Abstract
Radium-223 (223Ra) offers a new option for the treatment of bone metastases from prostate cancer. As cancer treatment progresses towards personalization, the potential for an individualized approach is exemplified in treatments with radiotherapeutics due to the unique ability to image in vivo the uptake and retention of the therapeutic agent. This is unmatched in any other field of medicine. Currently, 223Ra is administered according to standard fixed administrations, modified according to patient weight. Although gamma emissions comprise only 1% of the total emitted energy, there are increasing reports that quantitative imaging is feasible and can facilitate patient-specific dosimetry. The aim of this article is to review the application of imaging and dosimetry for 223Ra and to consider the potential for treatment optimization accordingly, in order to ensure clinical and cost effectiveness of this promising agent.
INTRODUCTION
Prostate cancer is the most common male cancer in the UK, and the second most common male cancer worldwide.1 The incidence of prostate cancer has increased by 155% in the past 40 years, in part due to increased detection from prostate-specific antigen testing, with the largest increase for males aged between 25 and 49 years. Diagnosis at Stage 4 occurs in 20–30% of cases when there may be bone involvement, in which case, life expectancy may be as low as 5 years.2
Radium-223 (223Ra) dichloride, approved by the United States Food and Drug Administration in 2013, offers a novel therapeutic treatment option for castration-resistant prostate cancer that has metastasized to the bone. Although not the first radiotherapeutic used for this purpose, samarium-153 ethylenediamine tetra(methylene phosphonic acid) (153Sm EDTMP) and strontium-89 (89Sr) chloride have long been administered, as have phosphorus-32, rhenium-186 (186Re) hydroxyethylidine diphosphonate (HEDP) and rhenium-188 (188Re) HEDP;3,4 223Ra is the first alpha emitter to be approved and the first to demonstrate a survival advantage. A number of clinical studies of 223Ra5–8 culminated in a Phase-3 clinical trial of 921 patients to evaluate the survival advantage of 223Ra in comparison with a placebo.9
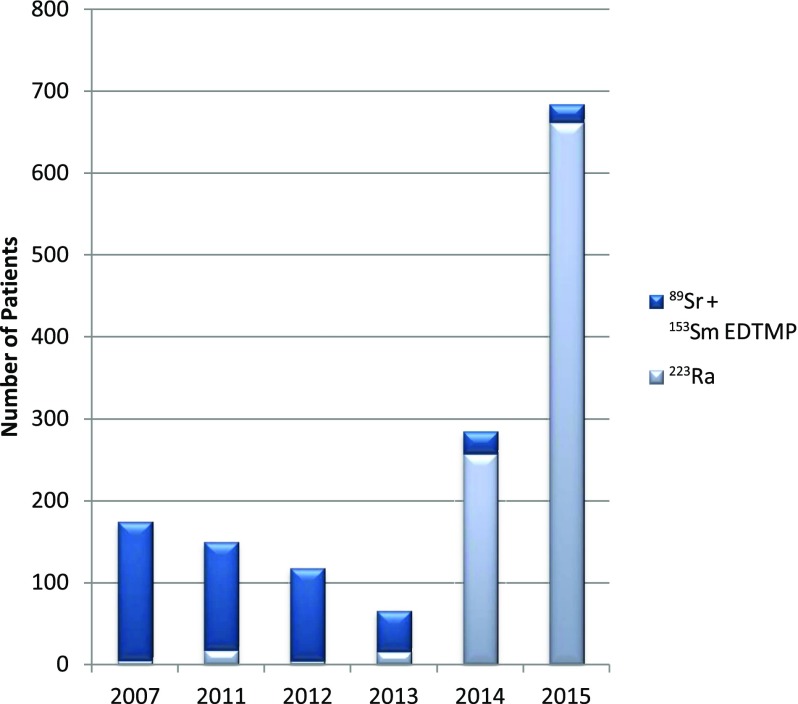
In the UK, 223Ra has become increasingly used following European Medicines Agency approval in 2013.10 From its initial use in 2007,11 223Ra was used in <5% of all treatments of bone metastases with radiopharmaceuticals in 2011 and 2012, but in 2015, it was used in >95% of treatments12,13 (Figure 1). The total number of patients with bone metastases treated with radiopharmaceuticals increased by nearly 400% from 2007 to 2015 due to 223Ra.
Figure 1.
Radiopharmaceutical treatments of bone metastases. 223Ra, radium-223; 153Sm EDTMP, samarium-153 ethylenediamine tetra(methylene phosphonic acid); 89Sr, strontium-89. Adapted from Rojas et al.13
Personalized treatment planning is of increasing interest in molecular radiotherapy, for which the theragnostic potential of radiotherapeutics can be utilized and for which there is increasing evidence of correlations between absorbed dose and outcome.14 A fully individualized plan requires quantitative imaging, internal dosimetry, predictions of effectiveness and toxicity, and evaluation and optimization of the planning parameters available. Financial aspects must also be taken into account to demonstrate that the potential for patient benefit and cost-effective use of drugs would outweigh the increased costs associated with image acquisition and dosimetry. An European Union directive (Euratom 59/2013) mandates the same level of treatment planning and verification for radiotherapeutics as for external beam radiotherapy,15 and there are increasing calls to implement routine image-based dosimetry.16
IMAGING
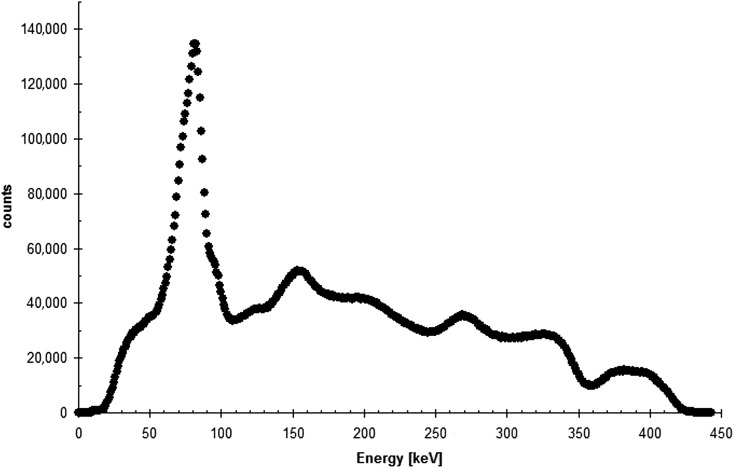
223Ra undergoes a complicated decay scheme, with a series of six daughter products, before decaying to stable lead. The total emitted energy is 28.2 MeV, of which 95% is from alpha emissions, 3.2% from beta particles and <2% from gamma emissions17 (Table 1). This results in a low signal which can present challenges for quantitative imaging, but nevertheless, introduces the potential for individualized biodistribution studies. Hindorf et al17 defined the imaging characteristics for 223Ra, identifying three energy peaks as suitable for imaging of the 10 photon energies emitted with a probability >1%. Optimal energy windows were set at 82, 154 and 270 keV, each with a 20% width (Figure 2). Camera sensitivity was found to be 69, 31 and 34 counts per second (cps) MBq−1 from the three windows, respectively. Although the most abundant photon emissions at around 82 keV are potentially contaminated by the lead X-ray emissions from the collimator, this did not prevent quantitative imaging. The full-width half-maximum spatial resolution of the system was 11 mm at all peaks, although the scatter was at a minimum for the 82 keV peak. This spatial resolution can lead to a marked partial volume effect for volumes <30 mm diameter. It was concluded that the 82-keV window is sufficient for quantitative imaging, with an effective mass attenuation coefficient of 0.071 cm2 g−1. A phantom study with clinically relevant activities and volumes demonstrated that activity could be quantified to within 10% for a large 200-ml volume and within 40% for a 0.5-ml volume.
Table 1.
Decay chain for radium-223 (223Ra). The relative proportions of the branched decay from bismuth-211 (211Bi) are 0.997 and 0.003 for 211Bi → thallium-207 (207Tl) and 211Bi → polonium-211 (211Po), respectively
| Radionuclide | Mode of decay | Abundance | Half-life |
|---|---|---|---|
| 223Ra → 219Rn | α | 100% | 11.43 days |
| 219Rn → 215Po | α | 100% | 3.96 s |
| 215Po → 211Pb | α | 100% | 1.78 ms |
| 211Pb → 211Bi | β- | 100% | 36.1 min |
| 211Bi → 211Po | β- | 0.276% | 2.14 min |
| 211Bi → 207Tl | α | 99.72% | 2.14 min |
| 211Po → 207Pb | α | 100% | 0.516 s |
| 207Tl → 207Pb | β- | 100% | 4.77 min |
| 207Pb → – | Stable | – | – |
207Pb, lead-207; 211Pb, lead-211; 219Rn, radon-219.
Figure 2.
Energy spectrum for radium-223 (acquired for a standard, using medium-energy collimators on a Philips Forte camera).
PHARMACOKINETICS AND DOSIMETRY
Internal dosimetry for the marrow and skeleton presents significant challenges due to microscopic energy deposition within the bone matrix.18,19 Although whole-body dosimetry can be assessed from either whole-body scans or from external retention measurements,20 dosimetry for red marrow can be obtained from imaging and from blood sampling and should take into account the activity in the extracellular fluid, the blood, the bone marrow cells, the bone and major organs of uptake.21 Models to generate absorbed fractions for alpha particles in cortical and trabecular bones are necessary to consider dosimetry at a microscopic scale.22–25 Correlations between the absorbed dose delivered to the red marrow and toxicity have been found for treatments with 153Sm EDTMP.26
Of particular relevance to alpha emitters, a relative biological effect (RBE) may be applied to account for the biological effect of the high linear energy transfer (LET) radiation with a value of 5 recommended by the US Department of Energy.27 For stochastic effects the International Commission on Radiological Protection (ICRP) recommends a radiation weighting factor of 20. Absorbed doses quoted in this review are for a RBE of 1, unless stated otherwise.
Absorbed doses were calculated according to the ICRP model for radium by Lassmann et al28 using the DOSEAGE software. The ICRP biokinetic model29,30 considers that 25% of the administered radium localizes in the bone, with preferential uptake in osteoblastic activity. This can offer both an analgesic effect and potentially a degree of tumour control. Daughter products are also taken into account. The bone endosteum was calculated to receive the highest absorbed doses at 7.5 × 10−7 Gy Bq−1 for alphas and 1.1 × 10−8 Gy Bq−1 for betas/gammas, with the red marrow receiving 7.2 × 10−8 Gy Bq−1 for alphas and 5.5 × 10−9 Gy Bq−1 for betas/gammas. The dose coefficients were also presented for radiation weighting factors of 5 and 20.
In a Phase-1 pharmacokinetic and biodistribution study, Carrasquillo et al31 performed an activity escalation study at 50, 100 and 200 kBq kg−1 of 223Ra in 10 patients. Rapid clearance of the 223Ra from the blood was found, with faecal excretion as the major route of elimination. Urinary excretion was minor. Few side effects were observed.
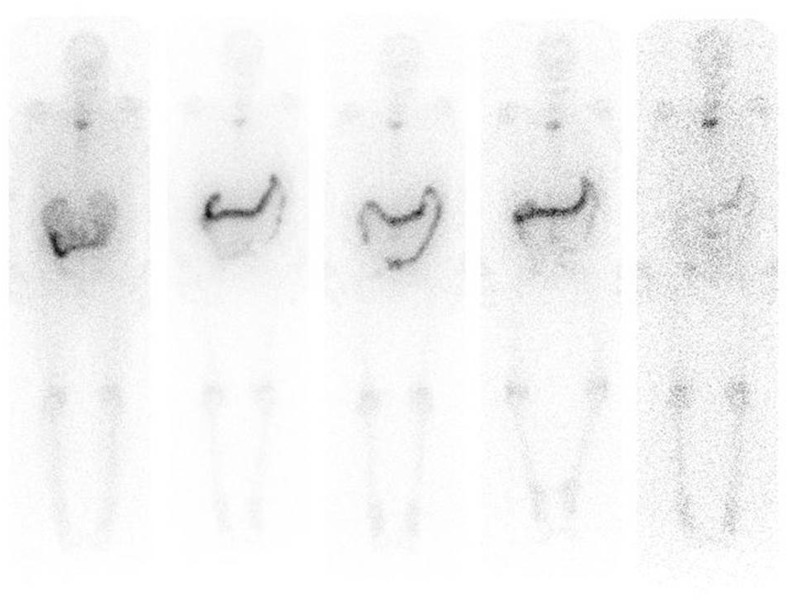
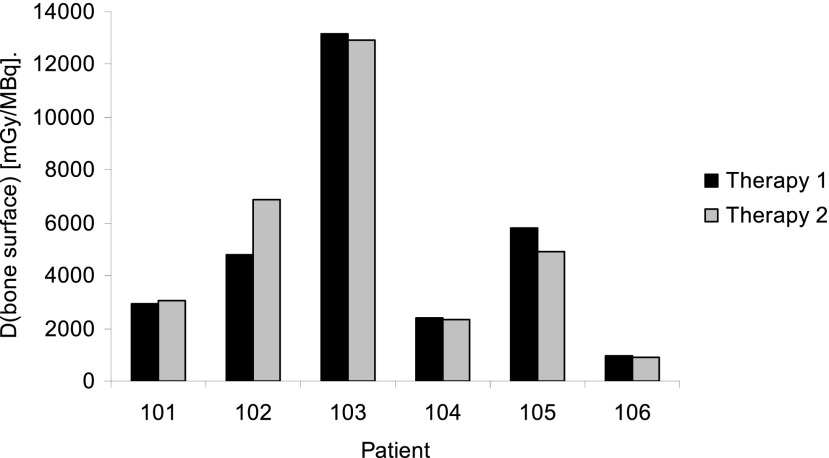
The biodistribution, pharmacokinetics and dosimetry of 223Ra were further studied in a Phase-I trial of 223Ra administered at 100 kBq kg−1 to six patients treated twice, 6 weeks apart.32 Dosimetry was evaluated from image data obtained according to the criteria determined by Hindorf et al17 from external whole-body counting,20 from blood sampling and from faecal and urinary excretion data, using the OLINDA/EXM software.33 The study confirmed that activity was quickly cleared from the blood and that the main route of excretion was via the gut (Figure 3). As predicted by the ICRP modelling study,28 the bone surfaces were observed to receive the greatest absorbed dose. Of particular note was that the range of absorbed doses delivered to bone surfaces was extremely large, ranging from 2.3 to 13.1 Gy MBq−1 from alpha emissions and 9–51 mGy MBq−1 from beta/gamma emissions. However, patients exhibited similar biodistribution and pharmacokinetic profiles for both 223Ra administrations (Figure 4). A lack of either severe gastrointestinal or severe myelotoxicity was assumed to be due to the very short path length of the alpha particles that do not uniformly irradiate the intestinal walls from the gut contents or the marrow from bone surfaces.
Figure 3.
Anterior scans of radium-223 at days 0, 1, 2, 3 and 6 following administration of 55 kBq/kg.
Figure 4.
Absorbed doses delivered to bone surfaces from six patients treated twice with 55 kBq kg−1 radium-223 with a 6-week interval.
A Phase-1 study was performed in Japan according to similar image acquisition parameters, as for the study by Chittenden et al34 for six patients who were administered a single injection of either 50 or 100 kBq kg−1. The absorbed doses delivered to osteogenic cells were found to be lower than that for the Chittenden et al study, at a mean of 0.76 Gy MBq−1. This may be due to the fact that two patients were considered as outliers due to the number or size of metastatic burden, although with the low numbers of patients recruited in each study, the values can only give an indication.
Pacilio et al35 performed tumour dosimetry on 24 lesions in a cohort of 9 patients. Patients were given six administrations of 50 kBq kg−1 at 4-week intervals. Tumours received absorbed doses ranging from 0.2 to 1.9 Gy. Whole-body planar imaging was performed for up to 24 days post-administration according to the recommendations of Hindorf et al,17 as outlined above. The potential benefit of incorporating data acquired from technetium-99m (99mTc) methylene diphosphonate (99mTc-MDP) bone scans was explored to facilitate delineation of target volumes following co-registration of the bone scans with the radium scans. A correlation between uptake on both imaging modalities was seen, indicating that the bone scans could provide an indication of 223Ra uptake. A Monte Carlo study with SIMIND36 and experimental measurements found good agreement with Hindorf for the spatial resolution. A mean absorbed dose of 0.7 Gy (range 0.2–1.9 Gy) was delivered to lesions. Notably, this trial included scans at later time points than those in the previous studies. The low count rate of 223Ra, in combination with the relatively long half-life, necessitates optimization of scan time points.
The absorbed doses delivered to bone surfaces from the most common radiotherapeutics for typical administrations are listed in Table 2.
Table 2.
The mean absorbed doses delivered to the bone surface and red marrow from commonly used radionuclides for typical administrations
| Target Volume | Total absorbed dose (Gy) |
||||
|---|---|---|---|---|---|
| 89Sra | 153Smb | 186Rec | 223Ra (ICRP)d | 223Ra (measured)e | |
| Bone surface | 2.6 | 17.6 | 1.8 | 17.3 | 54–303 |
| Red marrow | 1.7 | 3.9 | 1.7 | 1.7 | 4–23 |
223Ra, radium-223; 186Re, rhenium-186; 153Sm, samarium-153; 89Sr, strontium-89.
Values are based on administration levels in Lassmann and Nosske,28 Chittenden et al32 and Bodei et al.70
Fixed activity of 150 MBq.
Administered activity of 37 MBq kg−1, based on a 70-kg male.
Administered activity 1295 MBq.
Six administrations of 55 kBq kg−1, based on a 70-kg male.
Six administrations of 55 kBq kg−1, based on a 70-kg male.
EFFECTIVENESS
Pre-clinical studies found anti-tumour activity in rats.37 A Phase-1A study of single activity administrations ranging from 46 to 250 kBq kg−1 in patients with bone metastases from both prostate and breast cancer found pain relief in >50% of patients and a decline in alkaline phosphatase, although whether this correlated with the higher activity administrations was not reported.6 The Alpharadin in Symptomatic Prostate Cancer (ALSYMPCA) trial demonstrated an increased survival of 3.6 months, with minimal toxicity.9 Unfortunately, as no dosimetry was performed for these patients, there is as yet no evidence for correlations of absorbed dose with outcome.
TOXICITY AND RISKS
Considerations of marrow toxicity are complicated. The high LET of alpha particles and short path length (approximately 80 µm) induces a high cell kill in adjacent cells, but spares normal tissues beyond.38,39 Uptake of 223Ra on the bone surfaces will therefore not irradiate the marrow cavities uniformly. The largest uniform contribution to the absorbed dose delivered to the red marrow will be from the distribution of the radiopharmaceutical in blood following administration. This may account for a lack of expected toxicity. However, it has been observed that there is a spatial gradient of haematopoietic stem and progenitor cells with a larger concentration closer to the bone24 so that the radiosensitive cells of interest may receive higher absorbed doses. Uptake and marrow distribution will vary widely from patient to patient.
In a clinical Phase-1 trial, a single administration of up to 250 kBq kg−1 was given to 25 patients. Only grade 1 toxicity for thrombocytopenia was observed.6 In the Japanese study, thrombocytopenia was reported for 20% of EOD4 (“superscan” patients, as defined by intense uptake in the skeleton with little or no activity in the soft tissues) as opposed to 6% of patients receiving a placebo.34
The package insert for Xofigo40 states that 2% of patients administered with 223Ra on the ALSYMPCA trial experienced bone marrow failure (54% of whom required blood transfusions) or ongoing pancytopenia and that there were two deaths due to bone marrow failure. Four percent of patients receiving Xofigo (as opposed to 2% of those given a placebo) permanently discontinued therapy. Thrombocytopenia is cited as “very common” with an incidence of >1 in 10 patients. There has been no testing of the potential concomitant effects of toxicity for patients who subsequently receive chemotherapy.
To date, there have been no systematic studies to evaluate mid- to long-term risks associated with 223Ra due to the expected latency period, although in pre-clinical studies osteosarcomas were found in rats at clinically relevant administered activities.40 The issue of potential secondary malignancies for patients undergoing molecular radiotherapy has not been addressed but may become more relevant as trials promise increased survival. This may become particularly relevant for patients undergoing treatment for bone metastases from breast cancer.41
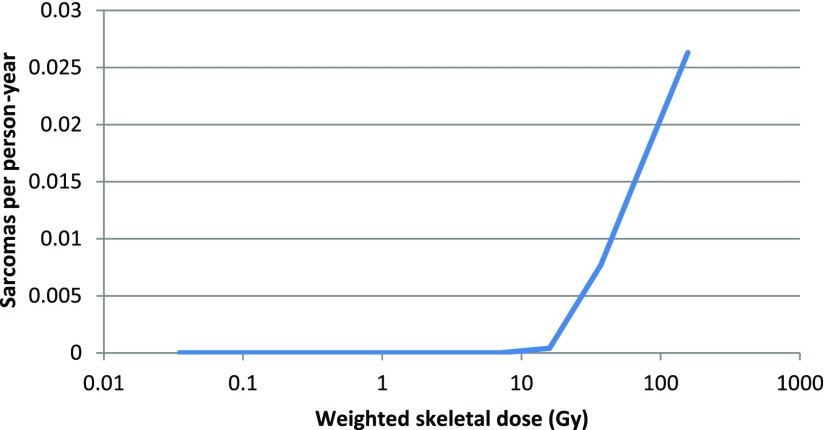
In the absence of long-term outcome data, it is interesting to note cases of toxicity from other radium products that have been used medicinally or for other purposes. Radium-224 was used to treat tuberculosis and ankylosing spondylitis in children and adults after World War II but was found to cause bone sarcomas and severe non-skeletal effects.42–44 The example of the “radium dial painters”, who ingested 226Ra from licking paint brushes whilst painting watch dials, is of particular interest. In this case, a retrospective analysis found a threshold skeletal absorbed dose of 10 Gy to the skeleton for the induction of bone sarcomas from 226Ra45 (Figure 5). In a study of 1634 dial workers, there were no cases of sarcoma below this threshold, whereas sarcomas were induced in 64 of the 264 cases who received >10 Gy. Although the challenges of retrospective dosimetry in a population, for whom dosimetry was not performed, render a degree of uncertainty on the actual value of the dose threshold, it is of note that this value is of the order of that seen in the clinical studies reported above.
Figure 5.
Incidence of bone sarcomas as a function of the absorbed dose to the skeleton. Adapted from Rowland.45
TREATMENT PLANNING
Treatment planning for molecular radiotherapy must operate with a different set of parameters than those used for external beam radiotherapy (EBRT). For a given radiotherapeutic, these parameters comprise the level and frequency of administrations. The prediction of an absorbed dose delivered from a first administration requires a study with either a low level administration of the radiotherapeutic or a surrogate tracer.
Adaptive treatment planning could consist of an initial nominal administration of 223Ra (currently 55 kBq kg−1), with dosimetry obtained from a series of quantitative scans acquired over a suitable time period, accounting for effective decay, to determine the biodistribution and retention. Subsequent administrations would be tailored to the individual patient, taking into account the absorbed doses delivered to healthy organs, particularly the gut, red marrow and bone endosteum. Blood sampling could also provide useful dosimetric information.
Murray et al46 demonstrated that baseline standardized uptake value (SUV) from a fluorine-18-fluoride scan correlated with 223Ra uptake and with the absorbed doses subsequently delivered. Response, as measured by a decrease in SUV, was seen as a function of the baseline SUV. Chittenden et al32 found a correlation between the absorbed doses delivered to the normal organs of the same patient, including bone surfaces, from two consecutive administrations of 223Ra despite a wide interpatient variation, indicating that an adaptive planning strategy may be investigated.
The rationale for repeated treatments is worthy of investigation. In EBRT, fractionation of treatment is based on differential sparing in late responding normal tissues relative to tumours. However, high LET radiation is not expected to exhibit this effect and in vitro experiments have shown that in human kidney cell lines, there is no tissue-sparing effect from administration of activity in two or three fractions.19,47 Nevertheless, marrow recovery benefits from an interval between treatments and continued administrations may prolong palliative effects.
A single study has investigated the reproducibility of imaging and dosimetry in up to six sequential treatments for four lesions in two patients using planar whole-body scans with three energy windows centred at the peaks identified above.48 In one patient, the uptake was imaged at between 30.0 and 34.5 counts/pixel/hour over the six cycles in one lesion and from 27.8 to 36.5 counts/pixel/hour in the second lesion. The half-life in the first lesion varied from 1.8 to 3.6 days and from 3.0 to 4.5 days in the second lesion. Variations in effective half-life resulted in a two-fold difference in the absorbed dose delivered to the different lesions, and a two-fold difference in the absorbed dose delivered to the same lesion over six cycles. A similar result was seen for the second patient. The authors conclude that owing to these variations in biokinetics and dosimetry of different patients and of different lesions, individualized treatment planning using dosimetry is necessary. This may be aided by correlations between uptake of 99mTc-MDP and 223Ra,35 as has also been seen for 153Sm EDTMP and 186Re etidronate.26,49,50 99mTc DMSA uptake has also been shown to correlate with that of 188Re DMSA.51
COSTS
Inevitably, a cost resource would be associated with the routine introduction of imaging and dosimetry. However, the cost of 223Ra, as for other emerging commercial radiotherapeutics, is in line with conventional cancer drugs and significantly greater than that for 153Sm or 89Sr for a full course of treatment. The cost of extra scans for dosimetry is therefore relatively low.
It follows that imaging and dosimetry calculations would be cost effective in the short term if unnecessary treatments could be identified and prevented, beyond that possible with diagnostic 99mTc-MDP or fluorine-18-fluoride imaging. This would enable alternative treatment strategies to be identified. Of particular relevance for this treatment is that the treatment course of 6 months is longer than that for many radiotherapeutics. As yet, there have been no studies to report the percentage of courses of 223Ra that are completed. The cost benefit would also be seen in the longer term if it were possible to deliver effective treatments in a shorter time frame that would mitigate further treatments. This hypothesis could also be tested in clinical trials. The cost of 223Ra relative to more established radiopharmaceuticals has a significant impact on the justification of resources for dosimetry although the health economics of radiopharmaceutical treatments, imaging and dosimetry have yet to be evaluated. Costs for imaging and dosimetry should therefore be considered in relation to patient benefit, the overall cost of the treatment and in relation to corresponding costs incurred for treatment planning for EBRT.
DISCUSSION
The challenges of dosimetry
The nature of alpha irradiation in a clinical context is not clearly understood. It has been noted that such irradiation from internal sources lies at an extreme from uniform irradiation from gamma rays52 and raises conceptual and practical challenges for dosimetry. Of particular relevance is the short range high LET that can necessitate the use of a relative biological weighting factor.
There are a number of complications and confounding factors that impede the accuracy with which dosimetry may be performed. These include the low gamma yield that produces poor qualitative information in comparison with conventional bone-seeking diagnostic agents, although it may be argued that for well-defined volumes of uptake, the reproducibility of quantitative information, as shown by phantom measurements and by the consistency of sequential measurements, can inform an individualized approach to treatment. The non-uniform cellular distribution of target cells and of 223Ra uptake and irradiation complicates interpretation of the macroscopically averaged absorbed dose in terms of the biological effect, and similarly, there are as yet no definitive evidence-based values for the RBE applicable in the context of therapy. This will only become apparent in time following investigative clinical trials and collection of absorbed dose–effect data. Nevertheless, preliminary evidence suggests that this is an area that is worthy of investigation, with a view to improving the palliative and therapeutic use of 223Ra, possibly in conjunction with other radiotherapeutics and “cold chemotherapeutics”.
The role of mean dosimetry can be questioned for alpha emitters, due to the high LET, culminating in the Bragg peak, and the short range that will result in a very localized deposition of energy.53 Nevertheless, macroscopic mean dosimetry is feasible in a clinical setting with patient-specific data, whereas microscopic considerations are necessarily limited to models with limited applicability to a given patient. The role of dosimetry is yet to be determined.54
Uncertainties
The challenges faced with quantitative imaging and dosimetry for 223Ra, although more pronounced, are not exclusive to alpha emitters. The most widely used radiopharmaceutical in molecular radiotherapy, iodine-131, also presents significant challenges due to the high-energy gamma emissions and the high activities administered. This incurs camera deadtime,55 which is not applicable to 223Ra. The various issues will undoubtedly continue as long as gamma cameras are designed exclusively for low-activity imaging of 99mTc.56 Similarly, although the heterogeneous absorbed dose distribution at a microscopic scale is critical to the relevance of alpha emitters,57–59 this characteristic is also highly relevant for beta emitters, that can have a mean path length of far <1 mm.
There are a number of sources of uncertainty inherent in the calculations of absorbed doses. The uncertainty regarding this value may make direct comparisons with other radionuclides challenging, although as a systematic factor it does not impede treatment planning. Similarly, the absorbed dose calculated for the endosteal layer is dependent on the thickness of this layer, as the absorbed dose delivered is inversely proportional to the mass. This has been quoted as between 10 and 50 µm.19 Again, this introduces only a systematic error that does not prevent treatment planning. The increasing use of 223Ra, with standardized administrations, offers the possibility to recruit a large patient cohort.
Harmonization of imaging, dosimetry and reporting
In addition to the need for harmonization of performing and reporting imaging and dosimetry,60 there is also a requirement for standardization of trial methodologies, reporting of trial outcomes and response criteria. Although using similar methodology, lower absorbed doses were reported for the Yoshida et al study34 than for the Chittenden et al study,32 possibly due to exclusion of the outliers in the former case that exhibited large tumour burdens. However, such “superscans”, expected in 10% of patients,8 do not constitute a contraindication for treatment.
Although there are no defined criteria by which to measure response,61 imaging of 223Ra offers the potential to evaluate response predictively according to tumour burden as has been demonstrated for 186Re HEDP62 and for whole-body diffusion-weighted MRI.63,64
Future prospects
The role of 223Ra as part of a multimodality approach to the patient pathway has yet to be investigated thoroughly. Alpha therapy is possibly best used as an adjunct to chemotherapy due to its strength at targeting microscopic deposits. The radiobiological considerations of alpha emitters offers the potential for concomitant administrations of complementary beta-emitting radiotherapeutics such as 188Re HEDP, 89Sr chloride, 153Sm EDTMP or lutetium-177 prostate-specific membrane antigen for which dosimetry is feasible.3,65–68
Treatment strategy is primarily pain relief, and although this mechanism is poorly understood,4 it can be linked to survival. 89Sr chloride, 153Sm EDTMP, 186Re HEDP and 188Re HEDP have similarly all been shown to have palliative effects, although no survival studies at the scale of the ALSYMPCA trial have been performed. The aim of treatment calls into question the treatment regimen itself. If pain palliation is the primary aim, it may be beneficial to administer lower levels of activity over a prolonged period. If an anti-tumour effect is intended, higher activities would be given, taking normal tissue toxicity into account.
SUMMARY
Dosimetry is increasingly used for all forms of treatment with radiotherapeutics that deliver radiation treatment. In the case of external-beam radiotherapy or brachytherapy, a lack of dosimetry-based personalized treatment planning and verification would be considered unsafe practice. Patients undergoing radiopharmaceutical treatment for bone metastases may receive higher absorbed doses to bone surfaces or marrow where the uptake is high, and those patients with a favourable prognosis may be exposed to unwarranted long-term risks.
There is now a pressing need for larger multicentre trials to investigate the dosimetry and to optimize treatment regimens. There is as yet little evidence for the absorbed doses delivered to metastatic deposits throughout the full course of six administrations or that the absorbed doses delivered to organs at risk over six administrations remain the same as those measured from one or two administrations.
223Ra is at the forefront of the resurgence of radiopharmaceuticals for cancer treatment.69 The potential for patient benefit as well as for adverse effects and the substantially increased costs relative to more established agents accentuates the need to ensure maximum effectiveness and cost benefit of clinical implementation. It is likely that the application of imaging and dosimetry to facilitate personalized treatment planning will help to ensure successful clinical and commercial results that will have a strong bearing on the continued development of other radiotherapeutics.
Footnotes
FUNDING: The authors acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden Hospital and the Institute of Cancer Research. Research funding was provided by Bayer Healthcare Pharmaceuticals and Algeta ASA.
REFERENCES
- 1. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence#heading-Five.
- 2. Available from: http://www.nhs.uk/Conditions/Cancer-of-the-prostate/Pages/Treatment.aspx.
- 3.Liepe K, Kotzerke J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl Med Commun 2007; 28: 623–30. doi: https://doi.org/10.1097/mnm.0b013e32825a6adc [DOI] [PubMed] [Google Scholar]
- 4.Rubini G, Nicoletti A, Rubini D, Asabella AN. Radiometabolic treatment of bone-metastasizing cancer: from 186rhenium to 223radium. Cancer Biother Radiopharm 2014; 29: 1–11. doi: https://doi.org/10.1089/cbr.2013.1549 [DOI] [PubMed] [Google Scholar]
- 5.Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007; 8: 587–94. doi: https://doi.org/10.1016/s1470-2045(07)70147-x [DOI] [PubMed] [Google Scholar]
- 6.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005; 11: 4451–9. doi: https://doi.org/10.1158/1078-0432.ccr-04-2244 [DOI] [PubMed] [Google Scholar]
- 7.Nilsson S, Strang P, Aksnes AK, Franzen L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012; 48: 678–86. doi: https://doi.org/10.1016/j.ejca.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 8.Parker CC, Pascoe S, Chodacki A, O'Sullivan JM, Germa JR, O'Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 2013; 63: 189–97. doi: https://doi.org/10.1016/j.eururo.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. doi: https://doi.org/10.1056/nejmoa1213755 [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency. Radium 223 Xofigo (223 Ra dichloride) summary of product characteristics. [updated 10 February 2015; accessed 9 April 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002653/WC500156172.pdf. 2015.
- 11.The British Institute of Radiology Molecular Radiotherapy Working Party. BIR report 23. molecular radiotherapy in the UK: current status and recommendations for further investigation. London, UK: The British Institute of Radiology; 2011. [Google Scholar]
- 12.Rojas B, Hooker C, McGowan DR, Guy MJ. Five years of molecular radiotherapy growth in the UK: survey results from 2007 to 2012. Nucl Med Commun 2015; 36: 761–5. doi: https://doi.org/10.1097/mnm.0000000000000306 [DOI] [PubMed] [Google Scholar]
- 13.Rojas B, Hooker C, McGowan DR, Guy MJ, Tipping J. Eight years of growth and change in UK molecular radiotherapy with implications for the future: Internal Dosimetry Users Group survey results from 2007 to 2015 Nucl Med Commun 2017; 38: 201–204. doi: 10.1097/MNM.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 14.Strigari L, Konijnenberg M, Chiesa C, Bardies M, Du Y, Gleisner KS, et al. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur J Nucl Med Mol Imaging 2014; 41: 1976–88. doi: https://doi.org/10.1007/s00259-014-2824-5 [DOI] [PubMed] [Google Scholar]
- 15.European Council Directive 2013/59/Euratom on basic safety standards for protection against the dangers arising from exposure to ionising radiation and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Off J Eur Union 2014; 57: 1–73. [Google Scholar]
- 16.National Cancer Research Institute (NCRI). CTRad: identifying opportunities to promote progress in molecular radiotherapy research in the UK 2016. Available from: https://www.ncri.org.uk/wp-content/uploads/2016/06/CTRad-promoting-research-in-MRT-UK-June-2016.pdf. [Google Scholar]
- 17.Hindorf C, Chittenden S, Aksnes AK, Parker C, Flux GD. Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl Med Commun 2012; 33: 726–32. doi: https://doi.org/10.1097/mnm.0b013e328353bb6e [DOI] [PubMed] [Google Scholar]
- 18.Hough M, Johnson P, Rajon D, Jokisch D, Lee C, Bolch W. An image-based skeletal dosimetry model for the ICRP reference adult male–internal electron sources. Phys Med Biol 2011; 56: 2309–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med 2010; 51: 311–28. doi: https://doi.org/10.2967/jnumed.108.058651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chittenden SJ, Pratt BE, Pomeroy K, Black P, Long C, Smith N, et al. Optimization of equipment and methodology for whole body activity retention measurements in children undergoing targeted radionuclide therapy. Cancer Biother Radiopharm 2007; 22: 243–9. doi: https://doi.org/10.1089/cbr.2006.315 [DOI] [PubMed] [Google Scholar]
- 21.Hindorf C, Glatting G, Chiesa C, Linden O, Flux G, Committee ED. EANM dosimetry committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging 2010; 37: 1238–50. doi: https://doi.org/10.1007/s00259-010-1422-4 [DOI] [PubMed] [Google Scholar]
- 22.Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys 2003; 85: 294–310. doi: https://doi.org/10.1097/00004032-200309000-00006 [DOI] [PubMed] [Google Scholar]
- 23.Watchman CJ, Bolch WE. Absorbed fractions for alpha-particles in tissues of cortical bone. Phys Med Biol 2009; 54: 6009–27. doi: https://doi.org/10.1088/0031-9155/54/19/023 [DOI] [PubMed] [Google Scholar]
- 24.Watchman CJ, Bourke VA, Lyon JR, Knowlton AE, Butler SL, Grier DD, et al. Spatial distribution of blood vessels and CD34+ hematopoietic stem and progenitor cells within the marrow cavities of human cancellous bone. J Nucl Med 2007; 48: 645–54. doi: https://doi.org/10.2967/jnumed.106.035337 [DOI] [PubMed] [Google Scholar]
- 25.Hamacher KA, Sgouros G. A schema for estimating absorbed dose to organs following the administration of radionuclides with multiple unstable daughters: a matrix approach. Med Phys 1999; 26: 2526–8. doi: https://doi.org/10.1118/1.598788 [DOI] [PubMed] [Google Scholar]
- 26.Pacilio M, Ventroni G, Basile C, Ialongo P, Becci D, Mango L. Improving the dose-myelotoxicity correlation in radiometabolic therapy of bone metastases with 153Sm-EDTMP. Eur J Nucl Med Mol Imaging 2014; 41: 238–52. doi: https://doi.org/10.1007/s00259-013-2552-2 [DOI] [PubMed] [Google Scholar]
- 27.Feinendegen LE. Meeting report: alpha-emitters for medical therapy—workshop of the United States Department of Energy, Denver, Colorado. Radiat Res 1997; 148: 195–201. [Google Scholar]
- 28.Lassmann M, Nosske D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging 2013; 40: 207–12. doi: https://doi.org/10.1007/s00259-012-2265-y [DOI] [PubMed] [Google Scholar]
- 29.ICRP. Publication 67. Age-dependent doses to members of the public from intake of radionuclides: part 2 ingestion dose coefficients. Ann ICRP 1992; 22: 1–67. [PubMed] [Google Scholar]
- 30.ICRP. Publication 103. The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP 2007; 2007: 37. [DOI] [PubMed] [Google Scholar]
- 31.Carrasquillo JA, O'Donoghue JA, Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, et al. Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2013; 40: 1384–93. doi: https://doi.org/10.1007/s00259-013-2427-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chittenden SJ, Hindorf C, Parker CC, Lewington VJ, Pratt BE, Johnson B, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra-dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med 2015; 56: 1304–9. doi: https://doi.org/10.2967/jnumed.115.157123 [DOI] [PubMed] [Google Scholar]
- 33.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005; 46: 1023–7. [PubMed] [Google Scholar]
- 34.Yoshida K, Kaneta T, Takano S, Sugiura M, Kawano T, Hino A, et al. Pharmacokinetics of single dose radium-223 dichloride (BAY 88-8223) in Japanese patients with castration-resistant prostate cancer and bone metastases. Ann Nucl Med 2016; 30: 453–60. doi: https://doi.org/10.1007/s12149-016-1093-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacilio M, Ventroni G, Cassano B, Ialongo P, Lorenzon L, Di Castro E, et al. A case report of image-based dosimetry of bone metastases with Alpharadin ((223)Ra-dichloride) therapy: inter-fraction variability of absorbed dose and follow-up. Ann Nucl Med 2016; 30: 163–8. doi: https://doi.org/10.1007/s12149-015-1044-9 [DOI] [PubMed] [Google Scholar]
- 36.Ljungberg M, Strand SE. A Monte Carlo program for the simulation of scintillation camera characteristics. Comput Methods Programs Biomed 1989; 29: 257–72. doi: https://doi.org/10.1016/0169-2607(89)90111-9 [DOI] [PubMed] [Google Scholar]
- 37.Henriksen G, Breistol K, Bruland OS, Fodstad O, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002; 62: 3120–5. [PubMed] [Google Scholar]
- 38.Turner PG, O'Sullivan JM. (223)Ra and other bone-targeting radiopharmaceuticals-the translation of radiation biology into clinical practice. Br J Radiol 2015; 88: 20140752. doi: https://doi.org/10.1259/bjr.20140752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henriksen G, Fisher DR, Roeske JC, Bruland OS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003; 44: 252–9. [PubMed] [Google Scholar]
- 40.Xofigo (radium Ra 223 dichloride) injection [prescribing information]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
- 41.Coleman R, Aksnes AK, Naume B, Garcia C, Jerusalem G, Piccart M, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat 2014; 145: 411–18. doi: https://doi.org/10.1007/s10549-014-2939-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiess H. Late effects of radium-224 injected in children and adults. In: Proceedings of the 9th International Conference on Health Effects of Incorporated Radionuclides Emphasis on Radium, Thorium, Uranium and their Daughter Products. Neuherberg, Germany; 2004.
- 43.Nekolla EA, Walsh L, Schottenhammer G, Spiess H. Malignancies in patients treated with high doses of 224Ra. In: Proceedings of the 9th International Conference on Health Effects of Incorporated Radionuclides Emphasis on Radium, Thorium, Uranium and their Daughter Products. Neuherberg, Germany; 2004.
- 44.Wick RR, Nekolla EA, Gössner W. Long term investigation of late effects in ankylosing spondylitis patients treated with 224Ra In: Proceedings of the 9th International Conference on Health Effects of Incorporated Radionuclides Emphasis on Radium, Thorium, Uranium and their Daughter Products. Neuherberg, Germany; 2004.
- 45.Rowland RE. Bone sarcoma in humans induced by radium: A threshold response? IARC monographs on the evaluation of carcinogenic risks to humans volume 78 ionizing radiation, part 2: some internally deposited radionuclides IARC press, Lyon, France; 2001. [PMC free article] [PubMed]
- 46.Murray I, Chittenden S, Denis-Bacelar A, Hindorf C, Parker C, Flux G. The potential of 223Ra and 18F-Fluoride imaging to predict bone lesion response to treatment with 223Ra-Dichloride in castration resistant prostate cancer. Eur J Nucl Med Mol Imaging 2011; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barendsen GW. Effects of different ionizing radiations on human cells in tissue culture. IV. Modification of radiation damage. Radiat Res 1964; 21: 314–29. [PubMed] [Google Scholar]
- 48.Kairemo K, Joensuu T, Rasulova N, Kiljunen T, Kangasmaki A. Evaluation of alpha-therapy with Radium-223-dichloride in castration resistant metastatic prostate cancer-the role of gamma scintigraphy in dosimetry and pharmacokinetics. Diagnostics (Basel) 2015; 5: 358–68. doi: https://doi.org/10.3390/diagnostics5030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bianchi L, Baroli A, Marzoli L, Verusio C, Chiesa C, Pozzi L. Prospective dosimetry with 99mTc-MDP in metabolic radiotherapy of bone metastases with 153Sm-EDTMP. Eur J Nucl Med Mol Imaging 2009; 36: 122–9. doi: https://doi.org/10.1007/s00259-008-0926-7 [DOI] [PubMed] [Google Scholar]
- 50.Israel O, Keidar Z, Rubinov R, Iosilevski G, Frenkel A, Kuten A, et al. Quantitative bone single-photon emission computed tomography for prediction of pain relief in metastatic bone disease treated with rhenium-186 etidronate. J Clin Oncol 2000; 18: 2747–54. doi: https://doi.org/10.1200/jco.2000.18.14.2747 [DOI] [PubMed] [Google Scholar]
- 51.Blower PJ, Kettle AG, O'Doherty MJ, Coakley AJ, Knapp FF Jr. (99m)Tc(V)DMSA quantitatively predicts (188)Re(V)DMSA distribution in patients with prostate cancer metastatic to bone. Eur J Nucl Med 2000; 27: 1405–9. [DOI] [PubMed] [Google Scholar]
- 52.Goodhead D. Status and problems of risk assessment for incorporated alpha-emitters. In: Edoeh U, Roth P, Paretzke HG. Proceedings of the 9th International Conference on Health Effects of Incorporated Radionuclides Emphasis on Radium, Thorium, Uranium and their Daughter Products; 2004.
- 53.Elgqvist J, Frost S, Pouget JP, Albertsson P. The potential and hurdles of targeted alpha therapy—clinical trials and beyond. Front Oncol 2014; 3: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lassmann M. Therapeutic use of alpha-emitters. In: Edoeh U, Roth P, Paretzke HG. Proceedings of the 9th International Conference on Health Effects of Incorporated Radionuclides Emphasis on Radium, Thorium, Uranium and their Daughter Products; 2004.
- 55.Guy MJ, Flux GD, Flower MA, Ott RJ, Papavasileiou P, Chittenden SJ. Practical scatter-independent gamma camera dead-time correction for iodine-131. Nuclear Science Symposium Conference Record, IEEE; 2000.
- 56.Bombardieri E, Aktolun C, Baum RP, Bishof-Delaloye A, Buscombe J, Chatal J, et al. Bone scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 2003; 30: 1. [DOI] [PubMed] [Google Scholar]
- 57.Akabani G, Kennel SJ, Zalutsky MR. Microdosimetric analysis of alpha-particle-emitting targeted radiotherapeutics using histological images. J Nucl Med 2003; 44: 792–805. [PubMed] [Google Scholar]
- 58.Humm JL, Roeske JC, Fisher DR, Chen GTY. Microdosimetric concepts in radioimmunotherapy. Med Phys 1993; 20: 535–41. doi: https://doi.org/10.1118/1.597049 [DOI] [PubMed] [Google Scholar]
- 59.Larsson E, Jonsson BA, Jonsson L, Ljungberg M, Strand SE. Dosimetry calculations on a tissue level by using the MCNP4c2 Monte Carlo code. Cancer Biother Radiopharm 2005; 20: 85–91. doi: https://doi.org/10.1089/cbr.2005.20.85 [DOI] [PubMed] [Google Scholar]
- 60.Lassmann M, Chiesa C, Flux G, Bardies M, Committee ED. EANM dosimetry committee guidance document: good practice of clinical dosimetry reporting. Eur J Nucl Med Mol Imaging 2011; 38: 192–200. doi: https://doi.org/10.1007/s00259-010-1549-3 [DOI] [PubMed] [Google Scholar]
- 61.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol 2004; 22: 2942–53. doi: https://doi.org/10.1200/jco.2004.08.181 [DOI] [PubMed] [Google Scholar]
- 62.Denis-Bacelar AM, Dearnaley DP, Divoli A, O'Sullivan JM, McCready VR, Johnson B, et al. Phase I/II trials of 186Re-HEDP in metastatic castration-resistant prostate cancer: post-hoc analysis of the impact of administered activity and dosimetry on survival. Eur J Nucl Med Mol Imaging 2016; 44: 620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blackledge MD, Collins DJ, Tunariu N, Orton MR, Padhani AR, Leach MO, et al. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: a feasibility study. PLoS One 2014; 9: e91779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Lopez R, Lorente D, Blackledge MD, Collins DJ, Mateo J, Bianchini D, et al. Volume of bone metastasis assessed with whole-body diffusion-weighted imaging is associated with overall survival in metastatic castration-resistant prostate cancer. Radiology 2016; 280: 151–60. [DOI] [PubMed] [Google Scholar]
- 65.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med 2016; 57: 1006–13. doi: https://doi.org/10.2967/jnumed.115.168443 [DOI] [PubMed] [Google Scholar]
- 66.Blake GM, Gray JM, Zivanovic MA, McEwan AJ, Fleming JS, Ackery DM. Strontium-89 radionuclide therapy: a dosimetric study using impulse response function analysis. Br J Radiol 1987; 60: 685–92. doi: https://doi.org/10.1259/0007-1285-60-715-685 [DOI] [PubMed] [Google Scholar]
- 67.Kwekkeboom D. Perspective on 177Lu-PSMA therapy for metastatic castration-resistant prostate cancer. J Nucl Med 2016; 57: 1002–3. doi: https://doi.org/10.2967/jnumed.115.171363 [DOI] [PubMed] [Google Scholar]
- 68.Pacilio M, Ventroni G, De Vincentis G, Cassano B, Pellegrini R, Di Castro E, et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting Ra-dichloride. Eur J Nucl Med Mol Imaging 2016; 43: 21–33. [DOI] [PubMed] [Google Scholar]
- 69. Thomson Reuter spotlight on radiotherapeutics: a pharma matters report. Available from: http://thomsonreuters.com/en/articles/2013/spotlight-on-radiotherapeutics.html.
- 70.Bodei L, Lam M, Chiesa C, Flux G, Brans B, Chiti A, et al. EANM procedure guideline for treatment of refractory metastatic bone pain. Eur J Nucl Med Mol Imaging 2008; 35: 1934–40. doi: https://doi.org/10.1007/s00259-008-0841-y [DOI] [PubMed] [Google Scholar]