Abstract
Objective
Severe sepsis survivors frequently experience cognitive and physical functional impairment. The degree of impairment and its association with mortality is understudied, particularly among those discharged to a skilled nursing facility (SNF). Our objective was to quantify the cognitive and physical impairment among severe sepsis survivors discharged to a SNF, and to investigate the relationship between impairment and long-term mortality.
Design
Retrospective cohort study
Setting
United States
Subjects
Random 5% sample of Medicare patients discharged following severe sepsis hospitalization, 2005–2009 (n=135,370).
Measurement and Main Results
Medicare data were linked with the Minimum Data Set (MDS); MDS-COGS was used to assess cognitive function, and the MDS ADL hierarchical scale was used to assess functional dependence. Associations were evaluated using multivariable logistic regression, Kaplan Meier curves, and Cox proportional hazards regression. Of 66,540 beneficiaries admitted to a SNF following severe sepsis, 34% had severe or very severe cognitive impairment, and 72.5% had maximal, dependence, or total dependence in ADL. Median survival was 19.4 months for those discharged to a SNF without having been in a SNF in the preceding 1 year, and 10.4 months for those discharged to a SNF who had spent time in a SNF in the prior year. The adjusted hazard ratio (HR) for death was 3.1 for those with very severe cognitive impairment relative to those who were cognitively intact (95% CI 2.9 to 3.2, p<0.001), and 4.3 for those with “total dependence” in ADLs relative to those who were independent (95% CI 3.8 to 5.0, p<0.001).
Conclusions
Discharge to a SNF following severe sepsis hospitalization among Medicare beneficiaries was associated with shorter survival, and cognitive impairment and ADL dependence were each strongly associated with shortened survival. These findings can inform decision-making by patients and physicians, and underscores high palliative care needs among sepsis survivors discharged to SNF.
Keywords: severe sepsis, elderly, survival, cognitive impairment, activities of daily living, critical illness, functional status, debility
Introduction
Severe sepsis affects over 750,000 Americans annually, with over 60% of cases occurring in those over 65 years of age (1, 2), and the number of survivors is growing because of increasing incidence and declining case fatality rates (3). Many survivors (4) experience reduced quality of life (5, 6) as a result of decline in physical function leading to debility (7–9) as well as new cognitive impairment (7, 10, 11), and are at increased risk of death in the months and years following sepsis (12).
More than one-third of all sepsis survivors are discharged to a skilled nursing facility (SNF) based upon the need for skilled nursing care, physical and occupational therapy, and/or speech-language pathology services following hospital discharge (13). Patients over age 65 are much more likely to be discharged to a SNF following sepsis hospitalization than are younger patients (14). The degree and impact of cognitive and functional impairment among severe sepsis survivors discharged to SNF settings are unknown. Studying older adults discharged to a SNF may add to our understanding of the magnitude of the challenges facing patients following severe sepsis hospitalization, and may highlight care needs of those patients. Furthermore, better knowledge of the association between cognitive and physical functional impairment and long-term survival could improve the understanding of prognosis in these patients.
Our primary outcome was to quantify the cognitive and physical functional impairment among a national random sample of Medicare beneficiaries who survived a hospitalization with severe sepsis and were discharged to a SNF, and to investigate the association between severity of acute illness and cognitive and physical functional impairment using care in an intensive care unit (ICU) and receipt of mechanical ventilation as markers of severity. We also examined the proportion of severe sepsis survivors discharged to a SNF, and investigated the relationship between the degree of cognitive and physical functional impairment at SNF admission and long-term mortality.
Methods
Data Sources
Enrollment, demographic, and claims data were obtained for a 5% random sample of Medicare beneficiaries from the Centers for Medicare & Medicaid Services (CMS) Chronic Conditions Data Warehouse (CCW) 2004–2009 (15). Beneficiaries aged 65 and older were included, and those who were in an HMO or received railroad retirement benefits were excluded. We identified hospitalizations that included an episode of severe sepsis 2005–2009 (data from 2004 was used to allow for one-year of pre-hospitalization data on all subjects) with a validated claims-based definition of severe sepsis that uses International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes to identify the presence of infection and acute organ dysfunction (2). Data from the CCW were linked with the CMS Long-Term Care Minimum Data Set 2.0 (MDS) (16), a standardized screening and assessment tool of health status mandated for all residents of Medicare and/or Medicaid certified SNFs. In the United States, SNF care includes short-term post-acute or rehabilitative care as well long-term residential care. For most patients discharged from the hospital setting, the goal is to return to the community and up to 100 days of care in a SNF is covered by Medicare. Long-term patients’ SNF care is paid out-of-pocket by patients and their families, by long-term care insurance, or Medicaid when personal resources are insufficient or have been exhausted. The University of Wisconsin Institutional Review Board determined this study to be exempt.
Variables
Patient-level variables from Medicare files included age, sex, race, Medicaid status as a marker of socioeconomic status, whether initial Medicare enrollment due to disability, and index hospitalization length of stay. Race was categorized as white, black, or other based on the beneficiary race code (17). Rurality was assessed with each patient’s residence by zip code, using the U.S. Department of Agriculture’s rural–urban commuting area codes (18, 19). Each patient’s CMS hierarchical condition category (CMS-HCC) score, calculated from all health insurance claims over the year before the index hospitalization, was included as a risk adjustment measure (20). Comorbid conditions were identified using Elixhauser methods, incorporating data from the index hospitalization as well as all hospitalizations and physician claims during the year prior (21). Respiratory failure requiring mechanical ventilation, a marker of increased severity of illness among those with severe sepsis, was identified by the presence of ICD-9-CM mechanical ventilation procedure codes 96.70, 96.71, or 96.72 (22). The presence of a diagnosis of dementia in the year prior to hospital admission was identified using an approach developed and validated in Medicare data and which has sensitivity of 85% and specificity of 89% (23, 24).
Cognitive function among those admitted to a skilled nursing facility was assessed using the MDS-Cognition Scale (MDS-COGS), a validated additive cognitive scale derived from Minimum Data Set (MDS) assessments (25–27). The MDS-COGS score incorporates data from 8 MDS variables (Supplemental Table 1), and was considered missing if data for all 8 variables was missing; otherwise missing variables were coded as zero for calculation of the score. This score (with range 0–10) was categorized in the standard way into 4 levels of cognitive function, “intact” (0–1), “moderate impairment” (2–4), “severe impairment” (5–8), and “very severe impairment” (9–10). Dependence in activities of daily living (ADL) was assessed using the MDS-ADL hierarchical scale, a validated hierarchical scale of dependency in activities of daily living based upon MDS items regarding independence in personal hygiene, toileting, locomotion, and eating, with higher scores indicate more impairment in ADL performance (Supplemental Table 2) (28–30). It was considered missing if data for all 7 variables was missing, otherwise missing variables were coded as zero for calculation of the score.
Statistical Analysis
Bivariate analysis compared differences between those discharged to a SNF and those discharged to all other destinations. Among those discharged to SNF settings, we compared those who had and had not been in a SNF in the preceding year. Bivariate analyses used χ2 for sex, age, race, Medicaid enrollment status, rural residence, diagnosis of dementia, indicator of an ICU stay, and indicator of the receipt of mechanical ventilation; Student’s t test for age; and Wilcoxon rank-sum tests for HCC score, hospital and ICU length of stay. Associations between hospitalization factors, patient demographics, and comorbid illness with severe or very severe cognitive impairment (the two most severe categories of MDS-COGS) or dependence or total dependence in ADL (the two most dependent categories in the ADL Hierarchical Scale) were evaluated using multivariable logistic regression. Model calibration was assessed with the Hosmer Lemeshow goodness-of-fit test, with p<0.05 considered evidence to reject the null hypothesis of good model fit. Odds ratios were converted to risk ratios, since the outcomes of interest were common (31). Survival by categories of cognitive and physical functional impairment were compared with Kaplan Meier curves, with statistical significance of differences by cognitive and ADL categories evaluated using the log-rank test (32). The risk of death following hospitalization was assessed using Cox proportional hazards regression models using robust variance estimates, and the assumption of proportionality was evaluated using log(-log) survival curves. For those with more than one hospitalization for severe sepsis, survival analyses were carried out using data from the first hospitalization. Multivariable models were adjusted for the following potential confounders a priori: age, sex, race, rurality, Medicaid eligibility, the receipt of mechanical ventilation during the hospitalization, admission to the ICU during the hospitalization, indicators for each of the 23 Elixhauser comorbidities with a prevalence in the cohort of >5%, the remainder of the Elixhauser comorbidities combined as a single indicator variable, and CMS-HCC risk score.
Results
Patient Characteristics
There were 135,370 subjects who survived 175,755 severe sepsis hospitalizations during 2005–2009, and following 66,540 of these hospitalizations, the subject was admitted to a SNF (Table 1, for which the level of analysis is each hospitalization). Those survivors discharged to a SNF tended to be older, were less likely to be male, more likely to be enrolled in Medicaid, slightly more likely to have been cared for in the ICU but no more likely to have received mechanical ventilation (7.3% for both groups). Those discharged to a SNF were also much more likely to have dementia (46.1% versus 27.3%, p<0.001). For 31,114 (46.8%) of those SNF admissions, the beneficiary had not been in a SNF in the year preceding hospitalization. Compared to those previously admitted to a SNF, those newly admitted were more likely to be white, much less likely to be receiving Medicaid benefits (19.6% versus 40.5%. p<0.001), much less likely to be diagnosed with dementia in the year preceding hospitalization (28.1% versus 61.9%, p<0.001), slightly more likely to have spent time in the ICU and more likely to have received mechanical ventilation during the hospitalization (8.6% versus 6.1%, p<0.001) (Table 1).
Table 1.
Demographics and Comorbid Illness, by Discharge destination
| Variable | Discharged to SNF (n=66,540) |
Other discharge destination (n=109,215) | P value | Newly discharged to SNF (n=31,114) | Discharged back to SNF (n=35,426) |
P value |
|---|---|---|---|---|---|---|
| Age at discharge, μ (sd) | 82.1 (7.7) | 79.1 (7.8) | <0.001 | 81.7 (7.6) | 82.4 (7.7) | <0.001 |
| Men, n (%) | 22,364 (33.6) | 44, 590 (40.8) | <0.001 | 11,033 (35.5) | 11, 331 (32.1) | <0.001 |
| Race | <0.001 | <0.001 | ||||
| White | 56,600 (85.1) | 91,113 (83.4) | 27.285 (87.7) | 29,315 (82.8) | ||
| Black | 7,420 (11.2) | 12,622 (11.6) | 2,751 (8.8) | 4,668 (13.2) | ||
| Other | 2,520 (3.8) | 5,480 (5.0) | 1,077 (3.5) | 1,510 (4.1) | ||
| Medicaid dual enrollment, n (%) | 20,452 (30.7) | 25,115 (23.0) | <0.001 | 6,111 (19.6) | 14,341 (40.5) | <0.001 |
| Rural residence, n (%) | 6,123 (9.2) | 12,383 (11.3) | <0.001 | 3,110 (10.0) | 3,013 (8.5) | <0.001 |
| # of Elixhauser comorbidities, μ (sd) | 5.9 (3.1) | 5.4 (3.1) | <0.001 | 4.7 (2.9) | 6.9 (2.8) | <0.001 |
| HCC score, median (IQR) | 2.8 (1.7, 4.3) | 2.5 (1.4, 2.5) | <0.001 | 2.0 (1.2, 3.1) | 3.6 (2.4, 5.1) | <0.001 |
| Dementia (Taylor) | 30,680 (46.1) | 29,786 (27.3) | <0.001 | 8,749 (28.1) | 21,931 (61.9) | <0.001 |
| Hospital length of stay, median (IQR) | 8 (5, 13) | 6 (4, 10) | <0.001 | 9 (5, 14) | 7 (5, 11) | <0.001 |
| Spent time in ICU | 32,040 (48.2) | 51,889 (47.5) | 0.01 | 14,900 (47.9) | 15,826 (44.7) | <0.001 |
| ICU days*, median (IQR) | 5 (3, 9) | 5 (3, 9) | <0.001 | 6 (3, 11) | 5 (3, 8) | <0.001 |
| Received mechanical ventilation | 4,834 (7.3) | 7,938 (7.3) | 0.98 | 2,690 (8.6) | 2,145 (6.1) | <0.001 |
| Mechanical ventilation ≥ 96h | 2,425 (3.6) | 4,408 (4.0) | <0.001 | 1,308 (4.2) | 1,117 (3.2) | <0.001 |
among those with ICU stay; though the median, 25th and 75th percentile ICU length of stay were the same for those discharged to SNF and those discharged to another destination, by the Wilcoxon ranksum test the ICU length of stay was significantly longer in those discharged to SNF.
Cognitive Impairment
Data necessary to calculate MDS-COGS was missing for 0.2% of subjects, and 98% of subjects had missing data for 2 or fewer of the component variables. Thirty-four percent of survivors had severe or very severe cognitive impairment upon SNF admission; those newly admitted were more likely to be cognitively intact compared to those who had been previously in the SNF (Supplemental Table 3). Those who received mechanical ventilation during hospitalization were more likely to have very severe cognitive impairment. In adjusted analyses, mechanical ventilation was associated with the outcomes of severe or very severe cognitive impairment (Risk Ratio 1.30, 95% CI 1.25 to 1.36, p<0.001), with the magnitude of this association being larger than the association between 10 additional years of age, male sex, or prior residence in a SNF and this outcome (Table 2).
Table 2.
Associations between demographic and clinical factors with cognitive and functional outcomes*
| Outcome Variable | ||||
|---|---|---|---|---|
| Severe or Very Severe Cognitive Impairment† | Dependence or Total Dependence in ADL‡ | |||
| Variable | Risk Ratio (95 % Confidence Interval) | P value | Risk Ratio (95 % Confidence Interval) | P value |
| Age (each additional 10 years) | 1.25 (1.22 to 1.27) | <0.001 | 1.08 (1.07 to 1.10) | <0.001 |
| Male sex | 1.11 (1.08 to 1.14) | <0.001 | 0.91 (0.93 to 0.97) | <0.001 |
| Race | ||||
| White | Referent | – | Referent | – |
| Black | 1.39 (1.35 to 1.44) | <0.001 | 1.21 (1.18 to 1.24) | <0.001 |
| Other | 1.30 (1.23 to 1.37) | <0.001 | 1.20 (1.16 to 1.25) | <0.001 |
| Medicaid dual enrollment | 1.17 (1.14 to 1.20) | <0.001 | 1.08 (1.05 to 1.10) | <0.001 |
| Rural residence | 1.12 (1.07 to 1.16) | <0.001 | 1.01 (0.97 to 1.04) | 0.64 |
| Dementia (Taylor) | 1.96 (1.93 to 2.00) | <0.001 | 1.19 (1.17 to 1.21) | <0.001 |
| Prior SNF in 1 year | 1.16 (1.13 to 1.19) | <0.001 | 1.15 (1.13 to 1.17) | <0.001 |
| Mechanical ventilation | 1.30 (1.25 to 1.36) | <0.001 | 1.31 (1.27 to 1.35) | <0.001 |
| ICU stay during hospitalization | 0.98 (0.95 to 1.00) | 0.10 | 1.18 (1.07 to 1.11) | <0.001 |
Multivariable logistic regression, with additional variables in the model including indicators for each of the 23 Elixhauser comorbidities with a prevalence in the cohort of >5%, the remainder of the Elixhauser comorbidities combined together as a single indicator variable, and CMS-HCC risk score;
From MDS-COG;
From ADL Hierarchical Scale categories;
Activities of Daily Living
Data necessary to calculate ADL hierarchical scale was missing for 9 subjects (0.01%), and more than 99.9% of subjects had missing data for 2 or fewer of the component variables. Most survivors had complete dependence in at least 1 ADL, and 72.5% had an ADL Hierarchal Scale score indicating Maximal, Dependence, or Total Dependence at SNF admission (Table 2). Those who received mechanical ventilation during a severe sepsis hospitalization were more likely to have total dependence in ADLs, both in those newly admitted to the SNF and those who had been in a SNF in the year preceding hospitalization. For each of the 7 ADLs assessed upon SNF admission, those newly admitted to the SNF were less dependent than those previously admitted to the SNF. Mechanical ventilation was associated with the outcome of Dependence or Total Dependence in ADLs (RR 1.31, 95% CI 1.28 to 1.35, p<0.001), with the magnitude of this association being larger than the associations between 10 additional years of age, prior residence in a SNF, or a diagnosis of dementia prior to the severe sepsis hospitalization and this outcome (Table 2).
Survival after Hospital Discharge
Discharge destination was associated with survival (Figure 1, log rank p<0.001). One-year mortality was 35.6% among those not discharged to SNF, 43.2% among those discharged to SNF who had not been in SNF in the year prior, and 52.8% among those discharged to SNF who had been in a SNF in the year prior. Among those discharged to a SNF, cognitive function at the time of SNF admission was strongly associated with survival (Figure 2a). Median survival for those with very severe cognitive impairment upon SNF admission (comprising 6.3% of the cohort) was 2.6 months, for those with severe cognitive impairment (27.7% of the cohort) was 7.0 months, and for those with moderate cognitive impairment (28.5% of the cohort) was 14.3 months, compared to 24.2 months for the 37.6% of the cohort who were cognitively intact (Figure 2a, p<0.01 across categories). In adjusted analyses the risk of death was 40% higher for those with moderate cognitive impairment, twice as high for those with severe cognitive impairment upon SNF admission, and over 3 times as high for those with very severe cognitive impairment relative to those who were cognitively intact (Table 3). Results were similar when restricted to those who were free of dementia and had not been in a SNF in the year prior to severe sepsis hospitalization (Supplemental Table 4).
Figure 1.
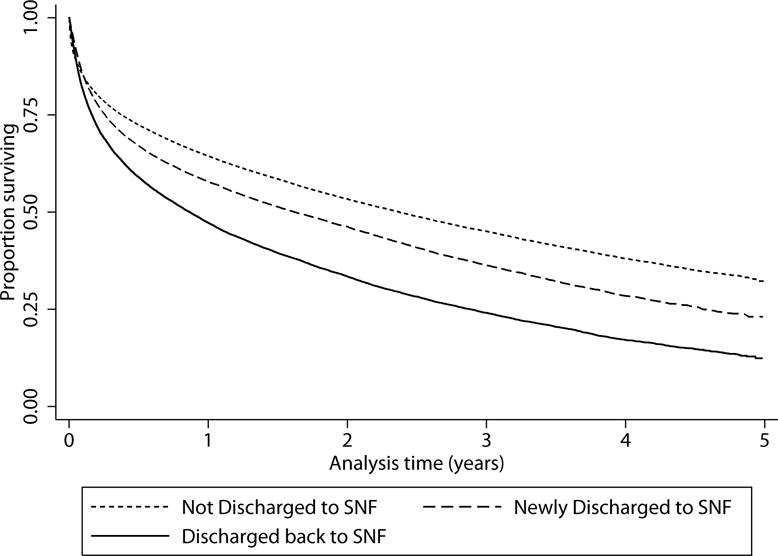
Survival by discharge destination. Kaplan Meier curve of survival following severe sepsis hospitalization, comparing those not discharged to a skilled nursing facility (SNF), those discharged to a SNF who were not in a SNF in the year prior to severe sepsis hospitalization, and those discharged to SNF who had been in a SNF in the year preceding severe sepsis hospitalization. Log-rank test for trend across categories p<0.001.
Figure 2a.
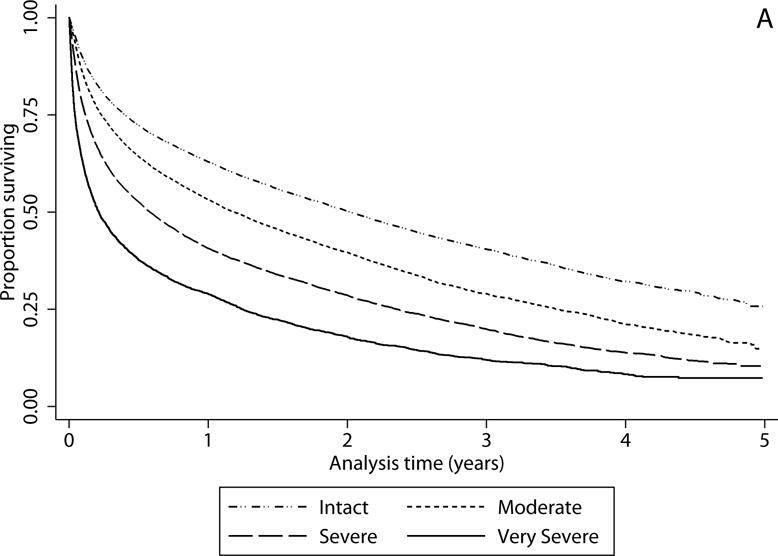
Survival by cognitive status. Kaplan Meier curves of survival after discharge from severe sepsis hospitalization among those discharged to skilled nursing facility (SNF) by cognitive at SNF admission. Cognitive status determined by MDS-COGS. Log-rank test for trend across categories of cognitive function p<0.001.
Table 3.
Association between cognitive status, ADL dependence and survival*
| Cognitive Status at SNF Admission | ADL Dependence upon SNF Admission | ||||
|---|---|---|---|---|---|
| Variable | Hazard Ratio (95 % Confidence Interval) | P value | Hazard Ratio (95 % Confidence Interval) | P value | |
| Cognitive status† | ADL category‡ | ||||
| Intact | Referent | – | Independent | Referent | – |
| Moderate dysfunction | 1.4 (1.3 to 1.4) | < 0.001 | Supervision | 0.98 (0.85 to 1.1) | 0.83 |
| Severe dysfunction | 2.0 (1.9 to 2.1) | < 0.001 | Limited | 1.2 (1.1 to 1.4) | <0.001 |
| Very severe dysfunction | 3.1 (2.9 to 3.2) | < 0.001 | Extensive | 1.5 (1.3 to 1.7) | <0.001 |
| Maximal | 1.8 (1.6 to 2.0) | <0.001 | |||
| Dependent | 2.4 (2.1 to 2.7) | <0.001 | |||
| Total dependence | 4.3 (3.8 to 5.0) | <0.001 | |||
| Mechanical ventilation | 1.1 (1.2 to 1.1) | 0.02 | Mechanical ventilation | 0.97 (0.93 to 1.02) | 0.26 |
| ICU stay during hospitalization | 1.03 (1.01 to 1.1) | 0.001 | ICU stay during hospitalization | 1.0 (0.98 to 1.02) | 0.99 |
| Age (each additional 10 years) | 1.3 (1.29 to 1.33) | < 0.001 | Age (each additional 10 years) | 1.3 (1.3 to 1.4) | <0.001 |
| Male sex | 1.3 (1.2 to 1.3) | < 0.001 | Male sex | 1.3 (1.3 to 1.4) | <0.001 |
| Race | Race | ||||
| White | Referent | – | White | Referent | – |
| Black | 0.87 (0.84 to 0.90) | < 0.001 | Black | 0.86 (0.82 to 0.89) | <0.001 |
| Other | 0.82 (0.78 to 0.88) | < 0.001 | Other | 0.80 (0.75 to 0.85) | <0.001 |
| Medicaid dual enrollment | 1.0 (0.97 to 1.02) | 0.80 | Medicaid dual enrollment | 1.01 (0.98 to 1.03) | 0.43 |
| Rural residence | 1.0 (1.0 to 1.07) | 0.07 | Rural residence | 1.05 (1.01 to 1.09) | 0.007 |
| Dementia (Taylor) | 0.96 (0.94 to 0.99) | 0.01 | Dementia (Taylor) | 1.09 (1.07 to 1.13) | <0.001 |
| Prior SNF in 1 year | 1.07 (1.04 to 1.10) | <0.001 | Prior SNF in 1 year | 1.06 (1.03 to 1.08) | <0.001 |
Multivariable cox proportional hazards regression; additional variables in the model included indicators for each of the 23 Elixhauser comorbidities with a prevalence in the cohort of >5%, the remainder of the Elixhauser comorbidities combined as a single indicator variable, and CMS-HCC risk score;
From MDS-COG;
From ADL Hierarchical Scale categories;
ADL dependence at the time of SNF admission was also strongly associated with long-term survival (Figure 2b). Median survival for those with the highest level of dependence by the ADL hierarchical scale upon SNF admission (comprising 12.6% of the cohort) was 2.3 months (95% CI 2.1 to 2.4 months), and for those with the second highest level of dependence in ADLs (33.5% of the cohort) was 8.7 months (95% CI 8.2 to 9.2 months) compared to 39.9 months for the 0.9% of the cohort who were independent in ADLs (95% CI 36.0 to 45.6 months) upon SNF admission (Figure 2b, p<0.01 across the 7 categories). In adjusted analyses, the risk of death was over twice as high for those who were “dependent” in ADLs upon SNF admission and over 4 times as high for those with “total dependence” in ADLs relative to those who were independent (Table 3). Results were similar when restricted to those who were not in a SNF and who dementia-free in the year prior to severe sepsis hospitalization (Supplemental Table 4).
Figure 2b.
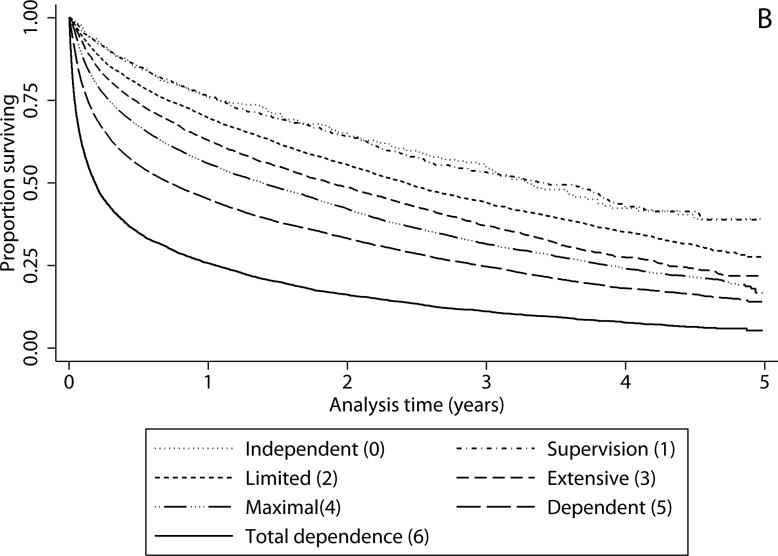
Survival by functional status. Kaplan Meier curves of survival after discharge from severe sepsis hospitalization among those discharged to skilled nursing facility (SNF) by level of independence in activities of daily living (ADL) at SNF admission. Categories of ADL dependence created using ADL hierarchical scale. Log-rank test for trend across categories of ADL dependence p<0.001.
Discussion
We determined that among the nearly half of Medicare beneficiaries surviving a hospitalization for severe sepsis who are discharged to a SNF, more than 1 in 3 had severe or very severe cognitive impairment, and nearly half had dependence in ≥4 ADLs or had total dependence for eating and/or locomotion. We found an association between discharge to a SNF following severe sepsis and shorter survival, which has been shown for other conditions (35). The 1-year mortality of sepsis survivors discharged to SNF was much higher than that of patients admitted to a SNF for post-acute care following hospitalization for other conditions (Almost 50% versus 26.1%) (36). Median survival for the most severely cognitively impaired survivors of severe sepsis (2.6 months), or those with the highest level of dependence in ADLs (2.3 months), was less than half that of patients with newly diagnosed metastatic non-small cell lung cancer or metastatic pancreatic cancer. Even among those without a diagnosis of dementia or a SNF stay in the year prior to hospitalization, cognitive and ADL dependence were each strongly associated with substantially higher mortality.
The magnitude of the association between poorer cognitive performance and greater ADL dependence at the time of admission to a SNF and shortened survival is striking. These data can inform physicians, patients, and their families’ as they weigh the potential benefits of rehospitalization, ongoing or recurrent life-sustaining therapy, and cardiopulmonary resuscitation against the substantial burdens of such therapies. These results demonstrate the need for palliative care services to be incorporated into SNF care, an aspect of care in the SNF setting that needs considerably improvement (37–39). In addition, many of these patients might have benefited from Hospice enrollment, which until 2016 has not been possible for patients receiving post-hospitalization care in a SNF using Medicare benefits (38, 40). Finally, the post-acute care Medicare SNF benefit often requires that patients receive rehabilitative services that, for patients whose trajectory suggests ongoing decline or those who are actively dying, may not be appropriate.
These findings also highlight the urgent need for approaches to minimize the risk of worsened cognitive impairment and new physical limitations that frequently develop during severe sepsis hospitalization. If novel management strategies in the hospital are developed to reduce these risks, such as effective approaches to prevent or treat delirium, or if novel therapies following such a hospitalization currently being investigated, such as cognitive and functional rehabilitation (41, 42), are discovered to effectively mitigate cognitive impairment and ADL dependence, longer-term survival may be improved as well.
There are several limitations to consider. This study is observational, and there is the risk that the associations we observed between factors such as mechanical ventilation and cognitive and functional outcomes and between cognitive and functional impairment and shortened survival may be confounded by variables not considered in these analyses, or not available in our data sources. We can only evaluate cognitive and functional status in those sepsis survivors discharged to a SNF, and so we cannot make comparisons with those who were discharged home. The association between severe sepsis hospitalization and functional impairment is bidirectional: pre-morbid functional impairment is a risk for poor outcomes following severe sepsis (43–45) and is likely a risk for severe sepsis itself (46), and severe sepsis (along with other critical illness syndromes) is a risk for cognitive and physical functional impairment following hospitalization (7, 8, 10). We cannot determine how much of the observed cognitive impairment and ADL dependence was newly acquired during the hospitalization. However, this question may be less relevant given how strongly such impairment is associated with poor outcomes including significantly shortened survival. A related limitation is that this study utilizes assessment of cognitive impairment and ADL dependence only at the time of SNF admission. Cohort studies of patients with critical illness syndromes have demonstrated that many survivors experience improvement in cognitive function in the months following discharge (11, 47), though the patients in these studies were generally younger. Recovery of physical function in older adults following hospitalization occurs in a minority of patients (9, 48, 49), and data suggest that older adults admitted to a SNF after hospitalization (particularly those with pre-hospitalization functional impairment) have a low likelihood of recovery (50, 51). Regardless, we do not know how much the impairment seen in this study may be transient. However, given the strength of association between a higher burden of cognitive impairment and ADL dependence and shorter survival, this uncertainty does not diminish the significance of these findings.
Future studies including an assessment of cognitive or ADL trajectory after (and ideally before) severe sepsis hospitalization may allow a fuller understanding of these associations with longer term outcomes, and could investigate for a differential impact of preexisting versus newly acquired impairment (9, 52). Prospective studies to differentiate those patients with newly acquired cognitive and other functional impairment who are most likely to recover are also needed, not only for prognostication to inform decision-making but also to allow for targeted interventional studies. In the meantime, physicians who care for older adults during and after severe sepsis should take these striking data into account as they counsel patients and families struggling with life after sepsis.
Conclusion
In this retrospective cohort study of Medicare beneficiaries surviving a hospitalization with severe sepsis, discharge to a SNF was associated with shorter survival. Among those discharged to a SNF, cognitive impairment and ADL dependence were each quite common and were each strongly associated with shortened survival. These findings can inform decision-making by patients and physicians, and underscores high palliative care needs among sepsis survivors discharged to SNF. They also highlight the urgent need for approaches to minimize the cognitive and physical functional impact of hospitalization for severe sepsis.
Supplementary Material
Acknowledgments
The authors thank Peggy Munson for Institutional Review Board assistance; Katie Ronk and Jeffrey Havlena for data management assistance; and Glenn Allen for analytic assistance.
Copyright form disclosure: Drs. Ehlenbach, Repplinger, Westergaard, Jacobs, Kind, and Smith received support for article research from the National Institutes of Health (NIH). Dr. Ehlenbach’s institution received funding from the NIH Dr. Gilmore-Bykovskyi’s institution received funding from National Hartford Centers of Gerontological Nursing Excellence. Dr. Kind’s institution received funding from the NIH, VA, CMS, and multiple non-profit foundations.
Funding/Support and Role of the Funder/Sponsor:
Dr. Ehlenbach is supported by funding from the National Institutes of Health, National Institute of Aging (K23 AG038352), funded by The Atlantic Philanthropies, The John A. Hartford Foundation, and the Starr Foundation. Additional support was provided by the University of Wisconsin School of Medicine and Public Health’s Health Innovation Program, the Community–Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research, and the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health (grant 1UL1RR025011). These funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest and Financial Disclosures
None of the authors have any financial relationships or potential conflicts of interest related to the subject of this manuscript.
Disclaimer
The contents of this article do not reflect Centers for Medicare & Medicaid Services policy.
References
- 1.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Cooke CR, Wunsch H, et al. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153(3):204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson S, Ruokonen E, Varpula T, et al. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 6.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlenbach WJ, Larson EB, Curtis JR, et al. Physical Function and Disability After Acute Care and Critical Illness Hospitalizations in a Prospective Cohort of Older Adults. J Am Geriatr Soc. 2015;63(10):2061–2069. doi: 10.1111/jgs.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante LE, Pisani MA, Murphy TE, et al. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140(5):1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 14.Gehlbach BK, Salamanca VR, Levitt JE, et al. Patient-related factors associated with hospital discharge to a care facility after critical illness. Am J Crit Care. 2011;20(5):378–386. doi: 10.4037/ajcc2011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. Chronic Condition Data Warehouse. 2015 [cited 2015 15 November]Available from: https://www.ccwdata.org/web/guest.
- 16.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4 Suppl):III50–59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 17.Arday SL, Arday DR, Monroe S, et al. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21(4):107–116. [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Agriculture Economic Research Service. Rural Classifications: Overview. 2014 [cited 2015 1 December]Available from: http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx.
- 19.Washington State Department of Health. Guidelines for Using Rural–Urban Classification Systems for Public Health Assessment. 2009 [cited 2016 28 January]Available from: http://www.doh.wa.gov/Portals/1/Documents/5500/RuralUrbGuide.pdf.
- 20.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ford DW, Goodwin AJ, Simpson AN, et al. A Severe Sepsis Mortality Prediction Model and Score for Use With Administrative Data. Crit Care Med. 2016;44(2):319–327. doi: 10.1097/CCM.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55(9):929–937. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DH, Jr, Ostbye T, Langa KM, et al. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmaier SL, Sloane PD, Guess HA, et al. The MDS Cognition Scale: a valid instrument for identifying and staging nursing home residents with dementia using the minimum data set. J Am Geriatr Soc. 1994;42(11):1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- 26.Hartmaier SL, Sloane PD, Guess HA, et al. Validation of the Minimum Data Set Cognitive Performance Scale: agreement with the Mini-Mental State Examination. J Gerontol A Biol Sci Med Sci. 1995;50(2):M128–133. doi: 10.1093/gerona/50a.2.m128. [DOI] [PubMed] [Google Scholar]
- 27.Gruber-Baldini AL, Zimmerman SI, Mortimore E, et al. The validity of the minimum data set in measuring the cognitive impairment of persons admitted to nursing homes. J Am Geriatr Soc. 2000;48(12):1601–1606. doi: 10.1111/j.1532-5415.2000.tb03870.x. [DOI] [PubMed] [Google Scholar]
- 28.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 29.Graney MJ, Engle VF. Stability of performance of activities of daily living using the MDS. Gerontologist. 2000;40(5):582–586. doi: 10.1093/geront/40.5.582. [DOI] [PubMed] [Google Scholar]
- 30.Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 32.Harrington DP, Fleming R. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- 33.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 34.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 35.Lai SM, Alter M, Friday G, et al. Stroke survival after discharge from an acute-care hospital. Neuroepidemiology. 1999;18(4):210–217. doi: 10.1159/000026213. [DOI] [PubMed] [Google Scholar]
- 36.Hakkarainen TW, Arbabi S, Willis MM, et al. Outcomes of Patients Discharged to Skilled Nursing Facilities After Acute Care Hospitalizations. Ann Surg. 2016;263(2):280–285. doi: 10.1097/SLA.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragon K, Covinsky K, Miao Y, et al. Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172(20):1573–1579. doi: 10.1001/archinternmed.2012.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller SC, Teno JM, Mor V. Hospice and palliative care in nursing homes. Clin Geriatr Med. 2004;20(4):717–734, vii. doi: 10.1016/j.cger.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Miller SC. Hospice and palliative care in nursing homes: challenges and opportunities for enhanced access. R I Med J (2013) 2015;98(4):26–29. [PubMed] [Google Scholar]
- 40.Huskamp HA, Stevenson DG, Chernew ME, et al. A new medicare end-of-life benefit for nursing home residents. Health Aff (Millwood) 2010;29(1):130–135. doi: 10.1377/hlthaff.2009.0523. [DOI] [PubMed] [Google Scholar]
- 41.Brummel NE, Jackson JC, Girard TD, et al. A Combined Early Cognitive and Physical Rehabilitation Program for People Who Are Critically Ill: The Activity and Cognitive Therapy in the Intensive Care Unit (ACT-ICU) Trial. Phys Ther. 2012 doi: 10.2522/ptj.20110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connolly B, Denehy L, Brett S, et al. Exercise rehabilitation following hospital discharge in survivors of critical illness: an integrative review. Crit Care. 2012;16(3):226. doi: 10.1186/CC11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chernow B. Variables affecting outcome in critically ill patients. Chest. 1999;115(5 Suppl):71S–76S. doi: 10.1378/chest.115.suppl_2.71s. [DOI] [PubMed] [Google Scholar]
- 44.Stump TE, Callahan CM, Hendrie HC. Cognitive impairment and mortality in older primary care patients. J Am Geriatr Soc. 2001;49(7):934–940. doi: 10.1046/j.1532-5415.2001.49184.x. [DOI] [PubMed] [Google Scholar]
- 45.Yende S, Iwashyna TJ, Angus DC. Interplay between sepsis and chronic health. Trends Mol Med. 2014;20(4):234–238. doi: 10.1016/j.molmed.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao KM, Lin TC, Li CY, et al. Dementia Increases Severe Sepsis and Mortality in Hospitalized Patients With Chronic Obstructive Pulmonary Disease. Medicine (Baltimore) 2015;94(23):e967. doi: 10.1097/MD.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 48.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrante LE, Pisani MA, Murphy TE, et al. Factors Associated with Functional Recovery Among Older ICU Survivors. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill TM, Gahbauer EA, Han L, et al. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64(12):1296–1303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buurman BM, Han L, Murphy TE, et al. Trajectories of Disability Among Older Persons Before and After a Hospitalization Leading to a Skilled Nursing Facility Admission. J Am Med Dir Assoc. 2016;17(3):225–231. doi: 10.1016/j.jamda.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox CE, Wysham NG. Untangling Health Trajectories among Patients with Sepsis. Annals of the American Thoracic Society. 2015;12(6):796–797. doi: 10.1513/AnnalsATS.201503-134ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


