Abstract
Background
Caloric restriction (CR) has been described to have cardioprotective effects and improve functional outcomes in animal models and humans. Chronic ischemic heart failure (HF) is associated with reduced cardiac sympathetic innervation, dysfunctional β-adrenergic receptor (β-AR) signaling and decreased cardiac inotropic reserve. We tested the effects of a long-term CR diet, started late after myocardial infarction (MI) on cardiac function, sympathetic innervation and β-AR responsiveness in a rat model of post-ischemic HF.
Methods and Results
Adult male rats were randomly assigned to MI or Sham operation and four weeks later were further randomized to a one-year CR or normal diet. One year of CR resulted in a significant reduction in body weight, heart weight and heart weight/tibia length ratio when compared to normal diet in HF groups. At the end of the study period, echocardiography and histology revealed that HF animals under the CR diet had ameliorated left ventricular remodeling compared to HF rats fed with normal diet. Invasive hemodynamic showed a significant improvement of cardiac inotropic reserve in CR HF rats compared to HF normal diet animals. Importantly, CR dietary regimen was associated with a significant increase of cardiac sympathetic innervation and with normalized cardiac β-AR levels in HF rats when compared to HF rats on the standard diet.
Conclusions
We demonstrate, for the first time, that chronic CR, when started after HF established, can ameliorate cardiac dysfunction and improve inotropic reserve. At the molecular level, we find that chronic CR diet significantly improves sympathetic cardiac innervation and β-AR levels in failing myocardium.
Keywords: heart failure, sympathetic nervous system, β-adrenergic receptors, caloric restriction, cardiac remodeling
INTRODUCTION
Despite improvements in diagnosis, management and treatment, heart failure (HF) still represents one of the greatest health care challenges worldwide for its high morbidity, mortality and economic impact1, 2. This syndrome represents the final common clinical event of numerous cardiovascular (CV) diseases, with coronary artery disease-dependent myocardial infarction (MI) being its most common cause in the western countries1. Despite some improvement in HF survival, the death rate remains high and current therapies are only able to slow disease progression. Hence, there is an urgency to identify new therapies to add to the HF therapeutic armamentarium in order to reduce the enormous mortality rate that this disease still poses.
Dietary interventions, including caloric restriction (CR), have been proven to counteract some CV risk factors, such as diabetes, hypertension and obesity that are known to promote atherosclerosis and the progression of cardiac dysfunction3–7. Indeed, pre-clinical and clinical studies have demonstrated that CR exerts several therapeutic effects on atherosclerosis by reducing circulating cholesterol levels, systemic inflammation and vascular fibrosis8–10. Furthermore, CR has also been shown to curb the maladaptive cardiac effects of hypertension in both genetic and surgical models of the disease, reducing the development of left ventricular (LV) hypertrophy and diastolic dysfunction11–15.
Importantly, the beneficial actions of CR on global metabolism are widely recognized and include a reduction of visceral fat mass, fasting insulin levels, and insulin resistance in diabetic and obese patients7. Further, CR has been described to exert direct cardioprotective effects3, 12, 16–19. Accordingly, intermittent fasting (IF), a specific CR regimen, protects the heart from ischemic injury, reduces cardiac remodeling and ameliorates LV function, when initiated before MI-induction17. In the same setting (IF started before MI), this CR regimen was associated with an increase in circulating adiponectin levels and a reduction in cardiac and systemic inflammation20. Moreover, short-term (6-week-long) IF has been shown to improve LV remodeling and survival in a rat model of post-ischemic HF via pro-angiogenic and anti-apoptotic mechanisms21. Interestingly, the beneficial effects of CR have been also studied in obese patients with HF at preserved ejection fraction (HFpEF) in a randomized clinical-trial, where CR combined with exercise training was able to increase peak oxygen consumption22. Of note, all of the aforementioned studies have shown the beneficial effects of CR on the post-MI heart only in the short-term. From a clinical point-of-view, however, it is evident that for chronic diseases such HF, a long-term and sustained CR regimen is potentially warranted to improve outcomes. Hence, the effects of a long-standing CR diet on cardiac function and structure in a model of ischemic HF has not been properly investigated. Thus, in the present study we have tested the effects of a 12-month CR regimen in a rat post-MI model where CR was started after HF establishment.
METHODS
The data, analytic methods, and study materials will be made available from the corresponding authors to other researchers upon request for purposes of reproducing the results or replicating the procedure. Expanded Methods are available in the Supplemental Material.
Experimental design
All animal procedures and experiments were performed in accordance with the guidelines of the Institutional Animal Care Committee of University Federico II of Naples, Italy. Two-month-old, male Sprague-Dawley rats (n=44) were randomly assigned to different surgical groups: 34 rats underwent surgically-induced MI and 10 rats received Sham operation (Figure 1A). Mortality rate was ~23.5% at 4-weeks post-MI. Four weeks post-MI, a time point when cardiac dysfunction was established, HF rats underwent echocardiographic evaluation and were further randomized to a one-year CR (IF dietary regimen that consists in a complete fasting day followed by a normal feed day), or normal diet (ND, standard rat diet) (Figure 1A). Sham-operated rats followed a ND regimen throughout the study period. Thus, the final population that started the dietary protocol consisted of 3 groups: Sham-operated rats fed with ND (Sham, n=10), HF rats fed with ND (HF-ND, n=13) and HF rats fed with CR dietary regimen (HF-CR, n=13). Twelve months after the beginning of the feeding protocol, echocardiography and hemodynamic analysis were performed in all groups. Then, rats were euthanatized for histological and molecular analysis. An expanded Methods section appears in Supplemental Material.
Figure 1. Long-term caloric restriction decreases body weight and accordingly heart weight in ischemic HF.
A, Overall design of the 13-months study. B, Body weight (BW) changes during the study period for all groups at 0-, 6- and 12-months after the beginning of the dietary protocol. C, Representative picture of HF-ND (right side) and HF-CR rats (left side) at the end of the study period (12 months after the beginning of the dietary protocol). Measures of (D) Heart weight (HW) and (E) HW normalized to tibia length (TL). F, Graph showing the direct correlation between HW and BW in HF groups (HF-ND group: black spots; HF-CR group: red spots). n. at the end of the study: Sham=10; HF-ND=11; HF-CR=12. Data are presented as mean±SEM *p<0.0001 vs HF-ND, **p<0.01 vs HF-ND, ***p<0.05 vs HF-ND #p<0.01 vs Sham, ##p< 0.001 vs Sham, ^p<0.0001 vs Sham. Two- or one-way ANOVA and Bonferroni test were used between groups.
In vivo experimental procedures
MI, echocardiography and hemodynamic analysis were performed as described23–25.
Plasma membrane levels of β-AR levels and Real-time PCR
Membrane proteins were isolated from LV samples26 to evaluate protein levels of β1-AR, β2-AR and β3-AR. Real-time PCR was performed as descrybed26.
Histological sectioning and staining
Masson’s Trichrome staining was used to evaluate LV fibrosis while capillaries were detected by Lectin Bandeiraea simplicifolia I staining. Immunofluorescence technique was performed using antibodies against Vesicular acetylcholine transporter (VaChT) and Dopamine β-hydroxylase (DβH)27.
Statistical analysis
All values in the text and figures are presented as means ± SEM. Statistical significance was determined by one-way or two-way ANOVA with Bonferroni post-hoc analysis or T-test as appropriate. All data were analyzed using GraphPad-Prism software version 7. Probabilities of 0.05 or less were considered to be statistically significant.
RESULTS
Effect of long-term caloric restriction on body weight and heart weight
As expected, at 4-weeks post-surgery, before the start of dietary intervention, the average body weight (BW) was similar among all study groups (Figure 1B and Table). Six months after the beginning of the CR diet protocol, average BW became significantly lower (p<0.001) in HF-CR rats compared to HF-ND rats (Figure 1B and Table). Of note, at this time-point HF-ND BW was significantly higher compared to Sham BW (p<0.05) (Figure 1B and Table). Twelve months after the beginning of different dietary regimens, HF-CR rats showed a significantly lower BW compared to HF-ND and Sham animals (respectively p<0.001 and p<0.0001) that was so evident on physical exam (Figure 1B–C). To analyze whether the different dietary protocols were able to influence the heart mass, we measured heart weight (HW), HW to tibia length (TL) ratio (HW/TL) and HW to BW ratio (HW/BW) at the end of the study period, in all groups. HW and HW/TL were significantly increased in HF-ND group compared to Sham group, consistent with an HF phenotype (p<0.001), while, HF-CR animals showed lower HW and HW/TL compared to HF-ND rats (p<0.01 and p<0.05, respectively) (Figure 1D–E and Table). As expected, HW/BW was increased in HF rats compared to Sham (p<0.001 vs HF-ND, p<0.0001 vs HF-CR) (Table) while HW/BW was not statistically different between HF-ND and HF-CR groups. Hence, we decided to analyze the correlation between HW and BW in all HF rats and we found that there was a direct correlation between these parameters (R2=0.6546, p<0.0001), suggesting that the decrease in BW in rats treated with a restricted dietary regimen was paralleled by a proportional decrease in HW (Figure 1F). The latter observation could also explain the reason why we did not find any difference between HF-ND and HF-CR groups in terms of HW/BW ratio (Table). Contrarily, HW/TL was significantly different between the two HF groups, as mentioned above, indicating that TL is not influenced by the dietetic regimen.
Table.
Long-term effects of caloric restriction on physical parameters and left ventricular function.
| Sham | HF-ND | HF-CR | |
|---|---|---|---|
| Physical data | |||
| BW(g) basal | 350±13.8 | 352±6.90 | 349±9.57 |
| BW(g) 6-months | 475±19.6 | 541±16.3* | 440±12.7† |
| BW(g) 12-months | 538±11.5 | 574±16.6 | 444±12.0‡,§ |
| HW(g) | 1.098±0.061 | 1.453±0.040‡ | 1.213±0.056∥ |
| HW/BW(g/Kg) | 2.045±0.113 | 2.540±0.061‡ | 2.724±0.077# |
| HW/TL(g/mm) | 0.2407±0.014 | 0.3166±0.008‡ | 0.2695±0.011** |
| AW(g) | 0.026±0.0029 | 0.037±0.0032* | 0.028±0.0017** |
| AW/BW(g/Kg) | 0.049±0.0056 | 0.066±0.0055 | 0.061±0.0032 |
| AW/TL(mg/mm) | 5.8±0.64 | 8.1±0.69* | 6.1±0.35** |
| Echocardiography (end of the study) | |||
| EF% | 68.6±3.09 | 30.7±0.92# | 33.5±0.99# |
| LVIDd, mm | 7.82±0.21 | 10.20±0.28# | 9.14±0.30*,** |
| LVIDs, mm | 5.04±0.44 | 8.63±0.23# | 7.62±0.26#,** |
| LVVol-d, µl | 329±19.8 | 595±34.6‡ | 470±35.4*,** |
| LVVol-s, µl | 127±25.7 | 437±19.7# | 336±36.2‡,** |
| LVAWd, mm | 1.57±0.11 | 0.99±0.03# | 0.97±0.02# |
| LVAWs, mm | 2.54±0.28 | 1.03±0.02# | 1.04±0.02# |
| LVPWd, mm | 1.53±0.13 | 1.89±0.09* | 1.85±0.06 |
| LVPWs, mm | 2.43±0.14 | 2.599±0.09 | 2.46±0.14 |
| HR, bpm | 349±10 | 315±9 | 319±10 |
| Hemodynamic data, basal | |||
| HR, bpm | 298±15 | 293±6 | 305±7 |
| LV+dP/dt, mm Hg/s | 7258±644 | 4135±299‡ | 5480±429* |
| LV−dP/dt, mm Hg/s | −7386±1191 | −3128±173†† | −4659±573* |
| LVEDP, mmHg | 5.934±0.97 | 11.61±0.81‡ | 9.9±0.68* |
| LVESP, mmHg | 130.1±4.07 | 99.32±5.63* | 125.6±9.86** |
| Hemodynamic data, ISO (333 ng/Kg BW) | |||
| HR, bpm | 361±9 | 352±8 | 359±6 |
| LV dP/dt, mm Hg/s | 13651±1814 | 6620±480‡ | 11017±657** |
| LV −dP/dt, mm Hg/s | −9870±1391 | −3961±146‡ | −6723±609*,** |
| LVEDP, mmHg | 5.33±1.21 | 9.77±1.93 | 6.72±1.88 |
| LVESP, mmHg | 139.8±7.23 | 109.8±4.17†† | 142.5±4.95∥ |
Body weight (BW), heart weight (HW), HW/BW ratio and HW/tibia length ratio (HW/TL), adrenal gland weight (AW), AW/BW ratio and AW/TL ratio were measured in all groups. Left ventricular (LV) ejection fraction (EF), internal diameter at diastole (LVIDd) and systole (LVIDs), anterior wall in diastole (LVAWd) and systole (LVAWs), posterior wall in diastole (LVPWd) and systole (LVPWs), Volume in systole (LVVol-s) and diastole (LVVol-d), and heart reate (HR) were evaluated in all groups. In vivo HR, LV + dP/dt, − dP/dt, end-diastolic pressure (EDP) and end-systolic pressure (ESP) were assessed under basal conditions and after maximal isoproterenol stimulation (ISO 333ng/Kg BW). n= 6 to 13, Data are presented as mean±SEM
p<0.05 vs Sham;
p<0.001 vs HF-ND;
p<0.001 vs Sham;
p<0.0001 vs HF-ND;
p<0.01 vs HF-ND;
p<0.0001 vs Sham;
p<0.05 vs HF-ND;
p<0.01 vs Sham.
One-way ANOVA and Bonferroni test were used between groups.
In vivo cardiac function over the course of the study period and biomarkers of heart failure
Four weeks after surgically-induced MI, echocardiographic studies revealed that LV ejection fraction (LVEF) was significantly reduced in both groups of HF rats compared to Sham, while internal diameter at diastole and systole (LVIDd and LVIDs) were significantly higher in HF rats compared to Sham, which are expected post-MI findings (Figure 2A–C). At the same time-point, cardiac function and structure, evaluated by echocardiography, were not different between the two HF groups, confirming a similar HF level between groups before the randomization to dietary interventions. Twelve months after the beginning of the CR or normal dietary programs in the HF rats, these groups still showed significantly impaired LV systolic function and increased LV diameters and volumes compared to Sham rats (Figure 2D–F and Table). However, impairment in cardiac systolic function, as expressed by LVEF, and adverse LV remodeling, as measured by parameters of ventricular dilatation, further progressed in HF-ND rats (p<0.0001 vs Sham at the end of the study), as observed by comparing measurements performed at 4 weeks post MI induction to measures obtained at the end of the study period. Importantly, 12 months of a CR diet regimen resulted in blunted HF progression, in fact, HF-CR rats showed increased cardiac function and reduced LV diameters and volumes compared to HF-ND rats (Figure 2E–F and Table).
Figure 2. Long-term caloric restriction decreases left ventricular dilatation in ischemic HF.
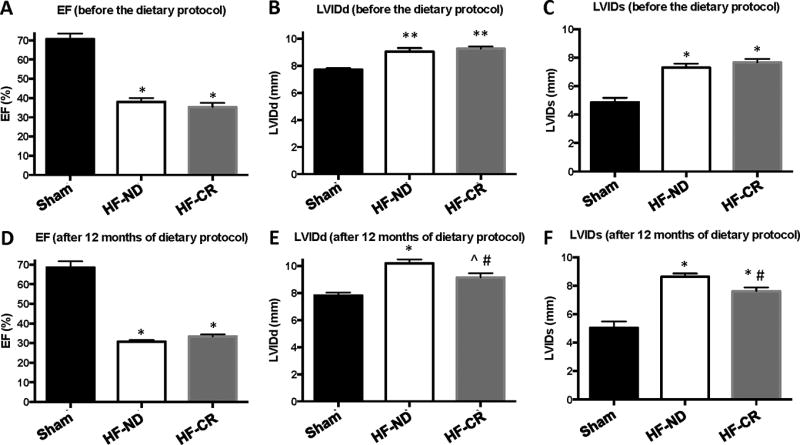
Ejection fraction (EF) (A), LV internal diameter at diastole (LVIDd) (B) and systole (LVIDs) (C), and heart rate (HR) (D) as measured by echocardiography before the dietary protocol (4 weeks after MI). EF (E), LVIDd (F), LVIDs (G) and HR (H) as measured by echocardiography at the end of the study period (12 months after the dietary protocol started). n = 6 to 11 per group. Data are presented as mean±SEM. * p<0.0001 vs Sham, **p<0.001 vs Sham, #p<0.05 vs HF-ND, ^p<0.05 vs Sham. One-way ANOVA and Bonferroni test were used between all groups.
At the end of the study period, LV catheterization and hemodynamic analysis showed a significant reduction in LV contractility and relaxation in HF-ND group compared to Sham confirming HF-dependent cardiac dysfunction and adverse remodeling (Figure 3 and Table). Of note, HF-CR rats showed a significant decrease in LV relaxation index compared to Sham animals (p<0.05), while +dP/dt was not different between HF-CR and Sham. Importantly, upon infusion of a maximal dose of isoprotenerol (ISO) (333 ng/Kg BW), rats undergoing CR showed a robust improvement of both LV contractility and relaxation compared to control diet HF rats. In fact, HF-CR group showed a significant increase in +dP/dt and a significant decreased in −dP/dt compared to HF-ND group (p<0.001 and p<0.05, respectively) (Figure 3 and Table). This latter finding suggests that a chronic CR diet is able to improve inotropic reserve of the failing heart.
Figure 3. Long-term caloric restriction enhances in vivo LV β-AR inotropic reserve in ischemic HF.
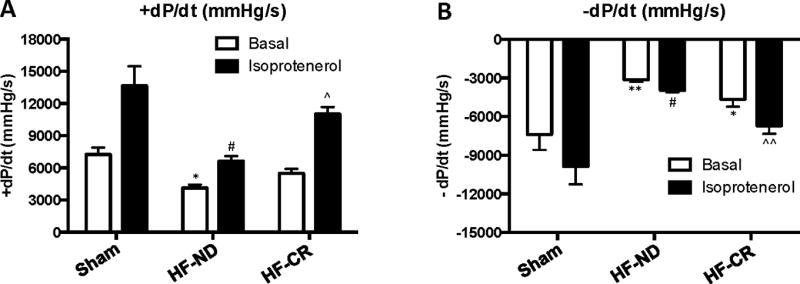
Average LV +dP/dt (A) and LV −dP/dt values (B) in the experimental groups (Sham, n=5; HF-ND, n=7; HF-CR, n=6) evaluated under basal conditions and after maximal isoproterenol stimulation (ISO). Data are presented as mean±SEM. *p<0.05 vs Sham at basal, **p<0.001 vs Sham at basal, #p<0.0001 vs Sham after ISO, ^p<0.001 vs HF-ND after ISO, ^^p<0.05 vs Sham and HF-ND after ISO. Two-way ANOVA analysis and Bonferroni test were used among groups.
Further, we assessed cardiac expression of specific genes known to play a role in HF pathogenesis and progression. At this regard, we measured the mRNA levels of atrial natriuretic factor (ANF), β-Myosin heavy chain (β-MHC) and Endothelin-1 in the LV of all groups via real-time polymerase chain reaction (RT-PCR) (Supplemental Figure 1). Consistent with functional and structural data (see below), ANF and β-MHC mRNA levels were markedly increased in HF-ND rats group compared with Sham rats (p<0.05) (Supplemental Figure 1). Interestingly, ANF levels were significantly reduced in HF-CR rats to levels similar to Sham animals (p<0.05) (Supplemental Figure 1). In addition, we found a trend to increase in Endothelin-1 mRNA levels in HF rats treated with CR diet when compared to HF-ND group (Supplemental Figure 1).
Effects of caloric restriction on cardiac β-AR level and adrenal catecholamine synthesis
Based on our in vivo findings, indicating that CR was able to ameliorate cardiac β-AR responsiveness in HF rats, we measured by Western blot β1-AR levels on plasma membranes isolated from the hearts of all groups. As expected, we observed a significant reduction in β1-AR plasma membrane levels in HF-ND hearts compared to Sham hearts (p<0.05) (Figure 4A–B). Importantly, 1 year of CR diet was able to completely prevent HF-related cardiac β1-AR down-regulation. Indeed, when compared with HF-ND group, HF-CR hearts showed a significant increase in β1-AR plasma membrane density that was equivalent to that observed in Sham hearts (Figure 4A–B). No differences were found in β2-AR and β3-AR membrane levels between Sham, HF-ND and HF-CR groups (Supplemental Figure 2).
Figure 4. Long-term caloric restriction restores β1-AR membrane level in ischemic HF.
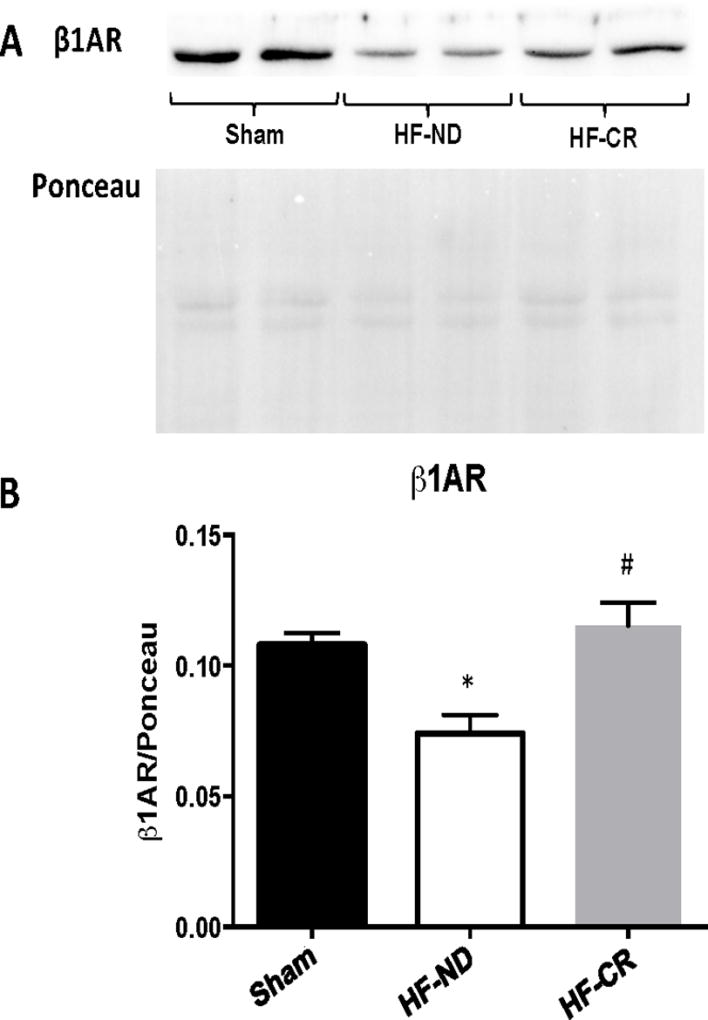
Representative western blot (A) and quantitative data (B) showing β1-AR protein levels in membrane extracted from cardiac lysates in the 3 experimental groups (Sham, HF-ND and HF-CR). Ponceau used as loading control. n = 6 to 10 per group. Data are presented as mean±SEM. *p<0.05 vs Sham, #p<0.01 vs HF-ND. One-way ANOVA analysis and Bonferroni test were used among groups.
Moreover, we measured adrenal mRNA levels of enzymes involved in the production of Catecholamines: Tyrosine hydroxylase (TH), Dopa decarboxylase (DDC), Dopamine β-hydroxilase (DβH) and Phenylethanolamine N-methyltransferase (PNMT). We found that CR treatment was able to decrease HF-related adrenal TH up-regulation to the levels observed in Sham animals (Supplemental Figure 3). In addition, there was a trend to decrease in adrenal mRNA levels of PNMT in the HF-CR group when compared to HF-ND group (Supplemental Figure 3). No differences were found between HF groups in adrenal DDC and DβH gene expression (Supplemental Figure 3). Accordingly, adrenal weight (AW) and AW/TL ratio were reduced in HF-CR rats to the levels observed in Sham animals (Table and Supplemental Figure 3). No differences in AW/BW ratio have been found between the 3 study groups (Table).
Effects of caloric restriction on cardiac fibrosis, capillary density and innervation
Masson’s Trichrome staining was performed on cardiac specimens obtained from all study groups at the end of the study period. Interstitial Fibrosis was measured in Sham animals as well as in the remote area (RA) and border zone (BZ) of HF groups (Figure 5A). As indicated in Figure 5B, HF-ND rats showed a vast amount of fibrosis that was negligible in Sham animals. Importantly, 12 months of a CR diet dramatically resulted in a robust reduction in cardiac fibrosis both in BZ and RA, as evident by comparing HF-CR to HF-ND hearts (p<0.0001) (Figure 5A–B). At a molecular level, we found that one year CR dietary treatment did not influence levels of pro-fibrotic genes like TGF-β, MMP-2 and TIMP-2 while it increased the levels of the anti-fibrotic TIMP-1 (P<0.05) in HF rats when compared to HF-ND rats (Supplemental Figure 4).
Figure 5. Long-term caloric restriction reduces left ventricular fibrosis in ischemic HF.
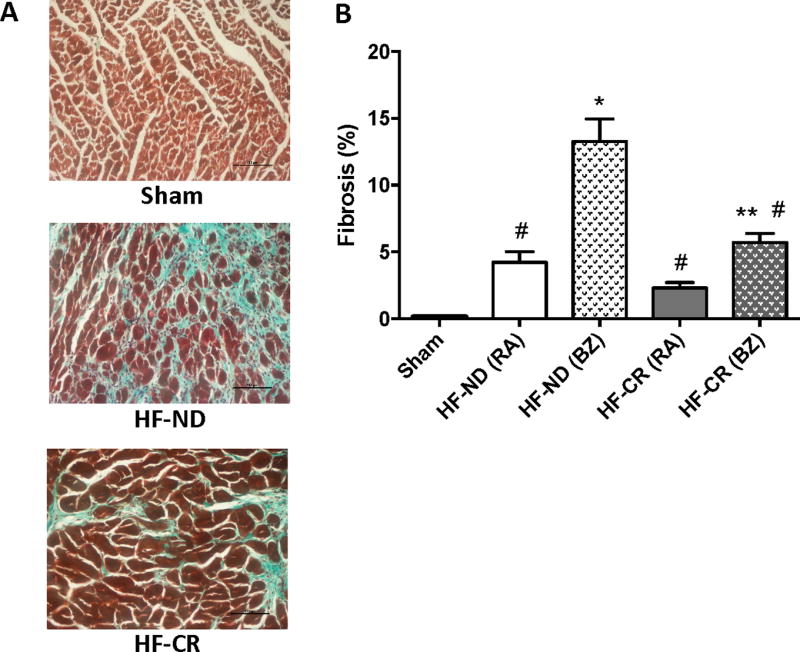
Masson-Trichrome staining denoting cardiac fibrosis. (A) Representative images of Sham as well as HF-ND and HF-CR in border zone (BZ). Black scale bar corresponding to 100 µm (B) Quantification of the % fibrosis in Sham as well as remote area (RA) and BZ of HF groups. N= 3 to 5 per group. Data are presented as mean±SEM. *p<0.0001 vs Sham, **p<0.05 vs Sham, #p<0.0001 vs HF-ND (BZ). One-way ANOVA analysis and Bonferroni test were used among all groups.
Moreover we evaluated cardiac angiogenesis at the end of the study period and found that capillary density was significantly increased (p<0.01) in HF-CR hearts compared with HF-ND in the RA (Supplemental Figure 5). There was a trend to increase in capillary density of the BZ of HF-CR hearts when compared to the BZ of HF-ND. (p=0.1452).
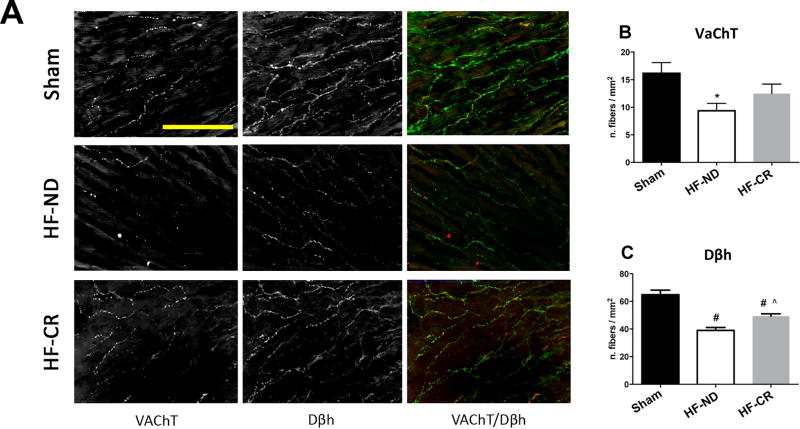
Furthermore, we evaluated adrenergic and cholinergic cardiac innervation by immunofluorescence in all study groups (Figure 6). HF-ND rats showed a significant decrease in both adrenergic and cholinergic nerves compared to Sham animals (respectively p<0.05 and p<0.0001) (Figure 6A, C). Remarkably, LV specimens from HF-CR animals revealed an enhancement in adrenergic innervation as suggested by Dβh staining (p<0.01) when compared to HF-ND group (Figure 6A, C). Of note, CR was not able to significantly affect cardiac cholinergic innervation.
Figure 6. Long-term caloric restriction improves left ventricular adrenergic fibers in ischemic HF.
Representative images (A) and quantitative data (B, C). Shown in (A) anti-vesicular acetylcholine transporter (VaChT), Dopamine β-hydroxylase (Dβh) stainings and merged color image (VaChT in red and Dβh in green) of the 3 experimental groups: Sham (top panels), HF-ND (middle panels) and HF-CR (bottom panels); images at full definition (300 dpi) and 40x magnification; yellow scale bar corresponding to 100 µm. Quantification of number of fibers/mm2 of VaCHT staining (B) and Dβh staining (C) in 3 groups. N=5 per each group. Data are presented as mean±SEM. *p<0.5 vs Sham, #p<0.0001 vs Sham, ^p<0.01 vs HF-ND. One-way ANOVA analysis and Bonferroni test were used among all groups.
DISCUSSION
In the present study, we have demonstrated that a long-term CR regimen in post-MI rats, started when HF was already established, is able to: a) improve HF-related maladaptive remodeling; b) enhance cardiac function and inotropic reserve; c) restore HF-related β1-AR down-regulation; d) reduce cardiac fibrosis; d) increase cardiac adrenergic innervation and capillary density.
Over the last decade, several pre-clinical and clinical studies have provided evidence indicating that CR is a safe and effective treatment for age-related CV diseases28. Importantly, it has been recently published that a two-year long CR diet can positively influence quality of life, global health, mood and sleep quality in healthy non-obese adults29. This latter finding is of particular interest since depression is known to negatively impact on morbidity and mortality of HF patients30, 31. Importantly, restricted dietary regimens have been shown to exert cardioprotective effect as shown in several models of cardiac dysfunction such as ischemia/reperfusion, MI and LV hypertrophy4, 11, 17, 18, 28, 32–36. The molecular mechanisms underlying these beneficial effects include, but are not limited to, a reduction in cardiac free-radical and reactive oxygen species production, a decrease in cardiac and systemic inflammation, an improvement in mitochondrial function, as well as an increase in adiponectin circulating levels and consequent activation of cardiac AMP-Activated Protein Kinase-endothelial nitric oxide synthase signaling pathway20, 28, 33, 34, 36–40. However, whether CR can be an effective long-lasting treatment in chronic HF has not been studied, yet. This is of crucial importance since HF is a chronic disease and current treatments are only effective at slowing disease progression. Ahmet et al. have investigated the short-term effects of CR in an ischemic model of HF (up to 10 weeks post-MI) and have found that a CR diet is associated only to a trend-to decrease in the end-diastolic volume compared to controls18. This latter finding may be explained by the fact that a dietetic treatment needs a longer period to exert its beneficial effects on cardiac structure and function. On the other hand, Katare and colleagues found that short-term IF regimen preserved cardiac volumes and improved LV function after MI21. Interestingly, our study indicates that one year of CR diet is able to reduce both systolic and diastolic LV diameters thus positively impacting on cardiac remodeling (Figure 2E–F and Table). Although, we are aware that a dietary regimen should be started as early as possible in order to prevent CV diseases or to slow their progression, it is recognized that, in the clinical practice, patients are motivated to start a dietetic treatment only when a debilitating chronic disease manifests itself. Therefore, we decided to randomize HF-rats to ad libitum or CR diets 4 weeks post-MI, a time point where cardiac dysfunction is already established.
CR can be performed either through daily reduction of caloric intake (15–60% less than ad libitum caloric intake without altering the levels of vitamins, minerals and amino acids) or by IF through the alternation of a complete fasting day with a feed day. Recently, IF has been proposed also in humans to obtain a BW reduction, since it seems to be easier to be followed and sometimes less frustrating than “traditional” CR dietary regimens, where subjects are not authorized to have a freely eating day. In particular, IF protocols proposed for humans consists in: 1) restricting energy intake on 1–3 day/week and eating liberally on the non-restricted days; 2) alternate day fasting regimen which includes a fast day (75% energy restriction) alternating with a ad libitum diet day7, 41. Of note, IF has been shown to induce a BW and fat mass reduction that was similar to those obtained with other CR diets41. Importantly, our study is the first indicating that a 12-month-long CR regimen is able to improve cardiac remodeling, as indicated by the decreased LV diameters/volumes and fibrosis observed in HF-CR compared to HF-ND rats. Moreover, our results indicate that the beneficial effects of CR on cardiac function are also evident on cardiac inotropic reserve, as indicated by the results of the in vivo hemodynamics after challenge with β-agonist isoproterenol. In order to confirm our results at molecular level, we have measured cardiac β1-AR membrane density in all study groups. β-AR dysfunctional signaling is recognized to be relevant pathogenic mechanisms in HF and reduced β-AR responsiveness has been described in several models of HF, as well as, in patients with HF42–45. Moreover, therapeutic intervention able to restore HF-related cardiac β-AR abnormalities has been shown to be therapeutically effective44. In our study, HF control hearts show reduced β1-AR plasma membrane density and, importantly, long-term CR is able to completely restore β1-AR down-regulation at levels similar to those observed in Sham hearts. CR did not affect plasma membrane levels of β2-AR and β3-AR in our post-ischemic HF model.
The adrenal medulla (the central part of the adrenal gland) is the major source of cathecolamine cirluating levels. In fact, almost all circulating Epinephrine levels together with a significant amount of circulating Norepinephrine levels derive from the adrenal medulla43. In our study, one year CR reduced adrenal TH levels as well as adrenal weight and adrenal weight/tibia length ratio to the levels of the Sham animals (Supplemental Figure 3). These data are particularly interesting since the rate-limiting step in Catecholamines’ biosynthesis is the hydroxylation of L-tyrosine to L-DOPA mediated by TH46. Given these results, we may speculate that CR diet might be able to blunt, at list in part, HF-related sympathetic nervous system hyperactivity.
Furthermore, in our study, long-term CR diet is associated with a reduction in cardiac fibrosis and increased capillary density (Figure 5 and Supplemental Figure 5). These findings are consistent with the reduction in HW and in LV diastolic and systolic diameters and, overall, confirms the improvement in LV remodeling obtained with CR. Moreover, in accordance with previous data showing positive effects of IF on cardiac fibrosis and angiogenesis in the short period, our results confirm that this therapeutic modality is effective also in the long-term. At molecular level, long-term CR did not affect pro-fibrotic genes like TGF-β, MMP-2 and TIMP-2 while resulted in upregulated levels of an anti-fibrotic gene like TIMP-147 in our model of ischemic HF (Supplemental Figure 4). This protective molecular mechanism can explain, at least in part, the tremendous decrease in fibrosis in the BZ of HF-CR rats compared to HF-ND rats. However, we cannot exclude that a dietary treatment is also able to blunt pro-fibrosis mechanisms (like TGF-β pathway) in an early phase of the dietetic regimen.
Functional and structural data have been confirmed by CR-induced changes in HF biomarkers. Long-term dietary treatment was able to significantly reduce HF-related up-regulation of cardiac ANF and to increase cardiac levels of Endothelin-1. Importantly, even a modest reduction in endothelin-1 gene expression has been shown to decrease cardiac function48.
Another pivotal characteristic of chronic HF is represented by cardiac sympathetic denervation that has been described both in pre-clinical models and in patients with HF and it has been related to the high risk of sudden cardiac death due to ventricular arrhythmias, observed in HF patients49. During HF, a dramatic loss in cardiac nerve fibers and, in particular, in sympathetic fibers has been described50, 51. This is due, at least in part, to cholinergic transdifferentiation of cardiac SNS cardiac nerve terminals52. As expected, we observed a dramatic loss of both cholinergic and adrenergic fibers in HF-ND rats compared to Sham animals. More importantly, CR resulted in a significant increase in sympathetic cardiac nerve fibers as evident by comparing HF-CR to HF-ND hearts. This result is consistent with previous findings that showed neuroprotective effects of CR in models of neuronal damage or cognitive impairment53.
Recently, a CR dietary program has been tested in obese older patients with clinically stable HF and preserved EF in a randomized clinical trial22. The authors found that CR was able to increase peak V̇O2 and to decrease LV mass, circulating C-reactive protein and cholesterol levels22. This latter investigation is consistent with the results of the present study and open to the idea to test if CR could be effective also in patients with ischemic HF and whether this dietary intervention may have an additive effect when combined with aerobic exercise training.
From a clinical point of view, we can hypothesize that CR could be associated with other pharmacological medications used in HF such as β-blockers and ACE inhibitors. Long-term β-blocker treatment is associated with weight gain and new onset diabetes54, 55. Consequently, a restricted dietary program could, at least in part, counteract the metabolic side effects of β-blockade in patients with HF. However, the concomitant utilization of β-blocker and CR diet needs further investigation since Azar et al. have shown that obese individuals treated with β-blockers exhibit significant impairments in weight loss and waist circumference reduction in response to a hypocaloric dietary intervention56.
Overall the findings of the present study are of particular interest since dietary interventions represent a cost saving therapeutic modality, whose favorable properties seem to show effectiveness comparable to that obtained with pharmacological interventions currently used in the clinical practice.
Study limitations
Due to the long-term nature of our study, it is difficult to obtain information regarding the mechanisms that are involved in the beneficial effects of CR in HF. In fact, the functional, molecular and structural observations that we have found at the end of the study period (13-months post-MI) may be the result of molecular mechanisms acting at the initial phase of the dietetic regimen. Moreover, we recognize that the lack of a Sham group fed with a CR diet could represent a limitation of the present study, but our interest was mainly focused on the long-term effects of CR in a post-ischemic model of HF, where altered β-AR signaling, increased cardiac fibrosis and denervation represent relevant pathogenic mechanisms that are currently therapeutic targets to curb HF progression. At this regard, Ahmet and colleagues characterized the outcomes of different long-term diets (CR vs ad-libitum) on cardiovascular fitness during aging. They showed positive effects of a long-lasting CR on LV diastolic function and remodeling in 24-month-old un-operated animals18. Compared to ad-libitum rats, 24-month-old CR rats showed reduced levels of cardiac fibrosis, attenuated diastolic dysfunction, but unchanged LV systolic function.
Conclusions
In summary, the present study reports that long-term CR, started when HF was already established, reverses cardiac dysfunction, attenuates LV remodeling and improves cardiac inotropic reserve in an experimental model of post-ischemic HF. As a contributing mechanism, we found that CR diet significantly reduces cardiac fibrosis and improves sympathetic cardiac innervation and β-AR membrane density in HF. Importantly, the beneficial effects of CR are strongly effective after 12 months of this regimen, providing evidence that this therapeutic approach is applicable long-term, although started when cardiac dysfunction was already evident. The true clinical potential of CR for a place in the HF therapeutic armamentarium awaits clinical validation in patients.
Supplementary Material
WHAT IS NEW?
We investigated for the first time the effects of a long-term caloric restriction (CR) regimen in an animal model of ischemic heart failure (HF).
CR improved cardiac inotropic reserve, as indicated by the results of the in vivo hemodynamics, and restored cardiac β1-adrenergic receptor plasma membrane density in a chronic HF model.
CR significantly curbed the dramatic loss in cardiac sympathetic nerve fibers in the failing hearts.
CR reverted cardiac maladaptive remodeling in ischemic HF reducing cardiac fibrosis and increasing capillary density.
WHAT ARE THE CLINICAL IMPLICATIONS?
For chronic diseases such HF, there is an urgency to identify new therapies that are successful if used for a long period.
We found that a long-term and sustained CR regimen is an effective treatment in a model of ischemic HF.
The findings of the present study are of particular interest since dietary interventions represent a cost saving therapeutic modality, whose favorable properties seem to show effectiveness comparable to that obtained with pharmacological interventions currently used in clinical practice.
The clinical potential of CR for a place in the HF therapeutic armamentarium awaits clinical validation in patients.
Acknowledgments
We thank Dr. Gennaro Pagano for the technical management of the dietary protocol.
Sources of Funding:
W. J. Koch is the W.W. Smith Endowed Chair in Cardiovascular Medicine and supported by NIH grants R37 HL061690, P01 HL091799, P01 HL075443, P01 HL108806, R01 HL085503 and R01 HL071818.
G. Rengo is supported by Ministry of Health (Italy) grant number: GR-2011-02346878 and by San Paolo Bank of Naples and University of Naples Federico II - STAR program 2016.
Footnotes
Disclosures: None.
Bibliography
- 1.Buggey J, Mentz RJ, Galanos AN. End-of-life Heart Failure Care in the United States. Heart Fail Clin. 2015;11:615–23. doi: 10.1016/j.hfc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Rengo F, Leosco D, Iacovoni A, Rengo G, Golino L, Borgia F, De Lisa G, Beneduce F, Senni M. Epidemiology and risk factors for heart failure in the elderly. Ital Heart J. 2004;5(Suppl 10):9S–16S. [PubMed] [Google Scholar]
- 3.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO Group WUSoMC. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 4.Melo DS, Costa-Pereira LV, Santos CS, Mendes BF, Costa KB, Santos CF, Rocha-Vieira E, Magalhães FC, Esteves EA, Ferreira AJ, Guatimosim S, Dias-Peixoto MF. Severe Calorie Restriction Reduces Cardiometabolic Risk Factors and Protects Rat Hearts from Ischemia/Reperfusion Injury. Front Physiol. 2016;7:106. doi: 10.3389/fphys.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGillicuddy FC, Roche HM. Nutritional status, genetic susceptibility, and insulin resistance--important precedents to atherosclerosis. Mol Nutr Food Res. 2012;56:1173–84. doi: 10.1002/mnfr.201100785. [DOI] [PubMed] [Google Scholar]
- 6.Coyan GN, Reeder KM, Vacek JL. Diet and exercise interventions following coronary artery bypass graft surgery: a review and call to action. Phys Sportsmed. 2014;42:119–29. doi: 10.3810/psm.2014.05.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–11. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002;123:1121–31. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–21. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 12.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–8. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1978;27:1711–4. doi: 10.1016/0026-0495(78)90256-1. [DOI] [PubMed] [Google Scholar]
- 14.Overton JM, VanNess JM, Casto RM. Food restriction reduces sympathetic support of blood pressure in spontaneously hypertensive rats. J Nutr. 1997;127:655–60. doi: 10.1093/jn/127.4.655. [DOI] [PubMed] [Google Scholar]
- 15.VanNess JM, Casto RM, Overton JM. Antihypertensive effects of food-intake restriction in aortic coarctation hypertension. J Hypertens. 1997;15:1253–62. doi: 10.1097/00004872-199715110-00009. [DOI] [PubMed] [Google Scholar]
- 16.Fontana L. Calorie restriction and cardiometabolic health. Eur J Cardiovasc Prev Rehabil. 2008;15:3–9. doi: 10.1097/HJR.0b013e3282f17bd4. [DOI] [PubMed] [Google Scholar]
- 17.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–21. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 18.Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–71. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–27. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–7. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–12. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rengo G, Zincarelli C, Femminella GD, Liccardo D, Pagano G, de Lucia C, Altobelli GG, Cimini V, Ruggiero D, Perrone-Filardi P, Gao E, Ferrara N, Lymperopoulos A, Koch WJ, Leosco D. Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br J Pharmacol. 2012;166:2348–61. doi: 10.1111/j.1476-5381.2012.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bathgate-Siryk A, Dabul S, Pandya K, Walklett K, Rengo G, Cannavo A, De Lucia C, Liccardo D, Gao E, Leosco D, Koch WJ, Lymperopoulos A. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–12. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–23. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 26.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356–65. doi: 10.1016/j.jacc.2010.08.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolano M, Provitera V, Caporaso G, Stancanelli A, Leandri M, Biasiotta A, Cruccu G, Santoro L, Truini A. Cutaneous innervation of the human face as assessed by skin biopsy. J Anat. 2013;222:161–9. doi: 10.1111/joa.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–89. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, Stewart T, Robinson L, Roberts SB Group CAoL-tEoRIoECPS. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern Med. 2016;176:743–52. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murberg TA, Bru E, Svebak S, Tveterås R, Aarsland T. Depressed mood and subjective health symptoms as predictors of mortality in patients with congestive heart failure: a two-years follow-up study. Int J Psychiatry Med. 1999;29:311–26. doi: 10.2190/0C1C-A63U-V5XQ-1DAL. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh RK, Ball S, Prasad V, Gupta A. Depression in heart failure: Intricate relationship, pathophysiology and most updated evidence of interventions from recent clinical studies. Int J Cardiol. 2016;224:170–177. doi: 10.1016/j.ijcard.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 32.Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–32. ix. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–17. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 34.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–55. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinmura K, Tamaki K, Ito K, Yan X, Yamamoto T, Katsumata Y, Matsuhashi T, Sano M, Fukuda K, Suematsu M, Ishii I. Indispensable role of endothelial nitric oxide synthase in caloric restriction-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308:H894–903. doi: 10.1152/ajpheart.00333.2014. [DOI] [PubMed] [Google Scholar]
- 36.Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;280:H2094–102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- 37.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–24. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–25. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 39.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–91. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 40.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 41.Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593–601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 42.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 43.de Lucia C, Femminella GD, Gambino G, Pagano G, Allocca E, Rengo C, Silvestri C, Leosco D, Ferrara N, Rengo G. Adrenal adrenoceptors in heart failure. Front Physiol. 2014;5:246. doi: 10.3389/fphys.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najafi A, Sequeira V, Kuster DW, van der Velden J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest. 2016;46:362–74. doi: 10.1111/eci.12598. [DOI] [PubMed] [Google Scholar]
- 45.Rengo G, Lymperopoulos A, Koch WJ. Future g protein-coupled receptor targets for treatment of heart failure. Curr Treat Options Cardiovasc Med. 2009;11:328–38. doi: 10.1007/s11936-009-0033-5. [DOI] [PubMed] [Google Scholar]
- 46.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 47.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–71. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 48.Hathaway CK, Grant R, Hagaman JR, Hiller S, Li F, Xu L, Chang AS, Madden VJ, Bagnell CR, Rojas M, Kim HS, Wu B, Zhou B, Smithies O, Kakoki M. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc Natl Acad Sci U S A. 2015;112:5141–6. doi: 10.1073/pnas.1504557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.J Boogers M, E Veltman C, J Bax J. Cardiac autonomic nervous system in heart failure: imaging technique and clinical implications. Curr Cardiol Rev. 2011;7:35–42. doi: 10.2174/157340311795677725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, Yagi T, Arai T, Sano M, Fukuda K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Autonomic Neuroscience-Basic & Clinical. 2010;156:27–35. doi: 10.1016/j.autneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Rengo G, Pagano G, Vitale DF, Formisano R, Komici K, Petraglia L, Parisi V, Femminella GD, de Lucia C, Paolillo S, Cannavo A, Attena E, Pellegrino T, Dellegrottaglie S, Memmi A, Trimarco B, Cuocolo A, Filardi PP, Leosco D, Ferrara N. Impact of aging on cardiac sympathetic innervation measured by (123)I-mIBG imaging in patients with systolic heart failure. Eur J Nucl Med Mol Imaging. 2016;43:2392–2400. doi: 10.1007/s00259-016-3432-3. [DOI] [PubMed] [Google Scholar]
- 52.Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–21. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pani G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin Cell Dev Biol. 2015;40:106–14. doi: 10.1016/j.semcdb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Winchester DE, Pepine CJ. Usefulness of Beta blockade in contemporary management of patients with stable coronary heart disease. Am J Cardiol. 2014;114:1607–12. doi: 10.1016/j.amjcard.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 55.Lee P, Kengne AP, Greenfield JR, Day RO, Chalmers J, Ho KK. Metabolic sequelae of β-blocker therapy: weighing in on the obesity epidemic? Int J Obes (Lond) 2011;35:1395–403. doi: 10.1038/ijo.2010.284. [DOI] [PubMed] [Google Scholar]
- 56.Azar M, Nikpay M, Harper ME, McPherson R, Dent R. Adverse Effects of β-Blocker Therapy on Weight Loss in Response to a Controlled Dietary Regimen. Can J Cardiol. 2016;32:1246.e21–1246.e26. doi: 10.1016/j.cjca.2015.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.