ABSTRACT
Non-arteritic anterior ischaemic optic neuropathy (NAION) and optic neuritis (ON) may be difficult to distinguish early in their disease courses. Our goal was to determine if specific magnetic resonance imaging characteristics differentiate acute NAION from ON. Neuroradiologists, masked to diagnosis, reviewed the diffusion-weighted imaging (DWI) and post-contrast enhancement (PCE) characteristics of the optic nerve in 140 eyes. PCE and DWI signals of the optic disc alone did not discriminate between NAION and ON. After taking age and sex into consideration, only DWI and PCE of the intraorbital segment of the optic nerve differentiated the two, with ON having the increased likelihood of these findings. Isolated PCE without DWI signal at the optic disc, however, was 100% specific for NAION. This may be the most specific way to radiographically differentiate between NAION and ON in the acute setting.
KEYWORDS: Diffusion-weighted imaging, magnetic resonanceimaging, non-arteriticanterior ischaemic optic neuropathy, optic neuritis, post-contrast enhancement
Introduction
Optic neuritis (ON) and non-arteritic anterior ischaemic optic neuropathy (NAION) are the two most common non-glaucomatous optic neuropathies in adults.1,2 ON is an acute inflammatory demyelination of the optic nerve, often associated with multiple sclerosis.3 NAION is the result of an impairment of perfusion of the prelaminar optic disc and is associated with the atherosclerotic vascular disease risk factors of hypertension, hyperlipidaemia, and diabetes mellitus.4 Although ON and NAION differ in their underlying pathophysiology, they share clinical features, especially early in their disease courses.
There are prognostic and therapeutic differences between ON and NAION that make differentiating between the two important. The Optic Neuritis Treatment Trial (ONTT) showed that patients with idiopathic ON have a 50% chance of developing multiple sclerosis (MS) within 15 years of presentation but have good long-term prognoses for visual recovery, with 72% having 20/20 vision at 15 years. These patients also experience a more rapid visual recovery when treated with intravenous (IV) steroids, which may also confer a protective effect from developing MS in the first 2 years.5 In contrast, patients with NAION are at no greater risk of developing MS than the general population, but the prognosis for visual recovery is not as favourable.6 There is also no confirmed evidence of improvement in visual acuity with steroid treatment in the setting of NAION. For these reasons, it is important to establish a diagnosis early in the disease process so that appropriate intervention can be instituted if indicated.
Magnetic resonance imaging (MRI) characteristics of ON have been well described, with enhancement and signal tau inversion recovery (STIR) signal abnormalities of the optic nerve often involving long segments of the intraorbital nerve with occasional intracranial extension. MRI characteristics of NAION have been less well described, but increased STIR signal has been detected in the retrobulbar optic nerve, usually without associated post-contrast enhancement (PCE) and without a predilection for a specific region.7
Diffusion MRI measures the molecular diffusion constant of water molecules as an apparent diffusion coefficient (ADC) and can detect the altered movement of water molecules resulting from disruption of the permeability of central nervous system structures. Historically, diffusion-weighted imaging (DWI) has been used to detect cytotoxic oedema and infarct in acute stroke, but by taking advantage of the highly anisotropic, tightly packed axons of the optic nerve, DWI may play a role in differentiating optic nerve pathologies.8 He et al. suggested that optic nerve DWI might help to differentiate acute ischaemic optic neuropathies from non-ischaemic optic neuropathies by showing diffusion restriction.9
DWI characteristics of NAION have not been robustly studied as a result of technical limitations of imaging, including limited spatial resolution due to the small size of optic nerves, motion artefact, and high signal from adjacent orbital fat and cerebrospinal fluid. There are a number of reports independently reviewing the imaging characteristics of ON and NAION with DWI and T1 post-contrast techniques.7,9–12 To date, however, none have compared the imaging characteristics of these two entities side by side or included a sufficient number of participants for the development of diagnostic imaging criteria.
The aim of this study was to determine essential imaging predictors of NAION and ON based on two hypotheses: (1) DWI signal would be a reliable predictor of NAION; and (2) the location of MRI findings would be useful in discriminating NAION from ON. By taking into account both the location and type of signal abnormality, optic nerve MRI may facilitate earlier differentiation of ON versus NAION and more timely intervention when indicated.
Participants and methods
This retrospective study was conducted at the Moran Eye Center, The University of Utah, Salt Lake City, Utah, USA. Institutional review board approval was obtained from The University of Utah Institutional Review Board, with a waiver of informed consent. All research adhered to the tenets of the Declaration of Helsinki and was HIPAA (Health Insurance Portability and Accountability Act of 1996) compliant.
Participants
Records of patients diagnosed with acute NAION and ON from 2003 to 2012 were reviewed. The diagnoses had been made by three experienced neuro-ophthalmologists (J.E.A.W., K.B.D., B.J.K.) and incorporated all aspects of the patient’s neuro-ophthalmic history and physical examination. All ON participants met clinical criteria for ON based on the ONTT. All NAION participants met clinical criteria for NAION based on the Ischemic Optic Neuropathy Decompression Trial (IONDT) (Supplementary Table 1).5,13
Patients 18 years of age and older who had undergone neuroimaging within 1 month of symptom onset/diagnosis were included. Participants with inadequate imaging were excluded. Eyes wherein the clinician could not definitively distinguish between ON and NAION, and cases of biopsy-proven giant cell arteritis, were excluded. If both eyes met the eligibility criteria, both eyes were included in the study.
Neuroimaging
Neuroimaging was performed at The University of Utah and referring facilities. All MRIs included standard T1- (T1WI) and T2- (T2WI) weighted imaging as well as fluid-attenuated inversion recovery sequences (FLAIR). All DWI sequences were obtained from MRI brain sequences. The majority (74/89; 83%) of participants had dedicated orbital imaging as part of the MRI evaluation. Orbit sequences included thin-section imaging through the orbits at 2.5- or 3-mm intervals with T1, T2, STIR, and post-contrast axial and coronal T1WI with fat saturation.
Image interpretation
Two neuroradiologists (K.L.S., J.S.M.) reviewed the MRI scans while masked to the clinical diagnosis and extraorbital findings. Optic nerve images were interpreted as having the presence or absence of PCE and DWI signals. The location of the abnormal signal along the optic nerve was also recorded as “disc” = within 2 mm of the intraocular optic disc, “intraorbital” = retrobulbar intraorbital segment, or “chiasm” = optic chiasm.
Statistical analysis
Data were summarised using frequency (%) for discrete variables (i.e., sex and eye). Mean and standard deviation were used for continuous variables (i.e., age and days from symptom onset to scan). The Fisher exact test for discrete variables and two-sample t test for continuous variables were used to compare the demographics and image characteristics between the two groups.
To calculate the odds ratio (OR) of having a clinical diagnosis of NAION versus ON, a generalised mixed-effects model with logit link, which accounts for the correlation of those participants who had both eyes included in the study, was used. Unadjusted ORs were reported along with confidence intervals and p values. Receiver operating characteristic (ROC) analysis was performed using a generalised logistic regression model to evaluate the MRI characteristics in differentiating between NAION and ON after adjusting for significant demographics, i.e., age and sex. The area under ROC curve, AUC, obtained from the generalised logistic regression analysis measures the probability that the presence of imaging characteristics, i.e., PCE and/or DWI at the optic disc and/or intraorbital segment, can correctly discriminate NAION and ON patients. An AUC of 1.0 represents a perfect classification; an AUC of 0.5 represents a valueless image characteristic. In general, a characteristic is excellent if 0.9 ≤ AUC ≤ 1.0; good if 0.80 ≤ AUC < 0.90; fair if 0.70 ≤ AUC < 0.80; poor if 0.60 ≤ AUC < 0.70; and failed if AUC < 0.60.
A p value < 0.05 was considered as statistically significant. All statistical analyses were performed with SAS for Windows (version 9.4; SAS Institute, Cary, NC, USA).
Results
Five hundred and ninety-four charts were reviewed, and 104 eyes from 89 patients met eligibility criteria. All but 2 eyes were symptomatic (1 NAION and 1 ON). Of these study eyes, 72 eyes (62 participants) were clinically diagnosed as NAION, and 32 eyes (27 participants) were diagnosed as ON.
Table 1 summarises participant demographics with imaging characteristics. The majority (74/89; 83%) of participants had dedicated orbital imaging as part of the MRI study protocol. NAION participants had significantly fewer positive optic nerve imaging findings than ON participants (p < 0.001 for PCE and p = 0.020 for DWI).
Table 1.
Demographic and imaging characteristics of participants with non-arteritic anterior ischaemic optic neuropathy and optic neuritis.
| Variable | NAION (n = 62) |
ON (n = 27) |
p value |
|---|---|---|---|
| Age (years), mean (± SD) [range] | 53.6 (± 9.4) | 37.5 (± 12.0) | <0.001 |
| [35–85] | [19–68] | ||
| Sex (males), n (%) | 39 (63%) | 6 (22%) | <0.001 |
| Multiple sclerosis, n (%) | 0 (0%) | 9 (33%) | <0.001 |
| Symptomatic bilateral eyes involved, n (%) | 10 (16%) | 5 (19%) | 0.77 |
| Days from symptom onset to neuroimaging,mean (± SD) [range] | 15.6 (± 10.3) | 10.4 (± 8.9) | 0.024 |
| [1–33] | [0–30] | ||
| Steroids prior to image, n (%) | 8 (13%) | 0 (0%) | 0.10 |
| Patients with positive post-contrast enhancement, n (%) | 24 (39%) | 22 (81%) | <0.001 |
| Unilateral, n | 21 | 20 | |
| Bilateral, n | 3 | 2 | |
| Days from symptom onset in at least one eye to scan with positive post-contrast enhancement (± SD) | 13.4 (± 9.5) | 8.7 (± 6.6) | 0.061 |
| Patients with positive DWI signal, n (%) | 12 (19%) | 12 (44%) | 0.020 |
| Unilateral, n (%) | 10 | 10 | |
| Bilateral, n (%) | 2 | 2 | |
| Days from symptom onset in at least one eye to scan with positive DWI (± SD) | 13.4 (± 8.6) | 6.6 (± 3.8) | 0.024 |
Note. DWI = diffusion-weighted imaging; NAION = non-arteritic anterior ischaemic optic neuropathy; ON = optic neuritis.
Imaging characteristics
Figures 1–4 show examples of PCE and DWI signal abnormalities in patients with NAION and ON, respectively. Table 2 shows the distribution of imaging findings in the different anatomical regions of the optic nerve. The regions of the optic nerves that showed PCE and DWI signals corresponded in both NAION and ON.
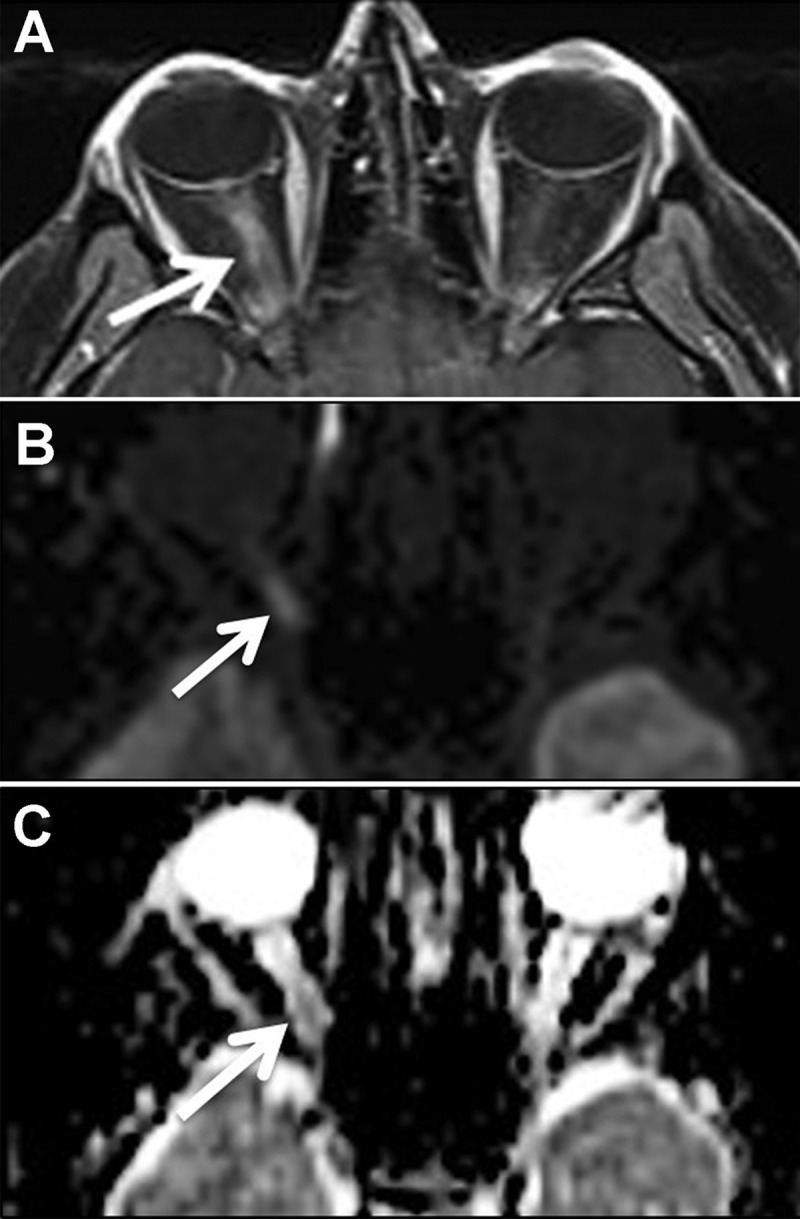
Figure 2.
Imaging findings in non-arteritic anterior ischaemic optic neuropathy. Focal enhancement (A) and diffusion restriction (B) are seen. The apparent diffusion coefficient (ADC) map (C) shows decreased signal at the optic disc and correlates with the diffusion restriction in the same location (arrowheads).
Figure 3.
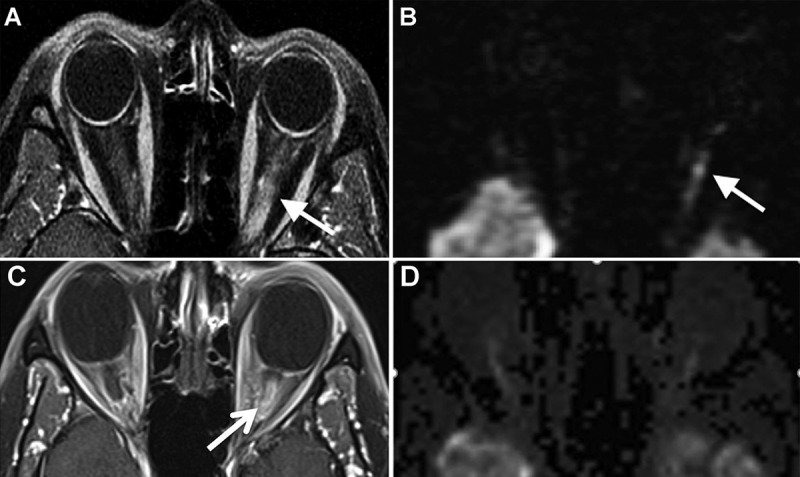
Imaging findings in optic neuritis. The axial post-contrast T1-weighted image (A) shows enhancement of the intraorbital segment of the left optic nerve (arrow). The diffusion-weighted image (B) shows hyperintensity within the left intraorbital optic nerve. The axial post-contrast T1-weighted image with fat saturation (C) shows marked enhancement and enlargement of the intraorbital segment of the left optic nerve (arrow). The corresponding diffusion-weighted trace image (D) shows no convincing areas of diffusion restriction within the nerve.
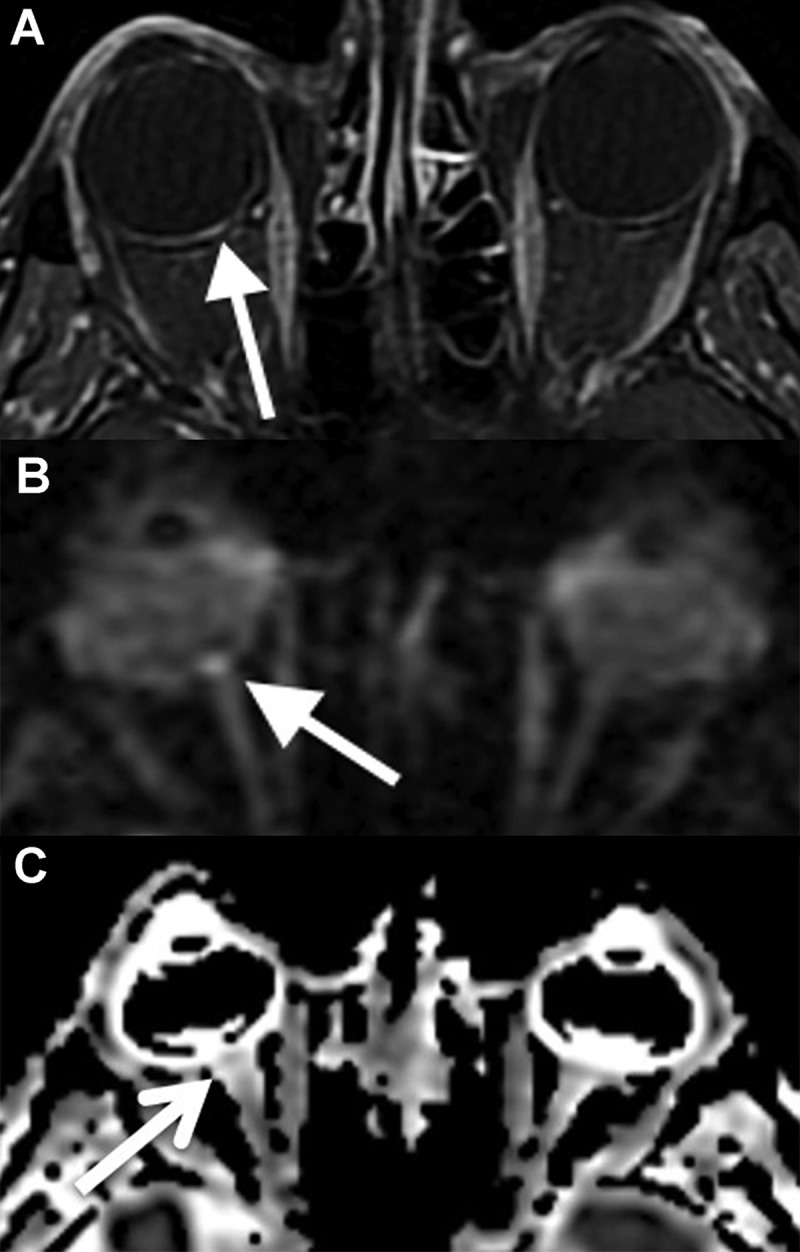
Figure 1.
Imaging findings in non-arteritic anterior ischaemic optic neuropathy. Focal enhancement at the optic disc on the post-contrast T1-weighted image (A) with fat saturation (arrows). The diffusion-weighted image (B) shows focal diffusion restriction at the optic disc (arrows). The hyperintensity seen in the left optic nerve is volume averaging, and not true diffusion restriction. (C, D) Focal enhancement at the right optic disc with associated diffusion restriction (arrows).
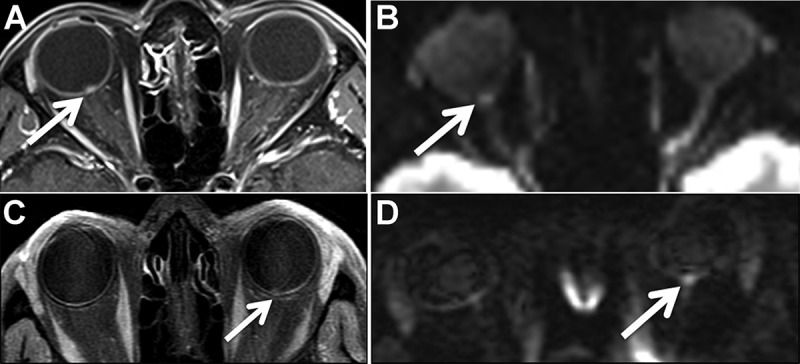
Figure 4.
Imaging findings in optic neuritis. The axial post-contrast T1-weighted image (A) shows enhancement and enlargement of the intraorbital segment of the right optic nerve. The axial diffusion-weighted trace image (B) shows associated hyperintensity in the same distribution (arrow). The apparent diffusion coefficient (ADC) map (C) shows decreased signal in the same region correlating with the area of diffusion restriction.
Table 2.
Summary of image characteristics in differentiating non-arteritic anterior ischaemic optic neuropathy versus optic neuritis.
| Image characteristic | NAION (n = 72) |
ON (n = 32) |
Unadjusted OR (NAION:ON) |
95% confidence interval for OR | p value |
|---|---|---|---|---|---|
| At any locations | |||||
| Post-contrast enhancement, n positive at any location (%) | 27 (38%) | 24 (75%) | 0.19 | 0.06–0.60 | 0.008 |
| DWI signal, n positive (%) | 14 (19%) | 14 (44%) | 0.31 | 0.10–0.98 | 0.047 |
| Post-contrast enhancement or DWI signal, n positive (%) | 32 (44%) | 24 (75%) | 0.25 | 0.08–0.80 | 0.022 |
| Post-contrast enhancement positive and DWI signal negative, n (%) | 18 (25%) | 10 (31%) | 0.67 | 0.21–2.15 | 0.47 |
| At optic disc | |||||
| Post-contrast enhancement, n positive (%) | 23 (32%) | 6 (19%) | 2.20 | 0.61–7.88 | 0.21 |
| DWI signal, n positive (%) | 12 (17%) | 6 (19%) | 0.91 | 0.23–3.65 | 0.88 |
| Post-contrast enhancement or DWI signal, n positive (%) | 26 (36%) | 6 (19%) | 2.58 | 0.73–9.08 | 0.13 |
| Post-contrast enhancement positive and DWI signal negative, n (%) | 14 (19%) | 0 (0%) | Infinity | N/A | N/A |
| At intraorbital segment | |||||
| Post-contrast enhancement, n positive (%) | 5 (7%) | 24 (75%) | 0.02 | 0.01–0.10 | <0.001 |
| DWI signal, n positive (%) | 3 (4%) | 14 (44%) | 0.06 | 0.01–0.29 | 0.002 |
| Post-contrast enhancement or DWI signal, n positive (%) | 8 (11%) | 24 (75%) | 0.04 | 0.01–0.15 | <0.001 |
| Post-contrast enhancement positive and DWI signal negative, n (%) | 5 (7%) | 10 (31%) | 0.14 | 0.03–0.64 | 0.014 |
Note. Disc = within 2 mm of the intraocular optic disc. DWI = diffusion-weighted imaging; NAION = non-arteritic anterior ischaemic optic neuropathy; ON = optic neuritis; OR = odd ratio.
Non-arteritic ischaemic optic neuropathy
Of 72 NAION eyes, 27 (38%) had PCE, and 14 (19%) had positive DWI signals. Forty of 72 (56%) NAION eyes had no abnormalities detected by either imaging modality. PCE was seen alone in 18 (25%) eyes, whereas DWI signal alone was detected in 5 (7%) NAION eyes. Nine NAION eyes had both PCE and DWI signals. Ten NAION participants had bilateral symptoms, of which 3 (30%) were found to have bilateral PCE, and 2 (20%) had positive DWI signals.
The majority of imaging findings seen with either imaging modality included the optic disc (23 of 27 [85%] PCE and 12 of 14 [86%] DWI) (Table 2). No imaging findings (0%) were seen at the chiasm. Of 27 positive PCE eyes, 22 (81%) had PCE at the optic disc alone, 4 (15%) eyes had PCE at the intraorbital segment alone, and only 1 (4%) eye had PCE at both locations. Similarly, of 14 positive DWI signal eyes, 11 (79%) had signals at the optic disc alone, 2 (14%) eyes had signals at the intraorbital segment alone, and only 1 (7%) eye had signal at both locations.
Optic neuritis
Of 32 ON eyes, 24 (75%) demonstrated positive PCE, whereas 14 (44%) eyes showed DWI signal abnormalities. All 14 eyes that showed positive DWI signals also had positive PCE. Five ON participants had bilateral symptoms, with only 1 (20%) showing bilateral PCE or DWI signal. In addition, the positive changes in either imaging modality all (100%) included the intraorbital segment, with none (0%) at the chiasm and 6 (19%) eyes involving the optic disc.
Differentiating NAION versus ON using MRI images only
Table 2 shows the results of imaging findings alone. An OR > 1 indicates that NAION is more likely, and an OR < 1 indicates ON is more likely. The presence of positive imaging findings at any location appears to favour the diagnosis of ON with either imaging modality (p < 0.05). Positive optic disc findings with each modality were not sensitive enough to distinguish between NAION and ON; however, eyes with PCE and negative DWI signal at the optic disc were all diagnosed with NAION (14 eyes). Positive findings at the intraorbital segment were predictive of ON over NAION by both modalities (OR = 42.19 [= 1/0.024] and p < 0.001 for PCE; OR = 17.33 [= 1/0.058] and p = 0.002 for DWI signal). Finally, whereas PCE could be seen in isolation, there was no instance in which the DWI signal was seen independent of PCE. Of the 51 patients with enhancing ON, 47 had orbit specific MR sequences, whereas 4 patients had brain MRI sequences only.
Differentiating NAION versus ON using MRI images after adjusting for participant demographics
There were significant differences in age (p < 0.001) and sex (p < 0.001) between NAION and ON (Table 1). The probability of diagnosing NAION or ON with each imaging modality or a combination of the two at each location, after adjusting for age and sex, are shown in Table 3. This model had a high AUC (0.94), indicating that older males are more likely to have NAION. After adjusting for age and sex effects (Table 3), only positive MRI findings along the intraorbital segment were able to improve the probability of differentiating NAION and ON (p < 0.05).
Table 3.
Differentiating non-arteritic anterior ischaemic optic neuropathy versus optic neuritis using magnetic resonance images after adjusting for significant demographic factors.
| Factor in the model | AUC* | p value |
|---|---|---|
| Basic model: Reference model | ||
| Age and sex | 0.94 | N/A |
| Any location model: Basic model+ | ||
| Post-contrast enhancement | 0.95 | 0.26 |
| DWI signal | 0.95 | 0.31 |
| Post-contrast enhancement or DWI signal | 0.95 | 0.44 |
| Post-contrast enhancement positive and DWI signal negative | 0.94 | 0.81 |
| Disc model: Basic model+ | ||
| Post-contrast enhancement | 0.95 | 0.14 |
| DWI signal | 0.94 | 0.81 |
| Post-contrast enhancement or DWI signal | 0.95 | 0.087 |
| Post-contrast enhancement positive and DWI signal negative | N/A | N/A |
| Retrobulbar intraorbital segment model: Basic model+ | ||
| Post-contrast enhancement | 0.98 | 0.002 |
| DWI signal | 0.95 | 0.018 |
| Post-contrast enhancement or DWI signal | 0.98 | 0.003 |
| Post-contrast enhancement positive and DWI signal negative | 0.97 | 0.061 |
Note. Disc = within 2 mm of the intraocular optic disc. DWI = diffusion-weighted imaging; NAION = non-arteritic anterior ischaemic optic neuropathy; ON = optic neuritis; OR = odd ratio.
*The area under ROC curve, AUC, measures the probability that presence of image characteristics, i.e., PCE and/or DWI at disc head and/or retrobulbar intraorbital segment, can correctly classify NAION and ON patients. An AUC of 1.0 represents a perfect classification; an AUC of 0.5 represents a worthless image characteristic. In general, a characteristic is excellent if 0.9 ≤ AUC ≤ 1.0; good if 0.80 ≤ AUC < 0.90; fair if 0.70 ≤ AUC < 0.80; poor if 0.60 ≤ AUC < 0.70; and failed if AUC < 0.60.
Discussion
Because NAION results from ischaemic injury to the optic disc and ON is a result of demyelination of the retrobulbar optic nerve, it was hypothesised that eyes with NAION would show more DWI signal characteristics and less PCE compared with ON. However, our study showed that the majority of NAION (56%) did not show any imaging abnormalities, and both entities demonstrated a higher proportion of cases with PCE than DWI signal. In fact, eyes with positive PCE and negative DWI signals at the optic disc were all diagnosed with NAION (14 eyes). The presence of PCE without DWI signal at the disc in NAION may be explained by the autoregulatory mechanism of luxury perfusion. Yovel et al. reported a case of NAION that demonstrated robust enhancement of the intraorbital optic nerve on post-contrast FLAIR imaging, which they attributed to luxury perfusion, which is “a vascular response to ischemia characterized by dilation of blood vessels and increased perfusion in a region surrounding an infarct.”12,14 It is possible that preferential shunting of blood flow to the ischaemic portions of the optic disc where there is a breakdown of the blood-brain barrier is the underlying mechanism of enhancement without DWI abnormality.
A delay in imaging may have played a role in finding less DWI positivity than expected, but the lack of dedicated orbital DWI imaging and technically difficult imaging parameters of the optic nerve likely played a bigger role. There was also a significant proportion of eyes with ON that showed abnormal DWI signal (14/32; 44%). This is felt to be related to (1) cytotoxic oedema (true diffusion restriction) and/or (2) vasogenic oedema (T2 shine through). Along the same lines, ischaemia-induced leakage of vessels supplying the optic disc in NAION could explain the enhancement. Although imaging findings localised at the optic disc were much more likely to represent NAION, the presence or absence of DWI signal or PCE at the optic disc alone was not able to differentiate between NAION and ON when adjusting for age and sex (Tables 2 and 3). Rather, Table 3 shows that in addition to age and sex, the presence of a retrobulbar intraorbital segment abnormality was an important factor in differentiating between ON and NAION and was more indicative of a diagnosis of ON. Additionally, we found that diffusion restriction and PCE could be seen anywhere along the length of the optic nerve in ON. This is consistent with what has been previously documented and indicates that the pathophysiology of ON is not localised.7,8,15
Al-Shafai and Mikulis were some of the first to describe the DWI characteristics of ischaemic optic neuropathy when they reported the case of a 56-year-old woman with subacute vision loss, optic nerve oedema, an afferent pupillary defect, and periorbital pain. MRI performed 2 days after symptom onset demonstrated diffuse DWI restriction along the intraorbital portion of the optic nerve that was correlated with ADC mapping. She was diagnosed with a combination of NAION and posterior ischaemic optic neuropathy10; however, no comment was made regarding post-contrast imaging in this patient.
The first group to compare the MRI imaging characteristics of NAION and ON was Rizzo et al. in 2002. PCE was seen in 31/32 cases and abnormal STIR signal in 27/32 cases of ON. By contrast, abnormal scans were seen in only 5/32 cases of NAION, with all 5 cases showing abnormal STIR signal and only 2 showing PCE. In ON, both enhancement and STIR abnormalities tended to include longer segments of the retrobulbar and intracranial optic nerve compared with NAION, but in neither ON nor NAION were these imaging abnormalities confined to the optic nerve head.7
There was a higher proportion of cases in our study with abnormal imaging findings in NAION when compared with the review by Rizzo et al.7 This is most likely due to advances in MRI technology that allow for improved spatial resolution of the optic nerve within the orbit, and particularly in the globe. In our study, there was a slight difference in time-to-imaging from the onset of vision loss between NAION and ON, with an average of 16 days for NAION and 10 days for ON. This difference is expected, as ON patients are younger and generally present with stereotypical symptoms of pain and painful eye movements with vision loss that warrant prompt imaging to rule out MS. This is opposed to NAION patients whose symptoms are less specific, potentially delaying neuroimaging. Time-to-imaging from symptom onset for participants with positive DWI signal in ON and NAION was, on average, 6 days earlier than those without. However, because imaging is not routinely ordered in the setting of NAION, it is possible that these patients were scanned after positive findings had resolved. Finally, there is more widespread use of MRI with DWI techniques currently, resulting in increased familiarity with this imaging modality and the ability to detect changes that may have been missed or overlooked in the past.
Limitations
Limitations of our study include its retrospective nature and lack of a standard imaging protocol (dedicated orbital vs. brain MRI) or timing for the MRIs reviewed in this study. Of the imaging studies included in this study, 21 cases were performed at The University of Utah whereas 36 cases were imaged at outside facilities with variable image quality due to differences between MRI protocols. There was also no standard protocol for treatment of patients, and the initiation of steroid therapy in patients with ON may have altered their imaging characteristics. Because many cases of typical NAION are never imaged, it is possible that characteristics of the participants in this study were influenced by a selection bias (more severe, younger, atypical pain). On the other hand, the participants we have studied are likely more reflective of the spectrum of disease one would encounter in a typical neuro-ophthalmic practice, which is often part of a tertiary referral centre.
The age of 18 was chosen for inclusion criteria because NAION is almost never seen below this age, and childhood ON has different demographic and aetiologic features from its adult counterpart. Therefore, the applicability of these results may be limited to adults. We specified imaging within the first month of symptom onset, as DWI imaging is generally seen in the acute phase of injury to white matter tracts and rarely beyond 1 month after insult. Imaging characteristics beyond 1 month of symptom onset cannot be characterised using our results.
Another limitation of our retrospective study design was that MRI protocols were not standardised and did not uniformly include orbital imaging. This, however, reflects the real-world issue that the study is meant to address, as many patients with acute optic neuropathy (NAION or ON) do not present to primarily to neuro-ophthalmologists familiar with these diagnoses. When practitioners who are unsure of the diagnosis order imaging to evaluate an optic neuropathy, we feel that the findings are robust enough to distinguish between NAION and ON even if dedicated orbital imaging is not performed. We feel that that this makes our study more generalisable to a real-world setting.
Conclusion
NAION and ON are common optic neuropathies that often have overlapping clinical profiles early in their disease courses. In acute cases or where the clinical profile does not help to differentiate NAION or ON, the characteristics of PCE and DWI may help differentiate these two entities. Our model takes into consideration age and sex in addition to the location of imaging findings. Positive findings at the intraorbital segment were predictive of ON over NAION by both modalities; PCE and negative DWI signal at the optic disc was consistent with a diagnosis of NAION.
Funding Statement
This work was supported in part by the National Institutes of Health (P30EY010608 [UTHealth]), Research to Prevent Blindness (Challenge Grant [UTHealth]), and the Hermann Eye Fund (UTHealth). No authors declare any conflicts of interest.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This work was supported in part by the National Institutes of Health (P30EY010608 [UTHealth]), Research to Prevent Blindness (Challenge Grant [UTHealth]), and the Hermann Eye Fund (UTHealth). No authors declare any conflicts of interest.
Supplemental data
Supplemental data for this article can be access on the publisher’s website.
References
- [1].Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT.. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 1997;123:103–107. doi: 10.1016/S0002-9394(14)70999-7. [DOI] [PubMed] [Google Scholar]
- [2].Rodriguez M, Siva A, Cross SA, O’Brien PC, Kurland LT.. Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology 1995;45:244–250. doi: 10.1212/WNL.45.2.244. [DOI] [PubMed] [Google Scholar]
- [3].Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014;13:83–99. doi: 10.1016/S1474-4422(13)70259-X. [DOI] [PubMed] [Google Scholar]
- [4].Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci 2009;16:994–1000. doi: 10.1016/j.jocn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- [5].Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, Savino PJ, Guy JR, Trobe JD, McCrary JA, Smith CH, Chrousos GA, Thompson S, Katz BJ, Brodsky MC, Goodwin JA, Atwell CA. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- [6].Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology 2008;115:298–305.e2. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rizzo JF 3rd, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2002;109:1679–1684. doi: 10.1016/S0161-6420(02)01148-X. [DOI] [PubMed] [Google Scholar]
- [8].Becker M, Masterson K, Delavelle J, Viallon M, Vargas MI, Becker CD. Imaging of the optic nerve. Eur J Radiol 2010;74:299–313. doi: 10.1016/j.ejrad.2009.09.029. [DOI] [PubMed] [Google Scholar]
- [9].He M, Cestari D, Cunnane MB, Rizzo JF 3rd.. The use of diffusion MRI in ischemic optic neuropathy and optic neuritis. Semin Ophthalmol 2010;25:225–232. doi: 10.3109/08820538.2010.518450. [DOI] [PubMed] [Google Scholar]
- [10].Al-Shafai LS, Mikulis DJ. Diffusion MR imaging in a case of acute ischemic optic neuropathy. AJNR Am J Neuroradiol 2006;27:255–257. [PMC free article] [PubMed] [Google Scholar]
- [11].Wang MY, Qi PH, Shi DP. Diffusion tensor imaging of the optic nerve in subacute anterior ischemic optic neuropathy at 3T. AJNR Am J Neuroradiol 2011;32:1188–1194. doi: 10.3174/ajnr.A2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yovel OS, Katz M, Leiba H. Magnetic resonance imaging of luxury perfusion of the optic nerve head in anterior ischemic optic neuropathy. J Neuroophthalmol 2012;32:256–258. doi: 10.1097/WNO.0b013e3182562afe. [DOI] [PubMed] [Google Scholar]
- [13].Dickersin K, Everett D, Feldon S, on behalf of The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful . JAMA 1995;273:625–632. [PubMed] [Google Scholar]
- [14].Friedland S, Winterkorn JM, Burde RM. Luxury perfusion following anterior ischemic optic neuropathy. J Neuroophthalmol 1996;16:163–171. doi: 10.1097/00041327-199609000-00001. [DOI] [PubMed] [Google Scholar]
- [15].Gass A, Moseley IF. The contribution of magnetic resonance imaging in the differential diagnosis of optic nerve damage. J Neurol Sci 2000;172(Suppl 1):S17–S22. doi: 10.1016/S0022-510X(99)00272-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


