualizing anatomical structures and accurately diagnosing diseases. Thus, modern CT scans should be performed by using as low radiation dose as possible, while maintaining adequate IQ. Recently, the development of iterative reconstruction (IR) algorithms has improved the IQ of LD musculoskeletal CT scans.2,3
Lumbar spine (L-spine) CT still plays an important role in the management of spinal trauma because it utilizes the three-dimensional information that plain lumbar radiographs cannot provide. Moreover, the inherently superior contrast resolution of L-spine CT allows surgeons to make accurate decisions regarding the best surgical approach. L-spine CT is also useful in the emergency department because it can be performed immediately to provide reliable and rapid anatomical and disease-related assessments.3
In the past few years, several studies concluded that low dose L-spine CT (LDCT) is acceptable as a replacement for plain L-spine radiography or standard dose (SD) L-spine CT (SDCT) that uses filtered back projection (FBP) reconstruction algorithms.4–6 Additionally, various studies have demonstrated better IQ and diagnostic efficacy with L-spine CT with IR algorithms than with CT with FBP for diagnosing spinal disorders in non-trauma patients.7–9 The utility of LDCT with IR for detecting osseous lesions has also been revealed.10–12 However, the diagnostic performance of LDCT with IR has not been compared with that of SDCT with IR in trauma patients.
The aim of the present study was to retrospectively compare the quantitative IQ, qualitative IQ and effective dose in trauma patients who underwent SDCT or LDCT. Furthermore, we sought to compare the relative diagnostic reliability of SDCT and LDCT in normal and fracture lesions of the L-spine. Although the LD protocol generally results in lower quantitative or qualitative IQ owing to image noise, we hypothesized that LDCT with the IR algorithm would show similar diagnostic reliability with a lower effective dose when compared to SDCT with the IR algorithm.
METHODS AND MATERIALS
Study population
The institutional review board approved this retrospective study, and the requirement for informed consent was waived. One author (Y.S.P) used our department’s electronic medical record and radiology information systems to identify eligible patients. The inclusion criteria for our study were as follows: adults (≥18 years) with L-spine trauma, patients who visited our Emergency Department and underwent L-spine multi-detector CT to evaluate their lower thoracic spine and L-spine fractures between November 2015 and September 2016, patients with L-spine fractures who were subsequently admitted to the orthopaedic department for fracture treatment, and patients without L-spine fractures who visited the orthopaedic outpatient clinic to confirm the “absence of acute fractures” within 1 month after undergoing L-spine CT in the Emergency Department. We initially enrolled 283 consecutive patients (age range, 19–80 years). Twelve of these patients were later excluded from the analysis, as they were lost to follow-up. Scans with significant metallic artefacts (n = 8) caused by previous surgeries were also excluded. The remaining 263 patients were included in the final analyses. Of these, 126 underwent SDCT between November 2015 and March 2016, while the remaining 137 patients underwent LDCT between April 2016 and September 2016. Before April 2016, the routine protocol (reference mAs, 300 [range, 200–300]; reference kVp, 120) for evaluating lower thoracic spine or L-spine fractures in our institution was SDCT. After April 2016, as agreed upon by emergency physicians and orthopaedic surgeons, the LD protocol (reference mAs, 150 [range, 80–150]; reference kVp, 120) was used because several studies concluded that LDCT is acceptable as a replacement for plain L-spine radiography or SDCT.4–8 Each patient’s body mass index (BMI) was also calculated from medical record data. Patients were categorized according to BMI, as follows: Group 1, underweight (<18.5 kg m–2) to the lower range of the normal weight (18.5–22.9 kg m–2); Group 2, the upper range of normal weight (23–24.9 kg m–2) or Group 3, overweight to extremely obese (≥25 kg m–2) (Figure 1).13
Figure 1.
Flow diagram of study population. L, lumbar; BMI, body mass index.*Underweight (<18.5 kg m–2) and lower range of the normal weight (18.5–22.9 kg m–2).†Upper range of the normal weight (23–24.9 kg m–2).‡Overweight (25–29.9 kg m–2) and obese (≥30 kg m–2).
Image acquisition
All CT examinations were obtained using a 64-slice, multiple-detector CT scanner (Ingenuity CT; Philips Medical Systems, Cleveland, OH). Scanning was performed from the upper endplate of the tenth thoracic vertebral body (T10) to the coccyx. The effective tube current-time product generally ranged between 200–300 mAs (reference, 300 mAs) and 80–150 mAs (reference, 150 mAs) for the SDCT and LDCT scans, respectively.7,8 The actual mAs was adjusted according to the patient’s body size using an automatic tube current modulation technique (Dose-Right; Philips Medical Systems, Cleveland, OH). The remaining parameters, which were identical for the two groups, were as follows: tube voltage, 120 kVp; collimation, 64 × 0.625 mm; matrix, 512 × 512; rotation speed, 0.5 s; and pitch, 0.609. For both CT scan types, reconstructions were performed with the sharp kernel and bone window (window, 2,500 Hounsfield unit [HU]; centre, 250 HU). Axial, sagittal and coronal images were reconstructed at a thickness of 3 mm with 3 mm increments. On both SDCT and LDCT images, image noise was reduced by using automated sequential IR of each image (iDose4 level 4algorithm; Philips Healthcare, Cleveland, OH).
Image analysis: quantitative IQ
In both groups, the quantitative IQ was evaluated in terms of the signal-to-noise ratio (SNR), which was determined by dividing the image signal (defined by the mean value of the CT Hounsfield number within the region of interest [ROI]) by image noise (defined as the standard deviation of the CT Hounsfield number measured in the background air outside of the patient; area of circle, 60 mm2).14,15 The SNR measurements were performed on a picture archiving and communication system by a board-certified radiologist (DHK) before subjective qualitative grading or diagnostic interpretation. This radiologist was blinded to patients’ information including their name, date of examination, BMI, clinical symptoms and qualitative IQ assessments. The following items were analysed in SDCT and LDCT images by drawing ROIs: mid-third lumbar vertebral (L3) body, both transverse processes at the level of the mid L3 body, spinous process, L3–4 intervertebral disc, thecal sac and both psoas muscles. The ROIs were drawn on axial images, except for the spinous process, which was drawn on the mid-sagittal image. All ROIs were measured three times, and the average value was used. For the transverse processes and psoas muscles, the SNR was calculated as the average values for the pair. The ROIs were drawn as large as possible, avoiding any obvious bony islands, fat infiltration or prominent noise.
Image analysis: qualitative IQ and pathological assessment
The qualitative IQ was independently assessed on a picture archiving and communication systemby one board-certified emergency physician (SH.L. reviewer 1) and one board-certified radiologist (H.H.J. reviewer 2) who were blinded to patients’ information and to image series data. Both reviewers independently reviewed all images and generated structured L-spine CT reports (Table 1). The qualitative IQ included evaluations of subjective image noise and diagnostic acceptability, which were based on a previously published scoring system.16 Image sharpness was also evaluated as part of the qualitative IQ by modifying the European guidelines on IQ for CT (EUR 16262); the following anatomical regions were scored for image sharpness using a 5-point ranking scale (Table 1): cortical/trabecular bones, intervertebral foramina/joints/pedicles, spinous/transverse processes, disc profile/end plates of vertebrae, adjacent soft tissues, sacro-iliac joints and any obscuring superimposed abdominal contents or gastrointestinal gas.17
Table 1.
Structured CT report for patients with suspected lumbar spinal fracture
| Analysed finding and rating | Description |
| Subjective image noise | |
| 1 | Little noise |
| 2 | Optimum noise |
| 3 | Noisy but permit evaluation |
| 4 | Too much noise |
| Image sharpness | |
| 1 | Structures are welldefined with sharp contour |
| 2 | Structures are seen but contours are not fully sharp |
| 3 | Structures can be seen, contours are sharp enough if clinical information can be retrieved |
| 4 | Structures can be visualized but not enough for diagnostic reporting, contours are blurred |
| 5 | Structures cannot be identified or defined |
| Diagnostic acceptability | |
| 1 | Fully acceptable |
| 2 | Probably acceptable |
| 3 | Only acceptable under limited condition |
| 4 | Unacceptable |
| Diagnosis | |
| 1 | Acute fracture |
| 2 | No acute fracture |
For the pathological assessments, the two reviewers independently selected the most appropriate diagnosis, i.e. acute fracture or no acute fracture. An old fracture or unfused apophysis was not considered an acute fracture.
Reference standard
A senior board-certified radiologist (S.J.Y.) and orthopaedic surgeon (J.G.S.) who were not blinded to the patients’ data and clinical progress notes determined the reference standards by interpreting the L-spine CT images that were acquired in the emergency department. They independently determined the most appropriate final diagnosis, i.e. acute fracture or no acute fracture. If discrepancies occurred, they reached agreement by consensus. Chart reviews and additional image examinations were available for determining the reference standard.
Radiation dose
The volume CT dose index and dose-length product were obtained from the final page of each examination, which was automatically generated by the system. The effective radiation dose (mSv) was calculated by multiplying the dose-length product by the conversion factor of 0.0129 (mSv × mGy−1 × cm−1).18
Statistical analysis
Statistical analyses were performed using the MedCalc software (Version 12.3.0, Mariakerke, Belgium). The statistical analysis included the following five components: (1) comparisons of the quantitative IQ and radiation doses using independent t-tests; (2) comparison of the qualitative IQ using the Mann Whitney U-test; (3) assessment of the diagnostic performance of SDCT and LDCT images for identifying the presence of fractures using Pearson’s chi-squared test or Fisher’s exact test for comparison with a reference standard; (4) comparison of the diagnostic performance between SDCT and LDCT images using DeLong’s test and (5) assessment of interobserver agreement using the intraclass correlation coefficient (ICC) or kappa value (κ) with 95% confidence intervals. The interobserver agreement was classified as follows: 0–0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement and 0.81–1.00, excellent agreement. Statistical significance was set at p < 0.05.
RESULTS
Patient demographics and dosage characteristics
The patient and dosage characteristics are described in Table 2. No significant differences were identified between LDCT and SDCT in terms of patient age, sex or BMI. The mAs, volume CT dose index, dose-length product and effective dose of LDCT were significantly lower compared to those of SDCT (p < 0.001).
Table 2.
Patient demographic and dosage characteristics
| Standard-dose CT | Low-dose CT (n = 137) | p-value | |
| Demographic characteristic | |||
| Male-to-female ratioa | 61:65 | 72:65 | 0.58 |
| Age (year) | 44.9 ± 15.4 | 43.8 ± 14.7 | 0.55 |
| Male | 42.4 ± 14.7 | 41.5 ± 13.8 | 0.72 |
| Female | 47.5 ± 16.0 | 46.0 ± 15.8 | 0.59 |
| BMIa | |||
| Group 1b | 35 | 34 | 0.68 |
| Underweight | 1 | 2 | 0.98 |
| Lower range of the normal weight | 34 | 32 | |
| Group 2c | 60 | 64 | 0.98 |
| Group 3d | 31 | 39 | 0.57 |
| Overweight | 28 | 34 | 0.73 |
| Obese | 3 | 5 | 0.84 |
| Dose characteristic | |||
| mAs | 237.3 ± 67.2 | 121.8 ± 34.1 | <0.001 |
| Volume CT dose index (mGy) | 11.9 ± 3.8 | 6.2 ± 2.8 | <0.001 |
| Dose-length product (mGy × cm) | 360.5 ± 59.7 | 188.4 ± 21.5 | <0.001 |
| Effective dose | 4.9 ± 2.3 | 2.1 ± 0.9 | <0.001 |
| BMI Group 1 | 3.6 ± 1.8 | 1.1 ± 0.4 | <0.001 |
| BMI Group 2 | 4.7 ± 2.1 | 2.0 ± 0.8 | <0.001 |
| BMI Group 3 | 5.7 ± 2.9 | 3.0 ± 1.4 | <0.001 |
Unless otherwise specified, data are means ± standard deviations. BMI, body mass index; CTDIvol, volume CT dose index; DLP, dose-length product.
aData are numbers of patients.
bUnderweight (<18.5 kg m–2) and lower range of the normal weight (18.5–22.9 kg m–2).
cUpper range of the normal weight (23–24.9 kg m–2).
dOverweight (25–29.9 kg m–2) and obese (≥30 kg m–2).
The causes of injury in the 263 patients included slipping and falling or falling in 119 (45.2%) patients, motor vehicle collision in 60 (22.8%) patients, motorcycle or bicycle accident in 43 (16.3%) patients, pedestrian accident in 25 (9.5%) patients and blunt injury in 16 (6.1%) patients. The mean duration between the trauma and CT scanning was 1 day (range: 0–3 days).
Based on the reference standard, 127 patients (48.3%) showed acute fractures, while no acute fractures were observed in the remaining 136 (51.7%) patients. In the 60 patients who underwent SDCT, 72 fractures (mean, 1.2 fractures per patient; range, 1–4 fractures) were observed and diagnosed as follows: compression (n = 38), transverse process (n = 18), spinous process (n = 6), sacral (n = 6), burst (n = 3) and coccyx (n = 1) fractures. In the 67 patients who underwent LDCT, 81 fractures (mean, 1.2 fractures; range, 1–3 fractures) were observed and diagnosed as follows: compression (n = 42), transverse process (n = 20), sacral (n = 9), spinous process (n = 6), coccyx (n = 2) and burst (n = 2) fractures.
Quantitative IQ assessment
For the vertebral body, transverse process and spinous process, the mean SNR of the LDCT images for the whole cohort was not significantly different from that of the SDCT images (p ≥ 0.1). Additionally, among BMI Groups1–3, the mean SNRs of the LDCT images were not significantly different from those of the SDCT images (p ≥ 0.2). In contrast, for the intervertebral disc, dural sac and psoas muscle, the mean SNRs of the LDCT images for the whole cohort and BMI Groups1–3 were significantly lower than were those of the SNRs of the SDCT images (p < 0.001; Table 3).
Table 3.
Quantitative image quality (signal-to-noise ratio) assessment according to BMI
| Standard-dose CT | Low-dose CT | p-value | |
| Vertebral body | 8.1 ± 2.4 | 7.9 ± 2.7 | 0.53 |
| BMI Group 1a | 8.2 ± 2.4 | 7.9 ± 2.2 | 0.59 |
| BMI Group 2b | 8.1 ± 2.9 | 8.0 ± 2.4 | 0.83 |
| BMI Group 3c | 8.0 ± 2.7 | 7.8 ± 2.6 | 0.75 |
| Transverse process | 4.6 ± 1.4 | 4.3 ± 1.5 | 0.10 |
| BMI Group 1 | 5.0 ± 1.8 | 4.6 ± 1.5 | 0.32 |
| BMI Group 2 | 4.6 ± 1.2 | 4.3 ± 1.4 | 0.20 |
| BMI Group 3 | 4.2 ± 1.4 | 4.0 ± 1.4 | 0.50 |
| Spinous process | 8.1 ± 4.1 | 7.8 ± 4.3 | 0.56 |
| BMI Group 1 | 8.5 ± 3.2 | 8.3 ± 4.2 | 0.82 |
| BMI Group 2 | 8.1 ± 4.3 | 8.0 ± 4.7 | 0.90 |
| BMI Group 3 | 7.8 ± 4.7 | 7.6 ± 4.1 | 0.85 |
| Intervertebral disc | 9.0 ± 2.5 | 4.5 ± 1.2 | <0.001 |
| BMI Group 1a | 9.2 ± 2.0 | 5.5 ± 1.3 | <0.001 |
| BMI Group 2b | 8.8 ± 3.1 | 4.2 ± 0.9 | <0.001 |
| BMI Group 3c | 7.7 ± 3.0 | 3.4 ± 1.4 | <0.001 |
| Dural sac | 2.5 ± 1.2 | 1.3 ± 0.5 | <0.001 |
| BMI Group 1 | 3.3 ± 1.7 | 1.6 ± 0.5 | <0.001 |
| BMI Group 2 | 2.2 ± 1.2 | 1.3 ± 0.4 | <0.001 |
| BMI Group 3 | 2.0 ± 1.4 | 1.2 ± 0.7 | <0.001 |
| Psoas muscle | 8.0 ± 4.1 | 4.8 ± 2.3 | <0.001 |
| BMI Group 1 | 8.9 ± 3.5 | 5.7 ± 2.2 | <0.001 |
| BMI Group 2 | 8.1 ± 4.7 | 5.0 ± 2.7 | <0.001 |
| BMI Group 3 | 7.2 ± 4.3 | 4.2 ± 2.1 | <0.001 |
Data are means ± standard deviations. BMI, body mass index.
aUnderweight (<18.5 kg m–2) and lower range of the normal weight (18.5–22.9 kg m–2).
bUpper range of the normal weight (23–24.9 kg m–2).
cOverweight (25–29.9 kgm–2) and obese (≥30 kg m–2).
Qualitative IQ assessment
The median scores of subjective image noise, image sharpness and diagnostic acceptability, as determined by the qualitative IQ, are described in Table 4. The median scores of subjective image noise from both reviewers were significantly higher in LDCT images than they were in SDCT images for BMI Group 3 (p < 0.001), but not for the whole cohort, BMI Groups 1 and 2 (p ≥ 0.22). The median image sharpness scores from both reviewers at the disc/end plate, adjacent soft tissue and abdominal structures were significantly higher in LDCT images than they were in SDCT images for both the whole cohort and BMI Group 3 (p ≤ 0.004). However, the median image sharpness scores at the cortex/trabecular bone, intervertebral foramina/joints/pedicles, spinous/transverse processes and sacro-iliac joints were not significantly different between the two CT scan types in the whole cohort or among the three BMI groups (p ≥ 0.08). With regard to diagnostic acceptability, no significant differences in the median scores were identified between SDCT and LDCT for either the whole cohort or the BMI groups (p ≥ 0.16).
Table 4.
Qualitative image quality assessment according to BMI
| Reviewer 1 | p | Reviewer 2 | p | |||
| SDCT | LDCT | SDCT | LDCT | |||
| Subjective image noise | 1 (0.4) | 1 (0.7) | 0.55 | 1 (0.4) | 1 (0.8) | 0.69 |
| BMI Group 1 | 1 (0.2) | 1 (0.5) | 0.28 | 1 (0.4) | 1 (0.5) | 0.36 |
| BMI Group 2 | 1 (0.2) | 1 (0.7) | 0.35 | 1 (0.2) | 1 (0.6) | 0.22 |
| BMI Group 3 | 1 (0.6) | 2 (0.5) | <0.001 | 1 (0.7) | 2 (0.7) | <0.001 |
| Image sharpness | ||||||
| Cortex, trabecular bone | 1 (0.2) | 1 (0.4) | 1.0 | 1 (0.3) | 1 (0.4) | 1.0 |
| BMI Group 1 | 1 (0.1) | 1 (0.4) | 0.16 | 1 (0.2) | 1 (0.5) | 1.0 |
| BMI Group 2 | 1 (0.2) | 1 (0.4) | 0.08 | 1 (0.2) | 1 (0.4) | 1.0 |
| BMI Group 3 | 1 (0.3) | 1 (0.4) | 1.0 | 1 (0.3) | 1 (0.5) | 1.0 |
| Intervertebral foramina, joints, pedicles | 1 (0.3) | 1 (0.4) | 1.0 | 1 (0.4) | 1 (0.5) | 0.08 |
| BMI Group 1 | 1 (0.2) | 1 (0.2) | 1.0 | 1 (0.2) | 1 (0.1) | 1.0 |
| BMI Group 2 | 1 (0.2) | 1 (0.3) | 1.0 | 1 (0.2) | 1 (0.4) | 0.19 |
| BMI Group 3 | 1 (0.5) | 1 (0.5) | 0.41 | 1 (0.7) | 1 (0.7) | 0.55 |
| Spinous and transverse process | 1 (0.1) | 1 (0.2) | 1.0 | 1 (0.1) | 1 (0.3) | 1.0 |
| BMI Group 1 | 1 (0.1) | 1 (0.2) | 1.0 | 1 (0.1) | 1 (0.1) | 1.0 |
| BMI Group 2 | 1 (0.1) | 1 (0.1) | 1.0 | 1 (0.1) | 1 (0.2) | 1.0 |
| BMI Group 3 | 1 (0.2) | 1 (0.5) | 0.30 | 1 (0.2) | 1 (0.5) | 0.30 |
| Disc, vertebral end plate | 1 (0.5) | 2 (0.6) | <0.001 | 1 (0.5) | 2 (0.6) | <0.001 |
| BMI Group 1 | 1 (0.2) | 1 (0.5) | 0.032 | 1 (0.3) | 1 (0.5) | 0.32 |
| BMI Group 2 | 1 (0.4) | 1 (0.6) | 0.032 | 1 (0.3) | 1 (0.7) | 0.31 |
| BMI Group 3 | 1 (0.6) | 2 (0.6) | <0.001 | 1 (0.6) | 2 (0.6) | <0.001 |
| Adjacent soft tissue | 1 (0.2) | 1 (0.7) | <0.001 | 1 (0.2) | 1 (0.6) | <0.001 |
| BMI Group 1 | 1 (0.2) | 1 (0.6) | 0.35 | 1 (0.2) | 1 (0.6) | 0.35 |
| BMI Group 2 | 1 (0.2) | 1 (0.7) | 0.04 | 1 (0.2) | 1 (0.6) | 0.22 |
| BMI Group 3 | 1 (0.3) | 2 (0.6) | <0.001 | 1 (0.3) | 2 (0.7) | 0.004 |
| Sacro-iliac joints | 1 (0.2) | 1 (0.4) | 0.01 | 1 (0.4) | 1 (0.4) | 1.0 |
| BMI Group 1 | 1 (0.1) | 1 (0.2) | 1.0 | 1 (0.4) | 1 (0.2) | 1.0 |
| BMI Group 2 | 1 (0.2) | 1 (0.4) | 0.08 | 1 (0.1) | 1 (0.4) | 1.0 |
| BMI Group 3 | 1 (0.2) | 1 (0.4) | 0.21 | 1 (0.2) | 1 (0.3) | 1.0 |
| Abdominal structures | 1 (0.3) | 1 (0.7) | <0.001 | 1 (0.3) | 1 (0.7) | <0.001 |
| BMI Group 1 | 1 (0.4) | 1 (0.7) | 0.47 | 1 (0.3) | 1 (0.7) | 0.13 |
| BMI Group 2 | 1 (0.1) | 1 (0.7) | 0.28 | 1 (0.1) | 1 (0.7) | 0.001 |
| BMI Group 3 | 1 (0.5) | 2 (0.6) | <0.001 | 1 (0.6) | 2 (0.7) | <0.001 |
| Diagnostic acceptability | 1 (0.5) | 1 (0.8) | 0.23 | 1 (0.4) | 1 (0.7) | 0.16 |
| BMI Group 1 | 1 (0.4) | 1 (0.5) | 1.0 | 1 (0.3) | 1 (0.5) | 0.32 |
| BMI Group 2 | 1 (0.6) | 1 (0.8) | 0.43 | 1 (0.6) | 1 (0.8) | 0.43 |
| BMI Group 3 | 1 (0.7) | 1 (0.8) | 0.59 | 1 (0.6) | 1 (0.9) | 0.60 |
Data are median and interquartile range in parenthesis. BMI, body mass index; LDCT, low-dose CT; SDCT, standard-dose CT.
Diagnostic performances of SDCT and LDCT
Table 5 describes the diagnostic performances of SDCT and LDCT for diagnosing fractures (both reviewers). SDCT exhibited slightly better diagnostic performance than did LDCT (sensitivity, 96.7–100%vs94–98.5%; specificity, 95.6–97.0%vs both 95.7%; accuracy, 96.0–98.4%vs94.9–97.1%). However, thereviewersnever identified any significant differences in the sensitivity, specificity and accuracy between SDCT and LDCT for the whole cohort (p ≥ 0.50). Furthermore, no significant differences in diagnostic performance, including diagnostic accuracy, were found between the two CT scan types among the BMI groups (p ≥ 0.49). Representative LDCT images of BMI Groups1–3 are shown in Figures 2–5.
Table 5.
Diagnostic performances of standard-dose (SD) and low-dose (LD) CT
| Reviewer 1 | p | Reviewer 2 | p | ||||
| SDCT | LDCT | SDCT | LDCT | ||||
| Total | |||||||
| Sensitivity | 96.7 (88.5–99.6) | 94.0 (85.4–98.4) | 0.77 | 100 (94.0–100) | 98.5 (92.0–100) | 0.96 | |
| Specificity | 95.6 (87.3–99.1) | 95.7 (88.0–99.1) | 0.70 | 97.0 (89.5–99.6) | 95.7 (85.0–99.1) | 0.96 | |
| PPV | 95.1 (86.5–98.3) | 95.5 (87.4–98.5) | 0.75 | 96.8 (88.5–99.2) | 95.7 (87.9–98.5) | 0.90 | |
| NPV | 96.9 (89.0–99.2) | 94.4 (86.8–97.8) | 0.77 | 100 (NA) | 98.5 (90.5–100) | 0.99 | |
| Accuracy | 96.0 (91.0–98.7) | 94.9 (89.7–97.9) | 0.50 | 98.4 (94.5–99.8) | 97.1 (92.7–99.2) | 0.77 | |
| BMI Group 1 | |||||||
| Sensitivity | 100 (78.2–100) | 94.1 (71.3–100) | 0.95 | 100 (78.2–100) | 100 (80.5–100) | 1.0 | |
| Specificity | 100 (83.2–100) | 100 (80.5–100) | 1.0 | 100 (83.2–100) | (80.5–100) | 1.0 | |
| PPV | 100 (NA) | 100 (NA) | 1.0 | 100 (NA) | 100 (NA) | 1.0 | |
| NPV | 100 (NA) | 94.4 (71.7–99.1) | 0.95 | 100 (NA) | 100 (NA) | 1.0 | |
| Accuracy | 100 (90.0–100) | 97.1 (84.7–99.9) | 1.0 | 100 (90.0–100) | 100 (89.7–100) | 1.0 | |
| BMI Group 2 | |||||||
| Sensitivity | 97.0 (84.2–99.9) | 97.1 (85.1–99.9) | 0.49 | 100 (89.4–100) | 100 (90.0–100) | 1.0 | |
| Specificity | 92.6 (75.7–99.1) | 93.1 (77.2–99.2) | 0.66 | 96.3 (81.0–99.9) | 93.1 (77.2–99.2) | 0.95 | |
| PPV | 94.1 (80.8–98.4) | 94.4 (81.7–98.5) | 0.65 | 97.1 (82.8–99.6) | 94.6 (82.1–98.5) | 0.95 | |
| NPV | 96.2 (78.3–99.4) | 96.6 (80.2–99.5) | 0.51 | 100 (NA) | 100 (NA) | 1.0 | |
| Accuracy | 95.0 (85.8–98.9) | 95.3 (86.6–98.9) | 0.73 | 98.3 (90.8–99.9) | 96.9 (88.7–99.5) | 0.94 | |
| BMI Group 3 | |||||||
| Sensitivity | 91.2 (61.5–99.8) | 86.7 (59.5–98.3) | 0.81 | 100 (73.5–100) | 93.3 (68.1–99.8) | 0.91 | |
| Specificity | 94.7 (74.0–99.9) | 95.8 (78.9–99.9) | 0.58 | 94.7 (74.0–99.9) | 95.8 (78.9–99.9) | 0.58 | |
| PPV | 91.2 (61.8–98.7) | 92.9 (65.4–98.9) | 0.57 | 92.3 (64.0–98.8) | 93.3 (67.2–99.0) | 0.53 | |
| NPV | 94.7 (73.3–99.2) | 92.0 (75.9–97.7) | 0.80 | 100 (NA) | 95.8 (77.6–99.4) | 0.89 | |
| Accuracy | 93.5 (78.1–99.1) | 92.3 (77.7–97.9) | 0.78 | 96.8 (84.2–100) | 94.9 (82.3–99.3) | 0.84 | |
Data are percentage and 95% confidence interval in parenthesis. BMI, body mass index; NA, not applicable; NPV, negative predictive value; PPV, positive predictive value;
Figure 2.
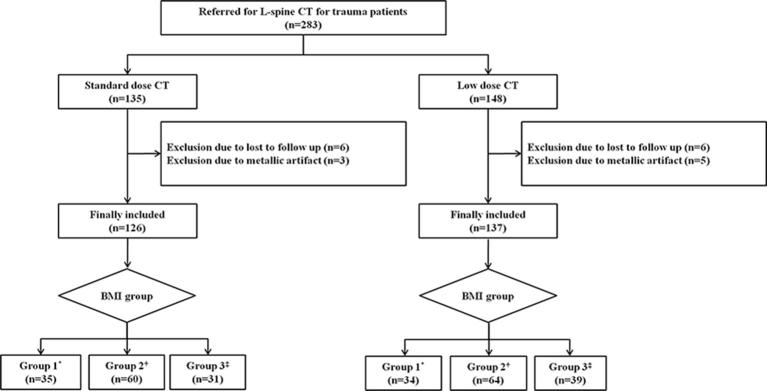
Low-dose CT images of a 31-year-old female with a history of slipping and falling down. The body mass indexand effective dose were 17.3 kg m–2 (underweight, body mass indexGroup 1) and 1.1 mSv, respectively. Sagittal (a) and axial (b) CT images show discontinuity of the anterior cortex of the T12 body (arrows, a and b), suggesting an acute compression fracture. Both reviewers assigned a score of 1 for diagnostic acceptability (fully acceptable) and diagnosed the fractures correctly. L2, body of the second lumbar vertebra.
Figure 5.
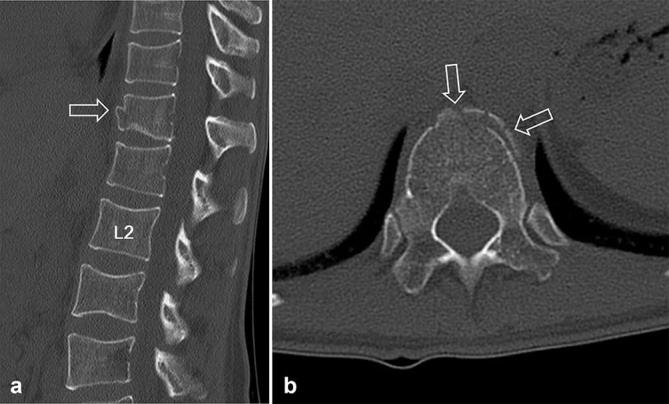
Low-dose CT images of a 27-year-old female with a history of motorcycle accidents. The body mass index and effective dose were 27.3 kg m–2 (overweight, body mass indexGroup 3) and 2.5 mSv, respectively. Sagittal (a) and axial (b) CT images show acute burst fracture in the L3 body (open arrow, a) and acute compression fracture in the L1 body (white arrows, a and b). Additionally, definite fracture line in the spinous process of the L4 (open arrowhead, a) is seen despite the image noise. Both reviewers assigned a score of 1 for diagnostic acceptability (fully acceptable) and diagnosed the fractures correctly.
Figure 3.
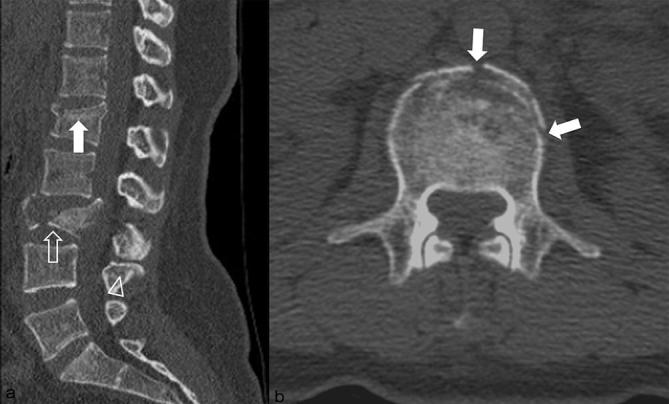
Low-dose CT images of a 48-year-old male with a history of falling down. The body mass index and effective dose were 20.9 kg m–2 (lower range of normal-weight, body mass indexGroup 1) and 1.2 mSv, respectively. Sagittal (a) and axial (b) CT images show acute fractures of the body (burst fracture, white arrow, a), left transverse process (open arrow, b) and lamina (open arrowhead, b) of the L2. Both reviewers assigned a score of 1 for diagnostic acceptability (fully acceptable) and diagnosed the fractures correctly.
Figure 4.

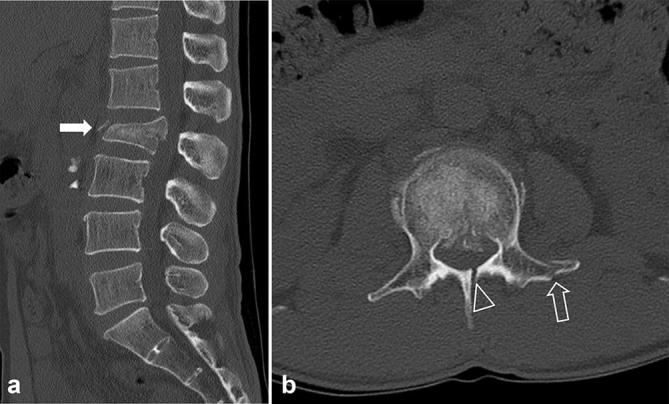
Low-dose CT images of a 30-year-old male with a history of falling down. The body mass index and effective dose were 23.5 kg m–2 (upper range of normal weight, body mass indexGroup 2) and 1.8 mSv, respectively. Sagittal (a) and axial (b) CT images show acute fractures of the body (burst fracture, white arrow, a), left transverse process (open arrow, b) and lamina (open arrowhead, b) of the L2. Both reviewers assigned a score of 1 for diagnostic acceptability (fully acceptable) and diagnosed the fractures correctly.
Interobserver agreement
The subjective image noise and image sharpness results from reviewers 1 and 2 showed excellent or good agreement for both SDCT (ICC = 0.892–0.985) and LDCT (ICC = 0.776–0.916). The diagnostic acceptability ratings of reviewers 1 and 2 exhibited excellent agreement for both SDCT (ICC = 0.930) and LDCT (ICC = 0.868). For diagnosing L-spine fractures, the interobserver agreement between the reviewers for SDCT and LDCT was excellent (k = 0.938 and 0.845, respectively). Furthermore, the agreement with the reference standard for reviewers 1 and 2 was excellent for both SDCT (k = 0.909 and 0.802, respectively) and LDCT (k = 0.971 and 0.858, respectively) (Table 6).
Table 6.
Interobserver agreement on qualitative image quality and diagnosis
| Standard-dose CT | Low-dose CT | |
| Qualitative image qualitya | ||
| Subjective image noise | 0.930 (0.901–0.951) | 0.891 (0.847–0.922) |
| Image sharpness | ||
| Cortex & trabecular bone | 0.896 (0.810–0.957) | 0.880 (0.815–0.952) |
| Intervertebral foramina, joints and pedicles | 0.912 (0.875–0.938) | 0.776 (0.714–0.840) |
| Spinous and transverse process | 0.985 (0.979–0.989) | 0.810 (0.734–0.865) |
| Disc & end plate of vertebra | 0.892 (0.847–0.924) | 0.849 (0.793–0.890) |
| Adjacent soft tissue | 1.0 (NA) | 0.916 (0.884–0.938) |
| Sacro-iliac joints | 0.959 (0.942–0.971) | 0.877 (0.831–0.910) |
| Abdominal structures | 0.983 (0.976–0.988) | 0.880 (0.835–0.912) |
| Diagnostic acceptability | 0.930 (0.901–0.951) | 0.868 (0.819–0.904) |
| Diagnosis | ||
| R1 vs R2 | 0.938 (0.853–0.971) | 0.845 (0.674–1.000) |
| R1 vs Reference standard | 0.909 (0.808–0.967) | 0.802 (0.614–0.990) |
| R2 vs Reference standard | 0.971 (0.914–1.000) | 0.858 (0.700–1.000) |
Data are intraclass correlation coefficients (qualitative image quality) or kappa values (diagnosis) and 95% confidence interval in parenthesis. NA, not applicable; R1, reviewer 1; R2, reviewer 2.
aAgreement between reviewer 1 and reviewer 2.
DISCUSSION
The present study did not identify any relevant differences between SDCT and LDCT scans in terms of the qualitative IQ of the bony structures among underweight, normal weight and overweight patients. Furthermore, among underweight, normal weight and overweight patients with trauma, LDCT exhibited equivalent diagnostic performance to SDCT, with a 47–69% reduction in the radiation dose.
As mentioned above, the radiation dose used in CT examinations is one of the most important safety concerns of the modern era.1,19 Use of an LD protocol was recommended at a National Institute of Biomedical Imaging and Bioengineering meeting, based on the principle of “first do no harm” and the concept of “ALARA”.20,21 Others have also advocated for reducing the CT radiation exposure, as it may help reduce the risk and incidence of cancer within the population.1,19 To help achieve this, the IR technique, which has long been used as a principal method for processing images in nuclear medicine, is now being applied to CT images, and this technique works in either the image space or the raw data space. In the past, this technique could not be applied in clinical situations owing to the large amount of CT imaging data required. However, recent advances in the processing power of computers have permitted the IR techniques to be utilized in the field of radiology.22 These techniques can reduce image noise by 31% in L-spine CT, while also preserving IQ and reducing the radiation dose by 52% compared with conventional FBP reconstruction techniques.9
The feasibility of using LDCT to evaluate intervertebral discs and disc herniation was investigated previously.4–9 Initially, researchers evaluated the feasibility of using LDCT with FBP compared with SDCT with FBP.4 After the introduction of IR algorithms as a potential method of reducing the radiation dose, SDCT with IR became the key for overcoming the image noise observed when using the FBP technique.9 Recently, several studies concluded that LDCT with IR was comparable to plain radiographs5,6 and SDCT with FBP for evaluating intervertebral discs and disc herniation.7,8 Here, the feasibility of using the LD protocol for L-spine CT with the IR algorithm in trauma patients was evaluated, and we found acceptable IQ and diagnostic efficacy with excellent or good interobserver agreement compared with SDCT with the IR algorithm.
In the present study, we also evaluated the effects of BMI on the quantitative IQ, qualitative IQ and diagnostic performance of SDCT and LDCT. The effects of BMI have been investigated previously in LD abdominal CT scans in renal colic patients.23,24 These studies concluded that LD abdominal CT scans were inadequate for obese patients because a high BMI leads to increased image noise, decreased IQ and decreased diagnostic performance.23,24 However, after the introduction of the IR algorithm, the diagnostic accuracy of LD abdominal CT scans for obese patients with renal colic was found to be equal to the accuracy for non-obese patients (non-obese patients, 95.7%; obese patients, 96.4%; p = 0.83).25 Similarly, our study demonstrated that LDCT scans were equally as applicable in obese patients with trauma as they were in underweight and normal weight patients. Specifically, the qualitative IQ in the bony structures, diagnostic acceptability and diagnostic performance were not significantly different between LDCT and SDCT scans among patients with different BMIs (although the mean SNR was different).
Our study has several limitations. First, although intra-individual comparison is the best methodology, we did not employ SDCT and LDCT in the same participants owing to ethical concerns about delivering a second radiation dose. Second, the degree of neural foramen stenosis and the amount of peridural fat can influence IQ, but these were not assessed in the present study. Third, the reference standard was considered as interpretation of the initial CT in emergency department, not MR or operative findings. Because few of the patients in our study underwent either MR or surgery. Fourth, we did not evaluate alternative diagnoses or complications related to the spinal fractures in our patients. This was mainly because the reasons behind the diagnostic performance of an alternative diagnosis or the complications from an LD protocol for patients with trauma were not a focus of our study. Fifth, patients were categorized according to BMI as underweight to the lower range of normal weight, upper range of normal weight or overweight to extremely obese because the numbers of underweight and obese patients were small in our study. Different categorizations may result in different diagnostic performances. Finally, we could not evaluate the L-spine CT images that were generated from reconstructions of abdominal CT images in multiple trauma patients as a different reconstruction kernel (smooth convolution kernel) was used. In the future, the diagnostic accuracy of LDCT should be further examined with prospective and MR correlation studies in a larger population.
In conclusion, the diagnostic performance of LDCT with IR for identifying an L-spine fracture was similar to that of SDCT with IR. These results suggest that LDCT with the IR algorithm can be used instead of conventional SDCT as the diagnostic imaging tool of choice when evaluating trauma patients with suspected L-spine fractures.
Contributor Information
Sun Hwa Lee, Email: sunhwa9@hanmail.net.
Seong Jong Yun, Email: zoomknight@naver.com.
Dong Hyeon Kim, Email: yji0310@naver.com.
Hyeon Hwan Jo, Email: ad-baby@hanmail.net.
Jae Gwang Song, Email: ysj850708@hanmail.net.
References
- 1.Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–84. [DOI] [PubMed] [Google Scholar]
- 2.Omoumi P, Verdun FR, Becce F. Optimization of radiation dose and imagequality in musculoskeletal CT: emphasis on iterative reconstructiontechniques (Part 2). Semin Musculoskelet Radiol 2015; 19: 422–30. [DOI] [PubMed] [Google Scholar]
- 3.Omoumi P, Becce F, Ott JG, Racine D, Verdun FR. Optimization of radiation dose and imagequality in musculoskeletal CT: emphasis on iterative reconstructiontechniques (Part 1). Semin Musculoskelet Radiol 2015; 19: 422–30. [DOI] [PubMed] [Google Scholar]
- 4.Bohy P, de Maertelaer V, Roquigny A, Keyzer C, Tack D, Gevenois PA. Multidetector CT in patients suspected of having lumbar disk herniation: comparison of standard-dose and simulated low-dose techniques. Radiology 2007; 244: 524–31. [DOI] [PubMed] [Google Scholar]
- 5.Alshamari M, Geijer M, Norrman E, Lidén M, Krauss W, Wilamowski F, et al. Low dose CT of the lumbar spine compared with radiography: a study on image quality with implications for clinical practice. Acta Radiol 2016; 57: 602–11. [DOI] [PubMed] [Google Scholar]
- 6.Alshamari M, Geijer M, Norrman E, Geijer H. Low-dose computed tomography of the lumbar spine: a phantom study on imaging parameters and image quality. Acta Radiol 2014; 55: 824–32. [DOI] [PubMed] [Google Scholar]
- 7.Yang CH, Wu TH, Chiou YY, Hung SC, Lin CJ, Chen YC, et al. Imaging quality and diagnostic reliability of low-dose computed tomography lumbar spine for evaluating patients with spinal disorders. Spine J 2014; 14: 2682–90. [DOI] [PubMed] [Google Scholar]
- 8.Yang CH, Wu TH, Lin CJ, Chiou YY, Chen YC, Sheu MH, et al. Knowledge-based iterative model reconstruction technique in computed tomography of lumbar spine lowers radiation dose and improves tissue differentiation for patients with lower back pain. Eur J Radiol 2016; 85: 1757–64. [DOI] [PubMed] [Google Scholar]
- 9.Gervaise A, Osemont B, Lecocq S, Noel A, Micard E, Felblinger J, et al. CT image quality improvement using adaptive iterative dosereduction with wide-volume acquisition on 320-detector C. Eur Radiol 2012; 22: 295–301. [DOI] [PubMed] [Google Scholar]
- 10.Ippolito D, Besostri V, Bonaffini PA, Rossini F, Di Lelio A, Sironi S. Diagnostic value of whole-body low-dose computed tomography (WBLDCT) in bone lesions detection in patients with multiple myeloma (MM. Eur J Radiol 2013; 82: 2322–7. [DOI] [PubMed] [Google Scholar]
- 11.Alshamari M, Geijer M, Norrman E, Lidén M, Krauss W, Jendeberg J, et al. Impact of iterative reconstruction on image quality of low-dose CT of the lumbar spine. Acta Radiol 2017; 58: 702–9. [DOI] [PubMed] [Google Scholar]
- 12.Vardhanabhuti V, Riordan RD, Mitchell GR, Hyde C, Roobottom CA. Image comparative assessment using iterative reconstructions: clinical comparison of low-dose abdominal/pelvic computed tomography between adaptive statistical, model-based iterative reconstructions and traditional filtered back projection in 65 patients. Invest Radiol 2014; 49: 209–16. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Obesity: preventing and managing the global epidemic— report of a WHO consultation of obesity. Geneva, Switzerland: World Health Organization, 1997. [PubMed] [Google Scholar]
- 14.Kilic K, Erbas G, Guryildirim M, Arac M, Ilgit E, Coskun B. Lowering the dose in head CT using adaptive statistical iterative reconstruction. AJNR Am J Neuroradiol 2011; 32: 1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 2010; 195: 649–54. [DOI] [PubMed] [Google Scholar]
- 16.Patro SN, Chakraborty S, Sheikh A. The use of adaptive statistical iterative reconstruction (ASiR) technique in evaluation of patients with cervical spine trauma: impact on radiation dose reduction and image quality. Br J Radiol 2016; 89: 20150082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongartz G, Golding SJ, Jurik AJ. European guidelines for multislice computed tomography: report EUR 16262 EN 2004. Luxembourg: European Commission, 2004. [Google Scholar]
- 18.Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010; 257: 158–66. [DOI] [PubMed] [Google Scholar]
- 19.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol 2008; 81: 362–78. [DOI] [PubMed] [Google Scholar]
- 20.McCollough CH, Chen GH, Kalender W, Leng S, Samei E, Taguchi K, et al. Achieving routine submillisievert CT scanning: report from the summit on management of radiation dose in CT. Radiology 2012; 264: 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boone JM, Hendee WR, McNitt-Gray MF, Seltzer SE. Radiation exposure from CT scans: how to close our knowledge gaps, monitor and safeguard exposure-proceedings and recommendations of the radiation dose summit, sponsored by NIBIB, February 24-25, 2011. Radiology 2012; 265: 544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willemink MJ, de Jong PA, Leiner T, de Heer LM, Nievelstein RA, Budde RP, et al. Iterative reconstruction techniques for computed tomography part 1: technical principles. Eur Radiol 2013; 23: 1623–31. [DOI] [PubMed] [Google Scholar]
- 23.Hamm M, Knopfle E, Wartenberg S, Wawroschek F, Weckermann D, Harzmann R. Low dose unenhanced helical computerized tomography for the evaluation of acute flank pain. J Urol 2002; 167: 1687–91. [PubMed] [Google Scholar]
- 24. Poletti PA, Platon A, Rutschmann OT, Schmidlin FR, Iselin CE, Becker CD. Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am J Roentgenol 2007; 188: 927–33. [DOI] [PubMed] [Google Scholar]
- 25.Gervaise A, Naulet P, Beuret F, Henry C, Pernin M, Portron Y, et al. Low-dose CT with automatic tube current modulation, adaptive statistical iterative reconstruction, and low tube voltage for the diagnosis of renal colic: impact of body mass index. AJR Am J Roentgenol 2014; 202: 553–60. [DOI] [PubMed] [Google Scholar]