Abstract
Objective:
Radiotherapy (RT) for synchronous bilateral breast cancer (SBBC) is technically very challenging. This study reports the clinical feasibility, dosimetry and safety of helical tomotherapy (HT) with simultaneous integrated boost (SIB) in patients treated with adjuvant radiotherapy for SBBC.
Methods:
21 women with SBBC treated with HT from January 2013 to June 2016 were retrospectively evaluated. Radiation lung toxicity was assessed using pulmonary function test (PFT) and high-resolution computerized tomography scan (HRCT) scan at baseline and 1 yearpost‐RT in 18 patients. Survival was calculated using Kaplan–Meier curves. Significance of the difference between pre- and post-RT PFT values was assessed using paired t-test.
Results:
The dose prescription was 50Gy to the breast, chest wall or regional nodes and 61Gy to the tumour bed as SIB, delivered in 25 fractions. Dosimetric outcome was excellent both for target volumes and normal tissues. Acute skin and oesophageal toxicities were minimal. Symptomatic radiation-induced pnuemonitis was not observed. Subclinical radiological Grade I–II changes were apparent in 14 patients. Only one patient developed Grade III radiological change whereas no change was documented for three patients. PFTs did not show any significant change in any of the measured parameters. At a median follow-up of 25 months, 3-year disease-free survival, overall survival and loco-regional control were 65.6%, 83.3% and 85.7% respectively.
Conclusion:
Women with SBBC can be safely treated with HT and this is not associated with adverse short- to intermediate term radiation toxicity.
Advances in knowledge:
This is the first report that establishes the safety of HT for adjuvant RT using SIB technique in SBBC.
INTRODUCTION
The occurrence of synchronous bilateral breast cancer (SBBC) is rare with a reported incidence ranging between 0.4% and 2.8% of all breast cancers.1,2 Radiotherapy in SBBC is technically challenging in view of the large and complex treatment volumes and proximity to critical structures. Target volumes typically involve bilateral breasts/chest wall with or without regional nodal irradiation (supraclavicular, axillary or internal mammary nodal region). Conventional tangents involving multiple field junctions may not cover target volumes adequately, especially in medially located tumours or if the target volume involves the internal mammary chain where electron/photon matching leads to significant dose heterogeneity.3 Moreover, delivering boost to tumour bed cavity either sequentially or with the simultaneous integrated boost (SIB) technique further increases the complexity of treatment planning as well as delivery. In our earlier study, we have demonstrated that helical tomotherapy (HT) has the ability to reduce high doses of radiation to the organs at risk (OARs) specifically while delivering SIB in the setting of SBBC.4 The use of HT for unilateral targets for breast cancer may lead to significant irradiation of the contralateral breast and lung with low doses owing to fan beam geometry.5,6 However, this disadvantage of HT is offset in the case of SBBC as targets on both sides need to be treated, which makes treatment planning simpler. This report describes the clinical use of HT with SIB for SBBC, covering the dosimetric results along with short-term clinical outcomes. To the best of our knowledge, this is the largest series of SBBC reported to date with all patients uniformly treated at a single institute. Secondly, this is the first report describing the use of HT involving the SIB technique for the clinical condition under consideration, although a smaller series has been reported with volumetric arc therapy.7
METHODS AND MATERIALS
Study design and patients
Women diagnosed to have SBBC and who had undergone bilateral breast/chest wall irradiation with or without regional nodal irradiation using HT technique between January 2013 and June 2016 were identified. In this retrospective audit, hospital medical records and other pertinent details were reviewed for patient demographics, family history, clinical management, radiotherapy treatment planning parameters and acute and late toxicities. Ethical clearance was obtained for the dosimetric study, the results of which formed the basis of adoption of HT for routine treatment of SBBC at our institute. The cohort constituted a total of 21 patients inclusive of 13 patients who had breast-conserving surgery (BCS) on both sides, 2 who had modified radical mastectomy (MRM) on both sides and rest of the 6women who had mastectomy on one side (3 left side and 3 right side) and breast conservation on the other side. Planning computerized tomography (CT) datasets were retrieved from the treatment planning system for reporting the dosimetric outcome.
Immobilization and volume delineation
All patients were immobilized using an individualized vacuum bag with both arms abducted above the head. Non-contrast CT scans with 2.75 mmslice thickness were taken from the level of mandible to mid-abdomen on the CT simulator (GE DISCOVERY IQ). Images were imported into the tomotherapy treatment planning system (Accuray Inc., Sunnyvale, CA, version 5.1.0). The clinical target volume (CTV) for the breast and/or chest wall was contoured with the help of a wire placed on the patient during planning CT scan. A 5 mm margin was grown from CTV to generate the PTV (planning target volume)_Breast. The PTV_Breast was cropped from the skin by 5 mm in case of BCS and 3 mm in case of MRM. The pectoral muscles were excluded from the CTV in BCS but included in MRM cases. In MRM cases, chest wall and ribs were included in advanced stage disease. Supraclavicular and internal mammary lymph nodes were drawn according to Radiation Therapy Oncology Group (RTOG) consensus guidelines and the respective PTVs grown by giving 5 mm margin isotropically.8 None of the patients received axillary irradiation. Furthermore, tumour bed (TB) was delineated with the help of seroma, surgical clips, post-operative changes and the metallic wire placed over the scar. A 5 mm margin was given to make PTV_boost, to be confined within the PTV_Breast. Organs at risk (OARs) such as the lung on each side, heart, oesophagus and spinal cord were contoured.
Helical tomotherapy planning
The details of the planning process have been published in the earlier report.4 The dose prescribed to the PTV_Breast and nodal PTVs (whole breast irradiation/chest wall ± regional nodal area) was 50Gy in 25 fractions whereas 61 Gy in 25 fractions was delivered as SIB to the PTV_Boost in case of BCS. The plan’s objectives were set with reference to the International Commission on Radiation Units criteria of 95% of the target volume getting covered with 95% of the prescribed dose with minimum spillage to the surrounding normal tissue. For OARs, the planning objectives were set as V20 Gy <25%, V30Gy <15% and V5 Gy <60% for both lungs and heart.
Plan evaluation
Evaluation of plans was based on dose volume histogram (DVH) analysis. For all the PTVs, the values of mean, minimum and maximum doses and V95%, V90% and V107% (the volumes receiving at least 95%, 90% or 107% of the prescribed dose) were recorded. For OARs, the analysis included the mean dose and a set of VXGy (OAR volume receiving at least XGy) such as V5, V10, V20, V30 and V40 for lungs and heart respectively. The homogeneity of the dose distribution for both primary PTV excluding boost volume and boost PTV was calculated by following formula:
where Imax is the maximum isodose inside the target and IR is the reference isodose. Ideally it should be 1.
Conformity of the dose distribution was calculated using the following formula
where TV is the target volume, TVPI is the target volume covered by the prescription isodose and VPI is the total volume covered by the prescription isodose. Ideally, it should be close to 1.
Monitoring of treatment toxicity
All patients were reviewed once a week during radiotherapy and patients were assessed for radiation dermatitis and oesophageal toxicity. Acute toxicity was graded according to RTOG criteria.11
Pre- and post-treatment evaluation of pulmonary function
All patients underwent baseline pulmonary function test (PFT) and high-resolution computerized tomography scan (HRCT) before initiation of RT and 1 year after completion of RT (if they were disease free) to assess the status of pulmonary function and to record late changes thereafter. On HRCT, lung alterations were scored according to the scoring system of Nishioka et al.12
In PFT, the following parameters were assessed after bronchodilator effect: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, forced expiratory flow at 25% (FEF 25) and 75 (FEF 75) of the vital capacity. FVC is a measure of lung volume; FEV1 reflects the mechanical properties of large and medium-sized airways and FEF 25 and FEF 75 are the measure of the average rate of airflow in the bronchioles and small airways. All these parameters are reduced in case of pulmonary fibrosis. All measurements are expressed as a percentage of the predicted values adjusted for age, gender, and height.
Statistical analysis
Statistical analysis was done using SPSS software version 18. Patient demographics were tabulated and reported as proportions. Similarly, dosimetric parameters were reported with appropriate values along with range. Significance of the difference between pre- and post-RT PFT values was assessed using paired t-test. HRCT changes were reported as numbers and percentage. Disease-related outcomes were analysed using Kaplan–Meier curves, and time to event analysis was done for locoregional control (LRC), disease-free survival (DFS) and overall survival (OS).
RESULTS
Patient and tumour characteristics
Clinicopathological characteristics and treatment strategies of 21 females with SBBC were studied. Patient and tumour characteristics are summarized in Tables 1 and 2. The median age was 52 years (Range: 29–70 years). Chemotherapy was anthracycline and taxane based in all patients. 4 patients had complete pathological response to neoadjuvant chemotherapy whereas 10 patients had partial response. Median time to start RT from the last intervention was 1 month. Radiation target volumes are described in Table 3. SIB was given in 18 patients. Three patients with BCS did not receive boost on any side owing to small tumour size and favourable histology. All hormone receptor-positive pre-menopausal females received adjuvant tamoxifen whereas post-menopausal females received aromatase inhibitors (letrozole/anastrozole) along with radiation.
Table 1.
Patient characteristics
| Characteristics | N (21) | % | |
| Age grouping | Age ≤52 years | 11 | 52.0 |
| Age > 52 years | 10 | 48.0 | |
| Family history | Positive | 9 | 42.8 |
| Negative | 12 | 57.2 | |
| Menopausal status | Pre-menopausal | 10 | 47.6 |
| Post-menopausal | 10 | 47.6 | |
| Peri-menopausal | 1 | 4.8 | |
| Focality of tumoura | Unifocal | 18 | 85.7 |
| Multifocal | 3 | 14.3 | |
| Clinical stageb | II | 9 | 42.9 |
| III | 12 | 57.1 | |
| Treatment sequence | NACT->Sx->RT | 11 | 52.4 |
| NACT->Sx->CT->RT | 3 | 14.3 | |
| Sx->CT->RT | 5 | 23.8 | |
| Sx->RT | 1 | 4.8 | |
| Missing | 1 | 4.8 | |
BCT, breast conservation therapy;CT, chemotherapy; MRM,modified radicalmastectomy; NACT,neoadjuvant chemotherapy;RT, radition therapy; Sx,sugery.
aFocality on either side.
bConsidering higher clinical stage on either side.
Table 2.
Tumour characteristicsa
| Parameter | N | % | |
| Histology | IDC on both sides | 19 | 90.5 |
| IDC on one side and other histology on contralateral side | 2 | 9.5 | |
| Grade | III | 18 | 85.7 |
| II | 3 | 14.3 | |
| ER status | Positive | 17 | 81.0 |
| Negative | 4 | 19.0 | |
| PR status | Positive | 15 | 71.4 |
| Negative | 6 | 28.6 | |
| Her2neu status | Positive | 8 | 38.0 |
| Negative | 13 | 62.0 | |
| PNI | Present | 0 | 0 |
| Absent | 21 | 100.0 | |
| LVE | Present | 9 | 43.0 |
| Absent | 12 | 57.0 | |
| EIC | Present | 6 | 28.6 |
| Absent | 15 | 71.4 | |
| Skin involvement | Present | 1 | 5.0 |
| Absent | 20 | 95.0 | |
| Margins | Negative | 21 | 100.0 |
| PNE | Present | 11 | 52.4 |
| Absent | 10 | 47.6 |
DCIS, ductal carcinoma in situ; EIC, extensive intraductal component; ER,estrogen receptor; IDC, infiltrating ductal carcinoma;LVE, lymphovascular emboli; PNE, perinodal extension; PNI, perineural invasion; PR, progesterone receptor.
aTumour characteristic positive on either side was considered overall positive and reported here.
Table 3.
Radiation target volumes
| Target volumes | N | % | |
| Primary | B/L Breasts | 13 | 61.5 |
| B/L chest wall | 2 | 9.5 | |
| Rt breast + Lt chest wall | 3 | 14.5 | |
| Lt breast + Rt chest wall | 3 | 14.5 | |
| Nodal | B/L SCF | 9 | 42.8 |
| Rt SCF only | 4 | 19.0 | |
| Lt SCF only | 5 | 23.8 | |
| Lt SCF+IMN | 1 | 4.8 | |
| None | 2 | 9.5 | |
| Boost | Both side | 10 | 47.6 |
| Left side | 4 | 19.0 | |
| Right side | 4 | 19.0 | |
| Not given | 3 | 14.4 |
IMN: internal mammary node, SCF, Supraclavicular fossa.
Dosimetric characteristics
Radiation treatment volumes of primary, boost and nodal region are summarized in Table 4. PTV coverage on both sides with 95% isodose was 94.4%. 95% coverage of nodal PTV and tumour bed PTV were 92.6% and 96.7% respectively. Spillage of the 107% vol of prescribed dose (50Gy) was 50 cc, which was confined to the area adjacent to the boost PTV. Homogeneity index for PTV was 1.09 for the right side and 1.11 for the left side, and for the tumour bed it was 1.1 for the right and 1.04 for the left side respectively. The mean conformity index was 0.71. The doses to the OARs are described in Table 5. Figure 1 depicts the target volumes and dose distribution in a case of bilateral BCT with SIB delivered on both sides.
Table 4.
Dosimetric parameters indicative of PTV coverage
| Volume | Right | Left | |
| PTV_Primary | Volume | 625cc (216.0–1157.0cc) | 648.3cc (308.4–1067.0cc) |
| V95 | 94.2%(92.7–99.7%) | 94.6%(91.6–99.7%) | |
| V90 | 98%(96.7–99.9%) | 98%(95.0–99.9%) | |
| V100 | 75.0%(44.2–97.0%) | 76.7%(45.5–97.0%) | |
| V107 | 49.0cc (0–133.5cc) | 52.0cc (2.0–126.0 cc) | |
| Mean dose | 51.2Gy (49.2–52.6 Gy) | 51.2Gy (49.5–52.0 Gy) | |
| Maximum dose | 60.0Gy (54.0–65.0 Gy) | 60.6Gy (55.6–66. 0 Gy) | |
| Minimum dose | 35.2Gy (27.0–41.2 Gy) | 34.0Gy (21.6–41.2 Gy) | |
| PTV_Nodal_SCF | Volume | 66.4cc (40.0–90.4cc) | 62.6cc (40.0–90.4cc) |
| V95 | 93.3%(90–100.0%) | 92%(89.0–99.9%) | |
| V90 | 98.4%(95.4–100.0%) | 98.6%(95.0–100.0%) | |
| V107 | 1.28cc (0–9.0cc) | 1.28cc (0–11.0cc) | |
| Mean dose | 50.5Gy (49.0–52.0 Gy) | 50.4Gy (49.0–52.0 Gy) | |
| Maximum dose | 54.5Gy (52.0–59.3 Gy) | 54.0Gy (52.3–57.4Gy) | |
| Minimum dose | 41.0 Gy (30.5–48Gy) | 42.2Gy (34.6–49.8Gy) | |
| PTV_TB | Volume | 85.6cc (25.5–160cc) | 84.8cc (37.7–142.2 cc) |
| V95 | 97.0%(91.8–99.94%) | 96.4%(92.6–99.7%) | |
| V90 | 99.7%(98.6–100%) | 99.8%(99.0–100.0%) | |
| V107 | 0.07cc (0–0.69.0 cc) | 0.2cc (0–1.6cc) | |
| Mean dose | 61.0Gy (57.0–62.5Gy) | 61.0Gy (56.6–62.4Gy) | |
| Maximum dose | 64.2Gy (60.0–65.6Gy) | 64.5Gy (59.8–66.4Gy) | |
| Minimum dose | 52.8Gy (43.0–57.0Gy) | 52.6Gy (49.0–56.7Gy) |
V95,V90,V100,V107—volume of PTV receiving 95%, 90%, 100% and 107% of prescribed dose respectively, recorded in overlap mode.
Table 5.
Dose and volume parameters for organs at risk
| Parameter | Right lung | Left lung | B/L total lungs | Heart |
| Mean Lung dose (range) | 10.8Gy(5.4–14.7Gy) | 9Gy(5.0–15.3 Gy) | 9.2Gy(5.8–13.2Gy) | 5.7Gy(2.2–10.8Gy) |
| V5Gy (range) | 48.6%(20.0–93.0%) | 45.2%(15.9–81.5%) | 46.8%(18.6–70.5%) | 31.2%(1.2–88.4%) |
| V10Gy (range) | 27.0%(10.5–47.0%) | 24.2%(7.4–48.7%) | 25.7%(14.6–39.3%) | 12.6%(0–31.0%) |
| V20Gy (range) | 14.6%(5.0–27.4%) | 12.6%(2.0–29.0%) | 13.3%(4.0–23.0%) | 4.2%(0–15.0%) |
| V30Gy (range) | 8.0%(0.9–17.9%) | 7.0%(0.5–16.8%) | 7.0%(0.8–13.4%) | 1.4%(0–8.3%) |
| V40Gy (range) | 2.8%(0–8.0%) | 2.5%(0–9.0%) | 2.5%(0–5.5%) | 0.4%(0–3.8%) |
Figure 1.
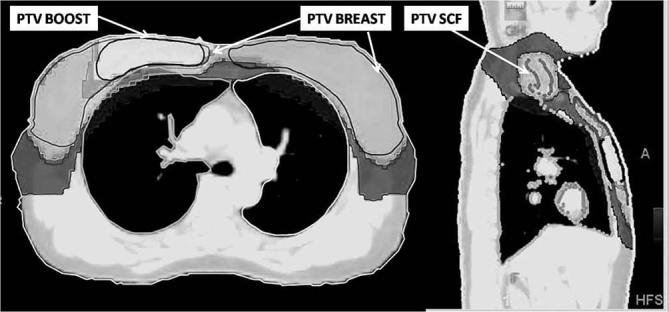
Target volumes and dose distribution in a case of bilateral breast conservation therapy with simultaneous integrated boost delivered on both sides. White: 95% dose wash of boost dose (58Gy);grey: 95% dose wash of breast/SCF dose (45 Gy) andblack: 50% dose wash of breast/SCF dose (25 Gy).
Treatment compliance and toxicity
Median duration of radiation was 5 weeks(5–7 weeks). 14 patients developed Grade I, 6Grade II and 1Grade III acute dermatitis during RT. Eleven patients developed Grade I, 3Grade II and 1Grade III acute dysphagia and the remaining 6 did not experience any dysphagia. This has been shown in Table 6. None of the patients had acute symptoms suggestive of radiation pneumonitis.
Table 6.
Acute toxicity of treatment as per Radiation Therapy Oncology Group scoring
| Toxicity | Dermatitis (N/percent) | Oesophageal (N/percent) |
| Grade 0 | – | 6 (28.5%) |
| Grade 1 | 14 (66.7%) | 11 (52.4%) |
| Grade 2 | 6 (28.5%) | 3 (14.3%) |
| Grade 3 | 1 (4.8%) | 1 (4.8%) |
| Total | 21 (100%) | 21 (100%) |
Compared with baseline, intermediate-term lung toxicity of 18 patients could be assessed with 1 year post-RT PFT and HRCT thorax. An elderly patient could not come for follow-up because of long distance and hence preferred follow-up at a local place. Two patients developed recurrence after a very short DFS (within 1 year) and hence could not be evaluated for lung toxicity. None of the patients developed clinical symptoms suggestive of radiation pnuemonitis on follow-up. Two patients had baseline postinfective bronchiectatic changes and another 2 had patchy fibrotic areas. One patient had Grade III post-RT fibrosis, 7 patients had Grade II lung changes, 7 patients had Grade 1 changes whereas 3 showed no radiological change. Parenchymal changes were mostly seen in apical lung corresponding to the junction of breast/chest wall and SCF field or in the area where the tumour bed was close to the ipsilateral chest wall. Grade III fibrosis was seen in a patient who had poorly controlled baseline asthma and atelectasis-like changes. Baseline PFT parameters of five patients were deviated from normal, though it was not clinically significant. Of the total 18 patients, 9 patients had drop in at least one of the FVC, FEV1 or FEF25-75values post-radiation, but the difference was not significant statistically. Out of these, only one patient had baseline asthma while the rest had normal baseline PFT values.
Disease-related outcome
Median DFS was 23 months (range: 10 to 47 months). During this study period, one patient developed regional (axillary recurrence), two developed distant recurrences and one patient developed both local and distant recurrence. Only one patient who developed distant (liver and skeletal metastasis) failure succumbed, whereas the rest of the females are alive. At a median follow-up of 25 months, 3-year DFS, OS and LRC were 65.6%, 83.3% and 85.7% respectively.
DISCUSSION
Various technical advancements in the field of radiation oncology have broadened the armamentarium and made treatment planning easy for different sites that are difficult to treat with conventional techniques. While treating with the conventional bitangential technique in the case of SBCC, dose heterogeneity at field junctions and increased hot spot over the large target volume are matters of concern. Moreover, setup difficulties with bitangential technique may occasionally amount to patient repositioning for the contralateral side. Hence intensity-modulated radiotherapy (IMRT) using HT offsets all these disadvantages and makes treatment delivery for SBCC much easier. It delivers a conformal and homogeneous dose to the complex target volumes as shown in our earlier dosimetric study, which was carried out in 10 patients without considering regional nodal irradiation.4 HT helps in achieving a differential dose distribution, thereby allowing delivery of higher dose to the tumour bed cavity and a lower spill to the remaining breast using the SIB technique. SIB technique was chosen to deliver boost as it results in significant reduction in the spillage of high dose volume to the remaining low-risk breast, decreased acute skin reactions as well as decreased overall treatment time.13–15 Among the earlier reports on the use of HT for locoregional RT in the setting of SBBC, SIB technique was employed in only one study.7,16,17
Kaidar-Person et al studied only nine patients, out of whichfour had recurrent disease and two underwent re-irradiation.16 All patients in their study received internal mammary nodal (IMN) irradiation either unilaterally or bilaterally and eight out of ninepatients received axillary irradiation as well. Moreover, differential dose and fractionation schedules were employed for locoregional as well as boost RT along with an accelerated regimen. As majority of patients had gross disease, bolus was also used. As expected, acute and late toxicities were much higher in their patient population, owing to the large treatment volumes as well as inappropriate case selection. Moreover, this study does not report the dosimetric criteria with respect to target volumes; only those for OARs have been described. Our policy is to consider IMN irradiation only if there are grossly visualized IMN nodes on pre-therapy imaging and axillary irradiation when there is incomplete axillary clearance or residual disease, owing to concerns of higher toxicities.18 The most notable difference is that we have employed HT for adjuvant RT and not in patients with gross disease. All patients were non-metastatic and had undergone optimal surgery and systemic therapy with curative intent. All patients were uniformly treated with single dose fractionation regimen using SIB technique. In our study, only one patient received unilateral IMN RT and none received axillary RT. In our patient cohort, HT was tolerated very well and females had much lower acute skin and oesophageal toxicities as well as late sequelae. Kemal Ekici et al have achieved encouraging results with tomotherapy in 14 patients treated in adjuvant setting.17 They used sequential boost (to a dose of 8–10 Gy in 4–5 fractions) for patients with breast conservation. However, the method of boost delivery has not been reported and the paper describes only short-term outcome limited to treatment tolerance and acute toxicity. Similar to the study by Kaider-Person et al, this study also does not report dosimetric indices for the target volumes except homogeneity index (HI) and conformity index (CI). In our study, we have reported much more detailed demographic, dosimetric and short/mid-term clinical outcome data of 21 patients consecutively treated in our institute. In the current study, acceptable target coverage was achieved with V95 for breast/chest wall PTV (Table 4). The spillage of the volume of 107% of the prescription dose to the breast/chest wall (i.e. 53.5Gy volume) was also within acceptable range and reflects the ability of HT to conform high dose to the tumour bed while restricting the same in the adjacent low-risk breast volume. Although the maximum absolute volume of 107% was up to 133 cc, it is only 11.5% of the whole breast volume. We calculated HI separately for both the tumour bed cavity and the remaining low-risk breast. HI was ~1 for both the volumes, which reflects good homogeneity in spite of differential dose distribution inside the target. The formula proposed by Paddick et al was chosen for calculating the CI as it is more appropriate in considering the geometric spatial overlap between the target and treated volumes.10
As expected in IMRT technique, low dose volumes were comparatively higher, mean V5 being 46.8% and 31.2% and mean V10 being 25.7% and 12.6% for lungs and heart respectively. It was noticed that the low dose spillage was higher in the two cases with bilateral mastectomy as compared to patients with breast conservation on at least one side. Nonetheless, the low dose volumes are much lower than that reported by the Italian investigators.7 It also probably reflects a learning curve in the planning process because the low dose constraints for IMRT planning are generally not validated. Hence subsequent to the initial few patients, attempt to achieve the dose constraint for the low dose spill was intentionally made, which reflected in lowering of this value in the remaining cases. However, the low dose constraint was still not achieved in four patients. At the same time, we could achieve significantly less high dose volumes, withmedian V30 being 7 and 1.4% and V40 being 2.5% and 0.4% for lungs and heart respectively. According to some studies, more the high dose volume more is the risk of development of radiation-induced malignancies.19 Significantly lower V40 and V30 volumes and acceptable low dose volumes in the current study, therefore, favour the treatment technique. However, as the relationship of radiation-induced secondary neoplasms (SN) within the low dose volumes is still not clear, concerns regarding SN will remain with any IMRT technique.20,21
Several studies have assessed post‐RT lung parenchymal changes using plain radiographs or conventional CT scan using the classification proposed by Arriagada et al.22 However, both of these modalities are less sensitive as compared to HRCT in the assessment of changes in lung parenchyma.23 We analysed the long-term radiological as well as functional abnormalities using HRCT and PFT parameters and also correlated it with the dosimetric data. Nishioka scoring was used for defining lung parenchymal changes in HRCT scan, which has been used traditionally in many studies and is a better way of assessing early post-RT lung parenchymal changes, especially in the absence of clinical symptoms.12,24,25 Nishioka scoring however defines Grade2 post-RT lung fibrosis as pulmonary parenchymal changes in less than 50% of irradiated area. We realized that parenchymal changes in even one or two axial CT slices had to be labelled as Grade2 changes, which is actually an overestimate and there can be a division in grading as early and late Grade2 changes (e.g. changes in <15% or15–50% of irradiated volume). Assuming that post-bronchodilator values are better as compared to prebronchodilator ones, we compared PFT parameters after bronchodilator effect. Although not clinically significant as reflected by the mean values, we observed more decrease in FEF 25 and FEF 75 values post-RT representing more small airway and bronchiolar changes, which is consistent with the findings of Marco Krengli et al.24 Not the least, it was very reassuring to find that HT was pulmonary safe with respect to the clinicoradiological and functional assessment, in the limited cohort studied here.
The main limitation of the study is the limited number of patients, which is related to the rarity of SBBC. Despite this, the current series comprising of 21 patients is the largest one reported so far. We have not reported the daily set up variations and other practical issues during treatment delivery as it was beyond the scope of this study. The short follow-up time also limits the assessment of safety of HT with respect to second malignancies and late toxicity for other organs such as heart and subcutaneous tissue.
CONCLUSION
Based on our preliminary results, HT can be considered a safe and well-tolerated treatment technique for females with SBBC. Excellent dosimetric outcomes are achieved with respect to PTV coverage and OAR sparing by using the SIB technique. However, limited number and lack of long-term clinical outcomes dowarrant further multi-institutional prospective studies for better assessment of outcomes and radiotherapy techniques for SBCC.
Contributor Information
Tabassum Wadasadawala, Email: twadasadawala@actrec.gov.in.
Shanu Jain, Email: drshanu.onco@gmail.com.
Siji Paul, Email: siji.menachery@gmail.com.
Reena Phurailatpam, Email: reena.ph@gmail.com.
Kishore Joshi, Email: kj61288@gmail.com.
Palak Popat, Email: dr.palakp@gmail.com.
Sandip Tandon, Email: tandonsp@tmc.gov.in.
Aruna Alahari, Email: arunaalahari@hotmail.com.
Rajiv Sarin, Email: drshanu.onco@gmail.com.
REFERENCES
- 1.Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH, Synchronous MJH. Synchronous, bilateral breast cancer: prognostic value and incidence. Breast 2003; 12: 83–8. doi: https://doi.org/10.1016/S0960-9776(02)00278-3 [DOI] [PubMed] [Google Scholar]
- 2.Mueller CB, Ames F. Bilateral carcinoma of the breast: frequency and mortality. Can J Surg 1978; 21: 459–65. [PubMed] [Google Scholar]
- 3.Dogan MH, Zincircioglu SB, Zorlu F. Comparison of various radiation therapy techniques in breast cancer where target volume includes mammaria interna region. Med Dosim 2009; 34: 42–50. doi: https://doi.org/10.1016/j.meddos.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Wadasadawala T, Visariya B, Sarin R, Upreti RR, Paul S, Phurailatpam R. Use of tomotherapy in treatment of synchronous bilateral breast cancer: dosimetric comparison study. Br J Radiol 2015; 88: 20140612. doi: https://doi.org/10.1259/bjr.20140612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauche O, Kirova YM. Helical tomotherapy in breast cancer treatment. Breast Cancer Manag 2014; 3: 441–9. doi: https://doi.org/10.2217/bmt.14.34 [Google Scholar]
- 6.Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, et al. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys 2009; 73: 1243–51. doi: https://doi.org/10.1016/j.ijrobp.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino A, Mazzola R, Naccarato S, Giaj-Levra N, Fersino S, Sicignano G, et al. Synchronous bilateral breast cancer irradiation: clinical and dosimetrical issues using volumetric modulated arc therapy and simultaneous integrated boost. Radiol Med 2017; 122: 464–71. doi: https://doi.org/10.1007/s11547-017-0741-y [DOI] [PubMed] [Google Scholar]
- 8.RTOG Breast cancer Contouring Atlas. http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx. Accessed Jan 29, 2017.
- 9.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993; 27: 1231–9. doi: https://doi.org/10.1016/0360-3016(93)90548-A [DOI] [PubMed] [Google Scholar]
- 10.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 2000; 93(Suppl. 3): 219–22. doi: https://doi.org/10.3171/jns.2000.93.supplement [DOI] [PubMed] [Google Scholar]
- 11.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Ttreatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. doi: https://doi.org/10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 12.Nishioka A, Ogawa Y, Hamada N, Terashima M, Inomata T, Yoshida S. Analysis of radiation pneumonitis and radiation-induced lung fibrosis in breast cancer patients after breast conservation treatment. Oncol Rep 1999; 6: 513–7. doi: https://doi.org/10.3892/or.6.3.513 [DOI] [PubMed] [Google Scholar]
- 13.Melchor MA, Soler M, Candela F, Cámara A, Martínez D. Comparison of two treatment regimes in left breast cancer: simultaneous integrated boost versus sequential boost. Int J Radiat Oncol Biol Phys 2011; 81: S266. doi: https://doi.org/10.1016/j.ijrobp.2011.06.456 [Google Scholar]
- 14.Lee HH, Hou MF, Chuang HY, Huang MY, Tsuei LP, Chen FM, et al. Intensity modulated radiotherapy with simultaneous integrated boost vs. conventional radiotherapy with sequential boost for breast Cancer - A preliminary result. Breast 2015; 24: 656–60. doi: https://doi.org/10.1016/j.breast.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Aly MM, Abo-Madyan Y, Jahnke L, Wenz F, Glatting G. Comparison of breast sequential and simultaneous integrated boost using the biologically effective dose volume histogram (BEDVH). Radiat Oncol 2016; 11: 16. doi: https://doi.org/10.1186/s13014-016-0590-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaidar-Person O, Kostich M, Zagar TM, Jones E, Gupta G, Mavroidis P, et al. Helical tomotherapy for bilateral breast cancer: clinical experience. Breast 2016; 28: 79–83. doi: https://doi.org/10.1016/j.breast.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 17. Ekici K, Gokce T, Karadogan I, Fatma Eraslan A, Akcay C, Temelli O, et al. Is helical tomotherapy-based intensity-modulated radiotherapy feasible and effective in bilateral synchronous breast cancer? A two-center experience. J BUON 2016; 21: 46–52. [PubMed] [Google Scholar]
- 18.Wadasadawala T, Bajpai J. Internal mammary nodal irradiation: the jury is still out. Clin Oncol 2016; 1: 1119 [Google Scholar]
- 19.Rubino C, Shamsaldin A, Lê MG, Labbé M, Guinebretière JM, Chavaudra J, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat 2005; 89: 277–88. doi: https://doi.org/10.1007/s10549-004-2472-8 [DOI] [PubMed] [Google Scholar]
- 20.Schulz U, Gokel JM, Poleska W. Soft tissue sarcomas after radiation treatment for breast cancer. three case studies and review of literature. Strahlenther Onkol 2000; 176: 144–9. doi: https://doi.org/10.1007/PL00002340 [DOI] [PubMed] [Google Scholar]
- 21.Dörr W, Herrmann T. Second primary tumors after radiotherapy for malignancies. Treatment-related parameters. Strahlenther Onkol 2002; 178: 357–62. [DOI] [PubMed] [Google Scholar]
- 22.Arriagada R, de Guevara JC, Mouriesse H, Hanzen C, Couanet D, Ruffie P, et al. Limited small cell lung cancer treated by combined radiotherapy and chemotherapy: evaluation of a grading system of lung fibrosis. Radiother Oncol 1989; 14: 1–8. doi: https://doi.org/10.1016/0167-8140(89)90002-9 [DOI] [PubMed] [Google Scholar]
- 23.Schratter-Sehn AU, Schurawitzki H, Zach M, Schratter M. High-resolution computed tomography of the lungs in irradiated breast cancer patients. Radiother Oncol 1993; 27: 198–202. doi: https://doi.org/10.1016/0167-8140(93)90074-I [DOI] [PubMed] [Google Scholar]
- 24.Krengli M, Sacco M, Loi G, Masini L, Ferrante D, Gambaro G, et al. Pulmonary changes after radiotherapy for conservative treatment of breast cancer: a prospective study. Int J Radiat Oncol Biol Phys 2008; 70: 1460–7. doi: https://doi.org/10.1016/j.ijrobp.2007.08.050 [DOI] [PubMed] [Google Scholar]
- 25.Yi A, Kim HH, Shin HJ, Huh MO, Ahn SD, Seo BK. Radiation-induced complications after breast cancer radiation therapy: a pictorial review of multimodality imaging findings. Korean J Radiol 2009; 10: 496. doi: https://doi.org/10.3348/kjr.2009.10.5.496 [DOI] [PMC free article] [PubMed] [Google Scholar]


