Abstract
Objective:
Diffuse pleural thickening (DPT) refers to extensive visceral pleural fibrosis with adhesion formation to the parietal pleura obliterating the pleural space. The radiological definition of DPT remains controversial with most of the literature requiring the presence of an obliterated costophrenic angle (CPA) for defining DPT. We conducted a study to investigate the variable distributions of DPT and associated lung function deficit.
Methods:
85 patients referred to a pleural clinic with suspected pleural thickening were screened for our study. Data were collected from 37 patients with DPT confirmed on CT by size criteria (≥3 mm thick, ≥5 cm wide and ≥8 cm in length), and 21 controls with pleural plaques but no other pleuroparenchymal pathology. 27 patients were excluded. Groups were matched to age, body mass index and smoking history.
Results:
The percentage of predicted forced vital capacity showed a gradual decline from 98.9% for the control group to 83.5% in the DPT without CPA obliteration group (p < 0.05), to 79.5% in the unilateral DPT group (p < 0.001) and 66.7% in the bilateral group (p < 0.001). Similar reductions were seen in the percentage of predicted total lung capacity in the DPT with no CPA obliteration group and the bilateral DPT group.
Conclusion:
Our study shows an incremental reduction in the forced vital capacity and total lung capacity in DPT without CPA obliteration, unilateral and bilateral DPT when compared with a matched control group.
Advances in knowledge:
Different distributions of DPT including no CPA obliteration can cause respiratory impairment, with bilateral DPT being the worst affected.
INTRODUCTION
Diffuse pleural thickening (DPT) refers to extensive fibrosis of the visceral pleura with frequent adhesions to the parietal pleura, leading to obliteration of the pleural space.1 It is usually the sequel to an exudative effusion and develops in approximately 7% of those with historical asbestos exposure.2,3
Unfortunately, there is still no universally accepted radiological definition for DPT. The International Labour Organization (ILO)4 bases their definition on obliteration of the costophrenic angle (CPA) by pleural thickening on a postero-anterior chest radiograph, and this definiton has been incorporated into the American Thoracic Society guidelines on diagnosis and initial management of non-malignant diseases related to asbestos.5 However, this definition ignores the gold-standard imaging modality for assessing the pleura—CT. Many authorities cite the definition of DPT on CT proposed by Lynch et al based on size criteria as continuous thickening of the pleura measuring ≥5 cm wide, ≥8 cm long craniocaudally and 3 mm in thickness, with or without CPA involvement.6 Regardless of the radiological definition, the important clinical implication of DPT is that it frequently leads to significant respiratory disability1,7,8 and, when related to asbestos exposure, is currently recognized as a compensable disease under the Industrial Injuries Disablement Benefit scheme in the UK.9
Our aim was to evaluate the impact of DPT defined by CT and correlate its distribution, specifically involvement of CPA, with chest radiographic findings and contemporaneous lung function testing.
METHODS AND MATERIALS
Data were collected from patients with suspected pleural thickening referred to a dedicated pleural clinic at a single UK centre, between September 2011 and October 2016. All patients included had DPT by size criteria (≥3 mm depth,≥5 cm axially and ≥8 cm craniocaudally), pleural thickening with tapered margins, pleuroparenchymal bands or rounded atelectasis on CT, and had contemporaneous chest X-ray and lung function. The study was approved by the Southwest Research Ethics Committee.
Data collected included patient demographics, asbestos exposure history including duration and level of exposure, smoking history and other comorbidities. Patients with breathlessness had their degree of breathlessness documented against the Medical Research Council dyspnoea score, where a score of 1 = not troubled by breathlessness except on strenuous exercise; 2= short of breath when hurrying on a level or when walking up a slight hill; 3 =walks slower than most people on the level, stops after a mile or so or stops after 15 min walking at own pace; 4 = stops for breath after walking 100 yards or after a few minutes on level ground; 5 = too breathless to leave the house or breathless when dressing/undressing.
CT scans
The CT scans were performed for clinical reasons using a range of multislice detector CT scanners (GE HD750 FREEdom Edition; GE Optima-General Electric, Boston, MA; Toshiba Aquilion CX; Toshiba, Tokyo, Japan; Philips Ingenuity; Phillips, Eindehoven, Netherlands). Standard departmental protocols were used with volumetric datasets acquired with or without contrast as indicated clinically. All datasets were available with orthogonal isotropic reconstructions at 1–1.25 mm collimation using soft-tissue and lung algorithms.
Lung function tests
All patients included in the study had full lung function tests, which included spirometry, lung volumes (by helium dilution and body plethysmography) and gas transfer (single-breath carbon monoxide diffusing capacity). All patients had their height and weight recorded at the time of their lung function test. Lung function tests within 12 months of the CT scan were used for analysis. Lung function testing was performed using the nSpire Health HDPFT 3000 (plethysmography and fast gas analyser) (nSpire Health Ltd, Hertford, UK) and Collins CPL (helium dilution and gas transfer). . Lung function indices were expressed as a percentage of predicted and standardized residual values. Predicted values were European reference values.
Control group
The control group comprised patients who were referred to the pleural clinic and were found to have asbestos-related discrete pleural plaques only. Patients in this group were matched for age, body mass index (BMI) and smoking status, to the DPT group.
Radiology review
Two experienced chest radiologists (with a collective experience of 34 years) independently reviewed all CT scans and chest radiographs. CT images were reviewed with orthogonal reformatted data and electronic callipers on a soft tissue algorithm using standard mediastinal window settings (40/400). Diffuse pleural thickening was assessed according to Lynch’s size criteria whereby to qualify as DPT the thickening needed to be more than 3 mm in thickness, 5 cm in width on axial CT slices and 8 cm in length craniocaudally. In addition to the size criteria, DPT was characterized morphologically by its tapered margin and ancillary signs of visceral pleural fibrosis, namely the presence of adjacent folded lung or pleuroparenchymal bands.10,11 These additional CT criteria were applied in order to exclude patients with large areas of confluent discrete plaques. The plain chest radiographs or CT scanograms were assessed for CPA obliteration and when present classified as unilateral (side specified) or bilateral.
Statistical analysis
Statistical analyses were carried out using the Stata statistical package, v. 14.2 (StataCorp LLC, College Station, TX). Patient demographics such as age, BMI and smoking history were expressed as means with standard deviations for each DPT group against the control group. Interobserver agreement when assessing chest radiographs and CT imaging was assessed using a weighted kappa coefficient. A p value less than 0.05 was considered statistically significant unless otherwise specified in the text. Distribution for normality was assessed using the Shapiro-Wilks test. Student’s t-test was used when comparing parametric data.
RESULTS
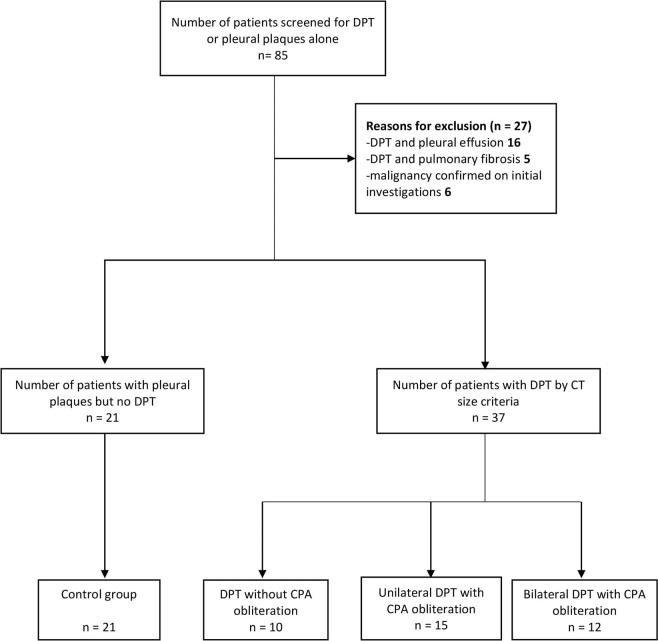
Eighty-five patients who were seen at the pleural clinic with suspected pleural thickening and or pleural plaques on their chest radiographs between September 2011 and October 2016 were included in the initial screening. Patients were excluded if they had significant pleural effusions at the time of the CT (n = 16), coexistent interstitial pulmonary fibrosis (n = 5) or a subsequent diagnosis of pleural malignancy (n = 6). Fifty-eight patients (37 with DPT and 21 with pleural plaques alone) were then evaluated, including full pulmonary function testing (Figure 1).
Figure 1.
Flow diagram of total number of patients screened and reasons for exclusion. CPA, costophrenic angle; DPT, diffuse pleural thickening.
The 37 patients who met DPT by CT size criteria were classified into three separate groups:
Group 1: DPT by CT criteria with no CPA obliteration on chest radiograph
Group 2: DPT by CT criteria with unilateral CPA obliteration on the chest radiograph
Group 3: DPT by CT criteria with bilateral CPA obliteration on the chest radiograph.
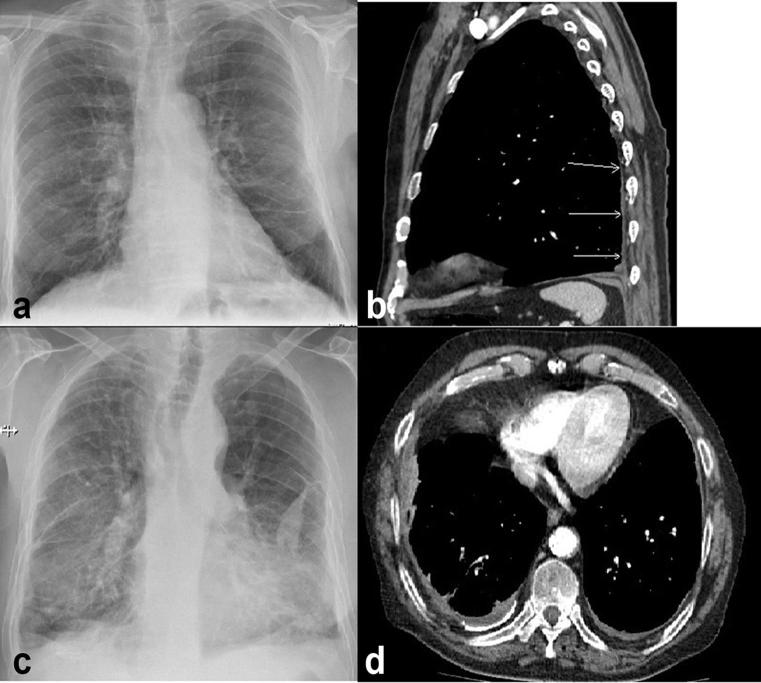
An example chest radiograph and CT of a patient with right-sided DPT but no CPA obliteration and bilateral DPT are shown in Figure 2.
Figure 2.
(a) Postero-anterior chest radiograph of a patient with right-sided diffuse pleural thickening (DPT) with no costophrenic angle obliteration. (b) Sagittal CT view of the same patient showing DPT involving the posterior pleura. (c) Patient with bilateral DPT and costophrenic angle obliteration. (d) Axial CT image confirming bilateral pleural thickening.
Overall, there was good interobserver agreement on radiological findings. There was moderate agreement for CPA obliteration on chest X-ray with a weighted kappa value of 0.79 and good agreement for DPT by size criteria on CT, with a weighted kappa value of 0.82. Discrepancies were resolved by consensus review of images.
A summary demographics table, including the control group (pleural plaques only) is shown in Table 1. Fifty-six of the patients were male (97%), the mean age was 69.6 years (95% CI: 67.3–71.9). The mean BMI was 29 kg m−2 (95% CI: 28–30) and the average pack year history was 22.9 (95% CI: 17.6–28.2). There was no significant difference in the ages, BMI, Medical Research Council grade or pack year history of smoking between the subgroups.
Table 1. Demographics by patient group.
| Demographic | Control group (n = 21) | No CPA obliteration (n = 10) | Unilateral DPT (n = 15) | Bilateral DPT (n = 12) | p-Value |
|---|---|---|---|---|---|
| Age (years)a | 69.4 (65.7–73.1) | 69.5 (64.9–74.0) | 70.2 (65.0–75.5) | 69.0 (62.0–76.1) | 0.99 |
| BMI (kgm−2)a | 28.2 (26.6–29.9) | 30.5 (27.4–33.6) | 28.7 (27.1–30.3) | 29.4 (26.6–32.2) | 0.45 |
| MRC gradea | 3.5 (2.9–4.2) | 3.9 (2.8–5.0) | 3.4 (3.5–4.3) | 4.6 (3.6–5.6) | 0.20 |
| Comorbidities | |||||
| COPD | 4/21 (19%) | 2/10 (20%) | 3/15 (20%) | 3/12 (25%) | |
| CABG | 1/21 (5%) | 1/10 (10%) | 4/15 (27%) | 2/12 (17%) | |
| Other | 3/21 (14%) | 0 | 3/15 (20%) | 1/12 (8%) | |
| Nil | 13/21 (62%) | 8/10 (80%) | 5/15 (33%) | 6/12 (50%) | |
| Smoking history (pyrs)a | 20.5 (9.8–31.2) | 24.2 (11.7–36.7) | 22.4 (12.6–32.2) | 27.0 (13.9–40.0) | 0.85 |
| Current smoker | 2/21 (9%) | 1/10 (10%) | 2/14 (14%) | 4/11 (36%) | |
| Ex-smoker | 13/21 (62%) | 8/10 (80%) | 10/14 (72%) | 5/11 (46%) | |
| Never smoker | 6/21 (29%) | 1/10 (10%) | 2/14 (14%) | 2/11 (18%) | |
BMI,body mass index; CABG, coronary artery bypass graft; CI, confidence interval; COPD,chronic obstructive pulmonary disease; CPA, costophrenic angle; DPT, diffuse pleural thickening; MRC, Medical Research Council; pyrs,pack years.
aValues in parentheses are the 95% CIs.
Most patients (53/58) had clear documentation of the predominant occupation that exposed them to asbestos (Supplementary Table 1; supplementary material available online). The largest proportions exposed were in construction work (12.1%), civil engineering (8.6%) or heating and insulation engineering (8.6%). The miscellaneous group 15/58 (25.9%) included factory workers, a dockworker, ship plater and a chemistry teacher.
Seven (18.9%) patients in the DPT cohort had no pleural plaques on CT. Of these seven patients, two have had a previous coronary artery bypass graft and one had a history of an eosinophilic pleural effusion, which was documented as the cause of their pleural thickening. The other four patients had no pertinent past history such as causative drugs or previous pneumonic illnesses to explain their pleural thickening. These four patients’ occupations were construction work, kitchen fitting, electrical wholesale and labourer (involved in cleaning pipes lagged with asbestos).
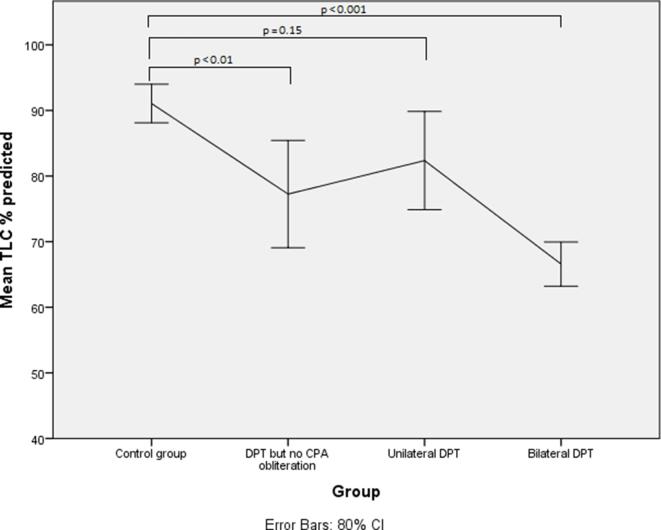
Lung function parameters with their means and standard deviations are shown in Table 2. The percentage of predicted forced vital capacity (FVC) showed a decline from 98.9% in the control group to 83.5% in the DPT with no CPA obliteration group (p = 0.045). Compared with the control group there were significant reductions in the FVC percentage of predicted in the unilateral DPT group 79.5% (p < 0.001) and bilateral DPT group 66.7% (p < 0.001) (Figures 3 and 4) . Similar differences are observed in the percentage of predicted values for total lung capacity (TLC) between the control group (91.1%) and those with DPT without CPA obliteration (77.2%;p < 0.01) and the bilateral DPT group (66.7%;p < 0.001) (Figures 5 and 6). However, this relationship is lost between the control group and the unilateral DPT group.
Table 2. Mean and standard deviations for lung function parameters.
| Parameter | Control group(n = 21) | DPT but no CPA involvement (n = 10) | Unilateral DPT (n = 15) | Bilateral DPT (n = 12) |
|---|---|---|---|---|
| FEV1 (L) | 2.5 ± 0.6 | 2.1 ± 0.5 | 1.9 ± 0.5 | 1.7 ± 0.4 |
| FEV1 % predicted | 86.9 ± 19.3 | 72.0 ± 15.7 | 65.7 ± 17.7 | 57.7 ± 10.0 |
| FVC (L) | 3.8 ± 0.7 | 3.0 ± 0.9 | 3.0 ± 0.6 | 2.5 ± 0.8 |
| FVC % predicted | 98.9 ± 15.2 | 83.5 ± 25.9 | 79.5 ± 13.0 | 66.7 ± 15.6 |
| FEV1/FVC ratio | 68.1 ± 13.1 | 69.9 ± 13.9 | 63.9 ± 14.0 | 64.9 ± 11.8 |
| TLC (L) | 6.2 ± 0.8 | 5.0 ± 0.9 | 5.4 ± 1.3 | 4.4 ± 0.6 |
| TLC % predicted | 91.1 ± 9.4 | 77.2 ± 16.4 | 82.4 ± 20.8 | 66.6 ± 8.6 |
| TLCo % predicted | 80.1 ± 23.3 | 62.8 ± 15.2 | 69.5 ± 17.5 | 61.4 ± 11.9 |
| KCO % predicted | 92.9 ± 24.9 | 96 ± 17.2 | 102.5 ± 22.4 | 104.8 ± 22.0 |
| BMI | 28.2 ± 3.6 | 31 ± 4.4 | 28.9 ± 2.8 | 28.5 ± 4.4 |
BMI, body mass index; CPA, costophrenic angle; DPT, diffuse pleural thickening; FEV1, forced expiratory volume in 1 s; FVC,forced vital capacity; KCO, diffusion coefficient; TLC,total lung capacity; TLCo, gas transfer.
Figure 3.
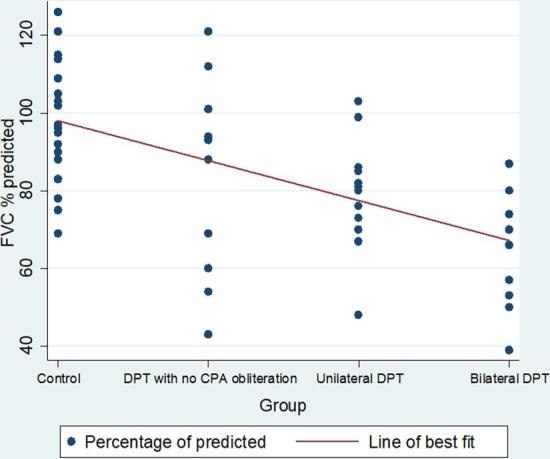
Trend of percentage of predicted FVC by group. CPA, costophrenic angle; DPT, diffuse pleural thickening; FVC, forced vital capacity.
Figure 4.
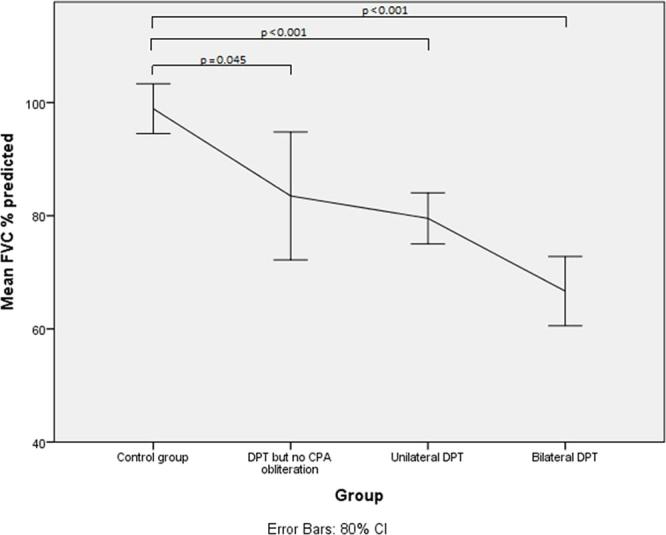
Mean FVC % predicted with confidence intervals
Figure 5.
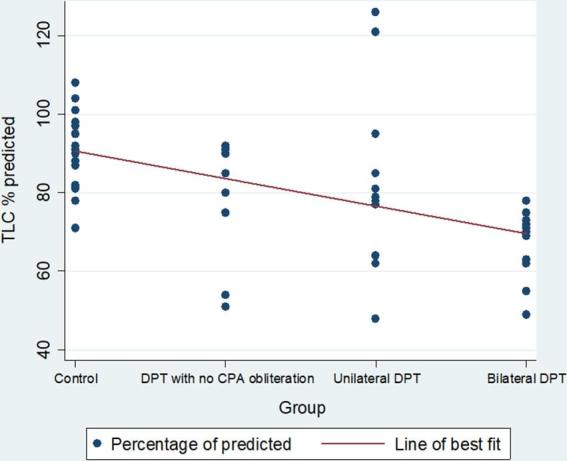
Trend of percentage of predicted TLC by group. CPA, costophrenic angle; DPT, diffuse pleural thickening; TLC, total lung capacity.
Figure 6.
Mean TLC % predicted with confidence intervals
A scatter plot of the individual percentage of predicted FVC and TLC values are shown in Figure 3 and 5.
DISCUSSION
To date, our study is the largest to examine the physiological impact of varied distributions of DPT using CT criteria and includes a significant number of patients in whom there is DPT without radiographic obliteration of the CPA on chest radiograph. Our data highlight the incremental deficit in lung function between bilateral DPT, unilateral DPT and DPT without radiographic obliteration of the CPA compared with a control group with asbestos-related pleural plaques only. We demonstrate a 33.2% reduction in the percentage of predicted FVC between the control group and those in the bilateral DPT group.
A number of previous studies have investigated the respiratory impairment caused by DPT.1,8,12–17 However, the reliance on a chest radiograph to make the initial diagnosis has prevented analysis of individuals in whom there is DPT without obliteration of the CPA. For instance, Singh et al predicate their hypothesis that DPT impacts lung function by involvement of the diaphragm based on a study of seven patients all of whom had CPA obliteration.16 More recently, a study by Ameille et al, designed to evaluate the impact of size-based criteria for DPT vs involvement of the costophrenic recess (n = 287) identified the poor correlation of size criteria on chest radiography with lung function.11 However, assessment of the true extent using chest radiography alone is very problematic and only allows for accurate craniocaudal extent of laterally sited pleural thickening. In this study, the authors acknowledge the superiority of CT over chest radiographs but aimed to provide data applicable to the current guidelines, which favour radiographs.11 The relative merits of CT-based size criteria over chest radiograph measurements is supported in a study by Fujimoto et al14 in which they found that size criteria using CT were predictive of respiratory deficit. Finally, a CT-based study by Copley et al8 demonstrated that pulmonary function deficits are proportional to the extent of DPT (defined in their cohort only as pleural thickening with a tapering margin; 5 out of 50 patients had DPT without obliteration of the CPA). A further shortcoming of the chest radiograph to diagnose DPT is the inability to discriminate between other pathologies that may mimic DPT. In our study, we excluded 16 patients with pleural effusions; six of these patients had small effusions that could easily be interpreted as pleural thickening on the chest radiograph in the absence of contemporaneous CT imaging.
It is unclear how the arbitrary size-based CT definition proposed by Lynch et al was reached;6 however, it remains widely cited in leading reviews and texts.18 In our study, we have refined the definition in order to exclude patients with confluent pleural plaques in two ways. First, the morphology of pleural thickening was assessed. Tapered margins are a feature of DPT compared with the shouldered margins of pleural plaques.8 Second, the presence of folded lung or pleuroparenchymal bands was assessed confirming that there was fibrosis of the visceral pleura rather than thickening of the parietal pleura alone.19
The mechanism of breathlessness in DPT remains controversial. The reduction in the lung function vital capacity has been attributed to the reduced expansion of the lower thoracic rib cage and reduced axial height of the rib cage.16 If the extent of the pleural thickening is significant, this in itself can cause restriction in the movement of the chest wall, a “lung en cuirasse” effect, despite the lack of extension over the diaphragm.20 In our study, we clearly demonstrate a significant difference in the predicted FVC and TLC in those with DPT but no CPA obliteration (Figure 4 and 6) when compared with a matched control group comprising patients who have evidence of previous asbestos exposure—pleural plaques, but no other underlying pleuroparenchymal pathology. The trend of the lung function demonstrates that patients with DPT but no CPA obliteration lie between the control group and the unilateral DPT group.
The current definitions of DPT based on chest radiography have been a pragmatic choice reflecting worldwide availability of this low-radiation, cost-effective technique. They also reflect the high level of interobserver agreement in defining obliteration of the CPA as opposed to size criteria on chest radiography.7,11 However, CT is now acknowledged as the gold standard for imaging the pleura. The Industrial Injuries Disablement Benefit currently uses the ILO’s classification of pneumoconiosis criteria when assessing patients’ eligibility for compensation, which requires “obliteration of the CP angle”.4 This study highlights that there is a group of patients with DPT who do not meet the current definitions laid out by the ILO, yet have significant respiratory impairment.
Particular care must be taken during the initial assessment of patients with new DPT. Many of these cases will require a confirmatory pleural biopsy to exclude underlying malignancy, particularly in the presence of concerning symptoms such as chest wall pain or weight loss, at presentation. Six patients (6/64, 9%) in our study who were referred with pleural thickening were discovered to have pleural malignancy after initial investigations (5 mesotheliomas and 1 metastatic breast cancer). Our standard operative procedure for DPT follow-up is a minimum of 2 years with interval CT imaging at 6, 12 and 24 months. This allows us to assess any progression and exclude a developing underlying pleural malignancy.
Our study is not without limitations. First, although this is the largest published series of patients with DPT on CT imaging, the number of subjects in each subgroup remains small. Second, although every effort was made to match the groups for age, BMI and cumulative smoking history, we were unable to adjust for the extent of emphysema present on CT imaging. We did, however, manage to exclude any patients with a coexisting pleural effusion or underlying pulmonary fibrosis.
In summary, this is the largest published series investigating the effects of varying distributions of DPT on respiratory function. Our study shows that there is a restrictive defect in the lung function of patients with DPT who have no CPA obliteration on chest radiograph compared with a matched control group. Those with bilateral and unilateral pleural thickening by size criteria on CT also had significant reductions in their lung function tests (FVC and TLC) compared with the plaque only group, with bilateral DPT being the most disabling condition.
REFERENCES
- 1.Yates DH, Browne K, Stidolph PN, Neville E. Asbestos-related bilateral diffuse pleural thickening: natural history of radiographic and lung function abnormalities. Am J Respir Crit Care Med 1996; 153: 301–6.https://doi.org/10.1164/ajrccm.153.1.8542134 [DOI] [PubMed] [Google Scholar]
- 2.Greillier L, Astoul P. Mesothelioma and asbestos-related pleural diseases. Respiration 2008; 76: 1–15.https://doi.org/10.1159/000127577 [DOI] [PubMed] [Google Scholar]
- 3.Jeebun V, Stenton SC. The presentation and natural history of asbestos-induced diffuse pleural thickening. Occup Med (Lond) 2012; 62: 266–8.https://doi.org/10.1093/occmed/kqs028 [DOI] [PubMed] [Google Scholar]
- 4.International Labour Office. Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses. Revised edition 2011. Geneva: International Labour Office; 2011. [Google Scholar]
- 5.American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004; 170: 691–715.https://doi.org/10.1164/rccm.200310-1436ST [DOI] [PubMed] [Google Scholar]
- 6.Lynch DA, Gamsu G, Aberle DR. Conventional and high resolution computed tomography in the diagnosis of asbestos-related diseases. Radiographics 1989; 9: 523–51.https://doi.org/10.1148/radiographics.9.3.2727359 [DOI] [PubMed] [Google Scholar]
- 7.McLoud TC, Woods BO, Carrington CB, Epler GR, Gaensler EA. Diffuse pleural thickening in an asbestos-exposed population: prevalence and causes. AJR Am J Roentgenol 1985; 144: 9–18.https://doi.org/10.2214/ajr.144.1.9 [DOI] [PubMed] [Google Scholar]
- 8.Copley SJ, Wells AU, Rubens MB, Chabat F, Sheehan RE, Musk AW, et al. Functional consequences of pleural disease evaluated with chest radiography and CT. Radiology 2001; 220: 237–43.https://doi.org/10.1148/radiology.220.1.r01jl27237 [DOI] [PubMed] [Google Scholar]
- 9.Industrial Injuries Advisory Council. Diffuse Pleural Thickening. 2016. Available from: https://www.gov.uk/government/publications/diffuse-pleural-thickening-iiac-report [Google Scholar]
- 10.Rudd RM. New developments in asbestos-related pleural disease. Thorax 1996; 51: 210–6.https://doi.org/10.1136/thx.51.2.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameille J, Matrat M, Paris C, Joly N, Raffaelli C, Brochard P, et al. Asbestos-related pleural diseases: dimensional criteria are not appropriate to differentiate diffuse pleural thickening from pleural plaques. Am J Ind Med 2004; 45: 289–96.https://doi.org/10.1002/ajim.10341 [DOI] [PubMed] [Google Scholar]
- 12.Kee ST, Gamsu G, Blanc P. Causes of pulmonary impairment in asbestos-exposed individuals with diffuse pleural thickening. Am J Respir Crit Care Med 1996; 154(3 Pt 1): 789–93.https://doi.org/10.1164/ajrccm.154.3.8810620 [DOI] [PubMed] [Google Scholar]
- 13.Schneider J, Arhelger R, Raab W, Hering KG. The validity of static lung compliance in asbestos-induced diseases. Lung 2012; 190: 441–9.https://doi.org/10.1007/s00408-012-9388-6 [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto N, Kato K, Usami I, Sakai F, Tokuyama T, Hayashi S, et al. Asbestos-related diffuse pleural thickening. Respiration 2014; 88: 277–84.https://doi.org/10.1159/000364948 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DA, Fuortes LJ, Galvin JR, Burmeister LF, Schmidt LE, Leistikow BN, et al. Asbestos-induced pleural fibrosis and impaired lung function. Am Rev Respir Dis 1990; 141: 321–6.https://doi.org/10.1164/ajrccm/141.2.321 [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Eastwood PR, Finucane KE, Panizza JA, Musk AW. Effect of asbestos-related pleural fibrosis on excursion of the lower chest wall and diaphragm. Am J Respir Crit Care Med 1999; 160(5 Pt 1): 1507–15.https://doi.org/10.1164/ajrccm.160.5.9806135 [DOI] [PubMed] [Google Scholar]
- 17.Nojima D, Fujimoto N, Kato K, Fuchimoto Y, Kiura K, Kishimoto T, et al. Pilot analysis of asbestos-induced diffuse pleural thickening with respiratory compromise. Acta Med Okayama 2015; 69: 261–6.https://doi.org/10.18926/AMO/53671 [DOI] [PubMed] [Google Scholar]
- 18.Gleeson F. Radiology: diagnostic : Light RW, Lee YCG, Textbook of pleural diseases. London, UK: CRC Press; 2016. 180–209. [Google Scholar]
- 19.Gevenois PA, de Maertelaer V, Madani A, Winant C, Sergent G, De Vuyst P. Asbestosis, pleural plaques and diffuse pleural thickening: three distinct benign responses to asbestos exposure. Eur Respir J 1998; 11: 1021–7.https://doi.org/10.1183/09031936.98.11051021 [DOI] [PubMed] [Google Scholar]
- 20.Corris PA, Best JJ, Gibson GJ. Effects of diffuse pleural thickening on respiratory mechanics. Eur Respir J 1988; 1: 248–52. [PubMed] [Google Scholar]