Abstract
Objective:
A Phase II study was designed to test the safety and efficacy of concurrent chemoradiotherapy with a modified target volumes delineation method for inoperable oesophageal cancer patients.
Methods:
All eligible patients were treated with concurrent chemoradiotherapy. The method of delineating target volume is as follows: Planning gross target volume (PGTV) was defined as the primary gross tumour volume (GTV-t) plus a 3 cm margin longitudinally and a 0.5 cm margin circumferentially, and positive lymph nodes(GTV-n) plus a 0.5 cm margin in all directions. Clinical target volume (CTV) was defined as PGTV plus a 0.5 cm margin in all directions and elective nodal region. Planning target volume (PTV) was defined as CTV plus a 0.5 cm margin in all directions. The dose of PGTV is 54–60 Gy in 27–30 fractions(2Gy per fraction). The dose of PTV is 48.6–54 Gy in 27–30 fractions(1.8Gy per fraction). The regimen consists of paclitaxel135 mgm−2on 1 day and DDP 25 mgm−2 on 3 days per 3 weeks. The patients received 2 cycles of chemotherapy during radiotherapy and 2–4 cycles of chemotherapy after radiotherapy.
Results:
34 patients were enrolled in this study. The median follow-up time was 20.9 months (range: 3.7–28.4 months) for all patients. The 1- and 2-year survival rates for all patients were 70.5 and 44.1%, respectively. Clinical complete response was observed in 21 patients(61.8%), cPR was observed in 9 patients(26.5%) and cSD was observed in 4 patients(11.7%).
Conclusion:
This modified method with concurrent chemotherapy could achieve better locoregional control rate. The 1- and 2-year survival rates of this method were close to the survival rates of the current methods widely adopted.
Advances in knowledge:
The modified target volumes delineation method can enhance locoregional control rate of concurrent chemoradiotherapy.
INTRODUCTION
Oesophageal cancer is the eighth most common cause of cancer-related deaths in the world.1 More than 60% of the oesophageal cancer patients are diagnosed at locally advanced stages which cannot be totally resected.2 Concurrent chemoradiation is a standard treatment for locoregional advanced unresectable disease.3 Nowadays, either three-dimensional conformal radiation therapy or intensity-modulated radiation therapy can be used in concurrent chemoradiation for oesophageal cancer.
Target volume delineation for the treatment plan in two textbooks4,5 is as follows: (1) gross tumour volume (GTV) consists of primary tumour(the wall thickness of more than 0.5 cm) and positive lymph nodes(longer than 1 cm); (2) clinical target volume (CTV) was derived by expanding standard GTV 3–5 cm in the cranio-caudal direction and 0.5–1 cm in the lateral and anterior/posterior directions; (3)standard GTV to CTV expansions for the positive lymph nodes are 0.5–1 cm in all directions; CTV should also include the adjacent nodal region; (4)expansion on the CTV by 5 mm can be applied to obtainplanning target volume(PTV). Button MR et al6 demonstrated that clinically acceptable radiotherapy fields, 3 cm superoinferior and 1.5 cm lateral margins from the GTV to the PTV, were adequate and not more extensive. The narrow RT fields used did not increase the risk of locoregional disease relapse.
In this study, we designed a Phase II study to test the safety and efficacy of concurrent chemoradiotherapy with a modified target volume delineation method for inoperable oesophageal cancer patients. Tumour response and 1- and 2-year survival rates were used to assess the effect of this method. We determined whether this method could increase locoregional control rate and 1- and 2-year survival rates.
METHODS AND MATERIALS
Oesophageal cancer patients who received concurrent RT with chemotherapy between January 2015 and August 2015 in the hospital were enrolled in this study. Inclusion criteria for this study were as follows: (1) oesophageal cancer confirmed by pathological biopsy; (2)patients without distant metastasis; (3) patients whose tumour was unsuitable for oesophagectomy; (4) patients having declined surgical treatment and (5) patients having signed the informed consent. Before definitive chemoradiotherapy, tumour staging was evaluated by CT scan or positron emission tomography scan, endoscopy, endoscopic ultrasound and barium swallow. This study was approved by the Ethics Committee of the hospital. Written informed consent was obtained from all patients before participation.
Radiotherapy
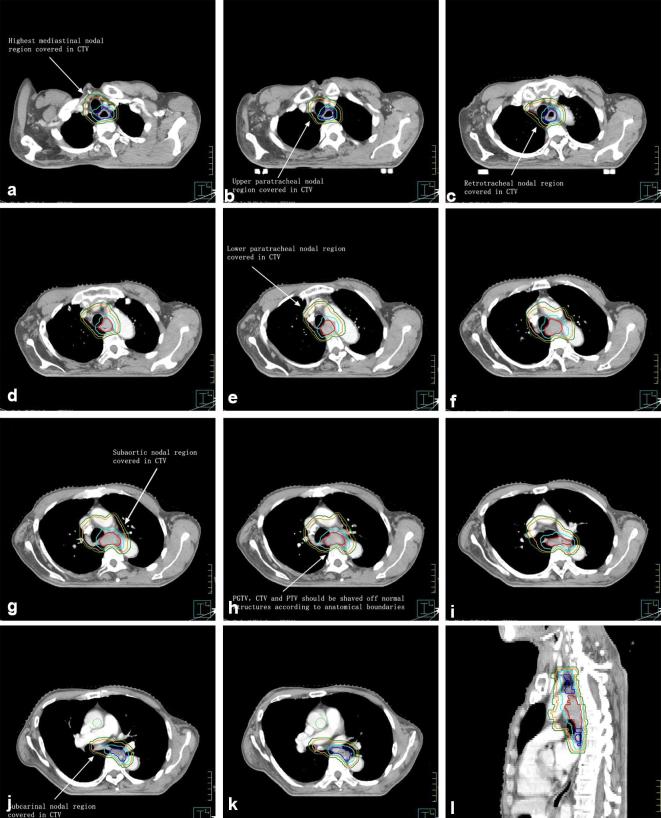
Delineation of target volumes is shown in Figure 1.
Figure 1.
GTV-t is in red line; 3 margin beyond GTV-t longitudinally is in blue line; PGTV is in sky-blue line; CTV is in orange line; PTV is in forest-green line. This 65-year-old male patient’s pathological type consisted squamous cell carcinoma. CTV, clinical target volume; GTV, gross tumour volume; PGTV, planning gross target volume; PTV, planning target volume. The order of pictures was from A to L.
The primary gross tumour volume (GTV-t) encompasses the primary tumour mass. The oesophageal wall thickness of more than 5 mm on CT scan, which is often considered abnormal, should be included in the GTV. The volume for positive lymph nodes (GTV-n) encompasses enlarged lymph nodes. Any lymph nodes of more than 10 mm in the short axis should be included in the GTV-n.
Standard GTV-t to planning gross target volume (PGTV-t) expansions for the primary tumour are 3 cm in the superior-inferior direction and 0.5 cm in the right-left direction and the anteroposterior direction. Standard GTV-n to planning gross target volume (PGTV-n) expansions for positive lymph nodes are 0.5 cm in the superior-inferior direction, the right-left direction and the anteroposterior direction. PGTV included both PGTV-t and PGTV-n.
Standard treatment margins from the PGTV to clinical target volume (CTV) are 0.5 cm in all directions. CTV was defined as PGTV plus a 0.5 cm PGTV to CTV margin in all directions and elective nodal region. CTV should be shaved off normal structures according to anatomical boundaries.
The 0.5 cm CTV to PTV margin can be applied to ensure adequate dose coverage to the CTV due to the set-up error.
The dose of PGTV is 54–60 Gy in 27–30 fractions (2Gy per fraction). The dose of PTV is 48.6–54 Gy in 27–30 fractions (1.8 Gy per fraction). The treatment goal was to deliver a dose of 54–60 Gy (2 Gy/fraction) to 95% of PGTV and a dose of 48.6–54 Gy (1.8 Gy/fraction) to 95% of the PTV simultaneously while potentially reduce dose to the heart, lung and other normal organs.
Planning dose constraints
More than 95% of the PGTV received an isodose line of 54–60 Gy. And more than 95% of the PTV received an isodose line of 48.6–54 Gy. The maximum point doses to the spinal cord were to be less than 40 Gy. For the liver, V60 was kept to be lower than 30%. For the heart, V40 was kept to be <30%. For the lung, V30, V20, V10 and V5 were kept to be <20%, <30%, <45% and <60%, respectively. The mean dose to both lungs was to be less than 20 Gy. For the stomach, V45 was kept to be lower than 50%. The maximum point doses to the stomach were to be less than 54 Gy.
All treatment plans were calculated by the Pinnacle treatment planning system (Philips, clinical version 9.2; Fitchburg, WI), with 6 MV beam from linear accelerator. RT was performed by volumetric-modulated arc therapy (Elekta Medical Systems, Stockholm, Sweden). Cone-beam CT (CBCT) was performed once a week.
Chemotherapy
The regimen consists of paclitaxel 135 mg m−2 on 1 day and DDP 25 mg m−2 on 3 days per 3 weeks. The patients received 2 cycles of chemotherapy during radiotherapy and 2–4 cycles of chemotherapy after radiotherapy.
Toxicity evaluation
During radiotherapy, treatment toxicities were assessed at least once a week. Blood routine,liver function and renal function were checked weekly. Electrocardiogram was checked pre, during and post treatment. Electrocardiogram was performed approximately every 1 month for the first 3 months after treatment, then every 6 months thereafter. The radiotherapy-related toxicities, including radiation oesophagitis, radio-induced tracheitis and pneumonia, haematologic toxicities and so on, were evaluated according to the RTOG/European Organization for Research and Treatment of Cancer(EORTC)classification criteria for early- and late-radiation reactions.
Tumour response assessment
Tumour response was evaluated by CT scan, endoscopy, endoscopic ultrasound and barium swallow. CT scan of the neck, chest and abdomen was performed approximately every 3 months for the first year after radiotherapy. The criteria of clinical response were defined by response evaluation criteria in solid tumours. Clinical complete response (cCR) was defined as the disappearance of target lesions by CT and by endoscopy for more than 4 weeks. Clinical partial response (cPR) was defined as a 30% reduction of target lesions at least for more than 4 weeks. Clinical progressive disease (cPD) was defined as more than a 20% increase of target lesions size or a new lesion. Clinical stable disease (cSD) was defined as neither shrinkage to qualify for cPR nor increase to qualify for cPD.
Statistical analysis
This sample size was chosen to accommodate an expected 30% rise of objective response rate (two-sided test; α = 0.05; β = 0.10). And thus at least 29 patients were needed to complete the study. Overall, survival rates were calculated using the Kaplan-Meier method. The data were calculated using SPSS 18.0.
RESULTS
Clinical characteristics
34 patients met the enrollment criteria and participated in this study. All patients did complete RT and concurrent chemotherapy. Those patients had been evaluated by surgeons and were unsuitable for surgical treatment. The median age was 67.5 years (range: 49–83 years). Karnofsky performance status of all patients was ≥80. Five patients were female. All other patients were male. Three patients’ pathological type was adenocarcinoma. All other patients’ pathological type was squamous cell carcinoma. Clinical characteristics are summarized in Table 1. The median V30, V20, V10 and V5 of both the lungs were 57.0, 39.5, 20.5 and 12.5% (range:32–60, 22–45, 8–25 and 4–18%), respectively. The median V40 of the heart was 22.0% (range: 0–29%). The median total PTV volumes were 565.6 cm3 (range: 110.8–832.7 cm3).
Table 1.
Clinical characteristics
| Characteristics | No. of Patients | (%) |
|---|---|---|
| Age | ||
| ≥60 | 26 | 76.5 |
| <60 | 8 | 23.5 |
| Sex | ||
| Male | 29 | 85.3 |
| Female | 5 | 14.7 |
| Karnofsky performance status(KPS) | ||
| 100 | 4 | 11.7 |
| 90 | 26 | 76.5 |
| 80 | 4 | 11.8 |
| Pathological type | ||
| Squamous cell carcinoma | 31 | 91.2 |
| Adenocarcinoma | 3 | 8.8 |
| T-staging | ||
| T1 | 1 | 2.9 |
| T2 | 7 | 20.6 |
| T3 | 12 | 35.3 |
| T4 | 14 | 41.2 |
| N-staging | ||
| N0 | 19 | 55.9 |
| N1 | 8 | 23.6 |
| N2 | 6 | 17.6 |
| N3 | 1 | 2.9 |
| Location of oesophageal cancer | ||
| Cervical | 2 | 5.9 |
| Upper-thoracic | 6 | 17.6 |
| Middle-thoracic | 17 | 50.0 |
| Lower-thoracic | 9 | 26.5 |
| Smoking | ||
| Yes | 14 | 41.2 |
| No | 20 | 58.8 |
Toxicities
Treatment-related acute toxicities are summarized in Table 2. Radiation-related heart damage, radiation-related lung injury were not observed in all patients. No electrocardiogram abnormalities were observed in all patients. Grade 1 radiation-related oesophagitis was observed in 5 patients. Grade 1 dermatological adverse events were observed in 4 patients. The haematological toxicity was as follows: 12 patients (35.3%) with Grade 1 white blood cell decreased, 9 patients (26.4%) with Grade 2 white blood cell decreased, 6 patients (17.6%) with Grade 3 white blood cell decreased, 1 patient (2.9%) with Grade 4 white blood cell decreased; 12 patients (35.3%) with Grade 1 neutrophils decreased, 8 patients (23.5%) with Grade 2 neutrophils decreased, 6 patients (17.6%) with Grade 3 neutrophils decreased, 1 patient (2.9%) with Grade 4 neutrophils decreased; 14 patients (41.2%) with Grade 1 haemoglobin decreased, 4 patients (11.8%) with Grade 2 haemoglobin decreased, no patient with Grade 3 or 4 haemoglobin decreased; 4 patients (11.8%) with Grade 1 platelet count decreased, 8 patients (23.5%) with Grade 2 platelet count decreased and no patient with Grade 3 or 4 platelet count decreased.
Table 2.
Treatment-related acute toxicities
| Grade | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| White blood cell count | 6 | 12 | 9 | 6 | 1 |
| Neutrophil count | 7 | 12 | 8 | 6 | 1 |
| Haemoglobin | 16 | 14 | 4 | 0 | 0 |
| Platelet count | 22 | 4 | 8 | 0 | 0 |
| Radiation-related heart damage | 34 | 0 | 0 | 0 | 0 |
| Radiation-related lung injury | 34 | 0 | 0 | 0 | 0 |
| Radiation-related oesophagitis | 29 | 5 | 0 | 0 | 0 |
| Radiation-related skin damage | 30 | 4 | 0 | 0 | 0 |
Treatment effect
The median follow-up time was 20.9 months (range: 3.7–28.4 months) for all patients, with no one lost to follow-up. The 1- and 2-year survival rates for all patients were 70.5 and 44.1%, respectively (Figure 2). cCR was observed in 21 patients (61.8%), who were diagnosed with endoesophagitis or oesophageal scars by endoscopy and biopsy after the treatment. And no residual disease of those 21 patients was discovered by endoscopy after the treatment. All target lesions of those 21 patients disappeared in CT. cPR was observed in 9 patients (26.5%), who were diagnosed with residual disease by endoscopy and biopsy after the treatment. More than 30% reduction of target tumours in 9 patients were observed in CT. cSD was observed in 4 patients (11.7%), who were diagnosed with residual disease by endoscopy and biopsy after the treatment. Less than 30% decrease of target tumours and less than 20% increase of target tumours in 4 patients were observed in CT. No patient was observed as having cPD.
Figure 2.
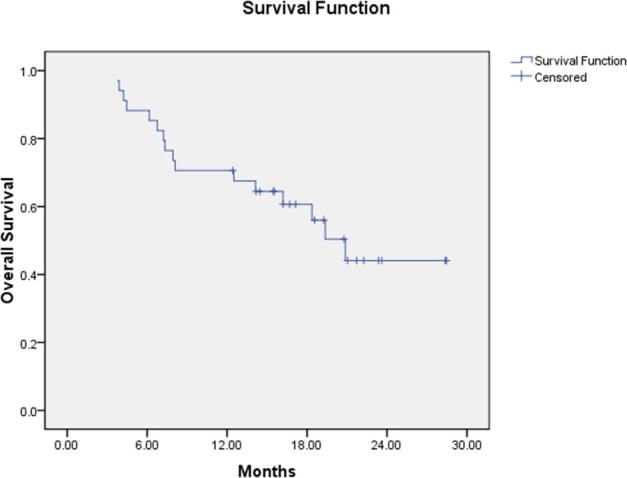
Kaplan-Meier overall survival curve for 34 patients.
DISCUSSION
We found that the 1- and 2-year survival rates in our study were close to those survival rates of other studies.7–11 Chen and colleagues7 reported that the 1-year survival rate of patients with oesophageal cancer following concurrent chemoradiotherapy was 72.5%. RTOG 02468 reported that the 1-year survival rates for all patients with locoregionally advanced oesophageal cancer after concurrent chemoradiation is 71%. RTOG 01139 reported that the 1-year survival rate of patients with localized oesophageal cancer receiving fluorouracil-based chemotherapy and concurrent radiotherapy was 75.7%. Nishimura Y and colleagues10 reported that 2-year survival rates of patients with oesophageal cancer following concurrent chemoradiotherapy in two different arms were 46 and 44%. Tomblyn MB and colleagues11 reported that 2-year survival rates within the patients with squamous-cell histology and with adenocarcinoma were 45.5 and 20.0%, respectively.
All patients with locoregionally advanced oesophageal cancer in RTOG 0246 received concurrent chemoradiotherapy.8 PTV was defined as having 3 cm superior and inferior margin beyond the edges of the grossly visible carcinoma and 2 cm lateral margin beyond the edges of the grossly visible tumour. The dose of PTV was 50.4 Gy in 28 fractions with 1.8 Gy d–1. Compared to our study, the total dose was lower than the total dose in our study. And, it was not to deliver a higher dose to PGTV or GTV. This means the primary tumour, positive lymph nodes and subclinical lesions were not treated by high dose.
RTOG 01139 used the three-dimensional planning technique to treat patients with localized oesophageal cancer. CTV included GTV, a 3-cm cephalad and caudad margin beyond GTV and locoregional lymph nodes. PTV was defined as having a 2 cm margin around CTV and 2 cm superior and inferior margin beyond the CTV. The total dose of PTV was 50.4 Gy in 28 fractions (1.8Gy per fraction). RTOG 0113 had no PGTV. And tumour in RTOG 0113 received the total dose of 50.4 Gy, which was lower than the dose mentioned in our study (54–60 Gy).
RTOG 94-0512 compared the effect of high-dose RT with the effect of standard-dose RT for oesophageal cancer. Conventional radiotherapy was delivered with the combination of anterior/posterior, oblique or lateral fields. The standard dose (50.4 Gy) was delivered to the radiation fields including primary tumour, 5-cm margin beyond the primary tumour longitudinally and ≥2 cm margin beyond the primary tumour circumferentially. High-dose arm was received a cone down of 14.4 Gy to a total dose of 64.8 Gy. In high-dose target volume, normal tissues, which was involving in 2 cm margin beyond the primary tumour longitudinally and ≥2 cm margin beyond the primary tumour circumferentially, received a total dose of 64.8 Gy. Compared to our study, RTOG 94-05 had no CTV and PGTV. And high-dose target volume was bigger than target volume of our study, which only included 0.5 cm beyond the primary tumour circumferentially. The dose in high-dose arm was higher than the dose mentioned in our study, which was 54–60 Gy.
A trial (RTOG 85-01)13 by Cooper and colleagues reported that 1-year survival rate of non-disseminated oesophageal cancer getting concurrent chemoradiotherapy would be 52%. In RTOG 85-01, radiation was delivered at 30 Gy/15 Fx in 2 Gy per fraction from the supraclavicular fossae to the oesophagogastric junction. Then it was followed by 20 Gy/10 Fx to the primary tumour length plus a 5-cm margin cephalad and caudad. The RT consisted of 50 Gy/25 Fx in 2 Gy per fraction over 5 weeks. The RT method of RTOG 85-01 was that the initial 30 Gy was delivered to the whole oesophagus from the supraclavicular fossae to the oesophagogastric junction and the final 20 Gy was delivered to a reduced volume including the primary tumour length plus a 5 cm margin cephalad and caudad. Compared to our study, RTOG 85-01 was not to deliver a higher dose (54–60 Gy) to GTV or PGTV. The primary tumour and subclinical lesion did not receive high dose.
The SCOPE1 trial14 used definitive chemoradiotherapy to treat patients with localized oesophageal squamous-cell cancer and adenocarcinomas. CTV was created by adding 2 cm manually along tumour superiorly-inferiorly and 1 cm laterally. PTV was then calculated by adding 1 cm superiorly-inferiorly and 0.5 cm laterally to CTV. Elective nodal irradiation was not done. The total dose of 50 Gy in 25 fractions was delivered to PTV with four radiotherapy fields by 3DCRT. The 2-year survival rate was 41.3% in the CRT plus cetuximab group and 56.0% in the CRT only group. Compared to our study, the SCOPE1 trial has no PGTV and did not deliver a higher dose (54–60 Gy) to tumour. But the 2-year survival rate in the CRT only group of the SCOPE1 trial was higher than the 2-year survival rate in our study. The patients in the CRT only group of the SCOPE1 trial received 2 cycles of intravenous cisplatin and oral capecitabine before radiotherapy; Then the patients received 2 cycles of chemotherapy concurrently with radiotherapy. The patients in our study received 2 cycles of intravenous cisplatin and paclitaxel with concurrent radiotherapy; then the patients received 2–4 cycles of chemotherapy after radiotherapy.
Those studies8,9,12–14 did not deliver a high dose to GTV and only did deliver a dose of approximately 50 Gy to 95% of PTV. Table 3 shows the target volume delineation methods and dose delivered in those references.
Table 3.
Difference target volume delineation methods and dose delivered in the references
| Study | Treatment regimen | Fields | Dose |
|---|---|---|---|
| Button MR et al6 | Definitive chemoradiotherapy | CTV = GTV + 1 cm margin laterally and 2.0 cm margin longitudinally PTV = CTV + 0.5 cm margin laterally and 1.0 cm margin longitudinally | PTV: 50 Gy/25f (2.0 Gy/1f/d) |
| Chen CZ et al7 | Definitive chemoradiotherapy | CTV = GTV+1.0 cm margin laterally and 2 cm margin longitudinally PTV = CTV + 0.5 cm in all directions | The dose of PTV: 50–74 Gy/25–37f (2 Gy/1f/d) |
| RTOG 02468 | Definitive chemoradiotherapy with selective surgical salvage | PTV = GTV+2.0 cm margin laterally and 3.0 cm margin longitudinally | The dose of PTV: 50.4 Gy/28f (1.8 Gy/1f/d) |
| RTOG 01139 | Definitive chemoradiotherapy | CTV = GTV+3.0 cm margin longitudinally + locoregional lymph nodes PTV = CTV + 2.0 cm margin in all directions | The dose of PTV: 50.4 Gy/28f (1.8 Gy/1f/d) |
| KROSG0101/JROSG02110 | Definitive chemoradiotherapy | CTV1 = GTV + locoregional lymph nodes CTV2 = GTV + 0.5 cm margin laterally and 1 cm margin longitudinally PTV1/PTV2 = CTV1/CTV2 + 1.0 cm margin longitudinally and 0.5 cm margin laterally | The dose of PTV1: initial 40 Gy/20f (2 Gy/1f/d) The dose of PTV2: final 20 Gy/10f (2 Gy/1f/d) |
| S041411 | Definitive chemoradiotherapy | CTV = GTV + 2.0 cm margin laterally and 5.0 cm margin longitudinally + locoregional lymph nodes PTV were left to the discretion of oncologist | The dose of PTV: 50.4 Gy/28f (1.8 Gy/1f/d) |
| RTOG 94–0512 | Definitive chemoradiotherapy | PTV1 = GTV + ≥2.0 cm margin laterally and 5.0 cm margin longitudinally+locoregional lymph nodes PTV2 = GTV + 2.0 cm margin longitudinally | In 50.4 Gy group, the dose of PTV1: 50.4 Gy/28f (1.8 Gy/1f/d) In 64.8 Gy group, the dose of PTV1: 50.4 Gy/28f (1.8 Gy/1f/d); and then the dose of PTV2: 14.4 Gy/8f (1.8 Gy/1f/d). |
| RTOG 85–0113 | Definitive chemoradiotherapy | PTV1 = the whole oesophagus from the supraclavicular fossae to the oesophagogastric junction PTV2 = GTV + 5.0 cm margin | PTV1: 30 Gy/15f (2.0 Gy/1f/d) PTV2: 20 Gy/10f (2.0Gy/1f/d) |
| SCOPE114 | Definitive chemoradiotherapy | CTV = GTV + 1 cm margin laterally and 2.0 cm margin longitudinally PTV = CTV + 0.5 cm margin laterally and 1.0 cm margin longitudinally | PTV: 50 Gy/25f(2.0 Gy/1f/d) |
In this study, since the total doses of PGTV and PTV were 54–60Gy and 48.6–54Gy, respectively, the primary tumour and subclinical lesion received high dose and, meanwhile, adjacent normal tissues received low dose. Our method of delineating target volumes was to improve radiation dose of PGTV or GTV while keeping radiation doses to adjacent normal tissues below tolerance. Meanwhile, the preventive dose was delivered to elective nodal region in order to prevent lymphatic metastasis.
There were some limitations in our study. Firstly, the most important one was that the tumour response was confirmed by CT and endoscopy, not by surgical pathology. So our study could not evaluate tumour pathological response after CRT, which was more precise than tumour clinical response. Jones DR et al15 demonstrate that CT is a poor diagnostic tool to assess the pathologic tumour response after CRT in oesophageal cancer patients. Endoscopic biopsy is not a sensitive predictor of residual cancer after chemoradiation in oesophageal cancer patients.16 Secondly, the number of patients was not enough in our study. Thirdly, there were no long-term outcomes in our study. Fourthly, the pathological type in our study predominantly consisted of squamous cell cancers.
In conclusion, we designed a Phase II study to test the safety and efficacy of concurrent chemoradiotherapy with a modified target volume delineation method for inoperable oesophageal cancer patients.
The 1- and 2-year survival rates in our study approached those survival rates of other studies. cCR was observed in 21 patients, cPR was observed in 9 patients and cSD was observed in 4 patients. In future, a study with a large number of patients should be performed to evaluate the effectiveness of this method to improve locoregional control and survival rate. The study should find a new method to assess tumour response of inoperable oesophageal cancer patients treated by concurrent chemoradiotherapy.
Contributor Information
Wenyi Zhang, Email: 53128408@qq.com.
Huifang Li, Email: 174334075@qq.com.
Xingxing Chen, Email: chenxing1819@126.com.
Meng Su, Email: smeng1989@163.com.
Ruifang Lin, Email: 174334075@qq.com.
Changlin Zou, Email: zcl19670115@163.com.
REFERENCES
- 1.Ilson DH, Minsky BD, Ku GY, Rusch V, Rizk N, Shah M, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer 2012; 118: 2820–7.https://doi.org/10.1002/cncr.26591 [DOI] [PubMed] [Google Scholar]
- 2.Yin L, Wu H, Gong J, Geng JH, Jiang F, Shi AH, et al. Volumetric-modulated arc therapy vs. c-IMRT in esophageal cancer: a treatment planning comparison. World J Gastroenterol 2012; 18: 5266–75.https://doi.org/10.3748/wjg.v18.i37.5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urba S. Esophageal cancer: preoperative or definitive chemoradiation. Ann Oncol 2004; 15(Suppl 4): iv93–6.https://doi.org/10.1093/annonc/mdh910 [DOI] [PubMed] [Google Scholar]
- 4.Lee NY, Lu JJ. Target volume delineation and field setup: a practical guide for conformal and intensity-modulated radiation therapy. Berlin: Springer; 2013. [Google Scholar]
- 5.Clifford Chao KS. Practical essentials of intensity modulated radiation 3rd. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 6.Button MR, Morgan CA, Croydon ES, Roberts SA, Crosby TD. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009; 73: 818–23.https://doi.org/10.1016/j.ijrobp.2008.04.062 [DOI] [PubMed] [Google Scholar]
- 7.Chen CZ, Chen JZ, Li DR, Lin ZX, Zhou MZ, Li DS, et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World J Gastroenterol 2013; 19: 1639–44.https://doi.org/10.3748/wjg.v19.i10.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swisher SG, Winter KA, Komaki RU, Ajani JA, Wu TT, Hofstetter WL, et al. A phase II study of a paclitaxel-based chemoradiation regimen with selective surgical salvage for resectable locoregionally advanced esophageal cancer: initial reporting of RTOG 0246. Int J Radiat Oncol Biol Phys 2012; 82: 1967–72.https://doi.org/10.1016/j.ijrobp.2011.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajani JA, Winter K, Komaki R, Kelsen DP, Minsky BD, Liao Z, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 2008; 26: 4551–6.https://doi.org/10.1200/JCO.2008.16.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura Y, Hiraoka M, Koike R, Nakamatsu K, Itasaka S, Kawamura M, et al. Long-term follow-up of a randomized Phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer (KROSG0101/JROSG021). Jpn J Clin Oncol 2012; 42: 807–12.https://doi.org/10.1093/jjco/hys112 [DOI] [PubMed] [Google Scholar]
- 11.Tomblyn MB, Goldman BH, Thomas CR Jr, Benedetti JK, Lenz HJ, Mehta V, et al. Cetuximab plus cisplatin, irinotecan, and thoracic radiotherapy as definitive treatment for locally advanced, unresectable esophageal cancer: a phase-II study of the SWOG (S0414). J Thorac Oncol 2012; 7: 906–12.https://doi.org/10.1097/JTO.0b013e31824c7bed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002; 20: 1167–74.https://doi.org/10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–7. [DOI] [PubMed] [Google Scholar]
- 14.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013; 14: 627–37.https://doi.org/10.1016/S1470-2045(13)70136-0 [DOI] [PubMed] [Google Scholar]
- 15.Jones DR, Parker LA Jr, Detterbeck FC, Egan TM. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 1999; 85: 1026–32.https://doi.org/10.1002/(SICI)1097-0142(19990301)85:5<1026::AID-CNCR3>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Cleary KR, Yao JC, Swisher SG, Roth JA, Lynch PM, et al. Significance of post-chemoradiation biopsy in predicting residual esophageal carcinoma in the surgical specimen. Dis Esophagus 2004; 17: 38–43.https://doi.org/10.1111/j.1442-2050.2004.00355.x [DOI] [PubMed] [Google Scholar]