ABSTRACT
The existence of vaccine-associated optic neuritis is essentially based on the temporal relationship between the administration of a vaccine and the development of optic neuritis in patients with no other known aetiologies for infectious or non-infectious inflammation that could account for the optic neuritis. Influenza vaccine (inactivated or live attenuated) is considered to be one of vaccines that could be related to optic neuritis. The authors describe a rare case of bilateral asymmetric optic neuritis with leptomeningeal enhancement on magnetic resonance imaging (MRI) in a previously healthy young woman who received inactivated influenza vaccination 2 weeks before the onset of symptoms.
KEYWORDS: Influenza vaccination, leptomeningealenhancement, MRI, optic neuritis
Introduction
The existence of vaccine-associated optic neuritis is essentially based on the temporal relationship between the administration of a vaccine and the development of optic neuritis in patients with no other known aetiologies for infectious or non-infectious inflammation that could account for the optic neuritis. Influenza vaccine (inactivated or live attenuated) is considered to be one of vaccines that could be related to optic neuritis. There are three different influenza vaccines (egg based, cell based, and recombinant) approved by the US Food and Drug Administration (FDA). The egg-based manufacturing process is the most common and has existed for more than 70 years. It is used to make both inactivated (killed) vaccine (flu shot) and live-attenuated vaccine (nasal spray). Attenuated (weakened) live viruses cannot cause flu illness. The weakened viruses are cold-adapted, which means they are designed to only cause infection at the cooler temperatures found within the nose. The live viruses in the nasal spray cannot infect the lungs or other areas where warmer temperatures exist. Cell-based influenza vaccine also uses egg-grown viruses but does not require large numbers of chicken eggs because the viruses are grown in animal cells and it takes slightly less time to manufacture cell-based influenza vaccines than egg-based vaccines. Recombinant influenza vaccine, approved in 2013, is the only 100% egg-free vaccine on the US market.
We describe a rare case of bilateral asymmetric optic neuritis with leptomeningeal enhancement on magnetic resonance imaging (MRI) in a previously healthy young woman who received inactivated influenza vaccination 2 weeks before the onset of symptoms.
Case report
A 23-year-old Indian woman presented to the neuro-ophthalmology clinic because of a 5-day history of right frontal headache and a 3-day history of blurred vision and pain on eye movement of the right eye. She recently arrived in the United States from India and as a requirement for college admission received an influenza vaccination 2 weeks prior to the development of symptoms. She had no other constitutional symptoms such as skin rash, fever, and muscle aches and no recent history of febrile illness or tick exposure. Initial ophthalmologic examination of the right eye showed reduced visual acuity (20/40), dyschromatopsia (4/10 on Hardy-Rand-Rittler [HRR] test), a visual field defect (constriction of peripheral visual field) on Humphrey automated perimetry, a relative afferent pupillary defect, and optic disc swelling suggestive of optic neuritis. The left eye was normal on examination, and neurologic examination was otherwise unremarkable. MRI of the brain and orbits with and without gadolinium showed enhancement of the right retrobulbar optic nerve and right hemispheric leptomeninges (Figure 1).
Figure 1.
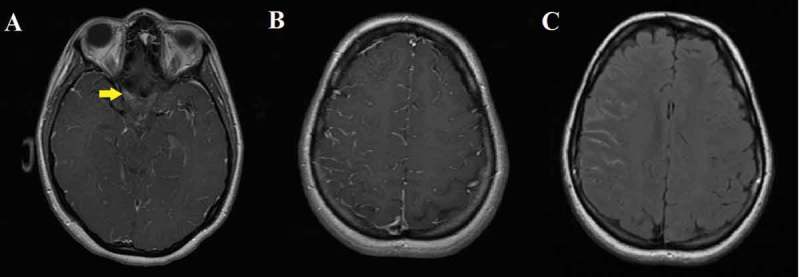
Magnetic resonance imaging (MRI) of the brain with and without gadolinium. (A) T1 axial with contrast showed enhancement of the right optic nerve (arrow). (B) T1 axial with contrast showed enhancement of the leptomeninges on the right frontoparietal lobes. (C) FLAIR axial showed increased intensity of the leptomeninges on the right frontoparietal lobes.
Given the atypical presentation of optic neuritis, further evaluation was performed. Serologic studies showed increased erythrocyte sedimentation rate (37 mm/h) and C-reactive protein (1.7 mg/L), mildly elevated immunoglobulin G4 (IgG4) (112.7 mg/dL) and low vitamin D; however, antinuclear antibody, antineutrophil cytoplasmic antibody, anti-Ro/La, C3/4, aquaporin 4 (AQ4) antibody, angiotensin-converting enzyme (ACE), Lyme, syphilis, human immunodeficiency virus (HIV), Bartonella, Brucella, and quantiferon gold were negative. Vitamins B1, B6, B12, and E levels were normal. Opening pressure on lumbar puncture was 23 cm H2O, and cerebrospinal fluid (CSF) showed leukocytosis (45/μL; mononuclear dominant), normal protein and glucose levels, no oligoclonal IgG, negative tests for cytomegalovirus, Epstein-Bar virus, herpes simplex virus, and varicella-zoster virus, and negative cultures for Mycobacterium and other fungi. Chest computed tomography was normal except for a small thyroid nodule. Positron emission tomography (PET) scan showed no lesion outside the brain and orbit. Her vision of the right eye started improving, and she deferred treatment and did not receive corticosteroid.
About 3 weeks after the initial visit, the patient developed mild pain and blurred vision in the fellow (left) eye. Repeat brain MRI with and without gadolinium showed new abnormal enhancement of the left optic nerve. Given the atypical presentation of the inflammatory optic neuritis and the involvement of fellow eye, she underwent meningeal and brain biopsies with craniotomy from the right frontal lobe, which showed reactive astrogliosis with lymphocytic infiltration without evidence of neoplasm, granulomas, or vasculitis. Her vision started improving at hospital day 4 and had improved to 20/20 in both eyes by the time of discharge. She deferred intravenous corticosteroid therapy but received oral corticosteroid for 14 days with tapering. A week after completing the course of oral corticosteroids, she experienced worsening of the vision in her right eye (counting fingers at 3 ft). Repeat brain and spinal MRI showed enhancement of the right optic nerve. Repeated rheumatologic work-up, infectious disease work-up, CSF analysis, and paraneoplastic antibody assay, including repeated AQ4 antibody, were unremarkable or negative. She received a 3-day course of intravenous corticosteroid, with minimal improvement of her vision, and then received 5-day plasmapheresis. Her vision of the right eye recovered to 20/25 in 2 weeks after the completion of the treatment and in 3 weeks after recurrence of vision loss.
Discussion
This is a rare case of idiopathic, atypical, sequential, bilateral optic neuritis with leptomeningeal enhancement on MRI in a young woman who had no previous medical history other than influenza vaccination about 2 weeks prior to the development of visual disturbance.
Numerous reports suggest that demyelination may be associated with various types of vaccine, including MMR (measles, mumps, and rubella), hepatitis, diphtheria, pertussis, tetanus and influenza vaccines. There are various case reports describing an association of optic neuritis and influenza vaccination based on the temporal relationship (Table 1).1–12 Both the inactivated and live-attenuated vaccine have been temporally related to the development of optic neuritis. However, reports of such cases should not be interpreted as a proof of cause. A recent review13 regarding adverse effects of vaccines stated that it is inadequate to accept or reject a causal relationship between influenza vaccine and optic neuritis. The temporal relationship between vaccine administration and the onset of acute optic neuritis in the absence of known infectious or non-infectious inflammatory aetiologies, coupled with some evidence from biological plausibility studies, suggests that the concept of an immune-mediated attack against optic nerves after vaccination in genetically susceptible individuals does exist, and the possible link should be considered. Here we present a case of optic neuritis that could be linked to alteration of immune system by influenza vaccination.
Table 1.
Case reports of unilateral or bilateral optic neuritis following influenza vaccination.
| Year of report | First author | Type of influenzavaccine | Age/Sex | Interval betweenvaccination andsymptom onset | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1971 | Wells2 | Inactivated | 26/M | 2 weeks | PO steroids | Full recovery in 2 months |
| 1977 | Bienfang3 | Inactivated | 27/M | 3 weeks | PO steroids | Full recovery in 3 weeks |
| 1979 | Perry4 | Inactivated | 58/M | 6 days | PO steroids | Full recovery in 12 months |
| 1980 | Cangemi5 | Inactivated | 38/M | 3 weeks | None | Optic atrophy at 3 months |
| 1982 | Macoul6 | Inactivated | 31/M | 3 weeks | None | Optic atrophy at 26 months |
| 1996 | Ray7 | Inactivated | 61/F | 3 weeks | IV steroids | Partial recovery in 12 months |
| 1997 | Hull8 | Inactivated | 59/F | 2 weeks | IV steroids | No improvement |
| 2010 | Tan9 | Inactivated | 55/F | 3 weeks | IV/PO steroids | Full recovery in 6 months |
| 2012 | Crawford10 | Live attenuated | 13/M | 2 weeks | IV steroids | Full recovery in 3 months |
| 2012 | Rubinov11 | Inactivated | 18/M | 2 weeks | IV/PO steroids | Full recovery in 3 months |
Note. PO = oral; IV = intravenous.
The exact pathophysiologic mechanism of post-vaccination optic neuritis is not clearly defined. It could be triggered by a common denominator such as an adjuvant14 used in the vaccine to enhance the antigen-specific immune response, which perhaps induces the release of inflammatory cytokines and interacts with Toll-like receptors and NLRP (NOD-like receptor protein) inflammasomes. Post-vaccination optic neuritis and other post-vaccination autoimmune phenomena could be understood as among the autoimmune/inflammatory clinical syndromes induced by adjuvants (ASIA),15 which include macrophagic myofasciitis, Gulf War syndrome, siliconosis, and other post-vaccination phenomena. Post-vaccination autoimmune responses are characterised by hyperactive immune responses to a common pathogenic denominator and accompanied by a similar complex of signs and symptoms. However, with some vaccines, the weakened or inactivated virus stimulates a strong immune response so no additional adjuvant is needed for protection against infection. In the United States, most vaccines against measles, mumps, rubella, chickenpox, rotavirus, polio, and seasonal influenza do not contain added adjuvants. In 2015, the US Food and Drug Administration (FDA) approved the first seasonal influenza vaccine containing adjuvant. Called FLUAD, this trivalent vaccine is produced from three influenza virus strains (two subtypes A and one type B), is manufactured using an egg-based process, and is formulated with the adjuvant MF59, an oil-in-water emulsion of squalene oil. It might be interesting to observe as data accumulate on this vaccine if it is associated with an increased occurrence of post–influenza vaccination autoimmune clinical syndromes, including optic neuritis.
Post–influenza vaccination optic neuritis is uncommon. The pathophysiology of development is presumably immune-mediated. Based on a review of most of the reported cases (including ours), optic neuritis usually develops around 2 to 3 weeks after influenza vaccination. Systemic corticosteroid therapy may facilitate recovery and prevent vision loss. To prove the causality between influenza vaccine and optic neuritis, extensive epidemiological studies would be required, but the rare occurrence of influenza vaccine–associated optic neuritis and the underreporting of such cases might be a limitation in collecting data to support such causality. Well-designed animal studies may help reveal the causality and understand the pathophysiologic mechanism of development of post–influenza vaccination optic neuritis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- [1].Stubgen JP. A literature review on optic neuritis following vaccination against virus infections. Autoimmun Rev 2013;12:990–997. [DOI] [PubMed] [Google Scholar]
- [2].Wells CE. A neurological note on vaccination against influenza. BMJ 1971;3:755–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bienfang BC, Kantrowitz FG, Noble JL, Raynor AM.. Ocular abnormalities after influenza immunization. Arch Ophthalmol 1977;95:1649. [DOI] [PubMed] [Google Scholar]
- [4].Perry HD, Mallen FJ, Grodin RW, Cossari AJ.. Reversible blindness in optic neuritis associated with influenza vaccination. Ann Ophthalmol 1979;11:545–550. [PubMed] [Google Scholar]
- [5].Cangemi FE, Bergen RL.. Optic atrophy following swine flu vaccination. Ann Ophthalmol 1980;12:857–863. [Google Scholar]
- [6].Macoul KL. Bilateral optic atrophy and blindness following swine influenza vaccination. Ann Ophthalmol 1982;14:398–399. [PubMed] [Google Scholar]
- [7].Ray CL, Dreizin IJ.. Bilateral optic neuropathy associated with influenza vaccination. J Neuroophthalmol 1996;16:182–184. [PubMed] [Google Scholar]
- [8].Hull TP, Bates JH.. Optic neuritis after influenza vaccination. Am J Ophthalmol 1997;124:703–704. [DOI] [PubMed] [Google Scholar]
- [9].Tan FU, Akarsu C, Gullu R, Tulay K.. Bilateral optic neuritis after influenza vaccination. Neuro-ophthalmology 2010;34:115–117. [Google Scholar]
- [10].Crawford C, Grasko MB, Raymond WR. 4th, Rivers BA, Munson PD. Reversible blindness in bilateral optic neuritis associated with nasal flu vaccine. Binocul Vis Strabolog Q Simms Romano 2012;27:171–173. [PubMed] [Google Scholar]
- [11].Rubinov A, Beiran I, Krasnitz I, Miller B.. Bilateral optic neuritis after inactivated influenza vaccination. Isr Med Assoc J 2012;14:705–707. [PubMed] [Google Scholar]
- [12].Pillar G, Toubi E.. Vaccination-induced bilateral optic neuritis: rare but existing [editorial]. Isr Med Assoc J 2012;14:690–691. [PubMed] [Google Scholar]
- [13].Stratton K, Ford A, Rusch E, Clayton EW, eds. Influenza vaccine In Adverse Effects of Vaccines: Evidence and Causalty. Washington DC: National Academies Press; 2012:310–313. [PubMed] [Google Scholar]
- [14].Agmon-Levin N, Kivity S, Szyper-Kravitz M, Shoenfeld Y.. Transverse myelitis and vaccines: a multi-analysis. Lupus 2009;18:1198–1204. [DOI] [PubMed] [Google Scholar]
- [15].Vera-Lastra O, Medina G, Cruz-Dominguez MP, Jara LJ, Shoenfeld Y.. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol 2013;9:361–373. [DOI] [PubMed] [Google Scholar]


