Abstract
RATIONALE
Previously, we reported that acute marijuana intoxication minimally affected complex cognitive performance of daily marijuana smokers. It is possible that the cognitive tests used were insensitive to marijuana-related cognitive effects.
OBJECTIVES
In the current study, electroencephalographic (EEG) signals were recorded as daily marijuana users performed additional tests of immediate working memory and delayed episodic memory, before and after smoking marijuana.
METHODS
Research volunteers (N=24), who reported smoking ~24 marijuana cigarettes/week, completed this study. Participants completed baseline computerized cognitive tasks, smoked a single marijuana cigarette (0%, 1.8%, or 3.9% Δ9-THC w/w), and completed additional cognitive tasks; sessions were separated by at least 72-hrs. Cardiovascular and subjective effects were also assessed throughout sessions.
RESULTS
Overall performance accuracy was not significantly altered by marijuana, although the drug increased response times during task performance and induced a response bias towards labeling “new” words as having been previously seen in the verbal episodic memory task. Marijuana reduced slow wave evoked potential amplitude in the episodic memory task and decreased P300 amplitude and EEG power in the alpha band in the spatial working memory task. Heart rate and “positive” subjective-effect ratings were increased in a Δ9-THC concentration-dependent manner.
CONCLUSIONS
Relative to previous findings with infrequent marijuana users, the frequent users in the current study exhibited similar neurophysiological effects but more subtle performance effects. These data emphasize the importance of taking into account the drug-use histories of research participants and examining multiple measures when investigating marijuana-related effects on cognitive functioning.
Keywords: EEG, EP, marijuana, cannabis, Δ9-THC, cognition, human, performance
Introduction
In recent years the impact of smoked marijuana intoxication on cognitive performance has received intense research attention, yet there remains little consensus regarding the neuropsychological consequences of marijuana intoxication (for review, see Iversen 2000). Some researchers have reported marijuana-related impairments in multiple cognitive domains (e.g., Lane et al. 2005; Ramaekers et al. 2006; Hunault et al. 2009), while others have failed to observe such effects (e.g., Heishman et al. 1997; Hart et al. 2001; D’Souza et al. 2008; Ramaekers et al. 2009). One potential source of the apparent incongruent findings is the marijuana-use histories of the research participants studied. For example, the average reported marijuana use of participants in the study by Ramaekers et al. (2006) was approximately three times per month, whereas most participants in the Hart et al. study reported smoking marijuana daily, averaging multiple marijuana cigarettes per smoking day. A plausible hypothesis is that infrequent marijuana smokers are more susceptible than frequent users to the cognitive-impairing effects of marijuana. In fact, a growing body of evidence suggests that frequent marijuana smokers are tolerant to many marijuana-related performance-impairing effects (e.g., Ward et al. 1997; Haney et al. 1999; Nordstrom and Hart 2006; Vadhan et al. 2007; Ramaekers et al. 2009).
Another likely source of inconsistent findings is the type of tasks used to assess cognitive functioning. Hart et al. (2001), for example, evaluated the effects of marijuana on complex cognitive performance in near-daily marijuana users. In general, cognitive performance was minimally affected: participants experienced greater difficulties inhibiting inappropriate responding following the high Delta-9-tetrahydrocannabinol (Δ9-THC) concentration cigarette and they required more time to complete several cognitive tests, but their test accuracy on a broad range of tasks was unaffected. It is possible that the cognitive tests used in that study were insensitive to many marijuana-related cognitive effects. For instance, most of the memory tests used a multiple-choice format, placing less demand on participants' memory than would other memory tasks. Indeed, in a set of other experiments, we employed more demanding memory tasks and found that acute marijuana smoking produced consistent episodic and working memory disruptions, which were accompanied by transient electroencephalographic (EEG) and evoked potential (EP) alterations (Ilan et al. 2004, 2005). One methodological concern related to these studies, however, was that research participants reported smoking marijuana on an infrequent basis - in some cases, as little as once a month – which might have increased their susceptibility to the cognitive-impairing effects of marijuana.
Given the above considerations, we felt that further examination of acute marijuana-related effects on cognitive functioning was warranted. One question of interest was to determine if cognitive task performance of frequent marijuana users would be impaired during marijuana intoxication when more demanding memory tasks are used as probes. Several researchers have demonstrated that smoked marijuana and oral Δ9-THC temporarily impairs memory function of infrequent users (Melges et al. 1970; Tinklenberg et al. 1970; Abel 1971; Miller and Cornett 1978; Curran et al. 2002; D’Souza et al. 2004), but the impact of marijuana on the memory of frequent users is less clear. Another question of interest was to determine if neurophysiological signals correspond with cognitive performance. EEG and EP signals recorded during a task provide an index of cognitive function that complements performance measures. By assessing neocortical function more directly such signals can provide insight to the effects of marijuana on the brain, as cognitive functions commonly affected by marijuana such as working memory and episodic memory rely crucially on cortical areas dense in cannabinoid CB1 receptors (Iversen, 2003). Recent studies have used EEG and EP signals to investigate potential alterations in information processing, learning, and sensory gating in heavy marijuana users relative to light or non-user controls (Edwards et al. 2009; Roser et al. 2010; Skosnik et al. 2008).
In addition, a previous investigation studied infrequent marijuana smokers and showed that acute marijuana smoking elevated ratings of euphoria that corresponded with significantly increased EEG alpha power, suggesting that specific transient EEG changes may reflect a neurophysiological correlate of marijuana-related rewarding effects (Lukas et al. 1995). Likewise, it is possible that there are neurophysiological markers of the cognitive effects produced by marijuana. Indeed, we reported that acute marijuana smoking impaired cognitive performance and altered EEG signals and EP amplitudes in infrequent marijuana smokers (Ilan et al. 2004, 2005). While such findings suggest that marijuana-associated performance deficits result from specific neuronal activity alterations, to date, there remains a dearth of studies assessing the direct effects of marijuana on brain activity during complex cognitive operations. Furthermore, there are no published data correlating neurophysiological effects with cognitive functioning of near-daily marijuana users following acute administration of smoked marijuana. Such investigations will contribute to a better understanding of the complex relationship between acute marijuana use and its impact on neurophysiology and cognitive function.
Methods
Participants
Twenty-four healthy research volunteers [mean age 25.8 ± 4.1 (± SD)] completed this 3-session, within-participant outpatient study: eleven were female (five Black, one Hispanic, five White) and thirteen were male (six Black, seven White). Participants' completed formal education ranged from 9 to 19 years (mean = 14.9). They were solicited via word-of-mouth referral and newspaper advertisement in New York City. All reported almost daily marijuana use (mean = 6.3 days/week [± 1.0]), averaging 4 cigarettes per smoking day. Twenty-one participants reported recent alcohol use (ranging between 0.25 and 7 drinks per week) and twelve reported regular tobacco cigarette use (ranging between 2 and 12 cigarettes per day). Nine participants reported recent caffeine use (ranging between 1 and 14 cups per week). Other reported drug use was infrequent. Urine toxicology screens confirmed this, as Δ9-THC was the only drug metabolite present during the screening process and before each experimental session.
Participants were enrolled into the study if they were healthy, as determined by physical and psychiatric examinations, electrocardiogram, and urine and blood chemistries. No participant met criteria for an Axis I psychiatric disorder, was taking a psychotropic medication, or had a history of a serious medical condition. They were within normal weight ranges according to the 1983 Metropolitan Life Insurance Company height/weight table (body mass index: 22.1 ± 3.5). Each female volunteer was given a serum pregnancy test during the screening process and a urine pregnancy test before each experimental session. Participants were told that the purpose of the study was to evaluate the effects of smoked marijuana on cognitive performance whilst their brain activity was being recorded. Each signed a consent form approved by the New York State Psychiatric Institute's Institutional Review Board. The consent form described the study and detailed any possible risks. At the end of their third session, participants were fully informed about experimental and drug conditions, and were compensated for their participation, They were compensated at a rate of $30/session; those who completed the entire study (3 sessions) were be paid an additional bonus of $30/session. Only one participant did not complete the study and this was due to personal reasons. Note that another participant was excluded from neurophysiological analyses because of abnormal epileptiform patterns throughout the EEG. Hence, data from twenty-three participants were used for all EEG and EP analyses, and data from twenty-four participants were used for all other analyses.
Procedure and Design
As a safety precaution, participants were provided with fare for public transportation following sessions. In this way, they would not be required to drive to and from the laboratory. After satisfying all study entry criteria, volunteers received a 3 – 4 hour training session designed to familiarize them with the daily routine and to provide practice on the cognitive battery. On a different day, they were provided instructions on how to smoke the marijuana cigarette using a paced puffing procedure (see below). During the session, they smoked a "sample" marijuana cigarette containing the largest Δ9-THC concentration (3.9%) that would be given during the study, and their cardiovascular (heart rate and blood pressure) and subjective responses were carefully monitored for any unusual responses to the study drug. No untoward events were noted. Subsequently, participants completed 3 experimental sessions, which were separated by at least 72 hours. During sessions, they smoked a single marijuana cigarette containing one of three Δ9-THC concentrations (0%, 1.8%, or 3.9% Δ9-THC w/w). Marijuana cigarettes were administered in a double-blind fashion and the sequence of Δ9-THC concentration order was balanced across participants using a Latin square design.
Experimental Session
Each session day, participants reported to the laboratory either at 0900 or 1200 hours and remained for approximately 4.5 hours. The session start time for all 3 sessions was constant for each participant. They were instructed to refrain from using any psychoactive drugs, with the exception of alcohol, caffeine, marijuana, and nicotine. To assure compliance with this requirement, at the start of each study session, participants gave a urine sample that was tested for several drug metabolites (amphetamines, cocaine, and morphine derivatives). Additionally, they provided a breath sample for the detection of recent alcohol use, and were required to pass a field sobriety test. No urine sample was positive for any drug metabolite, other than Δ9-THC, and no breath alcohol concentrations were detected for any participant.
Once enrolled into the study, participants selected a light meal from a list of food items, which was served to them before each test session. After meal consumption, participants were seated in front of a computer and monitor with a mouse manipulandum. Then, the EEG recording was initiated and continuously taken throughout the session. Baseline assessments of subjective effects, heart rate, blood pressure (Sentry II, Model 6100 automated vital sign monitor, NBS Medical, Costa Mesa, CA., USA), and cognitive performance (battery described below) were completed. Immediately following baseline measures, each participant smoked a single marijuana cigarette. Then, the measures taken at baseline were repeatedly obtained at predetermined time points throughout the remainder of the session (Subjective and Physiological: 7, 56, 100, 150, and 199 min; Cognitive: 15, 60, 110, 160, 200 min). Following each session, participants were required to pass a field sobriety test prior to being provided with public transportation fare and being excused.
Neurocognitive Assessment Battery and EEG Recording
A variant was used of a combined EEG and computerized cognitive assessment battery developed for testing cognitive neurophysiological effects of drugs (Gevins et al. 2002; Ilan et al. 2005; Smith et al. 2006; McEvoy et al. 2006; Meador et al. 2007). This 30-min computerized battery consisted of three tasks: Word Presentation, Working Memory and Word Recognition. Word Presentation (1 min). A sequential list of 24 words was displayed with an inter-stimulus interval (ISI) of 2000 msec and participants were instructed to indicate the number of syllables contained in each word by responding with mouse buttons labeled ‘1’ and ‘2.’ They were also informed that they would later be given a word recognition test for these words. Working Memory (4 min). Immediately following the Word Presentation phase, the working memory task was completed. In addition to providing a distraction-filled delay for the subsequent Word Recognition task, this task required sustained attention while imposing varying loads on working memory. During this spatial N-back task, a dot stimulus was displayed in one of six positions on each trial with a mean ISI of 4000 ms (range 3500–4500 ms). In the low-load phase of the task, participants were required to determine whether the spatial location of the dot on each trial matched the location of the dot on the previous trial. In the high-load phase, each dot was compared to the dot that appeared two trials previously. Word Recognition Memory (2 min). During this episodic memory task, participants were shown a sequential list of 48 words, and were asked to indicate whether each did or did not appear in the word presentation phase list displayed approximately 5 min earlier by responding old or new, respectively. Half the words were old and half were new, presented in random order with an ISI of 2000 ms. Different sets of words were used in each repetition of the task battery.
Two blocks of these three tasks were presented consecutively in each recording interval. The low-load version of the working memory task was presented in the first block and the high-load version in the second. The 24 words used in the first and second word presentation tasks were presented in identical order. In contrast, the first and second word recognition tasks employed different recognition lists, i.e., the same 24 old words appeared in both word recognition lists, but the 24 new words were different. Presentation of the same words in consecutive blocks allows for investigation of whether the experimental manipulations affects word list learning with repetition. The order of WM task difficulty preceding the recognition lists across blocks was fixed in order to facilitate analyses of recognition list learning from the first to the second blocks across subjects and conditions. Following the tasks, resting EEG was recorded for 90 sec in both eyes-open and eyes-closed conditions.
EEG was recorded from nine scalp locations (FP1, FP2, F3, FZ, F4, CZ, P3, P4, POZ) referenced to linked mastoids. Vertical and horizontal eye movements and blinks were monitored with electrodes placed above and lateral to each eye. EEG signals were sampled at 128 Hz and band-pass filtered from 0.1 to 35 Hz. Automated artifact detection was followed by application of adaptive eye contaminant removal filters. The data were then inspected visually and data segments containing possible residual artifacts were eliminated from subsequent analyses. Additional recording details were as in Ilan et al. (2004).
Subjective Effects
The computerized visual analog questionnaire consisted of a series of 100-mm lines labeled "not at all" at one end and "extremely" at the other end. The lines were labeled with "High," "Good Drug Effect," and "Bad Drug Effect."
Drug
During each session, participants smoked a single 1-gram marijuana cigarette (0, 1.8, 3.9% Δ9-THC w/w, provided by the National Institute on Drug Abuse), using a paced puffing procedure, which has been previously shown to produce concentration-dependent changes in heart rate and subjective-effects ratings (Foltin et al. 1987; Hart et al. 2001). Participants smoked 3 standardized puffs from the cigarette: each puff consisted of a 5-s preparation interval, followed by 5-s of inhalation, 10-s of breath-hold, and 40-s of exhalation and rest. In an effort to reduce expectancy effects, the contents of cigarettes were not visible; they were tightly rolled at both ends and were smoked through a hollow plastic cigarette holder. Twenty-four hours before administering cigarettes, they were removed from a freezer, where they were stored in an airtight container, and humidified at room temperature. On average, participants smoked approximately three quarters of the marijuana cigarette during each session.
Data Analysis
EEG power spectra were computed using 2 sec windows and averaged over the task interval. The theta band was measured from 4 to 7 Hz, alpha power was measured between 7 and 11 Hz, and beta power was measured from 13 to 18 Hz. EP analyses were restricted to trials on which the participant made a correct response. Waveforms were digitally smoothed with a low-pass filter using a half-power cutoff of 7 Hz, with the exception of a 20 Hz low-pass filter used for picking the P150. EPs in the Spatial N-back task were computed using epochs beginning 350 ms prior to stimulus onset and lasting 1350 ms, and measured relative to the 300 ms pre-stimulus baseline. The P300 peak was measured within a latency window of 290–570 ms, and the slow wave was measured between 400–700 ms. EPs in both phases of the verbal episodic memory task were computed using epochs beginning 750 ms prior to stimulus onset and lasting 1950 ms, and measured relative to a baseline occurring 700 to 500 ms before stimulus onset. Slow wave amplitude was measured using 100 ms windows between 400–750 ms, the N400 peak was measured between 325 and 445 ms, and the P150 peak was measured between 75–195 ms.
A 3 (dose) × 6 (time) repeated measures analyses of variance (ANOVA) with planned comparisons was used to determine the effects of Δ9-THC concentration on cognitive performance, neurophysiological measures, subjective-effect ratings, heart rate and blood pressure. For neurophysiological measures 3 (dose) × 6 (time) × 7 (electrode site) ANOVAs were initially conducted to determine if the effects of marijuana differed by electrode site. If no interactions between marijuana and electrode location were observed, further analyses focused on the site at which amplitude or power was largest (EPs: FZ for the P150, CZ for the P300 and N400, and POZ for the slow wave; EEG power spectra: FZ for the theta and beta bands, and POZ for the alpha bands). For all analyses, ANOVAs provided the dose and time error terms needed to calculate planned comparisons (Bonferroni's test) designed to answer the question of whether dependent variables varied as a function of Δ9-THC concentration condition. The following comparisons were performed at each time point: 0 vs 1.8%, 0 vs 3.9%, and 1.8 vs 3.9%. Results were considered statistically significant if p < 0.05, using Greenhouse-Geisser corrections where appropriate.
Results
Cognitive Effects
Episodic Memory
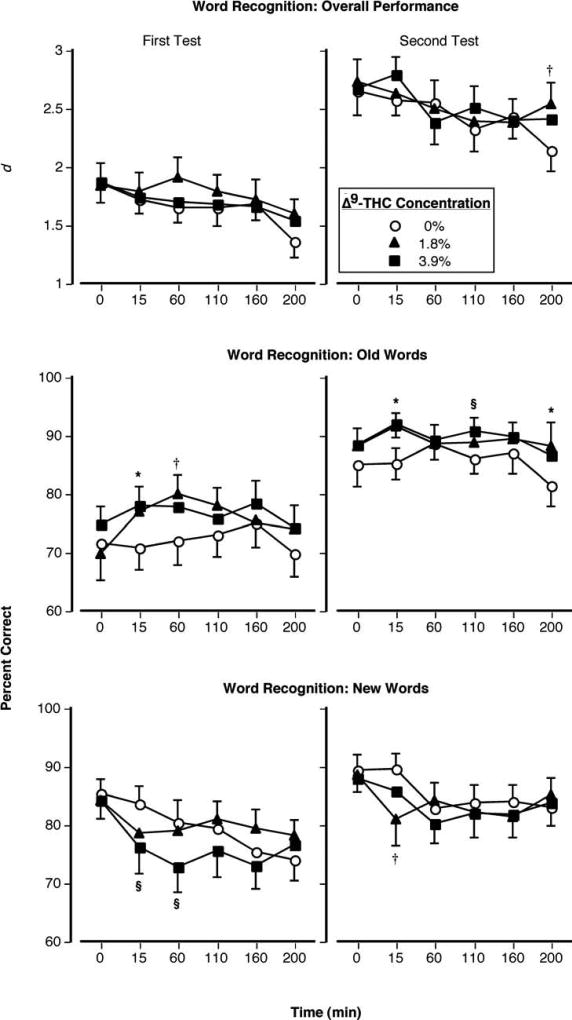
Figure 1 illustrates performance on the Word Recognition task as a function of Δ9-THC concentration and time. The repeated-measures ANOVA revealed no significant Δ9-THC concentration × time effect for episodic memory performance. The following describes results from the planned comparisons. Although marijuana produced only one significant effect on overall task performance: the 1.8% Δ9-THC cigarette improved performance 200 min after administration (p < 0.05: Figure 1, top right panel), it produced differential effects depending on whether the word was one previously viewed (old) or not (new). Marijuana induced a response bias during the recognition task such that participants were more likely to respond that a given word had been seen previously. As a result, response accuracy to old words was significantly increased (Figure 1, middle panels) and accuracy to new words was significantly decreased (Figure 1, bottom panels). These effects were most prominent 15 min after administration of active cigarettes. While the amount of time participants required to emit a response to old words was unaltered by marijuana, response time to new words markedly increased 15, 60, 110 min after smoking both active Δ9-THC concentrations (p < 0.05: data not shown).
Figure 1.
Top Panels: Overall accuracy performance on the episodic memory task as a function of Δ9-THC concentration and time. Middle Panels: Percentage of accurate identification of old words on the episodic memory task as a function of Δ9-THC concentration and time. Bottom Panels: Percentage of accurate identification of new words on the episodic memory task as a function of Δ9-THC concentration and time. Error bars represent one SEM. Overlapping error bars were omitted for clarity. An * indicates that the 1.8 and 3.9% Δ9-THC conditions significantly differed from the placebo condition (p < 0.05). An § indicates that the 3.9% Δ9-THC condition significantly differed from the placebo condition (p < 0.05). An † indicates that the 1.8% Δ9-THC condition significantly differed from the placebo condition (p < 0.05).
Working Memory
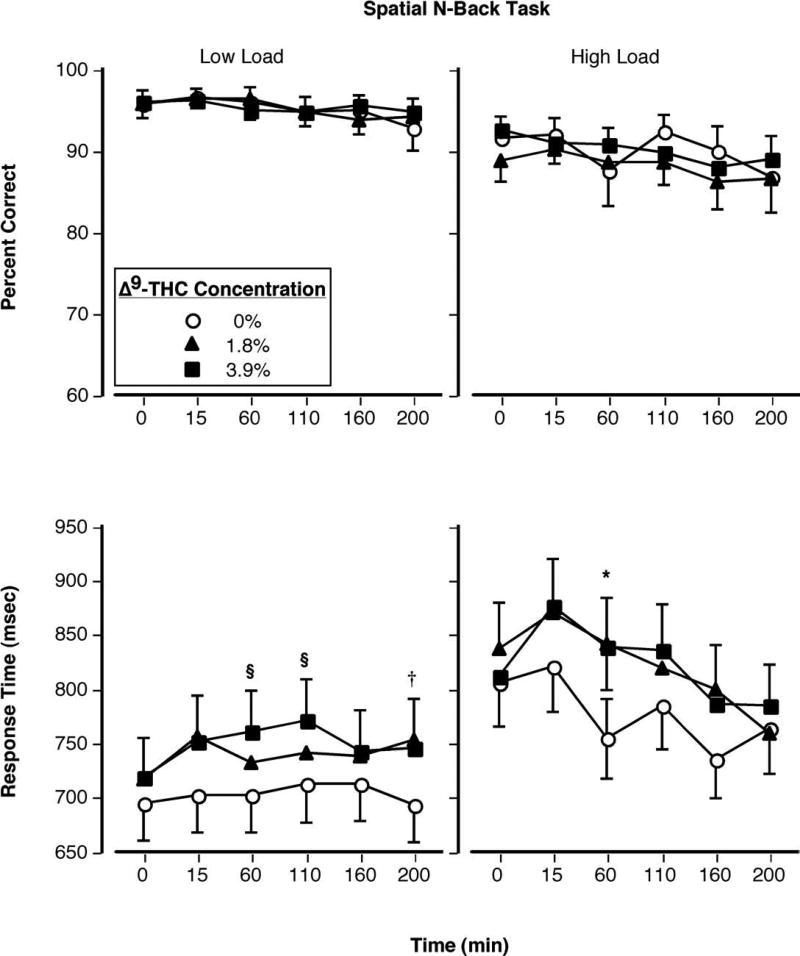
Figure 2 shows performance on the Spatial N-back task as a function of Δ9-THC concentration and time. Accurate responding during this task (during both low- and high-load versions) was unaffected by smoked marijuana (Figure 2, top panels), but response times significantly were increased (Figure 2, bottom panels). During the low-load version, this effect reached statistical significance 60 and 110 min post 3.9% Δ9-THC administration and 200 min post 1.8% Δ9-THC administration; during the high-load version, this effect was significant only 60 min after both active cigarettes (p < 0.05). However, the interaction between drug and task load level was not significant at any recording interval.
Figure 2.
Top Panels: Percentage of accurate responding on the working memory task during the low- (left) and high-load (right) versions as a function of Δ9-THC concentration and time. Bottom Panels: Mean amount of time participants required to complete the working memory task during the low- (left) and high-load (right) versions as a function of Δ9-THC concentration and time. Error bars represent one SEM. Overlapping error bars were omitted for clarity. An * indicates that the 1.8 and 3.9% Δ9-THC conditions significantly differed from the placebo condition (p < 0.05). An § indicates that the 3.9% Δ9-THC condition significantly differed from the placebo condition (p < 0.05). An † indicates that the 1.8% Δ9-THC condition significantly differed from the placebo condition (p < 0.05).
Neurophysiological Effects
Evoked Potentials
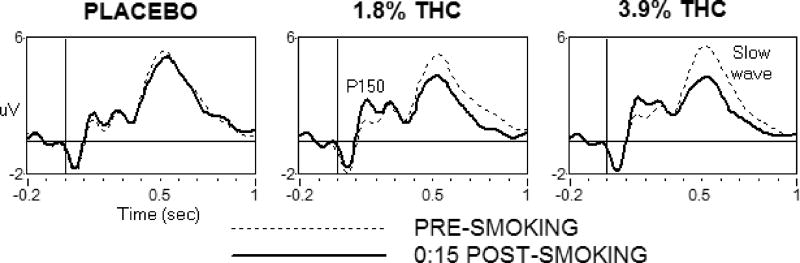
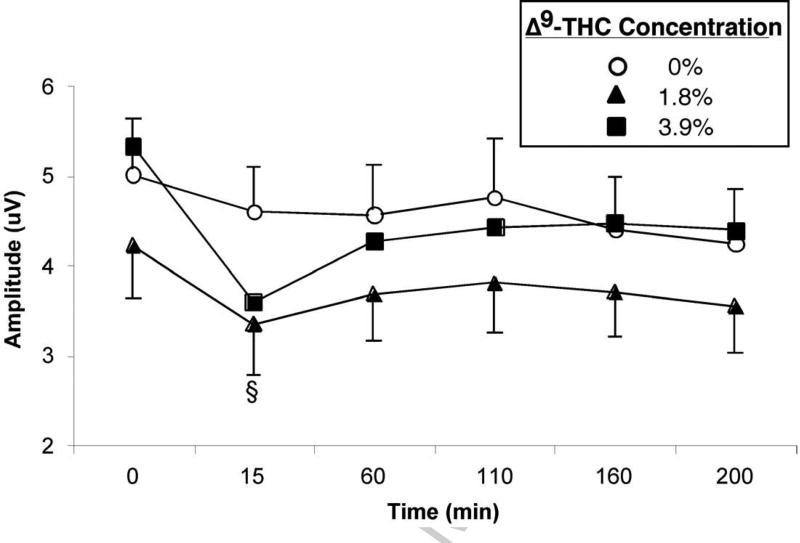
Figure 3 (top panel) shows the EPs during the Word Presentation and Recognition phases of the delayed episodic memory task. The most prominent feature of the EPs is a large positive slow wave from approximately 400–700 ms after the onset of the word, larger at centro-parietal-occipital sites than at frontal sites [F (1,22) = 13.91, p < 0.01]. Figure 3 (bottom panel) shows that in both phases, slow wave amplitude decreased in the 15 min post-smoking interval following the 3.9% Δ9-THC cigarette relative to the 1.8% Δ9-THC cigarette [F (1,22) = 13.99, p < 0.01] and placebo [F (1,22) = 19.00, p < 0.001]. This decrease after marijuana was larger at parietal sites than at fronto-central sites [F (1,22) = 9.81, p < 0.01]. Although a “memory-evoked shift” was observed, in which the slow wave amplitude was larger following recognition of old words than new words, its magnitude was not affected by marijuana. Also in both phases, marijuana (both active Δ9-THC conditions) significantly increased P150 amplitude 15 min after smoking: 1.8% = [F (1,22) = 6.80, p < 0.05] and 3.9% = [F (1,22) = 19.20, p < 0.001] (data not shown). In the Recognition phase, the amplitude of the fronto-central N400 decreased after 1.8% [F (1,22) = 6.43, p < 0.05] and 3.9% [F (1,22) = 6.22, p < 0.05] Δ9-THC (data not shown). The effects of marijuana on P150 and N400 amplitudes did not differ between the two active Δ9-THC conditions.
Figure 3.
Top Panel: Evoked potentials relative to a 200 ms pre-stimulus baseline at parietal-occipital site POz during the Presentation and Recognition phases of the episodic memory task, before (dotted line) and 15 min after (solid line) smoking. Amplitude of the slow wave decreased after smoking the 3.9% Δ9-THC cigarette, and amplitude of the P150 decreased after smoking both active Δ9-THC concentration cigarettes relative to placebo. Bottom Panel: Mean slow wave amplitude as a function of Δ9-THC concentration and time. Slow wave amplitude was often lower in the 1.8% Δ9-THC than placebo condition, but the change from pre-smoking baseline was significantly different from placebo only 15 min after smoking the 3.9% Δ9-THC cigarette. Error bars represent one SEM. Overlapping error bars were omitted for clarity. An § indicates that the 3.9% Δ9-THC condition significantly differed from the placebo condition (p < 0.05).
During the working memory task, P300 amplitude generally decreased after marijuana smoking, with the greatest decrease observed 60 min following drug administration (data not shown). This decrease was observed across the scalp and did not differ between electrode sites [F (12,264) = 1.62, p > 0.10]. In the low-load working memory phase, P300 amplitude decreased 60 min after smoking the 3.9% Δ9-THC cigarette only [F (1,22) = 5.06, p < 0.05], whereas in the more difficult high-load working memory phase P300 amplitude decreased 60 min after smoking the 1.8% Δ9-THC cigarette only [F (1,22) = 15.66, p < 0.01]. Marijuana did not significantly affect P300 latency or slow wave amplitude in the working memory task.
EEG
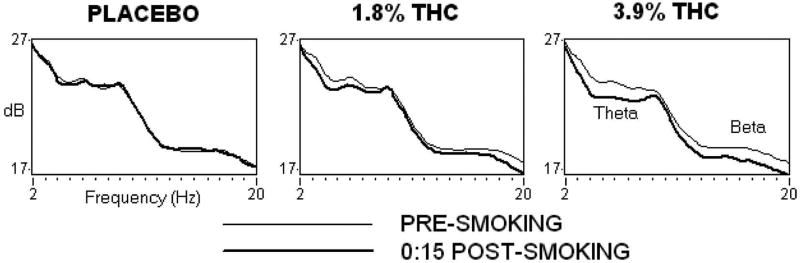
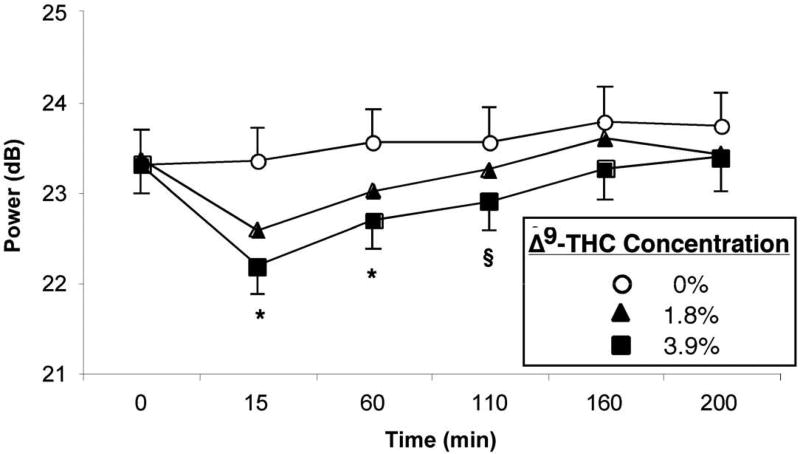
Figure 4 (top panel) shows the effects of marijuana on EEG spectral power. Both active Δ9-THC concentrations reduced theta band power across all task and resting conditions: 1.8% = [F (1,22) = 20.32, p < 0.001] and 3.9% = [F (1,22) = 63.33, p < 0.001]. The decrease produced by 3.9% Δ9-THC was significantly larger than that produced by 1.8% Δ9-THC [F (1,22) = 7.16, p < 0.05]. Reduced theta band power effects were largest 15 min after marijuana, but persisted until 60 and 110 min after smoking cigarettes containing 1.8% and 3.9% Δ9-THC, respectively. Beta power increased over the course of the recording day following placebo but decreased 15 min post-smoking marijuana cigarettes containing 1.8% [F (1,22) = 16.97, p < 0.001] and 3.9% [F (1,22) = 14.16, p < 0.01] Δ9-THC concentrations (data not shown). Beta power then increased systematically as the effects of marijuana dissipated, but the increase from baseline was greater in the placebo than the marijuana conditions through the 160 min post-smoking interval.
Figure 4.
Top Panel: EEG spectral power at midline frontal site Fz averaged over all task and resting conditions, before (light line) and 15 min after (dark line) smoking. Power in the theta and beta bands decreased after smoking both active Δ9-THC concentration cigarettes relative to placebo. Bottom Panel: Mean theta band power as a function of Δ9-THC concentration and time. Error bars represent one SEM. Overlapping error bars were omitted for clarity. An * indicates that the 1.8 and 3.9% Δ9-THC conditions significantly differed from the placebo condition (p < 0.05). An § indicates that the 3.9% Δ9-THC condition significantly differed from the placebo condition (p < 0.05).
Alpha power in the working memory task was similarly affected, increasing over the course of the day following placebo but less so following marijuana smoking. Alpha power tended to decrease after smoking marijuana relative to placebo, with the effect reaching statistical significance in the intervals 15 [F (1,22) = 5.82, p < 0.05], 160 [F (1,22) = 7.82, p < 0.05], and 200 minutes after smoking [F (1,22) = 6.25, p < 0.05] (data not shown). No significant differences between the two active marijuana conditions or the low- and high-load phases of the working memory task were observed.
Subjective and Physiological Effects
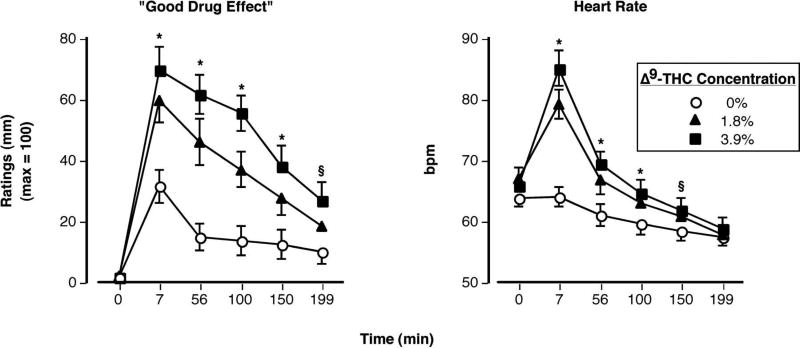
Figure 5 illustrates the effects of smoked marijuana on ratings of “Good Drug Effect” (left panel) and heart rate (right panel) as a function of Δ9-THC concentration and time. The repeated-measures ANOVA revealed a significant Δ9-THC concentration × time effect for ratings of “Good Drug Effect” [F (10,230) = 6.03, p < 0.0001]. These ratings, as well as ratings of “High” (data not shown), were significantly elevated in a Δ9-THC concentration-dependent fashion: both active concentrations were significantly different from placebo and 3.9% Δ9-THC cigarette produced greater ratings than the 1.8% Δ9-THC cigarette (p < 0.05). Similarly, a significant Δ9-THC concentration × time effect was observed for heart rate [F (10,230) = 14.76, p < 0.0001]. Relative to placebo, both 1.8% and 3.9% Δ9-THC significantly increased bpm (p < 0.0001). The active conditions did not significantly differ from each other; no significant effects of smoked marijuana on blood pressure were observed.
Figure 5.
Ratings of “Good Drug Effect” (left) and heart rate as a function of Δ9-THC concentration and time. Error bars represent one SEM. Overlapping error bars were omitted for clarity. An * indicates that the 1.8 and 3.9% Δ9-THC conditions significantly differed from the placebo condition (p < 0.05). An § indicates that the 3.9% Δ9-THC condition significantly differed from the placebo condition (p < 0.05).
Discussion
The present findings show that smoked marijuana produced minimal effects on episodic and spatial working memory of near-daily smokers. The overall response accuracy on the word recognition and working memory tasks was unaffected by marijuana, although smoked marijuana did increase the amount of time participants needed to complete these tasks. This pattern of effects is consistent with results previously reported by other researchers studying the acute effects of marijuana on cognitive performance of regular users (e.g., Heishman et al. 1997; Hart et al. 2001; Ramaekers et al. 2009). An important concern associated with earlier studies, however, was that the tasks used to probe memory functioning might have been insensitive to marijuana-related cognitive effects. The current data addresses this issue by showing that the performance of frequent marijuana users is similar across different memory tasks.
The finding that the current participants’ overall response accuracy on the episodic memory and working memory tasks was unaffected by marijuana stands in contrast to previous findings in occasional smokers who showed reduced accuracy on these same tasks after marijuana (Ilan et al. 2004, 2005). The frequent users in the current study did, however, exhibit some of the performance effects of marijuana seen in those previous studies, such as longer response times and a response bias to classify previously unseen words as “old” in the episodic memory task. In general, the overall pattern of performance results observed in regular users was less disruptive than what has been reported with more casual users.
In addition to performance measurements, the computerized cognitive assessment battery used in the current study recorded neurophysiological data, allowing further insight into the subtle manner in which marijuana may affect brain activity while performing cognition operations. Numerous effects of marijuana smoking were observed in measurements of EP amplitudes and EEG spectral power, with most peaking 15 min after smoking and some persisting for as long as 3.5 hours. Slow wave amplitude in the episodic memory task decreased following marijuana smoking, and P300 amplitude in the working memory task decreased as well. These EP findings are consistent with those previously observed in occasional smokers (Ilan et al. 2004, 2005), and suggest that specific processes of encoding and retrieval of verbal information were altered by marijuana.
On the placebo session, power in the alpha EEG band during performance of the working memory task increased over the course of the day. Generally, an increase over time in alpha power suggests that the task is becoming less difficult and/or more automated, such that fewer neurons firing synchronously are required to perform the task (Gevins et al. 2002). On both active drug sessions, however, alpha power decreased in the recording interval immediately following smoking, before gradually increasing over the next three hours. This suggests that marijuana alter the amount of sustained attention required in the working memory task, delaying the development of the type of automatization seen on the placebo session. A number of systemic EEG effects of marijuana were also apparent, present in all task conditions including passive resting. The pervasive reductions in power in the theta and beta bands are indicative of increased autonomic activity following marijuana smoking, consistent with the observed dose-dependent elevations in heart rate. As was the case with the decrease in EP amplitudes, the observed effects of marijuana on EEG spectral power are consistent with those previously seen in infrequent users (Ilan et al. 2004, 2005).
Overall, our data indicate that the effects of marijuana on memory performance of frequent users were more subtle than what has been previously observed in infrequent users performing the same tasks, but the neurophysiological and subjective-effect data are quite similar between the two types of participants. One possible explanation for this result is that the frequent users may have developed tolerance to marijuana-related impairing effects (e.g. Ward et al. 1997; Hart et al. 2001, 2002; Vadhan et al. 2007; Ramaekers et al. 2009). This observation is similar to findings showing that an acute dose of alcohol produces limited effects on cognitive functioning of regular alcohol drinkers (e.g., Hiltunen 1997). However, neurophysiological measures and subjective-effect data suggest that tolerance did not develop uniformly, as EEG and EP signals and subjective-effect ratings were systematically altered by Δ9-THC.
Regarding cognitive performance, the current participants may have developed ways of maintaining their level of task performance even when experiencing the euphoric and neurophysiologic effects of marijuana. This is consistent with the hypothesis of Schuster et al. (1966), who posited that tolerance develops to drug-related behaviors that are disruptive to an organism’s ability to meet performance requirements necessary for the delivery of reinforcers (e.g., cognition). These authors, on the other hand, suggested that tolerance would not develop to drug-related behaviors that facilitate or have no effect on an organism’s ability to satisfy necessary operations for reinforcement delivery (e.g., positive subjective effects). While others have reported findings with alcohol that are congruent with this hypothesis (Wenger et al. 1981), the current data with marijuana lend further support to this idea. That is, while accuracy on the working memory task was not significantly affected by marijuana, positive subjective effects (e.g., ratings of “good drug effect”) were dramatically increased. Together, the data highlight the importance of assessing multiple measures (e.g., cognitive, subjective ratings, neurophysiological, etc.) when characterizing the direct effects of marijuana on human cognition.
A potential criticism of the present study is that the Δ9-THC concentrations examined (1.8 and 3.9%) were lower than those available in the natural ecology, where the average Δ9-THC concentration in marijuana cigarettes has increased in recent years (ElSohly et al. 2000). Although a wider range of Δ9-THC concentrations is available outside of the laboratory, experienced marijuana smokers (like alcohol drinkers and other psychoactive drug users) self-titrate to the desired levels of intoxication regardless of the Δ9-THC concentration contained in a given cigarette. Another caveat of the current study is that even though the infrequent marijuana users assessed in the studies by Ilan and colleagues (Ilan et al. 2004, 2005) performed the same task battery as did the frequent users in the current study, the experimental protocols were not identical across studies. For example, in the current study, participants smoked three puffs from the cigarette over about a 3-min period, whereas in the study by Ilan et al. (2005), participants smoked five puffs over 10 minutes. There were also slight variations between the studies in terms of the Δ9-THC concentrations studied. The present study employed a slightly larger Δ9-THC concentration than the previous studies. Finally, it is important to note that the marked slowing of performance on both cognitive tasks after smoked marijuana could have important implications and may lead to behavioral problems in the natural ecology when rapid decisions are needed, e.g., certain workplace tasks and the operation of machinery and automobiles.
In summary, the current data suggest that frequent marijuana users may show fewer behavioral signs of disruption during intoxication than infrequent users, even when difficult memory tasks are used to assess cognitive performance. However, frequent and infrequent users may evince similar neurophysiological and subjective responses to marijuana smoking. The observation that frequent users’ response accuracy is not altered after marijuana smoking to the same extent it is for infrequent users – despite the similar CNS effects – suggests that near-daily marijuana smokers may have developed tolerance to some marijuana-related behavioral effects. The results underscore the value of examining multiple measures when attempting to understand drug-related effects on cognitive functioning. Finally, the data emphasize the importance of taking into account the drug-use histories of research participants when assessing cognitive responses to a drug, as frequent and infrequent users may respond similarly on one measure but differently on another measure after acute drug administration.
Table 1.
Demographic Information
| Mean (SD) | ||
|---|---|---|
| Age | 25.8 ± 4.1 | |
| Education | 14.9 ± 2.5 | |
| Completed formal education (Years) | ||
| 9–11 | 2 | |
| 12–14 | 7 | |
| 15–19 | 15 | |
| Race/Ethnicity | ||
| Black | 11 | |
| Hispanic | 1 | |
| White | 12 | |
| Sex | ||
| Female | 11 | |
| Male | 13 |
Acknowledgments
The technical assistance of Caroline Marvin and Gydmer Perez is gratefully acknowledged. This research was supported by grants from the National Institute on Drug Abuse to Drs. Carl L. Hart (DA-03476) and Alan Gevins (DA-12840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Abel EL. Marihuana and memory: Acquisition or retrieval? Science. 1971;173:1038–1040. doi: 10.1126/science.173.4001.1038. [DOI] [PubMed] [Google Scholar]
- Chung SS, McEvoy LK, Smith ME, Gevins A, Meador K, Laxer KD. Task-related EEG and ERP changes without performance impairment following a single dose of phenytoin. Clin Neurophysiol. 2002;113:806–814. doi: 10.1016/s1388-2457(02)00067-6. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, O'Donnell BF, Hetrick WP. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behav Neurosci. 2009;123:894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF., 3rd Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. Journal of Forensic Science. 2000;45:24–30. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy LK. Tracking the cognitive pharmacodynamics of psychoactive substances with combinations of behavioral and neurophysiological measures. Neuropsychopharmacology. 2002;26:27–39. doi: 10.1016/S0893-133X(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp WG, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Δ9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ. Acute alcohol tolerance in cognitive and psychomotor performance: influence of the alcohol dose and prior alcohol experience. Alcohol. 1997;14:125–30. doi: 10.1016/s0741-8329(96)00115-2. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Böcker KB, Schipper CM, Kruidenier M, Leenders ME, de Vries I, Meulenbelt J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC) Psychopharmacology. 2009;204:85–94. doi: 10.1007/s00213-008-1440-0. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology. 2004;176:214–222. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Iversen LL. The science of marijuana. New York: Oxford University Press; 2000. [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30:800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt R. Electroencephalographic correlates of marihuana-induced euphoria. Drug Alcohol Depend. 1995;37:131–40. doi: 10.1016/0376-8716(94)01067-u. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Gevins A, Loring DW, McEvoy LK, Ray PG, Smith ME, Motamedi GK, Evans BM, Baum C. Neuropsychological and neurophysiologic effects of carbamazepine and levetiracetam. Neurology. 2007;69:2076–2084. doi: 10.1212/01.wnl.0000281104.55418.60. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Fordyce M, Gevins A. Characterizing impaired functional alertness from diphenhydramine in the elderly with performance and neurophysiologic measures. Sleep. 2006;29:957–966. doi: 10.1093/sleep/29.7.957. [DOI] [PubMed] [Google Scholar]
- Mecklinger, Opitz B, Friederici AD. Semantic aspects of novelty detection in humans. Neurosci Lett. 1997;235:65–68. doi: 10.1016/s0304-3940(97)00712-x. [DOI] [PubMed] [Google Scholar]
- Melges FT, Tinklenberg JR, Hollister LE, Gillespie HK. Marihuana and temporal disintegration. Science. 1970;168:1118–1120. doi: 10.1126/science.168.3935.1118. [DOI] [PubMed] [Google Scholar]
- Meyer RE, Pillard RC, Shapiro LM, Mirin SM. Administration of marijuana to heavy and casual marijuana users. Amer J Psychiat. 1971;128:90–96. doi: 10.1176/ajp.128.2.198. [DOI] [PubMed] [Google Scholar]
- Miller LL, Cornett TL. Marijuana: Dose effects on pulse rate, subjective estimates of intoxication, free recall and recognition memory. Pharmacol Biochem Behav. 1978;9:573–577. doi: 10.1016/0091-3057(78)90205-8. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Hart CL. Assessing cognitive functioning in cannabis users: cannabis use history an important consideration. Neuropsychopharmacology. 2006;31:2798–2799. doi: 10.1038/sj.npp.1301210. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-Potency Marijuana Impairs Executive Function and Inhibitory Motor Control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Roser P, Della B, Norra C, Uhl I, Brune M, Juckel G. Auditory mismatch negativity deficits in long-term heavy cannabis users. Eur Arch Psychiatry Clin Neurosci. 2010 doi: 10.1007/s00406-010-0097-y. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Dockens WS, Woods JH. Behavioral variables affecting the development of amphetamine tolerance. Psychopharmacologia. 1966;9:170–182. doi: 10.1007/BF00404721. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O'Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33:1432–40. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Gevins A, McEvoy LK, Meador KJ, Ray PG, Gilliam F. Distinct cognitive neurophysiologic profiles for lamotrigine and topiramate. Epilepsia. 2006;47:695–703. doi: 10.1111/j.1528-1167.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- Tinklenberg JR, Melges FT, Hollister LE, Gillespie HK. Marijuana and immediate memory. Nature. 1970;226:1171–1172. doi: 10.1038/2261171b0. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Haney M, Gunderson EW, Foltin RW. Acute effects of smoked marijuana on decision-making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29:357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Ward AS, Comer SD, Haney M, Foltin RW, Fischman MW. The effects of a monetary alternative on marijuana self-administration. Behavioural Pharmacology. 1997;8:275–286. doi: 10.1097/00008877-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Wenger JR, Tiffany TM, Bombardier C, Nicholls K, Woods SC. Ethanol tolerance in the rat is learned. Science. 1981;213:575–577. doi: 10.1126/science.7244656. [DOI] [PubMed] [Google Scholar]