Abstract
Human adolescents engage in very high rates of unprotected sex. This behavior has a high potential for unintended, serious, and sustained health consequences including HIV/AIDS. Despite these serious health consequences, we know little about the neural and cognitive factors that influence adolescents’ decision-making around sex, and their potential overlap with behaviorally co-occurring risk behaviors, including alcohol use. Thus, in this review, we evaluate the developmental neuroscience of sexual risk and alcohol use for human adolescents with an eye to relevant prevention and intervention implications.
Keywords: Adolescent, Brain, Sexual risk, Alcohol use, HIV
Introduction
By the end of high school (age 18) [1], 47 % of American teens have had sexual intercourse and 34 % are sexually active. Just under half of those youth (41 %) use condoms, and almost a quarter (22 %) report substance use—most commonly alcohol—before having sex. While initiation of “adult” behaviors including sex and drinking is common and normative for this developmental stage [2, 3], unsafe engagement in these behaviors (i.e., unprotected sex) increases the chance of unintended, serious, and sustained health consequences, including unplanned pregnancy, sexually transmitted infections (STIs) and the human immunodeficiency virus (HIV) [4]. These risks are exacerbated when alcohol use is part of the equation [5]. Studies from our lab and others have shown that alcohol use is associated with greater likelihood of having sex and lower incidence of condom use among adolescents [6, 7]. Perhaps, in part, due to high rates of alcohol use during this timeframe [1], adolescents currently comprise one of the only segments of the population for whom acquisition of STIs/HIV is not decreasing, with 50 % of all new diagnoses occurring in this age group [1].
Despite these alarming statistics, we know relatively little about the neural and cognitive factors that influence adolescents’ sexual behavior. Given the rapid changes occurring in the brain at exactly this developmental stage [8], it seems natural to question whether these developmental shifts in neurocognitive structure and function could be leveraged in ways that guide the development of interventions to reduce adolescent risky sex. Remarkably, the extent of published neuroscience studies of sexual risk behavior in human adolescents is quite limited [9–13]. An inherent challenge in this work is that the cognitive processes involved in adolescent sexual decision-making are highly complex; involving everything from navigating emergent basic biological drives to procreate, the high potential natural rewards of the behavior, higher-order cognitive processes requisite within weighing costs/benefits, and charting new emotional, social, and affective waters.
Following a legacy of being characterized as “storm and stress” [14], there continues to be great controversy regarding whether the adolescent brain is adaptive (i.e., perfectly suited to initiate of behaviors important to growth and development) or predisposed towards poor and dangerous decision-making (i.e., an “immature” system unstoppably programmed for risk taking) [8, 15, 16]. The existing empirical data have generally fallen towards the side of an immature system that prohibits youths’ ability to inhibit risk behavior, a conceptualization that forms the foundation for several prevailing theories (e.g. “dual process” [17]; “triadic” [18]; “imbalance” [19]). Broadly, these theories suggest a developmental mismatch between reward and control systems, which results in adolescents’ biased selection of riskier decisions across myriad contexts.
Our conceptualization of the nature of the adolescent brain and related decision-making matters, as it translates to real-world variance in youths’ health risk behavior [20] and from a clinical perspective, is critical for informing intervention approaches to encourage safer behavior. Thus, the goal in this paper is to review and synthesize the cutting-edge empirical research in this area. We use these data to advance a preliminary conceptualization of the putative neurocircuitry of adolescent sexual risk. Finally, we compare this working model to those that have been advanced in the domain of alcohol use to highlight areas of consonance and dissonance between these co-occurring health risk behaviors.
The Neurocognitive Underpinnings of Sexual Decision-Making
Cognitive neuroscience provides an innovative and sophisticated tool to evaluate human behaviors, particularly complicated subtle ones. For example, in their landmark study, Bechara et al. were one of the first clinical research teams to shed light on why a subset of otherwise high-functioning individuals with typical intellect, memory, and problem solving skills [21], continued to chronically make decisions only informed by immediate (short-term) gains, even in the face of large, serious, and unremitting negative consequences. Ultimately, this team found that damage to a critical part of the frontal lobe (ventromedial prefrontal cortex) interfered with ability to consider future consequences. In other words, these studies suggested that these individuals were not “hypersensitive to reward” or “insensitive to punishment”, but rather, damage to this frontal region impaired the ability to learn from negative experiences and use that information to guide and reduce risky decision making (decisional “myopia”). Subsequently, investigators have learned that alterations in exactly these frontal regions are associated with variance in decision making across a wide array of risk behaviors from gambling, to alcohol use, and even to criminal culpability (e.g. [22–24]). While these studies have formed a foundation for understanding risk behavior for adults, the extension to adolescents, whose frontal regions are deeply entrenched in development, is only beginning.
At this point, the data are still a long way from providing a neural map of how and why adolescents make risky choices, and how developmental changes in the adolescent brain are associated with those choices. This is problematic, because this information is integral to guiding what providers can do to help adolescents make safer choices as they navigate entrée into adulthood. This disconnect is particularly felt in the context of sexual behavior, where very few studies have approached these questions.
Decisions About Sex in the Adult Brain
One avenue that has been employed to deconstruct sexual decision-making is to evaluate how people think about sex. In this area of research, adult participants have been invited to consider their relationship, their sexual partners, and/or to view sexual encounters. Paralleling foundational studies of risky decision-making, these studies indicate that when adults think about their romantic partner and/or observe sex (via erotica), prefrontal (dlPFC, mPFC), striatal (caudate, putamen), and mesocorticolimbic (dopaminergic) reward regions respond (e.g. anterior cingulate; ACC, nucleus accumbents; NAcc, insula) [25, 26]. Prefrontal regions are involved in executive control (assessing whether a reward is desired, how much the reward is desired, and how/whether one will pursue that reward) [27]. Well-established within the alcohol and broader addiction literature [28], these prefrontal regions are connected to striatal, mesocorticolimbic, and reward areas which are directly linked to, and arguably modulated by, the ventral tegmental area (VTA). This larger mesocorticolimbic system has been hypothesized to play a critical role in the assessment of the magnitude and valence of anticipated rewards (incentive salience and positive expectancies), along with related motivation and drive. Specifically, across various health risk behaviors (e.g. addiction, overeating, gambling), these regions underlie the overall experience of “wanting” [29], which often drives “seeking” of desired rewards. Extending this to adolescent sexual decision-making, it stands to reason that these very regions may be integral in youths’ evaluation of the valence and magnitude of the rewarding aspects of sex, the degree to which they want the rewards of sexual activity, and the subsequent seeking of sexual opportunities.
The Role of Neurodevelopment
As it develops, the adolescent brain is influenced by everything from cortical thinning including synaptic pruning and grey matter changes to myelination and white matter changes. Recently, scientists have begun to examine the contribution of salient neurochemicals (e.g. dopamine, GABA) and hormones (e.g. testosterone) [30, 31] to developing brain structure, connectivity, and function. These factors are particularly relevant to sexual decision-making, as adolescence is marked by a surge of hormones on the brain—driven by the hypothalamic–pituitary–gonadal (HPG) axis—which essentially “turns on” the switch from the pre-reproductive to the reproductive phase of life (c.f. [32]) underlying youths’ transition from general disinterest into fascination with sex.
Implication of Alcohol Use
While in many ways distinct, one behavior that highly co-occurs with sexual risk is alcohol use. A number of theory-based interventions to increase adolescent safer sexual behavior focus on improving intentions to use condoms both in non-drinking, as well as drinking situations [33–35]. Interestingly, even when young people report high intentions to engage in protected sex (e.g. intercourse with condoms), they have trouble translating planned intentions to actual in-the-moment behavior. In a study with a large sample of high risk adolescents, intentions to use condoms were significantly, though only moderately, related to condom use six months later (r = 0.40 and 0.43 for young women and young men, respectively [36]. Despite their clear behavioral parallels, the neurocognitive frameworks typically utilized for understanding in-the-moment adolescent decision-making about sexual intercourse and alcohol use have not been systematically evaluated. Thus, we are left questioning which neurodevelopmental processes may be most important. While there is a larger body of work in the adult literature [37], the empirical literature that has employed MRI and/or fMRI to evaluate these questions for youth is still in its infancy.
Learning as a Distinguishing Feature of Adolescent Sexual Decision-Making
“Seeking” in the realm of sexual decision-making does not necessarily mean “obtaining”. Rather, as observed in the mammalian literature [38] overlaid on top of straightforward reward-seeking and incentive salience, there is a highly-sophisticated (albeit somewhat clumsy) stretch of learning, experimenting, and conditioning associated with sexual activity, informed by essential contributions from sensory and social interactions. This iterative series of learning experiences enables youth to transition from the mesocorticolimbic-based drive that may underlie “wanting” sex, to their ability to find a willing partner, to successfully move into “having” sex [39]. This complexity is likely part of what sets sexual behavior apart from other types of risk behavior.
We suggest that this particular dynamic of learning brings youth sexual decision-making more in line with the fields of experimental cognition and learning in the developing brain [40]. Specifically, to develop basic though higher-order capacities around sex, some suggest that there is a critical period within adolescence where the brain is particularly sensitive and responsive to integrating new information (aka “love maps”) [39]. Animal models highlight that these connections are crucial to the development of healthy and positive expectations around sex [39]. Further, in terms of typical negotiations in heterosexual encounters, the exact skills that youth learn diverge by gender, setting the stage for different expectations and ultimately sexual behavior [41]. Notably, this work has been mostly explored in the animal literature. Thus, the exact neural substrates relevant for these emergent connections has yet to be established in human adolescents.
The Contribution of Self-Control
The development of neural networks underlying self-control is crucial to adolescent sexual decision-making [40]. A recent review by Casey points to the close coordination of three main regions in adolescent’s development of self-control, including the amygdala, the prefrontal cortex (PFC), and striatum [40]. Within this conceptualization, the PFC is important for synthesizing information about sensory input, reward availability and valence, and translating those data into behavioral action (or the stopping thereof). In application to sexual risk, speculatively, the PFC might process visual and sensory information about a potential sexual opportunity (an attractive girl; how she looks, how she smells, what she might feel like), what chance the adolescent has at successfully attracting her (is she available?), and whether or not the adolescent should initiate interaction (going to talk with her). Similarly, the limbic system, including the amygdala, is indispensible in assessing emotional valence, processing contextual cues, and creating new associations. Thus, this region might be important in determining how the youth feels about this potential partner (Are there feelings of love? lust? Is this a situation that lends itself to a single sexual event or a potential relationship? Has the adolescent’s previous experience led to positive/negative expectations around sex and relationships?) Finally, the striatum may predict gains and rewards. Through direct and indirect projections, these systems modulate each other to balance messages of reward, emotion, desire, learned expectation, and anticipation in adolescent sexual decision-making. In other words, this system might facilitate the adolescent’s ability to determine that a potential partner is high-reward, available, and, most importantly, interested! However, the adolescent himself might not be emotionally prepared for intercourse. Despite all motivational systems being set for “go”, there is an additional level of this neural system that should be able to modulate (stop) the drive for sexual behavior. One important component of this modulatory system is response inhibition; many have suggested that weaknesses in impulse control are associated with higher levels of risky sexual behavior. Importantly, while impulsivity is certainly a piece of the puzzle in adolescent sexual decision-making, the effect sizes are fairly modest (mean effect size = 0.18) [41]. We thus suggest that the neural substrates underlying self-control in the context of sexual decision-making are likely to include, but not be limited to, impulse control. There are potentially other self-regulatory functions (e.g. self-monitoring, attention, judgment, planning) [42] that underlie variability in risk behavior.
It is important to note herein the neurodevelopmental changes that could hinder the capacity of the control system to override the reward system [40]. For example, nucleus accumbens (NAcc) response to reward is heightened during adolescence as compared to childhood and adulthood [43–45]. This may, in part, reflect a dopamine-mediated response linked to increased dopamine receptor concentrations in the striatum (including nucleus accumbens) during adolescence [46]. Thus, one developmental question and challenge within adolescent clinical and neurocognitive research is how effective adolescents can be in their execution of self-control, while the PFC [47] and amygdala [44] continue their final stretch of development (through age 25 for adolescent males) [48].
Developmental Neurocognitive Differences by Gender
The amygdala is a highly featured structure in the developmental, adult, and non-human sexual decision-making literatures [49–51], but it seems to have less presence in the adolescent health behavior literature. At peak volume during adolescence, the amygdala is one of the only brain regions that has receptors for sex hormones [52]. Practically, this means that along with the contributions of social situations and context, sex hormones can directly modulate amygdalar activation, which in turn, influences communication to other salient decision-making regions [52]. In addition, the amygdala has direct projections to the hippocampus and hypothalamus [52]. Thus, the amygdala is not only involved in systems critical to learning, but also directly involved in systems that govern and oversee pubertal timing and the regulation of gonadal (estrogen, testosterone) and stress hormones (corticosterone) [40]. Thus, the amygdala is likely to play a particularly valuable role in biological drive and timing, the establishment of emotional learning, associations, and expectations around sex, and resultant sexual decision-making and behavior.
Often referred to as sexual dimorphism, gender differences in the brain and related behavior begin during puberty when gonadal steroids prepare the neuroendocrine systems for reproduction [53]. The impact of this shift is that neural organization underlying sexual decision-making may manifest differently for adolescent females versus males. For example, the human behavioral literature indicates that adolescent females seek sexual interactions to foster relationship factors (getting and/or keeping a partner), rather than for physical reward (as is the case for adolescent males) [3]. Studies with adult humans have also shown gender differences in willingness to engage in sexual encounters, differences in sexual arousal and the sexual double standard [54]. Further, human and animal studies show that sexual stimuli are processed differently depending on phase of the menstrual cycle for females, while androgen-based responsiveness and arousability are continuous and steady for males [39]. Human adolescent studies, including our own, reveal developmental gender differences across limbic structures, including the amygdala and hippocampus, as correlates of risky behavior [55, 56]. In the domain of risky sex, our work supports a strong negative relationship between amygdala volume and risky sex for girls; in contrast, we found no such relationship for boys.
Systematic Review Methodology
We sought to address the gap in our current understanding of the neural circuitry underlying adolescent sexual decision-making and behavior by systematically reviewing the existing published literature extending from the years 1989–2015. We followed PRISMA guidelines for systematic reviews (Liberati et al. 2009). As we were specifically interested in data addressing the interplay between sexual intercourse and the developing human adolescent brain, we required that studies include human adolescents (as defined as under 18 years of age). Empirical MRI/fMRI studies were required to have a sample size of at least 12 adolescent participants. All manuscripts were required to contain empirical data examining the relation between sexual risk and the brain function and/or structure. We began our search by examining “sexual intercourse” and “decision making” with “MRI” and/or “fMRI” as search terms. However, these terms were too limiting. Thus, we expanded our search to “adolescent”, “sexual”, “romantic”, “love”, “fMRI” and/or “MRI” on PubMed. This yielded 248 studies. Using decision points informed by recent key publications in the field [57], all publications were evaluated for fit against our inclusion criteria (see Table 1). There is an extensive body of literature on sexual orientation, sexual dimorphism, sexual/physical abuse and gender dysphoria. While critically important areas, questions addressing these literatures were not the focus of this review and were therefore excluded from this examination.
Table 1.
Selection criteria
| Criterion |
|---|
|
Emerging Empirical Evidence on the Neurocognitive Underpinnings of Adolescent Sexual Risk
These criteria narrowed the list from 248 studies in our original search to a total of N = 7 empirical studies on the neurocognitive correlates of adolescent sexual decision-making; four are published [9–12] and two are under review [55, 58] (see Table 2).
Table 2.
Overview of the N = 7 empirical studies on the neurocognitive correlates of adolescent sexual decision-making
| Manuscript (authors, journal, year) | N by gender | Age range | Nature of sample | Imaging modality | Method | Sexual outcome measure |
|---|---|---|---|---|---|---|
| Feldstein Ewing SW, Houck JM, and Bryan AD, Addictive Behaviors, 2015 | N males = 77 N females = 18 Total N = 95 |
14–18 | At-risk | fMRI | GoNoGo | Risky sex days (past month days of sexual intercourse minus days of condom use) |
| Feldstein Ewing SW, Ryman SG, Karoly H, Mayer AR, Ling J, Bryan AD, under review | N males = 94 N females = 38 Total N = 132 |
14–18 | At-risk | MRI | Freesurfer | Frequency of condom use; Frequency of intercourse |
| Ryman SG, Bryan AD, Thayer RE, Mayer AR, Ling J, Feldstein Ewing SW, under review | N males = 26 N females = 12 Total N = 38 |
14–18 | At-risk | MRI | Tract-based spatial statistics (TBSS) | Frequency of condom use; Frequency of intercourse |
| Goldenberg D, Telzer EH, Lieberman MD, Fuligni A, and Galvan A, Developmental Cognitive Neuroscience, 2013 | N males = 7 N females = 13 Total N = 20 |
15–17 | Not specified | fMRI | GoNoGo | Sexual riskiness rating (1 = condom and birth control, 2 = only condom, 3 = only birth control, 4 = withdrawal, 5 = none) |
| Hensel DJ, Hummer TA, Acrurio LR, James TW, and Fortenberry JD, Journal of Adolescent Health, 2015 | N males = 0 N females = 14 Total N = 14 |
14–15 | Adolescent primary care | fMRI | Risk decision making task assessing likelihood of: drinking the presented alcohol beverage, having sex with the presented man, eating the presented food, or purchasing presented household product | Daily diary of sexual behavior |
| Magnan RE, Callahan TJ, Ladd BO, Claus, ED, Hutchison KE, & Bryan AD, AIDS and Clinical Research, 2013 | N males = 205 N females = 79 Total N = 248 |
14–18 | At-risk | fMRI | Balloon Analogue Risk Task | Condom use; frequency of alcohol use during sex |
| Thayer, RE, Montanaro, E., Weiland, BJ, Callahan, TJ, and Bryan, AD, Current HIV Research, 2014 | N males = 175 N females = 64 Total N = 239 |
14–18 | At-risk | fcMRI | Resting state functional network connectivity in the dorsal default mode (dDMN), ventral default mode (vDMN), left executive control (LECN), and right executive control (RECN) networks | Risky sexual behavior (composite variable calculated as frequency of sex in the past 3 months multiplied by frequency of condom use (reverse coded) |
Goldenberg, Galvan, and colleagues [9] approached this question from the perspective of impulse control. Using a standard fMRI-based Go/NoGo task, they found a significant positive correlation between sexual riskiness (defined on a continuous scale of contraceptive use; 1 = condom and birth control to 5 = no contraception) and BOLD response in the Go > NoGo contrast in the superior frontal gyrus, inferior parietal lobe, insula, and middle frontal gyrus. They also found a significant negative correlation between sexual riskiness and neural activation (NoGo > baseline) in the superior parietal, lateral occipital, superior temporal cortex, insula, right inferior frontal gyrus, and in the NoGo > Go contrast, across the parietal and temporal cortex, superior, middle, and inferior frontal gyri, and the insula. This team summarized their findings as an overarching pattern of lower recruitment of frontal regions during this impulse control task for sexually riskier youth (youth who used less contraception).
Our team examined the role of sexual riskiness (number of unprotected sex days in the last 30) via a slightly different fMRI-based Go/NoGo task targeting response inhibition with sexually-active, substance using youth [12]. In the NoGo > Go contrast, we found a significant positive relationship between past month risky sex and activation within the right inferior frontal gyrus (IFG) and left middle occipital gyrus. We also found significant negative correlations between past month substance use (number of alcohol and cannabis days) and response within the left IFG and right insula. These results suggest a potentially differentiated pattern of brain response associated with adolescent risky sex versus alcohol and marijuana use.
In a different task with this same sample of youth, our team then examined neurocognitive correlates of alcohol-related risky sex [11] during an fMRI-based balloon analog risk task (BART). Using a priori regions of interest (ROIs) across the cerebellum, left posterior insula, right superior parietal lobe, and VTA, we found that greater alcohol-related sexual risk behavior (a latent variable comprised of frequency of binge drinking days, quantity/frequency of alcohol use, and sex concurrent with alcohol use) was inversely associated with BOLD response across these regions. Compellingly, BOLD response was a significant contributor, above and beyond the well-established behavioral risk factor of peer norms, to a latent variable of alcohol-related risky sex.
Our team recently extended this examination to network connectivity and brain structure. Using longitudinal data collected every three months across a 12-month time frame (for details, see 11) we investigated connectivity in the dorsal default mode network at baseline (dDMN) with cross-sectional and longitudinal alcohol use and sexual risk behaviors. We utilized a parallel process latent growth model that simultaneously modeled the trajectories of these behaviors, and included functional connectivity strength in the dDMN as an exogenous variable. We found positive associations between alcohol use and sexual risk; further, network functional connectivity strength of the dDMN was associated with initial and longitudinal alcohol use. Importantly, dDMN connectivity was not associated with initial or longitudinal variation in sexual risk behavior [10], which suggests different neural mechanisms underlying alcohol use and sexual risk. However, it is possible that associations were observed between functional connectivity and alcohol use, but not sexual behavior, because alcohol may be neurotoxic to developing adolescent brain structure and function, while sexual risk behavior is not.
We have begun exploring structural correlates of risky sexual behavior in adolescents. With a completely separate sample of adolescents, our team evaluated the association between sexual risk and brain volume as well as connectivity. We began by investigating tract-based spatial statistics (TBSS; [58], finding that sexual risk (defined as less frequent condom use) was associated with lower integrity of critical white matter fiber tracts connecting the subcortical frontal and reward structures [e.g. lower fractional anisotropy (FA) in the genu and body of the corpus callosum, anterior and superior corona radiata]. In this same sample, we also explored the role of brain volume [55], finding evidence of a significant volume by gender interaction. For adolescent girls, riskier sexual behavior (defined as less frequent condom use) was related to greater bilateral amygdalar and hippocampal volume; no such association emerged for boys.
An exciting new study, Hensel and colleagues [13] enrolled N = 14 14–15 year old girls to evaluate a variant of an fMRI visual cue exposure task. This task contained images of alcohol, male faces, food, and household products, and asked participants to assess the likelihood of engaging in the target activity (e.g. drinking the presented beverage, having sex with the presented man, eating the presented food, purchasing the presented household product). For each target category, decisions were operationalized as high-risk or low-risk. For example, the attractive male target was characterized as having many sexual partners and not using condoms (high-risk) or few sexual partners and using condoms (low-risk). Participants also completed detailed sexual behavior diaries. The authors observed greater activity during low-risk decision making across what they call a “cognitive affective network” including prefrontal and anterior cingulate regions. Yet they also found differences in activation based on type of decision. In the context of sexual decision making, greater activation was observed in the ACC for high-risk sexual decisions, while for low-risk sexual decisions, greater activation was found in the visual cortex. In addition, impulsivity scores were negatively related to activation in the fusiform gyrus and occipital cortex during high-risk sexual decisions.
Collectively, these findings suggest correlation between functional activation in frontal and reward areas (e.g. inferior frontal gyrus; IFG, superior frontal gyrus; SFG; anterior cingulate cortex; ACC) during response inhibition and risky decision making/reward tasks (Go/NoGo; Balloon Analogue Risk Task, BART; visual cue exposure) and risky sexual decision making. Further, there appears to be associations between white matter integrity across similar regions (frontal and reward) and sexual risk [58]. Importantly, few studies examined and/or controlled for co-occurring alcohol use. Thus, observed patterns may not be uniquely related to a propensity for sexual risk, but rather the potential neurotoxic impact of the highly co-occurring behavior of substance use on the developing brain [59]. On the other hand, supporting the dissociation between brain structure and function underlying substance use versus sexual risk, we did not find evidence of parallel relationships between functional response and/or resting state connectivity (dDMN) in adolescent alcohol use and sexual risk [10] but we did find these connections for initial levels and growth of alcohol use. Further, we found a significantly different pattern in the nature and regions of functional activation for sex versus for alcohol use [12] suggesting that alcohol use does not fully account for differences observed in the context of risky sex. Rather, the adolescent brain may have a unique pattern of response associated with decisions pertaining to risky sex, as measured in several different ways across the studies (e.g. likert scale; past month interview recall; daily diary).
In line with the adult literature in this area [37, 50], this early work also indicates salient gender differences in the neurocircuitry underlying adolescent sexual risk decision making. Interestingly, we found differences in brain structure in critical limbic areas (amygdala; hippocampus) and sexual risk behavior for adolescent females (only) when contrasted with adolescent males [55], and new work shows significant brain activation in the ACC associated with risky sex in a sample of adolescent females only [13].
Toward a Model of the Neurocircuitry of Adolescent Sexual Risk
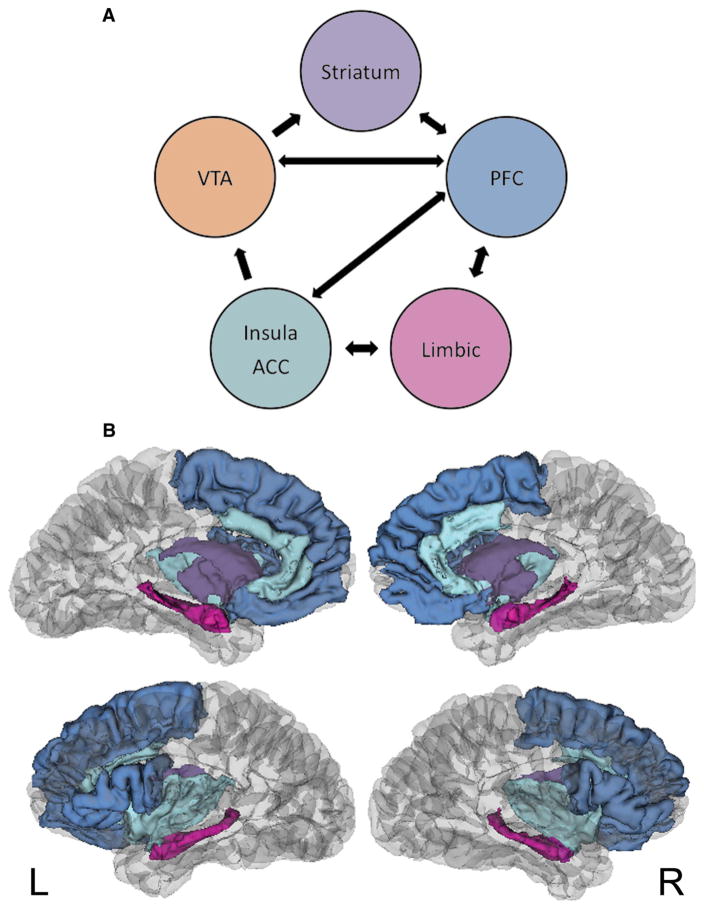
One way to advance research on the neurocognitive underpinnings of adolescent sexual decision-making is to synthesize the existing empirical data into a guiding conceptual framework (Fig. 1). Importantly, it underscores the preliminary nature of this field and the critical need for additional empirical studies to validate and extend these findings.
Fig. 1.
Working model of adolescent sexual decision-making. a Illustration of the neural circuitry in this system. Areas include the ventral tegmental area (VTA); striatum including the nucleus accumbens (NAcc); prefrontal cortex (PFC) including the dorsolateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), inferior frontal gyrus (IFG), superior frontal gyrus (SFG); limbic system including the amygdala (AMYG), hippocampus (HIPP), and hypothamalus (HYP); insula and anterior cingulate cortex (ACC), b Pictoral representation of these neural centers
This model is informed by emergent human adolescent studies [9–13, 55, 58], existing theories of the neuroscience of reward [28], adolescent risky decision-making [40], and animal/adult sexual decision-making [39]. We propose that the key centers in adolescent sexual decision making revolve around salient mesocorticolimbic reward and emotion regulation regions (projections from and between VTA, insula, striatum, PFC, and limbic systems including the amygdala, hippocampus, and hypothalamus). We suggest that these connections facilitate communication between prefrontal regions and critical reward, learning, and emotion centers (PFC projections to the VTA, insula, striatum, and limbic systems). We further propose that communication between key emotion regulation, reward, prefrontal, and gonadal and stress hormone centers (limbic projections to the insula, PFC, and hypothalamus) is one of the features that distinguishes and drives adolescent sexual risk decision making, as compared to other types of adolescent health risk.
While a compelling place to begin, it is critical to consider that this model may not be equivalent for both genders. In other words, based on emerging empirical work in this area [55], gender differences may exist within mesocorticolimbic reward and emotion regulation pathways (VTA, insula/ACC, striatum/NAcc, limbic systems), although the scope, size, and direction of these relationships has yet to be established. These developmental gender differences would be in line with the broader behavioral sexual decision-making literature, which suggests that adolescent girls are more likely than boys to have sex to bolster or advance emotional aspects of relationship development (to foster intimacy, love, and trust) [3]. While empirical studies in this area are needed, we posit that there may be neurocognitive and behavioral differences in adolescent male sexual decision-making across the dimension of reward, particularly in the early years of sexual activity. Relatedly, a recent meta-analysis by Dir and colleagues [41] supports a relationship between impulsivity and adolescent risky sex, with a significant moderational role of gender, such that the link between impulsivity and risky sex was significantly stronger for adolescent females versus males. One perspective is that impulsivity may be more relevant to adolescent female risky sexual decision-making, because of the greater stakes for girls, including more biological risk (increased risk of STIs/HIV and pregnancy), and the more socially- and emotionally-charged, often taboo, nature of sex for young girls [41, 60]. Thus, it is the cognitive and physical process of sexual decision making may be inherently more risky for young girls as compared with boys.
Model Overlap with Existing Neurocognitive Data for Adolescent Alcohol Use
One interesting question is whether this model is generally predictive of risk taking, or if it is specific to sexual risk behavior. In other words, how congruent might this model be with those applied to other adolescent health risk behaviors, including alcohol use? In some respects, we believe that there are areas of neural overlap between decision-making around adolescent alcohol use and sex, while in other ways, they are likely discrepant. It is likely that frontal systems that govern impulsive choices, reward-seeking, and immediate behavioral actions partially determine both behaviors. But in some aspects, sexual behavior is highly distinct from adolescent alcohol use. To that end, first and most importantly, evidence is accruing that alcohol use is neurotoxic for the developing brain [57, 59]; in stark contrast, there is no reason to believe that adolescent sexual behavior negatively impacts the developing brain. Thus, while the learning inherent in sexual decision-making may help establish synaptic links in salient neural regions, it is not likely to contribute to the aberrant neural circuitry that marks alcohol abuse and the eventual manifestation of addiction [28]. Second, while this work is very new, the literature suggests different patterns of functional brain response and connectivity across alcohol use and sexual decision-making [10, 61]. Third, the process of accomplishing alcohol use and sexual intercourse are quite different for adolescents. While it is inconvenient to obtain alcohol, if youth want to drink, it is possible to do it, often within hours of the desire. This is not true for sexual intercourse. Some youth find themselves waiting years between desiring intercourse and engaging in it. This stretch of learning that brings youth to successful sexual intercourse, we argue, is distinct and important to the neural substrates underlying sexual decision-making. Fourth is the connection to safer sex, including condom use. In some respects, the frontal neural networks that regulate motivated behavior to pursue sex in unsafe situations (e.g. knowing they do not have a condom, so they should not be having sex) may be those also engaged in drinking (e.g. knowing alcohol use is dangerous and illegal, so should not be drinking). However, in contrast to alcohol use, sexual risk is dyadic. For adolescent females in heterosexual sexual encounters, this extends further to having to make a decision about protective efforts for someone else’s body, and in a direction that generally runs contrary to natural sexual drives (to have sex without a condom). In terms of salient neural substrates, we thus argue that emotional decision-making regions, such as the amygdala, hippocampus, and related hypothalamic projections, are critically distinctive in this capacity.
While the reviewed studies suggest how sexual risk decisions may happen in the developing brain, we are still far from understanding how the adolescent brain may weigh decisions about risk in-the-moment, such as when youth are with a willing, but unprotected, sexual partner. Still, these studies offer preliminary information to help us understand how to improve youth’s capacity to engage prefrontal “brake” systems, and even potentially how to bring learning systems online to help youth engage in thoughtful considerations of potential risk/reward balances. Yet, we are still absent information to help guide youth in navigating the emotional landscape involved in sexual behavior and relationships; these data are relevant to helping youth make fully-informed and planned sexual decisions, quickly, in contexts that may pull for riskier decisions, particularly in emergent relationship contexts. Advances in developmental neuroscience must obtain empirical human data to fully and appropriately understand this phenomenon in order to effectively promote safer sexual behavior.
Potential Implications: Prevention, Intervention, and Policy
What are the implications of this developmental neuroscience perspective to prevention programming? While HIV/STI risk reduction interventions show promise, key challenges center around delaying sexual debut [62], and mitigating the widespread prevalence of unplanned pregnancy and STIs in this age group [63]. Our analysis of the burgeoning neurocognitive literature points to two specific needs in current programming. First, the vast majority of adolescent prevention programs—including our own—focus on social cognitive predictors of behavior (e.g. based on the Health Belief Model, the Theory of Reasoned Action/Planned Behavior, or Social Cognitive Theory). Major limitations of these rational cognitive theories include that they fail to include the role of affect and emotion in decision making [64], they do not allow for nonconscious processes [65] and they fail to incorporate the role of executive control and self-regulation [66]. Given that sexual activity is an emotionally laden, automatically rewarding behavior that requires strong self-regulation abilities to resist, it would seem these weaknesses of current intervention strategies based on rational cognitive models of behavior are worth recognizing. Further, the evidence supports the prominent involvement of the reward system, as compared to the control system, in adolescent sexual decision-making, so it stands to reason that these programs might be more successful if they incorporated explicit focus on the rewarding nature of sexual behavior and acknowledged this as a barrier to delaying sexual activity, limiting the number of sexual partners, or using effective preventive methods that are perceived as reducing pleasure (i.e., condoms). Second, existing interventions almost exclusively focus on individual decision-making. Yet, sexual behavior is inherently dyadic. Thus, translating programming for adolescents regarding how to engage relevant prefrontal, executive control networks in the context of dyadic decision-making is a critical next step. Third, adolescence is most often the period of life where both sexual and romantic relationships are initiated. For adolescent girls in particular, it is particularly important to look to the adult literature to incorporate and extend emotional decision-making components [67] into prevention programming to include the novel emotional and relational aspects of sexual behavior that young people are experiencing; an approach that has yet to be seen in adolescent female HIV/STI prevention programming.
Conclusions and Limitations
Adolescence is a time of considerable plasticity, both in terms of the brain and behavior. Thus, this is a opportune time to help youth initiate positive and protective health behaviors. One critical issue within adolescent examinations of risk behavior generally and sexual risk in particular revolves around ethics; in other words, there are greater advances in animal models of sexual risk and substance use behavior, and far fewer studies with human adolescents because many of these questions are ethically tricky to examine or manipulate. For example, we cannot randomize youth to sexual intercourse or not [3] nor to alcohol use or not [57], nor can we conduct the important laboratory-based work that directly examines the effect of alcohol intoxication on sexual risk decision-making [68]. Relatedly, the most commonly used stimuli to evaluate sexual decision making within MRI/fMRI studies with adults is erotica [26, 69], which is clearly not ethical for this age group. The resultant scientific challenge is how to approach the measurement of relevant neurocognitive and decision-making factors in adolescent sexual risk, and further, how to ask these questions while incorporating consideration of well-known biases in research on sexual risk (e.g. sexual double-standards, heterosexism).
The emergent nature of this work means that we cannot draw firm conclusions about the veracity of our working model, its empirical extension to and overlap with the adolescent alcohol literature, or the role of sexual dimorphism in the equation. However, future work will benefit from exploring the degree to which the pathways of sexual risk we have suggested are associated with aspects of sexual decision-making and risky sexual behavior. In addition, given that the majority of the existing studies have been conducted within adolescents residing in the United States, replication of this work in other countries is critical in order to ensure the generalizability of results across geographic and cultural regions. Despite its limitations, the sum of the existing work shows promise for understanding the nature of adolescent decision-making in the context of sexual behavior. Together, the data suggest that three regions (prefrontal cortical, reward, emotion/memory) may be critical in this equation. Of course, understanding the dynamic nature of these regions, the relevant role of co-occurring alcohol use, and the shifting landscape of hormonal change, with a particular eye to comparing this model across gender, are essential to eventually expanding this theoretical framework and for considering its implications for prevention, intervention, and public policy programming for the reduction of unplanned pregnancy and STI including HIV in this age group.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors have no competing financial or other conflicts of interest relating to the data included in the manuscript.
References
- 1.CDC. Youth risk behavior surveillance survey. MMWR Surveill Summ. 2013;66:1–172. [Google Scholar]
- 2.Shedler J, Block J. Adolescent drug use and psychological health: a longitudinal inquiry. Am Psychol. 1990;45(5):612–30. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- 3.Feldstein Ewing SW, Bryan AD. A question of love and trust? The role of relationship factors in adolescent sexual decision-making. J Dev Behav Pediatr. 2015 doi: 10.1097/DBP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC; National Center for HIV/AIDs VH, STD, and TB Prevention, editor. Sexually transmitted infections among young Americans. 2014. [Google Scholar]
- 5.Cooper ML. Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. J Stud Alcohol Drugs. 2002;14:101–17. doi: 10.15288/jsas.2002.s14.101. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ML. Does drinking promote risky sexual behavior? A complex answer to a simple question. Curr Dir Psychol Sci. 2006;15:19–23. [Google Scholar]
- 7.Bryan AD, Ray LA, Cooper ML. Alcohol use and protective sexual behaviors among high-risk adolescents. J Stud Alcohol Drugs. 2007;68(3):327–35. doi: 10.15288/jsad.2007.68.327. [DOI] [PubMed] [Google Scholar]
- 8.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore S-J. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–60. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg D, Telzer EH, Lieberman MD, Fuligni A, Galvan A. Neural mechanisms of impulse control in sexually risky adolescents. Dev Cogn Neurosci. 2013;6:23–9. doi: 10.1016/j.dcn.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thayer RE, Montanaro E, Weiland BJ, Callahan TJ, Bryan AD. Exploring the relationship of functional network connectivity to latent trajectories of alcohol use and risky sex. Curr HIV Res. 2014;12:293–300. doi: 10.2174/1570162x12666140721124441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnan RE, Callahan TJ, Ladd BO, Claus ED, Hutchison KE, Bryan AD. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. J AIDS Clin Res. 2013;4:217. [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addict Behav. 2015;44:80–7. doi: 10.1016/j.addbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensel DJ, Hummer TA, Acrurio LR, James TW, Fortenberry JD. Feasibility of functional neuroimaging to understand adolescent women’s sexual decision making. J Adolesc Health. 2015;56:389–95. doi: 10.1016/j.jadohealth.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall GS. Adolescence: Its psychology and relations to physiology, anthropology, sociology, sex, crime, religion, and education. I & II. New York: D. Appleton & Co; 1904. [Google Scholar]
- 15.Giedd JN. The digital revolution and adolescent brain evolution. J Adolesc Health. 2012;51:101–5. doi: 10.1016/j.jadohealth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sercombe H. Risk, adaptation, and the functional teenage brain. Brain Cogn. 2014;89:61–9. doi: 10.1016/j.bandc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Somerville LH, Jones R, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–11. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg L. The influence of neuroscience on US Supreme Court decisions about adolescents’ criminal culpability. Nat Rev Neurosci. 2013;14:513–8. doi: 10.1038/nrn3509. [DOI] [PubMed] [Google Scholar]
- 23.Noel X. Why adolescents are at risk of misusing alcohol and gambling. Alcohol Alcohol. 2014;49:165–72. doi: 10.1093/alcalc/agt161. [DOI] [PubMed] [Google Scholar]
- 24.Willoughby T, Good M, Adachi PJ, Hamza C, Tavernier R. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cogn. 2014;89:70–8. doi: 10.1016/j.bandc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Kawamichi H, Sasaki AT, Matsunaga M, Yoshihara K, Takahashi HK, Tanabe HC, et al. Medial prefrontal cortex activation is commonly invoked by reputation of self and romantic partners. PLoS One. 2013;8:e74958. doi: 10.1371/journal.pone.0074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn S, Gallinat J. Brain structure and functional connectivity associated with pornography consumption: the brain on porn. JAMA Psychiatry. 2014;71:827–34. doi: 10.1001/jamapsychiatry.2014.93. [DOI] [PubMed] [Google Scholar]
- 27.Donahue CH, Lee D. Dynamic routing of task-relevant signals for decision making in dorsolateral prefrontal cortex. Nat Neurosci. 2015;18:295–301. doi: 10.1038/nn.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Baler RD. Addiction science: uncovering neurobiological complexity. Neuropharmacology. 2014;76:235–49. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MJ, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Curr Biol. 2013;23:282–9. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luciana M, Segalowitz SJ. Some challenges for the triadic model for the study of adolescent development. Brain Cogn. 2014;39:118–21. doi: 10.1016/j.bandc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing? Dev Cogn Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis BJ. Determinants of pubertal timing: an evolutionary developmental approach. In: Ellis BJ, Bjorkland DF, editors. Origins of the social mind: evolutionary psychology and child development. New York: Guilford Press; 2005. pp. 164–88. [Google Scholar]
- 33.Stanton BF, Li X, Ricardo I, Galbraith J, Feigelman S, Kaljee L. A randomized, controlled effectiveness trial of an AIDS prevention program for low-income African-American youth. Arch Pediatr Adolesc Med. 1996;150:363–72. doi: 10.1001/archpedi.1996.02170290029004. [DOI] [PubMed] [Google Scholar]
- 34.Jemmott LS, Jemmott JB. Increasing condom use intentions among sexually active black adolescent women. Nurs Res. 1992;41:273–6. [PubMed] [Google Scholar]
- 35.Schmiege SJ, Broaddus MR, Levin ME, Bryan AD. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. J Consult Clin Psychol. 2009;77(1):38–50. doi: 10.1037/a0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broaddus MR, Schmiege SJ, Bryan AD. An expanded model of the temporal stability of condom use intentions: gender-specific predictors among high-risk adolescents. Ann Behav Med. 2011;42:99–110. doi: 10.1007/s12160-011-9266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Jannsen E, Heiman JR. The role of the anterior cingulate cortex in women’s sexual decision making. Horm Behav. 2009;1:42–7. doi: 10.1016/j.neulet.2008.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaus JG, Kippin TE, Coria-Avila GA. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- 39.Pfaus JG, Kippin TE, Coria-Avila GA, Gelez HA, Afonso VM, Ismail N, Parada M. Who, what, where, when (and maybe even why?) How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- 40.Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66(1–6):25. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- 41.Dir AL, Coskunipar A, Cyders MA. A meta-analytic review of the relationship between adolescent risky sexual behavior and impulsivity across gender, age, and race. Clin Psychol Rev. 2014;34:551–62. doi: 10.1016/j.cpr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Pharo H, Clark S, Graham M, Gross J, Hayne H. Risky business: executive function, personality, and reckless behavior during adolescence and emerging adulthood. Behav Neurosci. 2011;125:970–8. doi: 10.1037/a0025768. [DOI] [PubMed] [Google Scholar]
- 43.Heitzig MM, Villafuerte S, Weiland BJ, Enoch MA, Burmeister M, Zubieta JK, et al. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:3077–86. doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Galvan A, Hare TA, Parra CE, Penn J, Voss K, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 47.Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–9. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- 48.Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci USA. 2014;111:1592–7. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, et al. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behavior. PLoS One. 2014;9:e102419. doi: 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Lower sexual interest in postpartum women: relationship to amygdala activation and intranasal oxytocin. Horm Behav. 2013;63:114–21. doi: 10.1016/j.yhbeh.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alarcon G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–44. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav. 2013;64:64. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CF, Shah NM. Represting sex in the brain, one module at a time. Neuron. 2014;82:261–78. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefkowtiz ES, Shearer CL, Gillen MM, Espinosa-Hernandez G. How gendered attitudes relate to women’s and men’s sexual behaviors and beliefs. Sex Cult. 2014;18:833–46. doi: 10.1007/s12119-014-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldstein Ewing SW, Ryman SG, Karoly H, Mayer AR, Ling J, Bryan AD. Protected sexual intercourse and the adolescent brain: gender matters. under review. [Google Scholar]
- 56.Hammerslag LR, Gulley JM. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldstein Ewing SW, Blakemore S-J, Sakhardande A. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage. 2014;5:420–37. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryman SG, Bryan AD, Thayer RE, Mayer AR, Ling J, Feldstein Ewing SW. The association between protected sex and the adolescent brain. under review. [Google Scholar]
- 59.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bryan AD, Schmiege SJ, Magnan RE. Marijuana use and risky sexual behavior among high-risk adolescents: trajectories, risk factors, and event-level relationships. Dev Psychol. 2012;48:1429–42. doi: 10.1037/a0027547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldstein Ewing SW, Houck JM, Bryan AD. Examining the brain-behavioral interplay between adolescent substance use, sexual risk, and response inhibition. under review. [Google Scholar]
- 62.Robin L, Dittus P, Whitaker D, Crosby R, Ethier K, Mezoff J, et al. Behavioral interventions to reduce incidence of HIV, STD, and pregnancy among adolescents: a decade in review. J Adolesc Health. 2004;34:3–26. doi: 10.1016/s1054-139x(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 63.Kirby DB, Laris BA, Rolleri LA. Sex and HIV education programs: their impact on sexual behaviors of young people throughout the world. J Adolesc Health. 2007;40:206–17. doi: 10.1016/j.jadohealth.2006.11.143. [DOI] [PubMed] [Google Scholar]
- 64.Conner M, McEachan R, Taylor N, O’Hara J, Lawton R. Role of affective attitudes and anticipated affective reactions in predicting health behaviors. Health Psychol. 2015;34:642–52. doi: 10.1037/hea0000143. [DOI] [PubMed] [Google Scholar]
- 65.Sheeran P, Gollwitzer PM, Bargh JA. Nonconscious processes and health. Health Psychol. 2013;32:460–73. doi: 10.1037/a0029203. [DOI] [PubMed] [Google Scholar]
- 66.Sniehotta FF, Presseau J, Araujo-Soares V. Time to retire the theory of planed behavior. Health Psychol Rev. 2014;8:1–7. doi: 10.1080/17437199.2013.869710. [DOI] [PubMed] [Google Scholar]
- 67.Pachankis JE, Rendina HJ, Restar A, Ventuneac A, Grov C, Parsons JT. A minority stress-emotion regulation model of sexual compulsivity among highly sexually active gay and bisexual men. Health Psychol. 2014;34:829–40. doi: 10.1037/hea0000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons JS, Maisto SA, Wray TB, Emery NN. Acute effects of intoxication and arousal on approach/avoidance biases toward sexual risk stimuli in heterosexual men. Arch Sex Behav. 2015 doi: 10.1007/s10508-014-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wehrum-Osinsky S, Klucken T, Kagerer S, Walter B, Hermann A, Stark R. At the second glance: stability of neural responses toward visual sexual stimuli. J Sex Med. 2014;11:2720–37. doi: 10.1111/jsm.12653. [DOI] [PubMed] [Google Scholar]