ABSTRACT
Previously, it has been reported that melanopsin-mediated pupillary light response (PLR), measured with pupillometry, is reduced in patients with idiopathic intracranial hypertension (IIH), indicating the clinical utility of the tool in the diagnosis of IIH. In the current study, the authors aimed to measure the PLR in 13 treatment-naive patients with new-onset IIH and 13 healthy controls. In contrast to the previous report, which was based on patients with longstanding IIH (n = 13), the authors found no significant difference in the melanopsin-mediated PLR (p = 0.48).
KEYWORDS: IIH, ipRGCs, melanopsin, PIPR, pupillary light reflex, pupillometry
Background
Idiopathic intracranial hypertension (IIH) is a neurological disorder of unknown cause and characterised by increased intracranial pressure (ICP) without any clinical and laboratory signs of hydrocephalus or space-occupying lesion.1 In parallel with the obesity epidemic, the incidence of IIH is also increasing.2 Although IIH is a relatively rare disease, its socioeconomic impact is extensive due to the young age groups, high hospital admission, and increased morbidity rate.2
Chromatic pupillometry is a quick and non-invasive tool to investigate the afferent and efferent pathways of the pupillary light reflex (PLR).3 Whereas the afferent limb of the PLR consists of the melanopsin containing retinal ganglion cells (ipRGCs), the optic nerve, and the olivary pretectal nucleus (OPN), the efferent PLR signal is mediated by the parasympathetic fibres along the oculomotor nerve. By utilising different light wavelengths, it is possible to selectively stimulate rods, cones, and ipRGCs.4 The ipRGCs express the photopigment melanopsin, which shows maximum spectral sensitivity to blue light (λmax = 480 nm) and mediates important non-image-forming signals to different centres in the brain, including the area for circadian rhythm regulation, called the suprachiasmatic nucleus (SCN), and the OPN, which is the centre for PLR.4 The ipRGCs mediate pupillary constriction during and after light stimulation.5,6 The sustained pupillary constriction after light offset is termed post-illumination pupillary light response (PIPR), which is used as marker of ipRGCs in pupillometric studies.7,8 In addition to the ipRGCs, rod and cone photoreceptors contribute to the transient pupillary constriction to light exposure.5,6 By utilising the light sensitivity of the photoreceptors and the characteristic contribution of the rods, cones, and melanopsin to the dynamics of PLR, researchers have used chromatic pupillometry to differentiate the function of these photoreceptors.4,9–12
Pupillometric studies have shown reduced function of ipRGCs in various diseases, including retinitis pigmentosa, glaucoma, and non‐arteritic anterior ischaemic optic neuropathy (NAION).13–16
Recently, Park et al. reported abnormal rod- and melanopsin-mediated PLR in IIH patients.17 However, their study was performed on patients who had already received treatments. Hence, they investigated the longstanding effect of the intracranial hypertension on the ipRGCs.17 In this study, we investigated the rod-cone– and the melanopsin-mediated light response in treatment-naive and newly diagnosed IIH patients.
Materials and methods
This cross-sectional study complied with the tenets of the Helsinki Declaration and was approved by the Regional Committee on Health Research Ethics (protocol ID: H-3-2014-116). Written informed consent was obtained from all subjects prior to the enrollment.
Enrollment and neuro-ophthalmological work-up
We enrolled consecutively patients, referred for the evaluation of the IIH diagnosis to the Department of Ophthalmology or the Department of Neurology, Rigshospitalet, Glostrup, between February 2015 and March 2016. The eligibility criteria were age between 18 and 60 years and symptoms and clinical signs consistent with IIH.18 The exclusion criteria were previous history of IIH, secondary causes of increased ICP, use of any drugs influencing the PLR, refractive error >6 dioptres, and competing neuro-ophthalmological and systemic conditions affecting the PLR.
The diagnostic work-up and management of suspected IIH patients referred to our hospital are described in detail by Jensen et al.,19 but in summary it consisted of detailed neurological examination including fundoscopy followed by magnetic resonance imaging (MRI) and MR/computed tomography (CT) venography of the brain. When secondary causes of the intracranial hypertension were excluded, lumbar puncture in supine position with stretched legs including opening pressure measurement was performed. Patients with opening pressure above 25 cm H2O were referred to the Department of Ophthalmology for a detailed ophthalmological examination including test of visual acuity (Snellen) and slit-lamp and fundus examination. Spectral-domain optical coherence tomography (SD-OCT; Spectralis software, version 5.3, Heidelberg Engineering, Heidelberg, Germany) of the retina and optic nerve head was used to quantify the papilloedema and peripapillary retinal nerve fibre layer thickness (RNFLT).
Healthy age-matched controls were enrolled from capital region of Denmark during December 2015 and April 2016 to the Department of Ophthalmology, Rigshospitalet, Glostrup, Denmark.
Pupillometry
We used an automated binocular pupilometer (DP-2000 Human Laboratory Pupillometer; NeurOptics, Irvine, CA, USA) to elicit and record the PLR.3 The protocol consisted of 5 minutes dark adaptation, followed by monocular central-field stimulus with red (633 nm) and blue (463 nm) lights in 20 s. The illuminance level was at 100 lux, as measured to 300 CD/m2 during red light exposure and 332 CD/m2 under blue light illumination. Continuous binocular pupil measurements were performed before (10 s), during (20 s), and after (60 s) light stimulus (Figure 1). The pupillary measurements from the stimulated eye were used in the outcome calculation.
Figure 1.
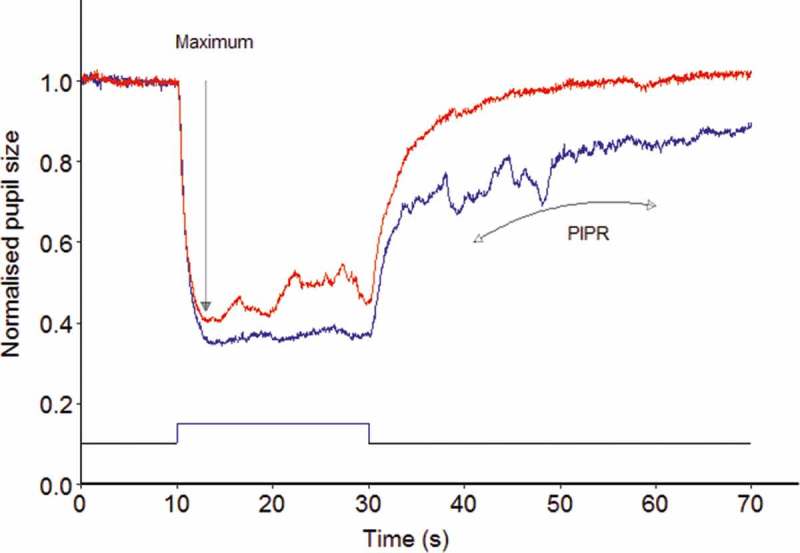
An example of pupil traces obtained with the red (633 nm) and blue (463 nm) light stimuli in a healthy control. Maximum indicates the maximal pupil constriction amplitude during light exposure, whereas PIPR corresponds to post-illumination pupillary response measured at 10–30 s following light offset.
The measured pupil diameters were imported into R statistical program (R version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria), and a custom algorithm was used to detect and remove blink artifacts. To adjust for the inter-subject pupil size variation, the pupil diameter was normalised, i.e., pupillary change was divided by baseline pupil size measured during the initial 10 s before light onset. Maximal pupil contraction was calculated as the mean of pupil contraction amplitude during (3–5 s) light stimulation, and since rods and cones are sensitive to red light and contribute to maximum pupillary constriction, it was regarded as “rod-cone marker” (Figure 1).12,16,20 Post-illumination pupillary light response after light offset has two phases, an early and a late phase. A recent study by Kostic et al. showed that rod photoreceptors contribute to sustained pupillary response 9.5 s after light offset; thus, the early phase of PIPR in our study was a mixed response of rod-cone and melanopsin photoreceptors and the late phase of PIPR was mainly mediated by PIPR.6 We calculated late phase of pupil re-dilation (PIPRLate) as mean pupil contraction amplitude from 10 to 30 s after light offset. And because the late PIPR is mainly controlled by the melanopsin-expressing ipRGCs, we used this parameter as melanopsin-mediated response.6,7
Visual field testing
Automated visual perimetry examination, using Octopus G-dynamic standard program (Haag-Streit AG, Koeniz-Berne, Switzerland), was performed to evaluate the effect of intracranial hypertension on the optic nerve. The Octopus perimeter was used, and the mean defect (MD) was reported. Correlations between MD and pupillary maximal contraction and late PIPR were calculated.
Statistics
Statistical analysis was performed using R statistical program (version 3.2.5). We used the data from the illuminated eye, which was chosen randomly. For all outcomes, means and standard deviations (SD) were reported. The primary outcome was the late PIPR to blue light, which is the marker of ipRGCs.7 Our secondary outcomes were maximal pupil contraction, peripapillary RNFLT, and perimetric MD. Peripapillary RNFLT was used to quantify papilloedema. Mann-Whitney test was performed due to the relatively small data size, which could not be assumed to be normally distributed. Pearson’s squared coefficient quantified the correlations between pupillary metrics, RNFL, perimetric MD, and intracranial pressure (ICP).
Results
Thirteen IIH patients without previous history of IIH and 13 healthy controls were included (Table 1). There was no significant difference in age and IOP between the two groups (p = 0.54 and p = 0.55, respectively). The body mass index (BMI) was significantly higher in the patient group (p = 0.00008), and the visual acuity was slightly decreased in the IIH patients (p = 0.03).
Table 1.
Demographics and clinical characteristics of IIH patients and healthy controls.
| Characteristic | Patients (n = 13) |
Controls (n = 13) |
p value |
|---|---|---|---|
| Sex, F:M | 12:1 | 13:0 | |
| Age, years (mean ± SD) | 32.6 ± 9.8 | 34.5 ± 10.5 | 0.54 |
| BMI, kg/m2 | 34.2 ± 6.1 | 23.4 ± 3.3 | 0.00008 |
| Visual acuity, Snellen | 1.0 ± 0.3 | 1.2 ± 0.1 | 0.03 |
| IOP, mm Hg | 14.6 ± 3.3 | 13.9 ± 3.05 | 0.55 |
| RNFLT | 265.3 ± 110.5 | 104.7 ± 11.6 | 0.000039 |
| Mean defect, dB | 7.2 ± 3.8 | NA | |
| Intracranial pressure (cm H2O) | 36.2 ± 7.6 | NA |
Note. BMI = body mass index; IOP = intraocular pressure; RNFL = retinal nerve layer fibre thickness.
The baseline pupil size prior to blue light stimulus was 6.94 ± 0.86 mm in healthy controls and 6.46 ±1.05 mm in patients with IIH, and there was no significant difference between the two groups in this parameter (p = 0.14). The mean maximal pupil contraction during blue light stimuli was 0.60 ±0.04 and 0.53 ± 0.14 in healthy controls and IIH patients, respectively, and there was no significant difference between the two groups regarding this parameter (p = 0.19; Figure 2). The mean PIPRLate after blue light exposure was 0.12 ± 0.06 in the control group and 0.10 ± 0.05 in patients, and we found no significant difference between patients and healthy controls (p = 0.48; Figure 2).
Figure 2.
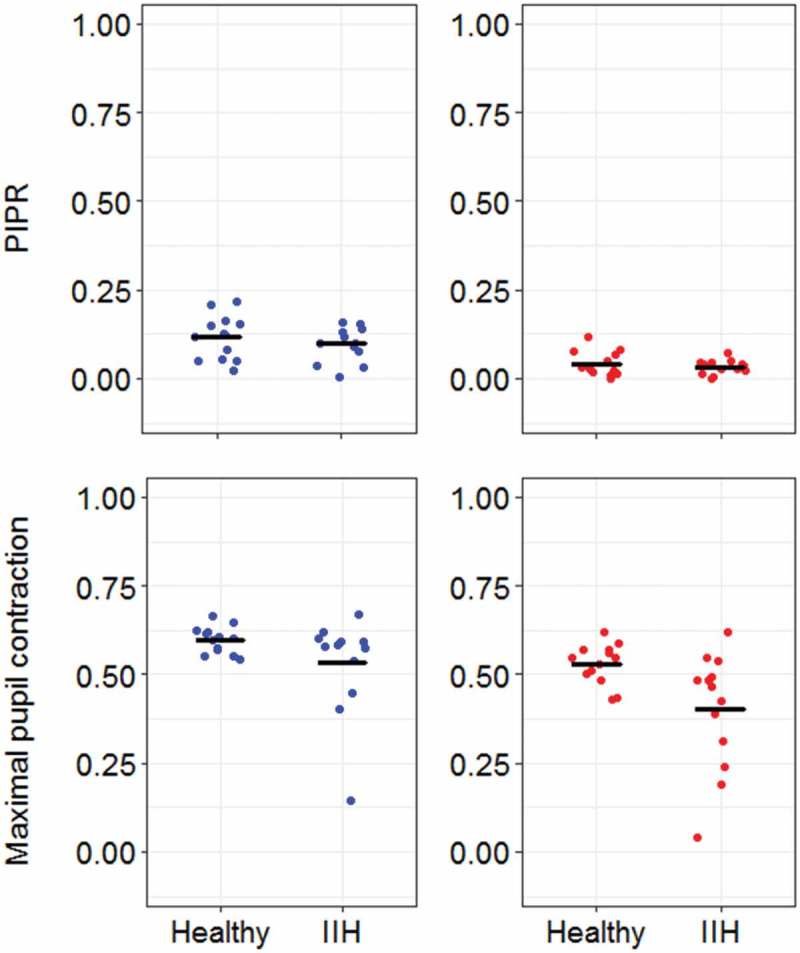
The post-illumination pupil response (PIPR) and the maximum pupil constriction to monocular blue and red light stimuli in healthy controls and patients with idiopathic intracranial hypertension (IIH). The blue and red colours indicate the stimulus colour, and the dark traces indicate the mean of PIPR and maximal pupil constriction. The PIPR and maximal pupil contraction are expressed relative to pupil size prior to light onset, i.e., a smaller value on the y-axis corresponds to pupil constriction relative to pupil size before light onset.
The PLR metrics to red light stimulus were also compared between healthy controls and IIH patients (Figure 2). The mean baseline pupil size was 6.78 ± 0.89 mm in healthy controls and 6.57 ± 1.00 mm in IIH patients; however, no significant difference in baseline pupil size was found between the two groups (p = 0.51). The maximal pupil contraction in the patient group was 0.53 ± 0.06, whereas it was 0.40 ± 0.16 in the control group, and statistical analysis showed that it was significantly reduced in the patient group (p = 0.01). The PIPRLate in the patient group was 0.04 ± 0.04, and in the control group it was 0.03 ± 0.02 (Figure 2). Hence, no significant difference in the PIPRLate was found between the two groups (p = 0.96).
To quantify the magnitude of melanopsin-mediated PIPRLate, we also calculated the difference between red light– and blue light–elicited PIPRLate (PIPR difference). In healthy controls, the mean PIPRLate after blue light stimulus was 0.12, whereas the mean PIPRLate after red light exposure was 0.04; hence, the blue light–elicited PIPRLate was 188% larger than the red light–elicited PIPRLate (p = 0.001). Similarly, the PIPR difference was also larger (201%) in the patient group (p = 0.001), indicating that our light stimulation protocol elicited melanopsin-mediated PIPR both in the healthy group and in the IIH patients.
The mean MD in IIH patients was 7.2 ±3.8 dB (normal limit <2 dB). Correlation analysis between the MD and the PIPRLate showed a clear tendency to significance (p = 0.059; Figure 3). However, we did not find any significant correlation between MD and the maximum pupil contraction in the IIH patients (p = 0.11).
Figure 3.

The correlation between post-illumination pupil response (PIPR) and visual field defects expressed as perimetric mean defect (MD) in the patient group. There was a trend toward significant correlation between PIPR and MD (p = 0.059).
The mean ICP in patients was 36.2 ±7.6 cm H2O, and no significant correlations between ICP and baseline pupil size (p = 0.52), maximum pupil contraction (p = 0.59), or PIPRLate (p = 0.75) were observed. All patients had bilateral papilloedema, and we did not observe any maculopathy in association with the papilloedema. The mean of RNFLT in the IIH patients was 265 ±110 µm, whereas in the healthy controls it was 105 ±12 µm; hence, the RNFLT was significantly larger in patients compared with controls (p = 0.00004; Figure 4). However, no significant correlation between ICP and RNFLT was found (p = 0.21).
Figure 4.
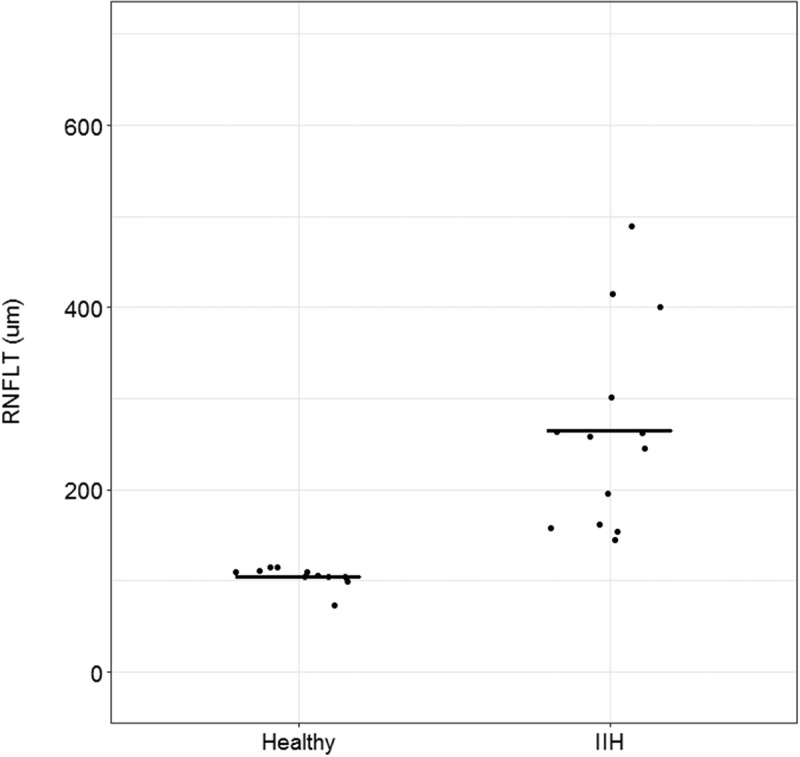
Peripapillary retinal nerve fibre layer thickness (RNFLT) in healthy controls and patients with idiopathic intracranial hypertension (IIH). The RNFLT was significantly larger in IIH patients compared with controls (p = 0.00004). The dark traces indicate the mean RNFLT in healthy controls and IIH patients.
Discussion
To the best of our knowledge, this is the first study to investigate the PLR evoked by rod-cones and ipRGCs in patients with newly diagnosed IIH and without previous treatment. Utilising monocular illumination of red and blue colours, we measured the PLR mediated by rod-cones and ipRGCs, respectively. We found no significant difference in blue light–elicited PIPRLate (ipRGC response). However, we found significant difference in the maximal pupil contraction amplitude evoked by red light stimulus between patients with IIH and healthy controls, indicating rod-cone dysfunction in the IIH group. The RNFLT was significantly larger in IIH patients compared with controls, demonstrating papilloedema in the patient group. The patient group showed visual field defects, and there was a trend toward correlations between MD and pupillometric data, which did not achieve statistical significance.
The ipRGCs are a special type of retinal ganglion cells, which are sensitive to blue light and regulate a number of non-visual functions, including the PLR and the circadian photoentrainment.21–23 Previous studies have shown reduced function of ipRGCs in certain optic neuropathies, i.e., glaucoma and non-arteritic anterior ischaemic optic neuropathy (NAION), whereas in other diseases such as autosomal dominant optic neuropathy and Leber’s hereditary optic neuropathy, these cells are reported to be preserved.13,15,24–26
The function of ipRGCs in IIH has previously been studied by Park and colleagues, showing reduced PIPR by means of pupillometry.17 Hence, our findings are contrary to the results reported by Park and colleagues. However, there was an important difference between our patients and the patient population used by Park et al. The majority (77%) of the patients reported by Park et al. were already treated with acetazolamide, weight loss, or implantation of ventriculoperitoneal shunt, indicating a long-term effect of intracranial hypertension on the optic nerve.17 The age, sex, and visual acuity were similar between our patients and the cohort reported by Park et al.17 In our patient cohort, the visual field analysis was normal (<2 dB) in one subject, mildly abnormal (2–5 dB) in five subjects, and moderately to severely abnormal (>5 dB) in eight patients, whereas in the study carried out by Park et al., the visual field was normal in one subject, mildly abnormal in four subjects, and moderately to severely abnormal in six patients.17 Our results on the correlation between perimetric MD and melanopsin-mediated PIPRLate were in agreement with the findings reported by Park et al.17 The reason that the MD and PIPR did not show significant correlation in our study might be because these photosensitive ganglion cells are known to be highly resistant to pathological conditions and retinal injury.27,28 Thus in patients with newly diagnosed IIH, the ipRGCs might not be damaged yet. Previous studies, involving other diseases, have demonstrated significant correlations between perimetry and pupil parameters, and the lack of correlation in the current study might be due to the range of optic nerve head dysfunction being too narrow.29 Another explanation could be that the perimetric defect may owe to degeneration of the conventional retinal ganglion cells or rod-cone photoreceptors, which are not as highly resistant as the melanopsin-expressing ganglion cells. Animal models have shown degeneration of optic nerve and retinal ganglion cells after only a week of increased ICP.30
Another important difference between our and Park et al.’s study was the expression of PIPR; we used the late redilation phase of PIPR, i.e., from 10 to 30 s after light offset, as marker of melanopsin, whereas Park et al. calculated the PIPR from 5 to 7 s following light offset. Consequently, the PIPR reported by Park et al. was relatively larger compared with the PIPR in our study. However, we calculated the difference between red light– and blue light–elicited PIPR (i.e., net PIPR) and showed significant difference between red- and blue-light PIPR, indicating that our pupillometry protocol was able to differentiate between rod-cone– and melanopsin-elicited PIPR. Moreover, the PIPR calculated during the late redilation phase of pupil response (PIPRLate) is a better marker of melanopsin-mediated response, as Kostic et al. recently reported that rod photoreceptors make significant contribution to PIPR up to 9.5 s after light exposure.6 Nevertheless, we also calculated the PIPR from 5 to 7 s after light offset, but there was still no significant difference between healthy controls and IIH patients (p = 0.58).
We showed reduced rod-cone–mediated pupil contraction amplitude in IIH patients, which is in agreement with the findings by Park et al., who also showed outer retina abnormality in a subgroup of patients with normal melanopsin response.17
Limitation
One important challenge in the current study was to quantify the exact time onset of the disease in our patient group. Due to its relatively low incidence, IIH is a diagnosis of exclusion, and the time span from the first, often rather unspecific, symptoms to the final diagnosis may vary considerably. However, the collaboration between the primary and tertiary sectors (Danish Headache Center and Department of Ophthalmology) is well established, making the communication and diagnostic work-up standardised and with relatively minimum time delay.
Conclusion
The pupillary light response–evoked ipRGCs is preserved in treatment-naive and newly diagnosed patients with IIH.
Funding Statement
This work was supported by grants from the Synoptik Foundation.
Acknowledgements
We thank the staff at Department of Neurology (N28), Rigshospitalet, Glostrup, for assisting in the process of patient recruitment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This work was supported by grants from the Synoptik Foundation.
References
- [1].Friedman DI. The pseudotumor cerebri syndrome. Neurol Clin 2014;32:363–396. doi: 10.1016/j.ncl.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [2].Friesner D, Rosenman R, Lobb BM, Tanne E.. Idiopathic intracranial hypertension in the USA: the role of obesity in establishing prevalence and healthcare costs. Obes Rev 2011;12:e372–e380. doi: 10.1111/j.1467-789X.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- [3].Ba-Ali S, Sander B, Brøndsted AE, Lund-Andersen H.. Effect of topical antiglaucoma medications on late pupillary light reflex, as evaluated by pupillometry. Front Neurol 2015;6:93. doi: 10.3389/fneur.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. . Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology 2009;116:1564–1573. doi: 10.1016/j.ophtha.2009.02.007. [DOI] [PubMed] [Google Scholar]
- [5].Keenan WT, Rupp AC, Ross RA, Somasundaram P, Hiriyanna S, Wu Z, Badea TC, Robinson PR, Lowell BB, Hattar SS. . A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. eLife 2016;5:e15392. doi: 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kostic C, Crippa SV, Martin C, Kardon RH, Biel M, Arsenijevic Y, Kawasaki A. . Determination of rod and cone influence to the early and late dynamic of the pupillary light response. Invest Ophthalmol Vis Sci 2016;57:2501–2508. doi: 10.1167/iovs.16-19150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adhikari P, Feigl B, Zele AJ.. Rhodopsin and melanopsin contributions to the early redilation phase of the post-illumination pupil response (PIPR). PLoS ONE 2016;11:e0161175. doi: 10.1371/journal.pone.0161175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adhikari P, Zele AJ, Feigl B.. The post-illumination pupil response (PIPR). Invest Ophthalmol Vis Sci 2015;56:3838–3849. doi: 10.1167/iovs.14-16233. [DOI] [PubMed] [Google Scholar]
- [9].Zhou W, Lou Y, Pan B, Huang J.. Reliability of field chromatic pupillometry for assessing the function of melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci 2015;56:2519. doi: 10.1167/iovs.15-16672. [DOI] [PubMed] [Google Scholar]
- [10].Wang B, Shen C, Zhang L, Qi L, Yao L, Chen J, Yang G, Chen T, Zhang Z. . Dark adaptation-induced changes in rod, cone and intrinsically photosensitive retinal ganglion cell (ipRGC) sensitivity differentially affect the pupil light response (PLR). Graefes Arch Clin Exp Ophthalmol 2015;253:1997–2005. doi: 10.1007/s00417-015-3137-5. [DOI] [PubMed] [Google Scholar]
- [11].Lorenz B, Strohmayr E, Zahn S, Friedburg C, Kramer M, Preising M, Stieger K. . Chromatic pupillometry dissects function of the three different light-sensitive retinal cell populations in RPE65 deficiency. Invest Ophthalmol Vis Sci 2012;53:5641–5652. doi: 10.1167/iovs.12-9974. [DOI] [PubMed] [Google Scholar]
- [12].Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC . Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 2011;52:6624–6635. doi: 10.1167/iovs.11-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herbst K, Sander B, Lund-Andersen H, Wegener M, Hannibal J, Milea D . Unilateral anterior ischemic optic neuropathy: chromatic pupillometry in affected, fellow non-affected and healthy control eyes. Front Neurol 2013;4:52. doi: 10.3389/fneur.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kankipati L, Girkin CA, Gamlin PD Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci 2010;51:2764–2769. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kankipati L, Girkin CA, Gamlin PD The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci 2011;52:2287–2292. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. . Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology 2011;118:376–381. doi: 10.1016/j.ophtha.2010.06.033. [DOI] [PubMed] [Google Scholar]
- [17].Park JC, Moss HE, McAnany JJ.. The pupillary light reflex in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 2016;57:23–29. doi: 10.1167/iovs.15-18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Friedman DI, Liu GT, Digre KB.. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- [19].Jensen RH, Radojicic A, Yri H.. The diagnosis and management of idiopathic intracranial hypertension and the associated headache. Ther Adv Neurol Disord 2016;9:317–326. doi: 10.1177/1756285616635987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schnapf JL, Kraft TW, Baylor DA.. Spectral sensitivity of human cone photoreceptors. Nature 1987;325:439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- [21].Provencio I, et al. A novel human opsin in the inner retina. J Neurosci 2000;20:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. . Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- [23].Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. . Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- [24].Tsika C, Crippa SV, Kawasaki A.. Differential monocular vs. binocular pupil responses from melanopsin-based photoreception in patients with anterior ischemic optic neuropathy. Sci Rep 2015;5:10780. doi: 10.1038/srep10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feigl B, Mattes D, Thomas R, Zele AJ.. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci 2011;52:4362–4367. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- [26].Ba-Ali S, Christensen, SK, Sander B, Rosenberg T, Larsen M, Lund-Andersen H. . Choroideremia: melanopsin-mediated postillumination pupil relaxation is abnormally slow. Acta Ophthalmol 2017. doi: 10.1111/aos.13394. [DOI] [PubMed] [Google Scholar]
- [27].La Morgia C, Ross-Cisneros FN, Sadun AA, Hannibal J, Munarini A, Mantovani V, Barboni P, Cantalupo G., Tozer KR, Sancisi E, Salomao SR, Moraes MN, Moraes-Filho MN, Heegaard S, Milea D, Kjer P, Montagna P, Carelli V. . Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain 2010;133:2426–2438. doi: 10.1093/brain/awq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Perez de Sevilla Muller L, Sargoy A, Rodriguez AR, Brecha NC. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina PLoS ONE 2014;9:e93274. doi: 10.1371/journal.pone.0093274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kelbsch C, Maeda F, Strasser T, Blumenstock G, Wilhelm B, Wilhelm H, Peters T . Pupillary responses driven by ipRGCs and classical photoreceptors are impaired in glaucoma. Graefes Arch Clin Exp Ophthalmol 2016;254:1361–1370. doi: 10.1007/s00417-016-3351-9. [DOI] [PubMed] [Google Scholar]
- [30].Nusbaum DM, Wu SM, Frankfort BJ.. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res 2015;136:38–44. doi: 10.1016/j.exer.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


