Abstract
In a limited number of studies modafinil has been shown to decrease food intake by laboratory animals and humans. The present study represents a secondary data analysis, in which the effects of modafinil on several measures of food intake were determined in humans living in a residential laboratory during simulated shift work. During this 23-day study, a wide selection of food items and beverages were freely available. During this double-blind, within-participant study, volunteers (N = 11) received oral modafinil dose (0, 200, or 400 mg) one hour after waking for three consecutive days under two shift conditions: day shift and night shift. Shifts alternated three times during the study, and shift conditions were separated by an "off" day. Modafinil (200, 400 mg) dose-dependently decreased total caloric intake by ~18% and ~38%, respectively, regardless of shift condition, without selectively altering the proportion of total calories derived from carbohydrate, fat and protein. Ratings of “Hungry” were also significantly decreased by both active doses, but only immediately before the lunch break period. In addition, tolerance to the anorexic effects of modafinil was not apparent, as these effects remained stable across the three days of modafinil dosing. These findings show that modafinil produced clear reductions in food intake and suggest that future prospective studies should examine the drug in obese participants.
Keywords: modafinil, food intake, human, hypophagia
Introduction
Modafinil, FDA-approved to promote wakefulness in patients with excessive daytime sleepiness, has been suggested to have a clinical profile comparable to that of stimulants for a number of indications. For instance, these agents have been shown to promote wakefulness (Broughton et al. 1997; Hirshkowitz and Black 2007; Boutrel and Koob 2004) and to reduce performance disruptions related to fatigue (e.g., Pigeau et al. 1995) and sleep deprivation (Hart et al. 2003a, 2006; Walsh et al. 2004; Eliyahu et al. 2007). Unlike the amphetamines, however, modafinil has a low abuse potential (Jasinski and Kovacevic-Ristanovic 2000; Jasinski 2000; Rush et al. 2002), produces relatively small changes in cardiovascular activity at clinical doses (e.g., Makris et al. 2004), and does not demonstrate signs of tolerance to its wake-promoting effects even after 12 months of treatment (Hirshwitz and Black 2007). These features make modafinil an intriguing compound to investigate for expanded therapeutic purposes. Indeed, modafinil has been suggested as a viable option for patients with Attention-Deficit Hyperactivity Disorder (ADHD) for whom traditional stimulant treatments have not been successful or well tolerated (Lindsay et al. 2006). This position is supported by growing evidence demonstrating that modafinil is an effective therapy for ADHD (e.g., Biederman et al. 2006; Boellner et al. 2006).
A recent area of interest related to therapeutic potentials of modafinil is the treatment of obesity. Although its exact neurobiological mechanisms are unknown, modafinil binds to both the dopamine and norepinephrine transporter at clinically relevant doses (Mignot et al, 1994; Madras et al, 2006); a large database suggests that the ability of the drug to enhance catecholaminergic activity is crucial to its currently approved therapeutic actions (e.g., Lin et al. 1992; Nishino et al. 1998; Wisor and Eriksson 2005). Modafinil has also been shown to increase serotonin (5-HT) turnover in several brain regions including the frontal cortex, the amygdala, and the dorsal raphe (Ferraro et al. 2002). These neurochemical actions are consistent with effects produced by many medications approved to treat obesity (see Nelson and Gehlert 2006 for review). For example, sibutramine, which has been indicated as a pharmacological obesity adjunctive treatment for a decade in the U.S., inhibits the reuptake of norepinephrine (NE) and 5-HT thereby enhancing the turnover of these neurotransmitters (Luque and Rey 1999).
Only a limited number of studies have directly assessed modafinil-related effects on food intake, although several investigators have documented decreased appetite as a common side effect of the medication (e.g., Turner 2006; Wigal et al. 2006). Nicolaidis and de Saint Hilaire (1993) examined feeding behavior of rats following modafinil treatment and found that the drug produced a U-shaped dose-response curve: feeding was decreased by the 20 and 40 mg/kg doses but was unaffected by the 10 and 80 mg/kg doses. The feeding decreases observed following the 20 and 40 mg/kg persisted for approximately eight hours. In the only study of human research participants that systematically examined modafinil-related effects on food intake over several hours, Makris et al. (2004) used an outpatient double-blind, laboratory design to compare the effect of single oral doses of modafinil (1.75, 3.5, 7.0 mg/kg) with oral d-amphetamine (0.035, 0.07, 0.14 mg/kg) during six hour sessions; placebo days were interspersed between active drug days. The researchers reported that modafinil produced food intake reductions comparable to those produced by d-amphetamine. However, modafinil-associated effects were not dose-dependent, as food intake was significantly decreased only by one dose (3.5 mg/kg or ~245 mg). Although these data are consistent with Nicolaidis and de Saint Hilaire (1993) findings in rats and with those reported by Jasinski 2000 in humans, it is also important to note that the study by Makris and colleagues was not designed to assess food intake over a 24-hr period or over consecutive days of modafinil administration. As a result, it is unclear whether modafinil-related effects on food intake will persist or whether rapid tolerance will develop following repeated dosing. Thus, the present study examined the influence of modafinil (0, 200, 400 mg) on food intake in human research participants living in a residential laboratory, where the pattern of food intake and ratings of “Hungry” were continuously measured over consecutive days. These participants were previously described in an investigation of modafinil-related effects on mood and cognitive performance of research participants during simulated shift work (Hart et al. 2006). Although this study represents a secondary data analysis, the experimental procedures employed afforded an opportunity to determine the effects of modafinil on several measures of food intake, including total daily caloric intake, meal size, and subjective ratings of hunger.
Methods
Participants
Eleven healthy research participants (mean age [± SD]: 25.2 ± 5.4) completed this 23-day inpatient study: five were females (four Black, one White) and six were males (one Asian, three Black, one Latino, one White). Prior to enrollment into the study, each participant signed a consent form that was approved by the Institutional Review Board of The New York State Psychiatric Institute (NYSPI) and passed comprehensive medical and psychological assessments. They also provided negative urine toxicologies, which confirmed the absence of illicit substance use. Additionally, no participant reported a history of dieting or eating anomalies, and all were within normal weight ranges according to the 1983 Metrapolitan Life Insurance company height/weight table (body mass index [± SD]: 24.3 ± 3.2 kg/m2). Finally, all participants indicated having previous experience working irregular shift schedules.
Participants were told that the objective of the study was to evaluate the effects of an FDA-approved medication on cognitive performance and mood of shift workers. However, they were not informed that modafinil was administered until the study conclusion, when experimental and drug conditions were fully explained.
Laboratory
Participants were housed in a residential laboratory at the New York State Psychiatric Institute in three groups of 3–4 individuals (Foltin et al 1996; Hart et al 2003a). The laboratory includes a common social area containing equipment used for recreational purposes, such as video games, television monitors for watching videotaped films, reading and art materials, board games, and free-weights for exercising. Each individual had a private bedroom with a bed, desk, Macintosh computer system, microwave, toaster, refrigerator, food preparation space, and a barcode scanner (Worthington Data Solutions, Santa Cruz, California) for food requesting and reporting. Cameras and microphones located throughout both the social and private areas, but not in the bathroom or dressing areas, allowed for the continuous observation of behavior. Communication between the staff and participants was minimal and primarily by way of a network system composed of the computers in each of the bedrooms and the control room computer.
Design
Table 1 shows that this residential study consisted of six 3-day blocks of sessions, during which participants “worked” on two different shifts: day and night shift. Work consisted of completing computerized task batteries (described in Hart et al. 2006). During the day shift, they were awakened at 0815 and went to bed at 2400; during the night shift, they were awakened at 0015 and went to bed at 1600. Shifts alternated three times during the study, and shift conditions were separated by an "off" day, during which participants were not on a work schedule but were required to go to bed 8.25 hrs prior to the next shift as they had done during other days. Two groups of participants (N = 7) began on the night shift and one group (N = 4) began on the day shift. Placebo or modafinil (200, 400 mg) was administered once per day, one hour after waking (0915 on the day shift and 0115 on the night shift). In order to minimize potential social confounding effects, presentation of modafinil doses were counterbalanced between and within groups of participants (i.e., participants in each group did not receive the same dosing order). All participants experienced 6 dose/shift combinations: placebo + day and night shift, low dose + day and night shift, and high dose + day and night shift. Finally, days 8 and 16 were "drug washout" days during which participants received placebo modafinil before being switched to another drug condition.
Table 1.
Study Design
| Study day | Shift condition | Modafinil (mg) |
|---|---|---|
| 1–3 | Day | 0 |
| *4 | Off | Off |
| 5–7 | Night | 200 |
| *8 | Night | 0 |
| 9–11 | Night | 400 |
| *12 | Off | Off |
| 13–15 | Day | 400 |
| *16 | Day | 0 |
| 17–19 | Day | 200 |
| *20 | Off | Off |
| 21–23 | Night | 0 |
Note.
Indicates days that were not included in data analyses
Modafinil dose order was counterbalanced across participants
Shift condition order was varied across participants
Modafinil dosing times: 0915 during the day shift and 0115 during the night shift, i.e., 1-hr after waking
Procedure
The procedures have been detailed elsewhere (Hart et al. 2006). Briefly, participants were admitted into the residential laboratory on the day before study commencement in order to be further acquainted with study procedures. On experimental days, they were awakened at 0815 or at 0015, depending on the work-shift condition. At 0920 (0120), after receiving modafinil, participants were weighed (but not informed of their weight) and were given a food box containing a wide variety of food items as well as diet and non-diet beverages. Frozen meal items, illustrated in a booklet, and additional amounts of any food item could be obtained upon request. Subsequently, time was allotted for breakfast, after which they completed 8 hrs of computerized task batteries separated by several 15-min breaks and a 1.5 hrs lunch period. Following completion of task batteries, participants began a recreation period, during which time they had access to the activities available in the social area, including two films shown at 1800 (1000) and at 2100 (1300). At 2330 (1530) they were asked to return the food boxes with any left over items. During “off” days when the transition was being made from day to night shift, participants had access to the food boxes from 0805 to 1530, and from 0100 to 2330, when the switch was from night to day shift. Lights were turned off at 2400 (1600) for an 8.25 hrs sleep period.
Food Monitoring
Food items were available ad libitum during both the work and recreational periods. Meals that required preparation time (e.g., frozen meals), however, were not permitted while tasks were being administered. Additionally, participants had no access to food from the end of the recreation period, when the food boxes were collected, until the following work period. Consumption of food items was closely examined. Participants were required to specify the substance and portion of any item they ate or drank by scanning custom-designed barcodes and were informed that independent observers would continuously monitor their food intake. Research monitors in the control room electronically acknowledged and kept hand-written records of each food request and report. Moreover, wrappers for each food item were color-coded by participant and their trash was examined daily to confirm the accuracy of their reports and of the observers’ records. These procedures do not alter total daily intake and are sensitive to methodologies influencing amounts and patterns of intake (Foltin et al. 1988, 1992; Haney et al. 1997).
Subjective reports of appetite were measured several times throughout each day via a computerized visual analog questionnaire that consisted of a 100-mm line labeled 'not at all' at one end and 'extremely' at the other end. The line was labeled with 'I feel Hungry,'
Drug
Modafinil hydrochloride tablets (200 mg) (Provigil® Cephalon, Inc., West Chester, PA) were repackaged by the Pharmacy Department of the New York State Psychiatric Institute by placing tablets into white #00 opaque capsules and adding lactose filler. Placebo consisted of white #00 opaque capsules containing only lactose. All capsules were administered double blind.
Data Analysis
Data from off and drug washout days (days 4, 8, 12, 16, 20) were not included in the analyses. The food intake data were based on the participants' computerized scanned reports of food intake, and verified by trash examination. Total caloric intake, gram intake of carbohydrate, fat, and protein, proportion of caloric intake from each macronutrient (estimated as kcal from g-intake using Atwater factors [McLaren 1976]), mean number of eating occasions, inter-meal interval, and eating occasion size were measured. Mean number of eating occasions and eating occasion size were determined using a minimal inter-occasion interval of 10 minutes: an eating occasion was defined as beginning with the first report of consuming an item and ending when there was a pause of greater than 10 minutes between food reports. Eating occasion parameters and the percent of energy intake derived from each of the three macronutrients were analyzed based on data obtained for the entire day.
Data were analyzed using a three-factor repeated measures analyses of variance (ANOVA): the first factor was modafinil dose (placebo, 200, 400 mg), the second factor was shift condition (day, night), and the third factor was day within condition (1, 2, 3). For all analyses, ANOVAs provided the error terms needed to calculate planned comparisons that were designed to answer three questions: (1) is food intake altered as a function of shift condition; (2) does modafinil decrease food intake regardless of shift condition; and (3) are modafinil-related effects diminished over time? To evaluate shift condition-related alterations, each day of placebo was compared to the corresponding night of placebo (e.g., the first day of placebo during the day shift versus the first night of placebo during the night shift. To evaluate the effects of modafinil on food intake, each day of each drug condition was compared to a corresponding day of another drug condition (e.g., the first day of 200 mg modafinil during the day shift versus the first day of placebo during the day shift). Modafinil-related effects during night shift work were evaluated similarly. To determine if tolerance developed to the effects of modafinil on food intake, the first day of each dosing condition was compared with the third day of each dosing condition (e.g., the first day of 200 mg modafinil during the day shift versus the third day of 200 mg modafinil during the day shift). In addition, food intake data was analyzed as a function of time of day: morning, afternoon and evening. For the sake of brevity, however, collapsed data for the entire day are discussed primarily because drug effects were similar for both analyzes.
Subjective ratings of “Hungry” were analyzed by using three-factor repeated-measures ANOVA: the first factor was modafinil dose (placebo, 200, 400 mg), the second factor was shift condition (day, night), and the third factor was time of day (10 time points: baseline to bedtime). The planned comparison was designed to answer the question of whether modafinil decreased ratings of “Hungry” throughout the day. Data were considered statistically significant at p < 0.05, using Huynh-Feldt corrections where appropriate.
Results
Effects of Shift Condition on Food Intake
Food intake varied little between the day and night shifts when participants received placebo. Mean total caloric, carbohydrate, and fat intake were significantly increased during the first night that participants worked on the night shift compared with the first day of the day shift by 29%, 16%, and 57%, respectively (p < 0.03 for all measures). No other significant effects of shift condition were observed.
Effects of Modafinil on Food Intake and Subjective Reports of Hunger
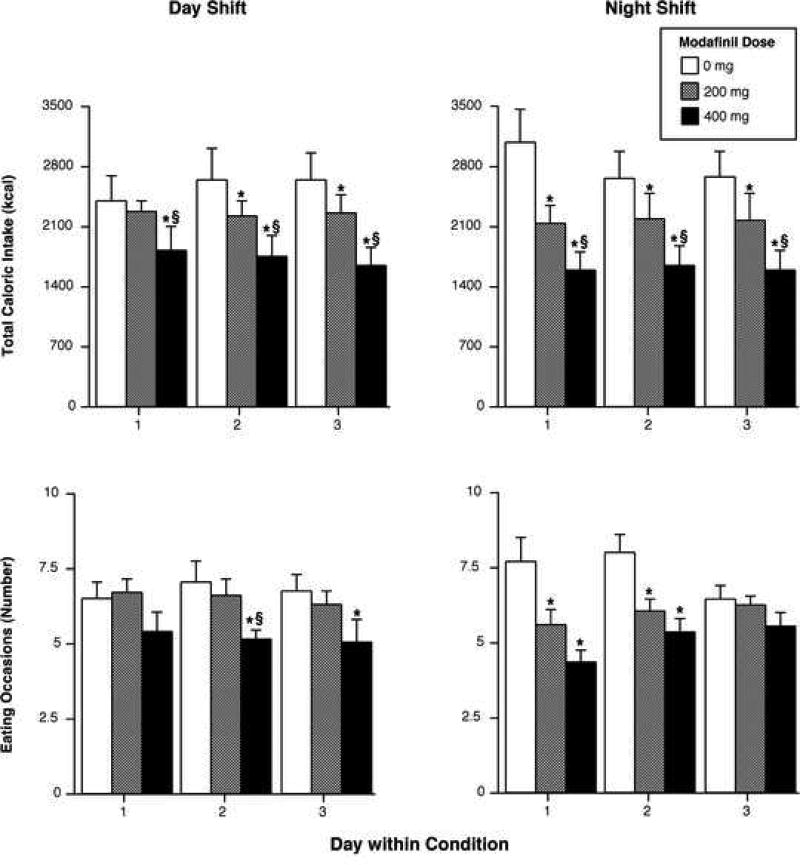
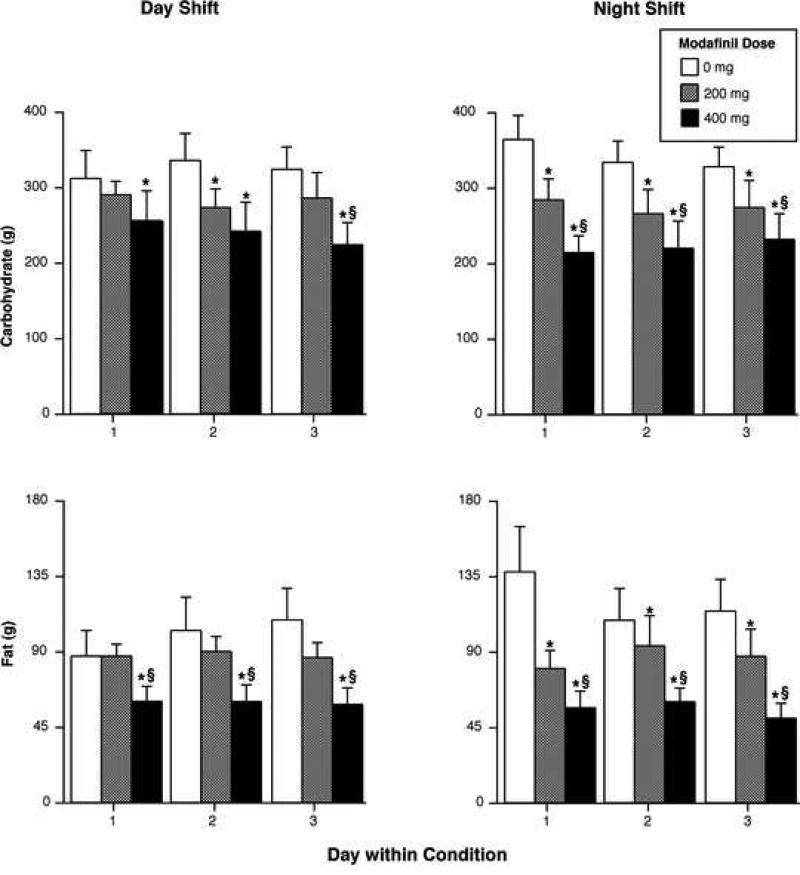
Figure 1 illustrates the effects of modafinil on selected food intake measures during the day (left panels) and night shifts (right panels). During both shift conditions, modafinil produced dose-related reductions in total caloric intake. On average, across all three days, the 200-mg dose decreased total caloric intake by ~18% and the 400-mg dose decreased total caloric intake by ~38%. In addition, the 400-mg dose caused significantly greater caloric intake reductions than the 200-mg dose during both shift conditions across all days (p < 0.005). Partially consistent with total caloric intake results, the larger modafinil significantly reduced the mean number of eating occasions during both shift conditions (p < 0.005), while the smaller dose produced significant reduction only during the night shift (p < 0.02). When the data were collapsed across shift conditions, relative to placebo, the 200-mg dose decreased the number of eating occasions per day by approximately 1 meal, and the 400-mg dose decreased the number of eating occasions by approximately 2 meals. The active doses did not significantly differ in terms of number of eating occasions. Regarding mean meal size, significant reductions were only observed on the third night of the night shift: both active doses differed from placebo (p < 0.03: data not shown). Additionally, the 400-mg dose increased the interval between eating occasions on the third day of the day shift and on both the first and third nights of the night shift (p < 0.03 for all measures). Finally, total intake of fat, carbohydrate and protein showed a similar pattern to total caloric intake, indicating that modafinil did not specifically alter intake of any macronutrient, but decreased intake of all three macronutrients (Figure 2; protein data not shown). Compared with placebo, the 200-mg dose decreased fat, carbohydrate and protein intake by 21% (p = 0.09), 17% (p < 0.03) and 17% (p < 0.04), respectively; the 400-mg dose reduced fat, carbohydrate and protein intake by 49% (p < 0.006), 31% (p < 0.0008) and 37% (p < 0.0003), respectively. Macronutrient reductions produced by the larger modafinil dose were significantly greater than those produced by the smaller dose (p < 0.05).
Figure 1.
Upper Panels: Total kilocalories consumed as a function of modafinil dose (placebo, 200, 400-mg) and day within condition. Lower Panels: Number of eating occasions as a function of modafinil dose (placebo, 200, 400-mg) and day within condition. Left panels display results from the day shift and the right panels from the night shift. Error bars represent one SEM. An * indicates significantly different from placebo (p < 0.05). An § indicates significantly different from 200 mg (p < 0.05).
Figure 2.
Grams of carbohydrate (upper panels) and fat (lower panels) intake as a function of modafinil dose (placebo, 200, 400-mg) and day within condition. Left panels display results from the day shift and the right panels from the night shift. Error bars represent one SEM. An * indicates significantly different from placebo (p < 0.05). An § indicates significantly different from 200 mg (p < 0.05).
Relative to placebo, ratings of “Hungry” were significantly decreased by modafinil (200 and 400 mg) only at one time point throughout the day and only when participants were on the day shift (p < 0.03). This reduction occurred at approximately 1200, which was shortly before the lunch break period.
Discussion
The major finding from this study was that modafinil produced clear reductions in total daily caloric intake by research participants subjected to abrupt changes in work-shift schedules. On average, modafinil (200 and 400 mg) decreased daily caloric intake by ~18 and ~38%, respectively and this effect remained relatively constant across all three days of dosing conditions during both the day and night shifts. Modafinil also reduced subjective ratings of “Hungry” during day shift work immediately before the lunch period, but this was the only effect of the drug on subjective reports of appetite. Although others have reported that an acute single dose of modafinil reduced food intake (Jasinski 2000; Makris et al. 2004), the present data are the first to show that the medication, administered over consecutive days, systemically decreased caloric consumption by humans living in a residential laboratory where behavior was continually monitored. The current data also show that when placebo was administered, changes in work-shift schedules produced limited effects on food intake. This observation is consistent with other studies assessing the effect of simulated shift work on food intake by research participants (e.g., Hart et al. 2003a, b).
Modafinil produced dose-dependent decreases in total caloric intake regardless of shift condition. In general, this finding is consistent with data from Jasinski (2000), who examined modafinil-related effects on food intake following a single meal and reported that 200, 400, and 800 mg modafinil decreased caloric consumption by 10, 20, and 60%, respectively. The percentage of energy intake reductions caused by modafinil (200 and 400 mg) in the Jasinski study was lower than those observed in the current study. One possible explanation for this apparent difference is that Jasinski (2000) examined food intake only following the lunchtime meal, whereas in this study food intake was assessed throughout the entire day. In addition, the present dose-related effects of modafinil on food consumption are not entirely congruent with results reported by Makris et al. (2004). These researchers found that while the 490-mg dose reduced food consumption by 22%, only the 245-mg dose produced statistically significant reductions in consumption (31%). The reason for this apparent inconsistent finding is unclear, but it should be noted that when the mean daily gram intake of solid foods alone was analyzed by Makris et al. (2004), they found that this measure was significantly reduced by both the 200 and 400-mg modafinil doses.
The behavioral mechanism(s) underlying modafinil-related effects on total caloric intake remains to be elucidated, but selective effects of the medication on macronutrient intake can be excluded because the proportion of total calories derived from carbohydrates, fats and proteins was not altered. Modafinil decreased the number of eating occasions and it is possible that this contributed substantially to the observed effects on overall caloric intake. On average, participants engaged in one or two fewer eating occasions while being maintained on modafinil (200 and 400 mg, respectively) compared with placebo. Another factor that contributed to the overall effect of modafinil on total caloric intake was meal size. For example, on night three of both active modafinil conditions, the only night that the number of eating occasions was not significantly decreased by modafinil, meal size was significantly reduced. In general, these results concur with those reported by Chapelot et al. (2000) who found that the anti-obesity medication sibutramine produced similar reductions on measures of food intake in non-obese human research participants.
It is possible that the effects of modafinil on food intake were due to an adverse pharmacological effect. Data from subjective-effect ratings completed throughout each day (Hart et al. 2006) indicate that this possibility seems unlikely because modafinil did not significantly alter ratings that would be indicative of untoward effects. For example, the medication did not decrease ratings of “Alert” or “Energetic,” or increase ratings of “Can’t concentrate” or “Confused.” Furthermore, modafinil enhanced cognitive performance in several domains during both day- and night-shift work, although the effects were more pronounced during the night shift. Together, these observations argue against the likelihood of modafinil-associated food intake effects resulting from nonspecific pharmacological adverse events.
Modafinil occupies catecholamine transporters enhancing the activity of CNS dopamine, NE, as well as 5-HT, which are established mechanisms through which many anti-obesity medications exert their therapeutic actions (Cooke and Bloom 2006; Nelson and Gehlert 2006). Hence, it is tempting to conclude that these neurochemical features played a role in decreasing food intake in the current study. Because modafinil exerts multiple additional neurochemical actions and because a comparator anorectic agent with a well-known neurochemical profile was not included in the current study, such explanations are speculative and suggest directions for future research.
The current findings should be considered in light of several possible limitations. For example, the generality of these results to an obese patient population is unknown because the data were obtained using healthy research volunteers with no history of weight-related disorders. Although other researchers have reported data demonstrating the utility of employing non-obese research participants when examining the effects of appetite suppressants on food intake (e.g., Chapelot et al. 2000; Batterham et al. 2002), future studies should examine modafinil-associated anorexic effects in a clinical sample. Another related potential caveat is that while tolerance to modafinil-related anorexic effects was not found following three consecutive days of the medication, it is possible that tolerance might be observed with a longer period of modafinil administration. Additional studies should investigate modafinil-associated effects on food intake over a more extensive period of time.
In conclusion, these data are the first to demonstrate that repeated administrations of modafinil decreased total caloric intake and ratings of “Hungry” by human research participants living in a residential laboratory that models the natural ecology, i.e. unlimited quantities of a wide selection of food items. Additionally, these results show that tolerance to modafinil-related hypophagic effects is not apparent after consecutive dosings. Taken together, these findings suggest that future studies should investigate the effects of modafinil in a clinical population prospectively using a comparator anti-obesity medication.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grant #DA-03746. We gratefully acknowledge the efforts of Matthew Kirkpatrick, who read an earlier version of the manuscript and made helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of interest: The authors declare, that except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Biederman J, Swanson JM, Wigal SB, Kratochvil CJ, Boellner SW, Earl CQ, Jiang J, Greenhill L. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116(6):e777–784. doi: 10.1542/peds.2005-0617. [DOI] [PubMed] [Google Scholar]
- Boellner SW, Earl CQ, Arora S. Modafinil in children and adolescents with attention-deficit/hyperactivity disorder: a preliminary 8-week, open-label study. Curr Med Res Opin. 2006;22(12):2457–2465. doi: 10.1185/030079906X148300. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27(6):1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- Broughton RJ, Fleming JA, George CF, Hill JD, Kryger MH, Moldofsky H, Montplaisir JY, Morehouse RL, Moscovitch A, Murphy WF. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 1997;49(2):444–451. doi: 10.1212/wnl.49.2.444. [DOI] [PubMed] [Google Scholar]
- Chapelot D, Marmonier C, Thomas F, Hanotin C. Modalities of the food intake-reducing effect of sibutramine in humans. Physiol Behav. 2000;68(3):299–308. doi: 10.1016/s0031-9384(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5(11):919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- Eliyahu U, Berlin S, Hadad E, Heled Y, Moran DS. Psychostimulants and military operations. Mil Med. 2007;172(4):383–387. doi: 10.7205/milmed.172.4.383. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002;68(1):107–112. doi: 10.1002/jnr.10196. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11(1):1–14. doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Rolls BJ, Moran TH, Kelly TH, McNelis AL, Fischman MW. Caloric, but not macronutrient, compensation by humans for required-eating occasions with meals and snack varying in fat and carbohydrate. Am J Clin Nutr. 1992;55(2):331–342. doi: 10.1093/ajcn/55.2.331. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Comer SD, Fischman MW. Effect of fluoxetine on food intake of humans living in a residential laboratory. Appetite. 1996;27(2):165–181. doi: 10.1006/appe.1996.0043. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Fischman MW, Foltin RW. Alprazolam increases food intake in humans. Psychopharmacology (Berl) 1997;132(3):311–314. doi: 10.1007/s002130050350. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2006;31(7):1526–1536. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Nasser J, Foltin RW. Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berl) 2003a;169(1):42–51. doi: 10.1007/s00213-003-1464-4. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW. Zolpidem-related effects on performance and mood during simulated night-shift work. Exp Clin Psychopharmacol. 2003b;11(4):259–268. doi: 10.1037/1064-1297.11.4.259. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study. CNS Drugs. 2007;21(5):407–416. doi: 10.2165/00023210-200721050-00004. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14(1):53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23(3):149–156. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591(2):319–326. doi: 10.1016/0006-8993(92)91713-o. 1992. [DOI] [PubMed] [Google Scholar]
- Lindsay SE, Gudelsky GA, Heaton PC. Use of modafinil for the treatment of attention deficit/hyperactivity disorder. Ann Pharmacother. 2006;40(10):1829–1833. doi: 10.1345/aph.1H024. [DOI] [PubMed] [Google Scholar]
- Luque CA, Rey JA. Sibutramine: a serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity. Ann Pharmacother. 1999;33(9):968–78. doi: 10.1345/aph.18319. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and nonrepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Makris AP, Rush CR, Frederich RC, Kelly TH. Wake-promoting agents with different mechanisms of action: comparison of effects of modafinil and amphetamine on food intake and cardiovascular activity. Appetite. 2004;42(2):185–195. doi: 10.1016/j.appet.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Gehlert DR. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29(1):49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- Nicolaidis S, De Saint Hilaire Z. Nonamphetamine awakening agent modafinil induces feeding changes in the rat. Brain Res Bull. 1993;32(2):87–90. doi: 10.1016/0361-9230(93)90060-o. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mao J, Sampathkumaran R, Shelton J. Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online. 1998;1(1):49–61. [PubMed] [Google Scholar]
- Pigeau R, Naitoh P, Buguet A, McCann C, Baranski J, Taylor M, Thompson M, MacK II. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature. J Sleep Res. 1995;4(4):212–228. doi: 10.1111/j.1365-2869.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13(2):105–15. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Turner D. A review of the use of modafinil for attention-deficit hyperactivity disorder. Expert Rev Neurother. 2006;6(4):455–468. doi: 10.1586/14737175.6.4.455. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27(3):434–439. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Biederman J, Swanson JM, Yang R, Greenhill LL. Efficacy and safety of modafinil film-coated tablets in children and adolescents with or without prior stimulant treatment for attention-deficit/hyperactivity disorder: pooled analysis of 3 randomized, double-blind, placebo-controlled studies. Prim Care Companion J Clin Psychiatry. 2006;8(6):352–360. [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132(4):1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]