Abstract
Objective
Chronic subdural haematoma (CSDH) is a common neurosurgical problem, and treatment includes evacuation of the haematoma by burr hole drainage. Commonly, these procedures are performed under local anaesthesia, general anaesthesia or, recently, with monitored anaesthesia care (MAC). We compared dexmedetomidine- and propofol-based sedation along with scalp nerve block for burr hole evacuation of CSDH.
Methods
In this prospective randomised study, 62 patients were divided into the following two groups of 31 patients each: Group D and Group P. Group D received dexmedetomidine 1 μg kg−1 over 10 minutes as a loading dose, followed by 0.2–0.7 μg kg−1 hr−1. Group P received propofol 1 mg kg−1 over 10 minutes as a loading dose, followed by 1–3 mg kg−1 hr−1. The heart rate (HR) and blood pressure were measured at different intervals. The recovery parameter and satisfaction score were also recorded.
Results
There were no significant differences noted in the demographic profile. A significant decrease in HR compared to preoperative value was seen in Group D compared to Group P. Blood pressure values were statistically significantly lower in both study groups, compared to preoperative values during the whole procedure and after surgery (p<0.05). Time to achieve modified Aldrete score of 9–10 was not significantly different between the groups (p=0.354). Surgeon satisfaction was significantly better in Group D compared to Group P (p<0.05), but patient satisfaction was similar between the groups (p=0.364).
Conclusion
Dexmedetomidine-based sedation compared to propofol, along with scalp block for MAC in patients undergoing burr hole evacuation of CSDH is associated with haemodynamic stability and greater surgeon satisfaction.
Keywords: Dexmedetomidine, propofol, monitored anaesthesia care, chronic subdural haematoma
Introduction
Chronic subdural haematoma (CSDH) is a common neurosurgical emergency presenting to the operating room for evacuation of the haematoma by burr hole (1, 2). Even though such cases were performed under local infiltration or monitored anaesthesia care (MAC) (3–7), they presented with unique anaesthetic challenges. The need for immobility, stable perioperative haemodynamics, attenuation of response to painful stimuli, the prevention of straining and brain bulge as well as early responsiveness are of paramount importance for a good outcome. Perioperative haemodynamic variations manifesting as hypertension or hypotension may be associated with cerebral oedema, reaccumulation of haematoma, infarction, seizures and intracerebral haemorrhage (8). Local infiltrative anaesthesia alone is not comfortable to the patient, and perioperative agitation and non-cooperation produce inadequate surgical conditions for the surgeon. General anaesthesia usually has a higher risk in the elderly patients, especially in those with coexisting systemic disease (7). Presently, MAC is frequently used in various procedures, and its safety and efficacy have been proven by numerous studies (9–13). A combination of scalp nerve block and sedation is a reliable alternative (14).
A number of sedatives have been tried in MAC, with varying degree of success. Commonly used sedatives include midazolam, propofol, fentanyl and dexmedetomidine (9–13). The midazolam and fentanyl combination frequently used as a part of MAC has been found to be associated with an increased incidence of respiratory depression (15, 16). Dexmedetomidine, a highly selective α2-adrenoceptors agonist, has sedative, hypnotic, anxiolytic, sympatholytic and analgesic properties, without producing respiratory depression (17). Propofol has been utilised because of its quick-in, quick-out property and producing a clear-headed and faster recovery. These properties make them potentially useful for the sedation of patients requiring regional blockade. The aim of this prospective study was to compare the efficacy and safety of dexmedetomidine- and propofol-based sedation along with scalp nerve block for burr hole evacuation of chronic SDH.
Methods
After the Institutional Ethics Committee approval and written informed consent from the patients or their legally acceptable relatives, this prospective, randomised study was started. Randomisation was done with sealed-envelope technique with patients divided in 1:1 fashion between the groups. The trial was registered with the Clinical Trial Registry of India. www.ctri.nic.in (ref: CTRI/2015/01/005478).
A total of 62 patients 20–65 years of age, ASA physical status I–III, and scheduled for burr hole and evacuation of CSDH under MAC were enrolled. Patients with a history of known sensitivity to study drugs, Glasgow coma scale <12, predicted difficult airway, history of drug or alcohol abuse, oral anticoagulant use (warfarin), impaired kidney or liver functions, haematological or endocrine disorder and any degree of heart block were excluded from the study. They were divided into two groups of 31 patients each as follows:
Group D: Dexmedetomidine loading dose 1 μg kg−1 over 10 minutes, followed by maintenance dose at a rate of 0.2–0.7 μg kg−1 hr−1.
Group P: Propofol loading dose 1 mg kg−1 over 10 minutes, followed by maintenance dose at a rate of 1–3 mg kg−1 hr−1.
In the operating room after establishing intravenous (i.v.) access, monitors such as electrocardiography, blood pressure and pulse oximetry were applied (Philips IntelliVue MP 20 Monitor). Bispectral index (BIS) value was also monitored by using a single-channel sensor (BIS QuatroTM, Mansfield, MA, USA) in a frontal temporal montage. Before the study drug infusion, scalp block was given with 2% lignocaine with adrenaline on the proposed side of the operation. First, the supraorbital and supratrochlear nerves were blocked with 2 mL of the drug. The auriculotemporal nerves and greater auricular nerves were blocked with 5 mL of the drug. Lastly, the greater, lesser and third occipital nerves were blocked with 3 mL of the drug. After the scalp nerve block, study drugs prepared by an independent anaesthesiologist were started with an infusion pump. All the patients received oxygen (2–4 L min−1) by a nasal prong throughout the procedure.
An experienced neurosurgeon then commenced the procedure. During the procedure, sedation was maintained with the study drug infusion to maintain a target BIS 60 to 80. Requirement of more than three attempts to restrain the patient, BIS>80 or surgeon dissatisfaction was managed with additional boluses of propofol (additional 0.25 mg kg−1 bolus was administered, and the infusion rate increased) or dexmedetomidine (0.25 μg kg−1 bolus was administered and the infusion rate increased). Failures in achieving satisfactory operating conditions despite an increased drug dosage were managed with the administration of general anaesthesia. At the completion of the procedure, study drug infusion was stopped. The modified Aldrete score (MAS; 0–10) was applied to evaluate recovery. Patients were discharged from the recovery room after attaining a MAS of 9–10. Time taken to achieve this score was recorded. After completion of the procedure, overall satisfaction of the neurosurgeon was recorded using a 100 mm Visual Analog Scale (VAS). At discharge, the patient satisfaction was also noted using a similar VAS. During the procedure, the following complications were observed, recorded and treated accordingly: any desaturation or apnoea was recorded when the SpO2 dropped to <92% or recorded cessation of respiration for 15 s or more, respectively, and was managed by supporting the airway and/or assisting ventilation. Hypotension was considered when the mean arterial pressure (MAP) decreased by more than 30% of the baseline MAP and was managed by fluid bolus and/or vasopressors. Bradycardia was considered when the heart rate (HR) was less than 50 beats min−1 and was managed with atropine 0.6 mg applied i.v., whereas a HR and MAP more than 30% from the baseline were considered as tachycardia and hypertension. Other complications such as coughing, gagging, hiccough, nausea and vomiting were also recorded.
HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and oxygen saturation (SpO2) were recorded preoperatively, after study drug administration, during procedure at 5, 10, 15, 20, 25 and 30 minutes and 5 minutes after the end of the procedure.
After the initial pilot observations, it was decided that a 10% of difference in HR should be the minimum detectable difference of means in the two groups. The standard deviation (SD) of residual was 12% of average difference of the two groups. The alpha value was 0.05, and the power of the study was 0.80. Thus, the calculated sample size was 25 patients per group. Taking into account possible dropouts from the two groups, we decided to enroll a total of 60 patients.
Statistical analysis
GraphPad Prism 7.0 statistical software was used for the statistical analysis. Patient characteristic data were analyzed with Student’s t-test for continuous variables and chi-squared test for categorical variables. Intergroup comparisons of haemodynamic parameters were done with Student’s t-test. A repeated measure analysis of variance with the post hoc Tukey test was used for comparing means for haemodynamic variables in intragroup comparison with preoperative parameters. Satisfaction score was analyzed using the Mann-Whitney U test. A P-value of <0.05 was considered to be statistically significant.
Results
A total of 68 patients were screened, of which 62 patients were included in the study, and 59 (95.2%) completed the study (Figure 1). Two patients in Group D required vasopressors because of a history of hypotension during surgery, and one patient in Group P developed intraoperative airway obstruction, which was relieved by nasal airway placement. These three patients were excluded from further statistical analysis. There were no significant differences between the study groups with respect to demographic data and duration of surgery (p>0.05) (Table 1).
Figure 1.
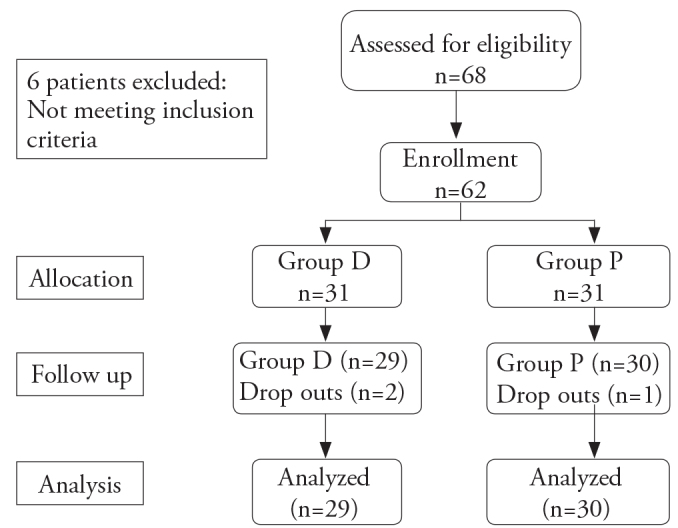
Study design
Table 1.
Demographic data
| Group D | Group P | p | |
|---|---|---|---|
| Mean age (yrs) | 56.1±7.2 | 53.6±9.3 | 0.238 |
| Male/Female | 26/5 | 24/7 | 0.748 |
| Weight (kg) | 63.4±9.3 | 66.0±11.9 | 0.342 |
| Duration of surgery (Min.) | 51.7±8.9 | 54.6±10.5 | 0.245 |
Data are presented as either mean values±SD or by absolute numbers.
There was no significant difference noted in the preoperative haemodynamic parameters between the two groups. In Group D, there was a significant decrease in HR after the administration of the study drug (p<0.05), while it was not significant in Group P (p=0.398). There was a significant decrease in HR in Group D during the whole procedure, compared with preoperative HR, while it was not significant in Group P at any time interval. There was a statistically significant difference in HR between study groups after study drug administration and at 5, 10 and 15 minutes during the procedure (Figure 2).
Figure 2.
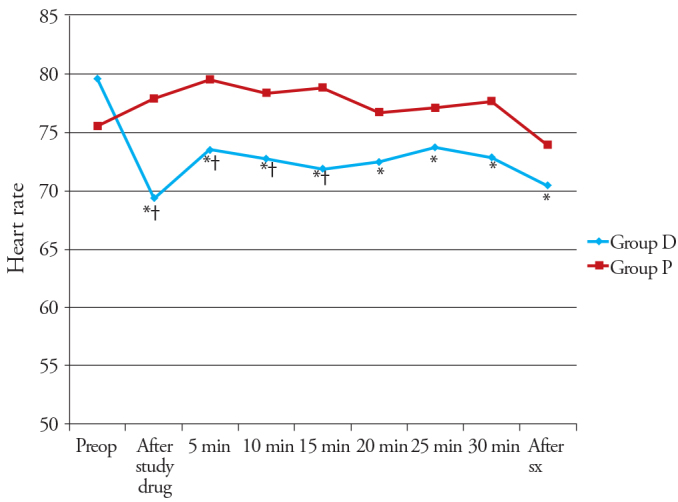
Changes in the heart rate (HR) observed in the two groups during the study period
*p<0.05 within group (vs preoperative value), †p<0.05 compared with group P
Systolic blood pressure and DBP values were significantly decreased in both the study groups compared to preoperative values during the procedure, except SBP value in Group P at 10, 15 and 20 minutes during the procedure. There was a significant difference in SBP, between both the study group during the study procedure from the 15th minute onwards, while DBP values were significant at only 15- and 25-min intervals during the procedure (Figure 3).
Figure 3.
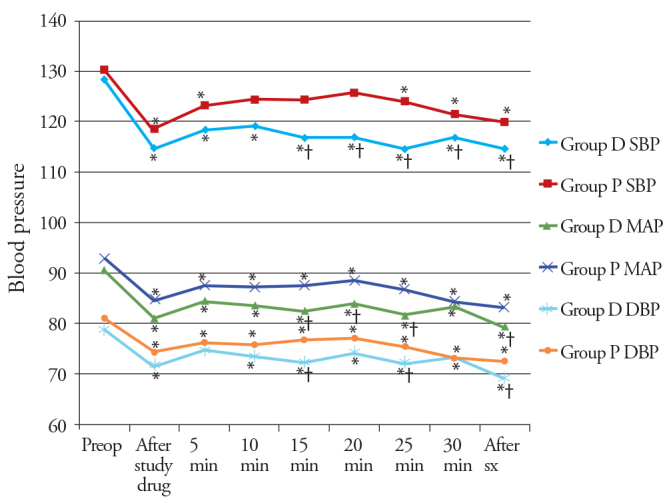
Changes in the blood pressure observed in the two groups during the study period
*p<0.05 within group (vs preoperative value), †p<0.05 compared with group P.
MAP values were statistically significantly decreased in both the study groups compared with preoperative values during the whole procedure and after surgery (p<0.05). There was a significant difference in MAP values, between the study groups during the study procedure at 15–25 min interval and after surgery (Figure 3). HR and blood pressure changes were within 15% of the preoperative values. There were no significant differences in SpO2 in both the groups.
Time to achieve MAS to 9–10 was not significantly different between the groups (p=0.354). Surgeon satisfaction was significantly better in Group D than in Group P (p=0.037), but patient satisfaction was similar between the groups (p=0.364). (Table 2) Hypotension was noticed in two patients (6.45%) in Group D, which was controlled by the administration of mephentermine 0.5 mg i.v. twice. One patient (3.22%) in Group P had airway obstruction, which was relieved by placement of the nasal airway.
Table 2.
Comparison of postoperative variables
| Group D (n=29) | Group P (n=30) | p | |
|---|---|---|---|
| Time to Aldrete score 9–10 (Min.) | 8.5±3.1 | 7.8±3.1 | 0.354 |
| Surgeon satisfaction score | 93.4±10.1 | 87.3±11.7 | 0.037 |
| Patient satisfaction score | 90.7±8.8 | 87.67±11.3 | 0.364 |
Data are presented as mean values±SD
Discussion
The findings of the present study suggest that the use of dexmedetomidine along with a scalp block for MAC in patients undergoing burr hole evacuation of CSDH is associated with haemodynamic stability, good operating conditions and greater surgeon satisfaction.
Intraoperative patient movement, coughing and airway-related problems during MAC may obstruct surgery, and they show inadequate sedation. Drugs maintaining adequate sedation and having haemodynamic stability with minimal depression of respiration are preferred. Bishnoi et al. (6) compared dexmedetomidine and midazolam-fentanyl combination for MAC and demonstrated a lesser number of intraoperative movement, faster postoperative recovery and better surgeon satisfaction with the use of dexmedetomidine for CSDH evacuation under MAC.
The use of dexmedetomidine had a higher incidence of bradycardia and hypotension, and this is dose related (18, 19). This haemodynamic derangement can be minimised by administering the drug as an infusion. Drug dosage selection in our study was based on previous studies in patients undergoing different surgeries, where no significant movement or haemodynamic variations were noted (6, 7, 9 and 13). Sriganesh et al. (10) also used the same dose of propofol and dexmedetomidine for cerebral angiography in patients with SAH and noticed that a more frequent dose adjustment is required for preventing patient movement. In our study, BIS-guided infusion rate of dexmedetomidine and propofol to maintain desired level of sedation decreased the dose of sedative, frequent dose adjustment and drug-related side effects.
Hypertension, movement during surgery and bleeding, all pose disturbance to the microscopic nature of the surgery. In this study, both the drugs (propofol and dexmedetomidine) had better haemodynamic control with minimum fluctuations during whole surgery. Dexmedetomidine has a property of decreased sympathetic outflow, catecholamine levels and also additional vagal mimetic effect. This explains lower HR and MAP in Group D compared with that in Group P. The anaelgesic property of dexmedetomidine reduces sympathetic stimulation which also reduces MAP. These results confirm that dexmedetomidine has an advantage over propofol in providing a better surgical field. Similar effects were also observed by Sriganesh et al. (10). Srivastava et al. (20) have also noticed this property of dexmedetomidine for maintaining haemodynamic stability in patients for microscopic spine surgery and observed that dexmedetomidine is a useful adjuvant to decrease bleeding when a bloodless operative field is required.
The real issues with dexmedetomidine include its haemodynamic impacts, as the drug often produces bradycardia and hypotension. In our study, bradycardia (3.33%) and hypotension (6.66%) were likewise observed. The bradycardia and hypotension that happened in the dexmedetomidine group were unsurprising from the known properties of α2 agonists, and have been affirmed by past studies (18, 19).
In our study, there were no significant differences noted between the study groups in terms of the patient satisfaction because of titration of sedative agents guided by BIS. However, surgeon satisfaction was better in the dexmedetomidine group than in propofol group, because dexmedetomidine group patients had less coughing, movement and bleeding during surgery. Our study had similar effects as noted by Sriganesh et al. (10) and Bishnoi et al. (6). Parikh et al. (13) also demonstrated that satisfaction score was better in the dexmedetomidine group than the midazolam-fentanyl group for tympanoplasty under MAC. They suggested that it is due to the difference in the quality of sedation of both the drugs.
In a study by Sriganesh et al. (10), there was no significant difference in recovery time between the propofol and dexmedetomidine groups. Postoperative recovery time was significantly lower in the dexmedetomidine group in comparison with midazolam-fentanyl group, demonstrated by Bishnoi et al. in burr hole surgery for CSDH. The delay in recovery time in the patients from Bishnoi et al.’s study (6) may have been because of an effect of a combined use of midazolam and fentanyl. In our study, there was no significant difference observed between the groups in MAS because of the titration of sedative agents guided by BIS. Thus, immediate neurological examination was possible in both the groups in our study at the completion of the surgery.
There are some limitations to our study. 1) This study included only patients with GCS greater than 12. The impacts of dexmedetomidine on the cardiovascular system may be useful in high-risk patients. Further investigations need to be carried out recruiting high-risk patients. 2) A larger sample size and the target-controlled infusion of propofol and dexmedetomidine could be used for more titrated sedation in a tightly controlled environment, as the slightest movement by the patient has an adverse impact on the quality of microscopic surgery. 3) Due the physical appearance of the propofol, this study was not completely drug blinded. 4) Our study did not utilise advanced monitoring, such as capnography. Capnography is more sensitive than oximetry or visual assessment for identifying apnoea in deeply sedated patients.
Conclusion
Dexmedetomidine along with scalp block provides better operating conditions, stable haemodynamics and a greater surgeon satisfaction compared with propofol. The use of dexmedetomidine-based sedation protocol for procedures under MAC is suitable and should be incorporated as a first-line anaesthetic technique for treating such patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Apollo Hospitals.
Informed Consent: Written informed consent was obtained from patients and patients’ relatives who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Design - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Supervision - V.K.S., S.A., S.K.; Resources - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Materials - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Analysis and/or Interpretation - V.K.S., S.A., S.K.; Literature Search - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Writing Manuscript - V.K.S., S.A., S.K., S.Khan., S.S., R.K.; Critical Review - V.K.S., S.A., S.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Chan DY, Woo PY, Mak CH, Chu AC, Li CC, Ko NM, et al. Use of subdural drain for chronic subdural haematoma? A 4-year multi-centre observational study of 302 cases. J Clin Neurosci. 2017;36:27–30. doi: 10.1016/j.jocn.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan IA, Mack WJ. Minimally Invasive Surgical Approaches for Chronic Subdural Hematomas. Neurosurg Clin N Am. 2017;28:219–27. doi: 10.1016/j.nec.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Guzel A, Kaya S, Ozkan U, Ufuk Aluclu M, Ceviz A, Belen D. Surgical treatment of chronic subdural haematoma under monitored anaesthesia care. Swiss Med Wkly. 2008;138:398–403. doi: 10.4414/smw.2008.12121. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Feng L, Bai F, Zhang Z, Zhao Y, Ren C. The Safety and Efficacy of Dexmedetomidine vs. Sufentanil in Monitored Anesthesia Care during Burr-Hole Surgery for Chronic Subdural Hematoma: A Retrospective Clinical Trial. Front Pharmacol. 2016;7:410. doi: 10.3389/fphar.2016.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu XP, Liu C, Wu Q. Monitored anesthesia care with dexmedetomidine for chronic subdural hematoma surgery. J Neurosurg Anesthesiol. 2014;26:408–9. doi: 10.1097/ANA.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 6.Bishnoi V, Kumar B, Bhagat H, Salunke P, Bishnoi S. Comparison of Dexmedetomidine Versus Midazolam-Fentanyl Combination for Monitored Anesthesia Care During Burr-Hole Surgery for Chronic Subdural Hematoma. J Neurosurg Anesthesiol. 2016;28:141–6. doi: 10.1097/ANA.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 7.Surve RM, Bansal S, Reddy M, Philip M. Use of Dexmedetomidine Along With Local Infiltration Versus General Anesthesia for Burr Hole and Evacuation of Chronic Subdural Hematoma (CSDH) J Neurosurg Anesthesiol. 2017;29:274–80. doi: 10.1097/ANA.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 8.Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93:48–54. doi: 10.1097/00000542-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Alhashemi JA. Dexmedetomidine vs midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]
- 10.Sriganesh K, Reddy M, Jena S, Mittal M, Umamaheswara Rao GS. A comparative study of dexmedetomidine and propofol as sole sedative agents for patients with aneurysmal subarachnoid hemorrhage undergoing diagnostic cerebral angiography. J Anesth. 2015;29:409–15. doi: 10.1007/s00540-014-1952-1. [DOI] [PubMed] [Google Scholar]
- 11.Kaygusuz K, Gokce G, Gursoy S, Ayan S, Mimaroglu C, Gultekin Y. A comparison of sedation with dexmedetomidine or propofol during shockwave lithotripsy: a randomized controlled trial. Anesth Analg. 2008;106:114–9. doi: 10.1213/01.ane.0000296453.75494.64. [DOI] [PubMed] [Google Scholar]
- 12.Khalil M, Al-Agaty A, Asaad O, Mahmoud M, Omar AS, Abdelrazik A, et al. A comparative study between propofol and dexmedetomidine as sedative agents during performing transcatheter aortic valve implantation. J Clin Anesth. 2016;32:242–7. doi: 10.1016/j.jclinane.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Parikh DA, Kolli SN, Karnik HS, Lele SS, Tendolkar BA. A prospective randomized double-blind study comparing dexmedetomidine vs. combination of midazolam-fentanyl for tympanoplasty surgery under monitored anesthesia care. J Anaesthesiol Clin Pharmacol. 2013;29:173–8. doi: 10.4103/0970-9185.111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garavaglia MM, Das S, Cusimano MD, Crescini C, Mazer CD, Hare GM, et al. Anesthetic approach to high-risk patients and prolonged awake craniotomy using dexmedetomidine and scalp block. J Neurosurg Anesthesiol. 2014;26:226–33. doi: 10.1097/ANA.0b013e3182a58aba. [DOI] [PubMed] [Google Scholar]
- 15.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Robin C, Trieger N. Paradoxical reactions to benzodiazepines in intravenous sedation: a report of 2 cases and review of the literature. Anesth Prog. 2002;49:128–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY MAC Study Group. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach AT, Murphy CV, Jones GM, Cook CH. The relationship between dexmedetomidine dosing and hypotension. J Trauma Acute Care Surg. 2012;72:799. doi: 10.1097/TA.0b013e3182475408. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach AT, Blais DM, Jones GM, Burcham PK, Stawicki SP, Cook CH, et al. Predictors of dexmedetomidine-associated hypotension in critically ill patients. Int J Crit Illn Inj Sci. 2016;6:109–14. doi: 10.4103/2229-5151.190656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava VK, Mishra A, Agrawal S, Kumar S, Sharma S, Kumar R. Comparative Evaluation of Dexmedetomidine and Magnesium Sulphate on Propofol Consumption, Haemodynamics and Postoperative Recovery in Spine Surgery: A Prospective, Randomized, Placebo Controlled, Double-blind Study. Adv Pharm Bull. 2016;6:75–81. doi: 10.15171/apb.2016.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


