Abstract
Distress intolerance (DI), a trait-like individual difference reflective of the inability to endure aversive affective states, is relevant to multiple forms of psychopathology, but its relations to theoretically-relevant neurobiological systems has received little attention. Altered cognitive control-related neurobiology has been theorized to underlie individual differences in DI, but little empirical work has been conducted. To test this hypothesis, baseline data from a large community sample with elevated high levels of emotional psychopathology and co-morbidity was utilized (N = 256). Participants completed a complex Go/No-go task while EEG was recorded, and P2, N2, and P3 amplitudes were measured. Based upon prior findings on the relations between these components and response inhibition, a core cognitive control function, we hypothesized that DI would predict reduced No-go N2 and P3 amplitude while controlling for current anxious/depressive symptom severity (i.e., negative affect). Peak amplitudes from the raw data and principal components analysis were used to quantify amplitude of ERP components. Partially consistent with predictions, high DI was independently associated with reduced No-go N2 peak amplitude in the raw ERP data, and was significantly related to a frontal positivity factor in the N2 time window across No-go and Go trials. Contrary to predictions, no relations between DI and the P3 were found. Overall, results support the theorized relevance of cognitive control-linked neurobiology to individual differences in tolerance of distress over and above distress severity itself, and suggest specific relations between DI and alterations in early controlled attention/conflict-monitoring but not response inhibition or response inhibition-related sequelae.
Keywords: distress intolerance, response inhibition, P2, N2, P3, cognitive control
Introduction
Distress intolerance (DI), defined as the capacity to withstand aversive affective states, is theorized to be a trait-like individual difference variable that contributes to multiple forms of psychopathology (Leyro, Zvolensky, & Bernstein, 2010). High DI has been prospectively associated with greater risk of relapse/treatment dropout in individuals with substance use disorders (Brown et al, 2009; Daughters et al., 2005; Strong et al., 2012) and greater symptom severity in individuals with diagnosed unipolar mood and anxiety disorders (Allan, Macatee, Norr, & Schmidt, 2014; Macatee, Capron, Guthrie, Schmidt, & Cougle, 2015; Macatee, Capron, Schmidt, & Cougle, 2013). Further, high DI has been cross-sectionally and prospectively linked to ADHD symptoms (Cummings et al., 2013; Seymour, Macatee, & Chronis-Tuscano, in press) as well as Borderline and Antisocial Personality Disorder (Bornovalova et al., 2008; Daughters, Sargeant, Bornovalova, Gratz, & Lejuez, 2008; Sargeant, Daughters, Curtin, Schuster, & Lejuez, 2011). DI appears to be an important person-level moderator of response to negative affect with relevance to externalizing and internalizing psychopathology; however, theoretical understanding of DI is limited and its underlying neural mechanisms remain unclear.
Extant research has largely studied DI using behavioral (i.e., latency to quit a distressing task; Lejuez et al., 2003) and self-report (i.e., perceived ability to tolerate distress; McHugh & Otto, 2012) indicators, whereas DI’s neurobiological referents generally remain unexplored, limiting understanding of the construct and cross-discipline translation of research findings. One candidate construct that may underlie DI’s relationship with internalizing and externalizing psychopathology is cognitive control, defined as the ability to bias and dynamically adjust ongoing processing in response to goals and task-related demands, and thus encompasses inhibition of inappropriate responses, working memory updating, and task-set shifting (Friedman & Miyake, 2017). Theoretically, the ability to tolerate negative affect requires inhibition of inappropriate behavioral response tendencies (e.g., smoking a cigarette to relieve anxiety during a quit attempt) as well as cognitive interference control (e.g., disengaging covert attention from anxious/cigarette-related intrusions to prevent elaborative processing), both of which are thought to be core cognitive control functions (i.e., response inhibition and working memory updating, respectively; Friedman & Miyake, 2017). Indeed, an extant neurobiological theory of DI highlights the importance of response inhibition impairment to the construct (Trafton & Gifford, 2011), and a number of authors have speculated that covariance among internalizing and externalizing disorders is attributable to common impairment in cognitive control-related processes (e.g., Beauchaine & Thayer, 2015; Carver, Johnson, & Timpano, 2017; Snyder, Hankin, Sandman, Head, & Davis, in press).
In addition to theoretical plausibility, there is some indirect empirical support for a link between DI and cognitive control. Although high DI has been associated with most disorders across the internalizing and externalizing spectrums, evidence is particularly robust for distress disorders (e.g., MDD and GAD; Banducci, Bujarski, Bonn-Miller, Patel, & Connolly, 2016; Cougle, Timpano, Fitch, & Hawkins, 2011; Ellis, Vanderlind, & Beevers, 2013; Macatee et al., 2013, 2015, 2016; Renna et al., in press), a latent cluster of internalizing disorders characterized by pervasive, dysregulated negative affect (Watson, 2005; Waszczuk, Kotov, Ruggero, Gamez, & Watson, 2017), and substance use disorders (Brown et al, 2009; Daughters et al., 2005; Hasan, Babson, Banducci, & Bonn-Miller, 2015; Strong et al., 2012), whereas data are less robust for fear disorders (e.g., Keough, Riccardi, Timpano, Mitchell, & Schmidt, 2010; Laposa, Collimore, Hawley, & Rector, 2015; Macatee, Albanese, Allan, Schmidt, & Cougle, 2016; Norr et al., 2013), a latent cluster of internalizing disorders defined by excessive fear and avoidance (Watson, 2005; Waszczuk et al., 2017). Correspondingly, distress and substance use but not fear disorders have been linked to greater cognitive control impairment across self-report and neurophysiological measures; further, worse cognitive control enhanced the relationship between threat sensitivity and internalizing disorder symptom severity (Nelson, Strickland, Krueger, Arbisi, & Patrick, 2016; Venables et al., 2017), analogous to DI’s association with multiple indices of internalizing disorder severity (e.g., functional impairment, treatment response) in clinical samples (Banducci, Connolly, Vujanovic, Alvarez, & Bonn-Miller, 2017; McHugh et al., 2014; Michel, Rowa, Young, & McCabe, 2016; Williams, Thompson, & Andrews, 2013) as well as its moderating effect on the relation between daily stress and distress/substance use disorder symptoms (Macatee et al., 2013, 2016; Volz, et al., 2014). These data suggest that cognitive control and DI may be related, but direct evidence of an association between these constructs is needed.
Only two studies known to the authors have assessed DI’s theorized relationship with behaviorally-indexed cognitive control functioning (Bagge, Littlefield, Rosellini, & Coffey, 2013; Ledgerwood, Alessi, Phoenix, & Petry, 2009). In a sample of recent suicide attempters, Bagge and colleagues (2013) found a significant association between worse cognitive control as assessed by the Immediate Memory Task (Dougherty, Marsh, & Mathias, 2002), but not the Go-Stop task (Dougherty, Mathias, & Marsh, 2003), and lower persistence on a behavioral measure of DI. However, in a combined sample of community participants and pathological gamblers with and without a history of substance use disorder, worse cognitive control as assessed by the Go-Stop task (Ledgerwood et al., 2009), but not the Immediate Memory Task, was significantly associated with increased likelihood of quitting on a behavioral measure of DI. Thus, impaired performance on cognitive control tasks appears to be linked to DI, though associations with specific tasks are inconsistent, which may be attributable to covert compensatory processes normalizing task performance (e.g., see Eysenck, Derakshan, Santos, & Calvo, 2007). Brain activity during cognitive control tasks may be more sensitive to individual differences in DI, but little empirical research has explored DI’s relationship with neural indices of cognitive control, precluding stronger conclusions about DI’s hypothesized relationship with cognitive control impairment.
There is some evidence from a recent fMRI study demonstrating an association between DI and activation in cognitive control-related brain regions (Daughters et al., 2016). In a sample of substance users, Daughters and colleagues (2016) found a positive association between greater persistence on a behavioral DI task and task-elicited activation in the right insula, anterior cingulate cortex (ACC), bilateral medial frontal gyrus (MFG), right inferior frontal gyrus (IFG), and right ventromedial prefrontal cortex (vmPFC), as well as greater functional connectivity between the right MFG and a vmPFC/subgenual ACC cluster. All of these regions have been implicated in cognitive control functioning (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Boes et al., 2008; Japee, Holiday, Satyshur, Mukai, & Ungerleider, 2015; Menon & Uddin, 2010; Niendam et al., 2012; Shackman et al., 2011), providing support for cognitive control impairment as a possible underlying mechanism of DI. However, DI was measured using a behavioral task that requires constant updating and manipulation of working memory, leaving open the possibility that task persistence was positively associated with cognitive control-linked neural activation partially due to the specific demands of the task rather than individual differences in DI.
Only one study known to the authors has used a behavioral DI measure free from overt cognitive control demands (i.e., cold pressor task; McHugh et al., 2011) and assessed its relationship with neural activity elicited by a separate cognitive control task. In a sample of healthy older adults, Zhou, Kemp, Despres, Pebayle, and Dufour (2015) used electroencephalography (EEG) methodology to measure event-related potentials (ERPs) during a go/no-go task, a commonly-used task to measure response inhibition, a core cognitive control function (Friedman & Miyake, 2017), and its neural referents (Simmonds, Pekar, & Mostofsky, 2008). The N2, a frontal negative deflection occurring between 200 and 300ms post-stimulus, and P3a, a frontal-central positive deflection occurring between 300 and 500ms post-stimulus, are ERPs sensitive to trial type (i.e., NoGo vs. Go) in response inhibition tasks and are thought to reflect two stages of cognitive control processing. Functionally, the N2 is thought to reflect conflict-monitoring or pre-motor response program updating, whereas the P3a is more closely linked to actual motor inhibition and its evaluation (Huster, Enriquez-Geppert, Lavallee, Falkenstein, & Herrmann, 2013). Typically, reduced No-go N2 and P3a amplitudes have been interpreted as reflective of decreased signaling of the need for response inhibition and worse implementation of response inhibition/inhibition-related sequelae, respectively (Huster et al., 2013; Polich, 2007); indeed, reduced No-go N2 and P3a amplitudes have been linked with worse go/no-go task performance (Falkenstein, Hoormann, & Hohnsbein, 1999; Fan et al., 2016; Nakata, Sakamoto, & Kakigi, 2012; Pliszka, Liotti, & Woldorff, 2000) as well as DI-related psychopathology (e.g., Kim, Kim, Yoo, & Kwon, 2007; Luijten, Littel, & Franken, 2011; Ruchsow et al., 2008a, 2008b; Singh & Basu, 2009). Zhou and colleagues (2015), however, found a significant, positive relationship between behaviorally-measured DI and the P3b but not the P3a on No-go trials, a parietal positivity in the 300–500ms time window thought to reflect target/memory processing rather than cognitive control (Polich, 2007), and no relations between N2 amplitude and DI were observed. Given the putative cognitive control functions of the N2 and P3a (Huster et al., 2013; Folstein & Van Petten, 2008), these findings are inconsistent with cognitive control as an underlying mechanism of DI. However, Zhou and colleagues (2015) used a go/no-go variant with task parameters that reduce cognitive control demands and associated ERPs (i.e., N2, P3a) (e.g., easy stimulus discrimination, 50% no-go stimuli, long response window; Donkers & van Boxtel, 2004; Polich & Comerchero, 2003; Wessel, in press), leaving open the possibility that neural activity elicited by go/no-go tasks with greater cognitive control demands may be more sensitive to individual differences in DI.
Overall, the extant literature is somewhat supportive of the neural substrates of cognitive control as an underlying neural mechanism of individual differences in DI. However, some limitations of the existing studies preclude firmer conclusions. First, only one report (Zhou et al., 2015) explicitly examined DI’s relationship with response inhibition-elicited neural activity, and this study utilized a sample of healthy older adults. Given DI’s conceptualization as an individual difference relevant to multiple forms of psychopathology (Leyro et al., 2010), it is important to explicitly test DI’s relationship with the neural substrates of response inhibition in a large, clinical sample. Further, Zhou and colleagues (2015) used a go/no-go task with minimal cognitive control demands, which may explain the null associations between DI and the No-go N2 and P3a; utilization of a go/no-go task requiring greater cognitive control may be more sensitive to individual differences in DI. Second, extant work on neural processes has exclusively used behavioral measures of DI. Although both self-report and behavioral DI measures have been linked to the same clinical phenomena in prior work (e.g., relapse, craving; Hasan, Babson, Banducci, & Bonn-Miller, 2015; McHugh & Otto, 2011; Strong et al., 2012; Volz et al., 2014), multiple studies have found that self-report and behavioral indices of DI are modestly or non-significantly associated with one another (Kiselica, Rojas, Bornovalova, & Dube, 2015; McHugh & Otto, 2011; McHugh et al., 2011), leaving open the possibility that perceived versus behaviorally-assessed DI are differentially associated with response inhibition-related neural activity, which may contribute to current debates on the nature and measurement of the DI construct (Ameral, Palm Reed, Cameron, & Armstrong, 2014; Glassman et al., 2016; Kiselica et al., 2015; McHugh & Otto, 2011). Relatedly, self-report assessments of DI are not confounded with simultaneous cognitive control requirements as are some behavioral measures of DI (e.g., Daughters et al., in 2016), allowing for measurement of DI and cognitive control-related neural activation in distinct tasks.
Finally, a significant limitation of existing studies concerns the question of DI’s specificity to response inhibition-related neutral activity. It is important to evaluate if DI, a construct defined as the perceived or actual ability to withstand negative affect rather than the frequency/intensity of negative affect itself (Leyro et al., 2010), is related to altered response inhibition-elicited neural activity independent of the variance accounted for by current distress. Given high DI’s relationship with current negative affect (e.g., depressive/anxiety symptoms; Allan et al., 2014; Kiselica et al., 2015; Macatee, Albanese, Allan, Schmidt, & Cougle, 2016) and the latter’s relationship with altered neural activation during response inhibition tasks (Kaiser et al., 2003; Righi, Mecacci, & Viggiano, 2009; Ruchsow et al., 2008; Sehlmeyer et al., 2010), testing the association between DI and inhibition-related neural activity while controlling for current depressive/anxiety symptoms would ensure that any observed relationship between neural activity and the inability to tolerate distress is not more parsimoniously accounted for by current distress intensity/frequency. Such a test would rigorously evaluate the relevance of cognitive control’s neural substrates specifically to individual differences in the ability to withstand distress rather than the degree of current distress severity.
The current investigation was conducted to address the aforementioned limitations by evaluating the N2 and P3 during a response inhibition task in a large, clinically-defined sample. Baseline data collected in the context of a clinical trial for anxiety/mood disorder risk factors was utilized to test study hypotheses. Among other tasks, participants completed a response inhibition task (i.e., complex go/no-go) while EEG data was recorded. In contrast to static go/no-go tasks, complex go/no-go tasks require working memory updating as well as response inhibition (Simmonds et al., 2008), placing multiple demands on cognitive control and thus expected to be maximally sensitive to the hypothesized relationship between cognitive control and DI. Consistent with prior research on the relations among No-go N2 amplitude, No-go P3a amplitude, go/no-go task performance, and DI-related psychopathology (Falkenstein et al., 1999; Fan et al., 2016; Kim et al., 2007; Luijten et al., 2011; Nakata et al., 2012; Pliszka et al., 2000; Ruchsow et al., 2008a, 2008b; Singh & Basu, 2009), we predicted that greater perceived DI would be associated with reduced No-go P3a amplitude and decreased (i.e., less negative) No-go N2 amplitude. Further, we predicted that these associations would be independent of current depressive/anxiety symptoms.
Methods
Participants
The sample comprised 280 people who completed a diagnostic interview as well as self-report measures and neurocognitive tasks prior to their participation in an IRB-approved clinical trial targeting risk factors for anxiety and mood disorders (clinical trial #: NCT01941862).
Eligibility criteria included being 18 years of age or older and elevating at least one of several risk factors for anxiety and depressive-related disorders (i.e., >= 9 on Anxiety Sensitivity Concerns – Cognitive Subscale; Albanese et al., in press; >= 9 on Perceived Burdensomeness subscale/>= 21 on Thwarted Belongingness subscale of Interpersonal Needs Questionnaire; Van Orden, Cukrowicz, Witte, & Joiner, 2012; >=1 on Depressive Symptom Inventory – Suicidality Subscale; Glischinski, Teismann, Prinz, Gebauer, & Hirschfeld, 2016). Exclusion criteria was as follows: history of neurological conditions or visual impairments, current Bipolar or other psychotic-spectrum disorders, and severe suicide risk requiring immediate hospitalization.
Several individuals were removed from analyses due to participant non-compliance (n = 4) or technical problems (n = 12). Individuals with performance at or below 50% (i.e., correct responses were at or below chance) on either Go or No-go trials were also removed (n = 8). This resulted in 256 people with EEG data for analysis, though only 254 of these participants completed at least some of the self-report measures and diagnostic interview (M age = 35.45, SD = 16.09; 57.1% female). The majority of the sample identified as White (60.2%), with 25.6% identifying as Black, 2.8% Asian, .4% Pacific Islander, .4% American Indian, and 10.6% other (e.g. bi-racial). Further, 28.3% of individuals identified as veterans (n = 72). Primary diagnoses for the sample can be found in Table 1. 90.2% of the sample were diagnosed with at least one disorder, and 63% were diagnosed with two or more disorders (M diagnoses = 2.20, SD = 1.54, range: 0 – 8). Of note, 56.3% of the sample were diagnosed with a primary anxiety or related disorder (i.e., obsessive-compulsive disorder, post-traumatic stress disorder) and 23.2% were diagnosed with a primary unipolar mood disorder (i.e., major depressive disorder, persistent depressive disorder).
Table 1.
Diagnostic Frequencies and Percentages of Sample
| Primary Diagnosis | Frequency | Percent |
|---|---|---|
| None | 25 | 9.8 |
| Panic Disorder without Agoraphobia | 11 | 4.3 |
| Specific Phobia | 3 | 1.2 |
| Social Anxiety Disorder | 40 | 15.7 |
| Social Anxiety Disorder, Performance Only | 1 | .4 |
| Obsessive-Compulsive Disorder | 3 | 1.2 |
| Hoarding | 2 | .8 |
| Posttraumatic Stress Disorder | 39 | 15.4 |
| Generalized Anxiety Disorder | 31 | 12.2 |
| Anxiety Disorder, NOS/Other Specified Anxiety Disorder | 10 | 3.9 |
| Major Depressive Disorder | 29 | 11.4 |
| Bipolar I Disorder | 8 | 3.1 |
| Depressive Disorder, NOS/Other Specified Depressive Disorder | 2 | .8 |
| Adjustment Disorder | 1 | .4 |
| Eating Disorder, NOS | 1 | .4 |
| Trichotillomania | 2 | .8 |
| Axis II Probable | 3 | 1.2 |
| Persistent Depressive Disorder | 28 | 11.0 |
| Alcohol Use Disorder | 2 | .8 |
| Cannabis Use Disorder | 1 | .4 |
| Hallucinogen/PCP Use Disorder | 1 | .4 |
| Opioid Use Disorder | 1 | .4 |
| Cocaine Use Disorder | 2 | .8 |
| Somatic Symptom Disorder | 2 | .8 |
| Illness Anxiety Disorder | 2 | .8 |
| Other Specified Trauma | 2 | .8 |
| Obsessive-Compulsive Disorder, NOS | 1 | .4 |
| Unable to assess | 1 | .4 |
| Total | 254 | 100.0 |
Self-report measures
Distress Intolerance Index (DII).
The DII (McHugh & Otto, 2012) is a 10-item self-report measure designed to assess individual differences in the perceived ability to tolerate distressing affective states (e.g., “I can’t handle feeling distressed or upset”). Items from three DI measures (i.e., Distress Tolerance Scale; Simons and Gaher, 2005; Anxiety Sensitivity Index; Reiss, Peterson, Gursky, & McNally, 1985; Frustration-Discomfort Scale; Harrington, 2005) that consistently demonstrate the strongest loadings on a latent DI factor comprise the DII (McHugh & Otto, 2012). Items are scored on a 5-point Likert scale ranging from 0 (very little) to 4 (very much) such that higher scores represent less perceived ability to tolerate distressing affective states. The DII has demonstrated good test-retest reliability (Cakir, 2016), strong internal consistency (Cakir, 2016; McHugh & Otto, 2011; Szuhany & Otto, 2015), and superior convergent validity with behavioral measures of DI relative to other tolerance measures (McHugh & Otto, 2011; McHugh et al., 2016; Seo & Kwon, 2016; Szuhany & Otto, 2015; but see Williams, Vik, & Wong, 2015). In the current study, the DII demonstrated excellent internal consistency (α =.94).
Beck Depression Inventory – II (BDI-II).
The BDI-II (Beck et al., 1996) is a 21-item self-report measure designed to assess symptoms of depression. For each item, participants were asked to select one of four statements the best represents how they have felt over the past two weeks. These statements are scored using a four-point Likert scale ranging from 0 to 3 in which higher scores reflect greater depression severity. The BDI-II has demonstrated strong psychometric properties in previous research, such as good internal consistency and test-retest reliability (Beck et al., 1996). In the current study, the BDI-II demonstrated excellent internal consistency (α = .93).
Beck Anxiety Inventory (BAI).
The BAI (Beck et al., 1988) is a 21-item self-report measure designed to assess anxiety severity. Participants are asked to rate the extent to which they have experienced a number of symptoms in the previous week using a four-point Likert scale ranging from 0 (not at all) to 3 (severely). Previous research has demonstrated that the BAI is psychometrically sound, with good internal consistency, test-retest reliability, and construct validity (Beck et al., 1988; Fydrich, Dowdall, & Chambless, 1992). In the current study, the BAI demonstrated excellent internal consistency (α = .93).
Clinician Administered Measure
Structured Clinical Interview for DSM-V, Research Version (SCID-5-RV).
All psychiatric diagnoses were determined using the SCID-5-RV (First, Williams, Karg, & Spitzer, 2015). The SCID-5-RV was administered by highly trained doctoral level therapists with extensive training in SCID-5-RV administration and scoring, including reviewing training tapes, observing live administrations, and conducting practice interviews with other trained therapists. In addition, all SCID-5-RV results were reviewed by a licensed clinical psychologist to ensure accurate diagnoses. In the current study, a subsample of subjects were used for reliability coding which yielded excellent interrater reliability (κ = .86) (Schmidt, Norr, Allan, Raines, & Capron, 2017).
Experimental Procedure
Prior to enrolling in the treatment trial, participants completed a baseline session during which they completed the structured clinical interview and a packet of self-report questionnaires. Following this, they were scheduled for a neurophysiological assessment session. All data used in the present study represent baseline, pre-treatment functioning.
Neurophysiological testing was conducted in a dimly lit, sound-attenuated room. Stimuli were presented using a Dell OptiPlex 780 computer running E-Prime version 2.0.8.90. Stimuli were presented on a 21” CRT color monitor at a viewing distance of 100 cm, subtending a visual angle of 3.5°. The recording session consisted of several different tasks, including the Go/No-go task used in the present study. Total recording time lasted between 2.5 and 3 hours.
Experimental Task
Go/No-go Complex Paradigm.
Go/No-go tasks are commonly used paradigms designed to assess individual differences in response inhibition by requiring participants to respond to frequent signals (i.e., Go trials) and infrequently withhold a response when presented with pre-determined and distinct signals (i.e., No-go trials; Simmonds et al., 2008). Though many versions of this task exist, we utilized a complex Go/No-go task in which two different letters are presented serially in an alternating pattern and participants are required to press the corresponding button, either left or right, for each letter that is different than the previous letter (i.e., Go trials; Garavan, Ross, Kaufman, & Stein, 2003; Garavan, Ross, Murphy, Roche, & Stein, 2002; Hester et al., 2004; Kelly et al., 2004). No-go trials, in which participants are required to withhold a response, are signaled by a two-letter repeat (e.g., the fifth stimulus in the sequence X-Y-X-Y-Y-X). This variant of the Go/No-go task assesses response inhibition in the context of increased working memory demand, and has been found to activate right lateralized prefrontal-parietal circuits in addition to the regions recruited in Go/No-go tasks with static No-go stimuli (i.e., pre-SMA, left fusiform gyrus) (Aviyente, Tootell, & Bernat, 2017; Simmonds et al., 2008).
More specifically, our task included seven blocks comprised of 18 Go trials (75%) and 6 No-go trials (25%) for a total of 126 Go trials and 42 No-go trials. A distinct set of letters were used for each block. Prior to the beginning of each block, instructions regarding what button to press for each letter and performance on the previous block were presented. Each Go and No-go signal (i.e., the letters) were presented for 296 ms immediately followed by a blue fixation point during a response window which lasted 1150 ms. Feedback was then presented for an additional 1000 ms. Inter-trial intervals were marked by a blue fixation point presented on the screen for 900 ms.
Stimulus Delivery and EEG Measurement.
ERP data were collected using a Dell OptiPlex 780 computer and Neuroscan Acquire software. Two 64-channel Neuroscan SynAmps RT amplifiers and a BrainVision actiCap 96-channel cap were used to measure EEG responses (1000 Hz sampling rate, with an online analog bandpass filter of 0.05 – 100 Hz). The midline electrode AFz was used as the ground and FCz was used as an online reference electrode.
Offline, the data were re-referenced to the averaged mastoids (electrodes TP9 and TP10). Horizontal electrooculogram (EOG) activity was recorded from electrodes placed lateral to the outer canthus of each eye, while vertical EOG activity was recorded from electrodes placed above and below the left eye. Electrodes were filled using high-chloride (10%) Abrasive Electrolyte-Gel (EasyCap). All impedance values were below 10 kohms throughout the recording session.
Data Preprocessing
Data were first downsampled to 250 Hz, then high-pass (0.1 Hz; ripple = .05 dB, attenuation = 80 dB) and low-pass (40 Hz; ripple = .01 dB, attenuation = 40 dB) FIR filters were applied. Given that FCz was the online reference, it was regenerated offline using the average reference assumption so that it could be used in analyses. Next, the Fully Automated Statistical Thresholding for EEG artifact Rejection algorithm (FASTER; Nolan, Whelan, & Reilly, 2010), an EEGLAB plugin, was used for artifact detection and rejection. The FASTER algorithm provides researchers with enhanced efficiency of data processing and greater standardization of artifact rejection parameters compared to traditional visual inspection methods (Hatz et al., 2015; Nolan, Whelan, & Reilly, 2010). Moreover, the FASTER algorithm has demonstrated equal or superior reliability when compared to traditional visual inspection methods of artifact rejection (Hatz, et al., 2015; Nolan et al., 2010). FASTER uses a z-score threshold of ± 3 to detect artifactual data within individual channels, epochs, independent components, and within-epoch channels (FASTER procedure is summarized in more detail in the Supplemental material) (M # rejected trials = 5.27, SD = 2.16, range: 1–12; M # interpolated channels = 3.51, SD = 1.50, range: 0–9).
Measurement of ERP Amplitude
For the present study, only electrophysiological data from correct trials (i.e., correct Go and correct No-go trials) were used. For each subject, correct No-go and Go trials were averaged to create No-go and Go grand-average waveforms. Consistent with prior studies, the N2 and P3 were examined at Fz and Cz based upon prior literature reporting a maximal response inhibition effect on the N2 and P3 at these channels, respectively (Folstein & Van Petten, 2008; Groom & Cragg, 2015; Huster et al., 2013; Watson & Garvey, 2013). Sample grand averaged No-go and Go waveforms as well as difference waves are displayed in Figure 1. N1, P2, N2, and P3 peaks are visible in the raw waveforms, though only the P3 component is present in the difference waves. Unexpectedly, the N2 peak was positive and there was no apparent effect of trial type on the N2, though similar patterns have been observed in some prior literature using comparable complex Go/No-go tasks (Littel et al., 2012; Maij, van de Wetering, & Franken, in press; Rietdijk, Franken, & Thurik, 2014). Further, inspection of subject-level grand-averaged waveforms revealed greater variability and generally smaller amplitudes for the N2 relative to P2 and P3, which may have obscured the inhibition effect on the N2 in the sample grand-averaged waveforms displayed in Figure 1.
Figure 1.
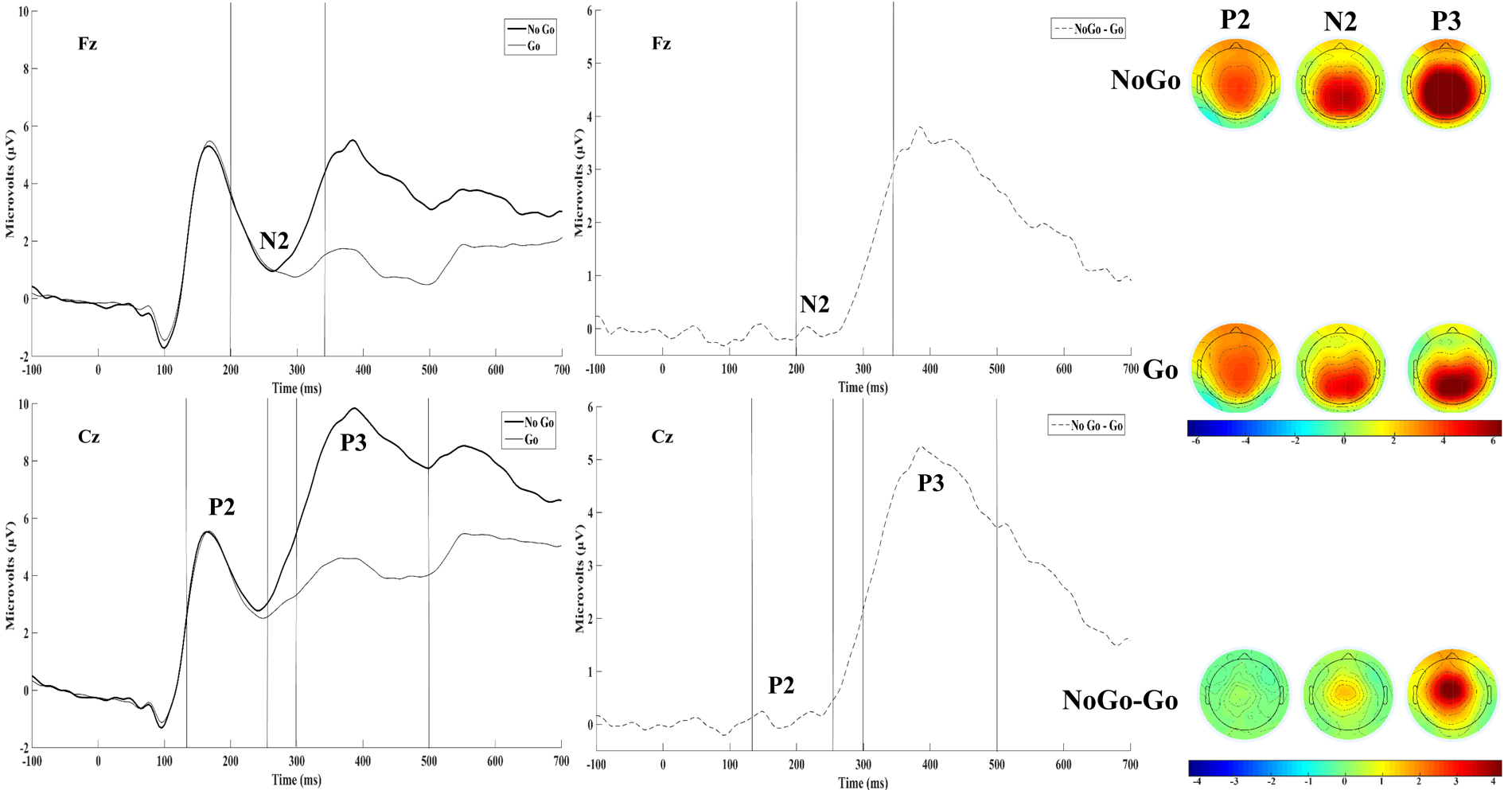
Grand-averaged (N=256) No-go and Go ERPs are displayed adjacent to their corresponding difference waves at Fz and Cz. Topographical maps of the mean amplitude in the P2 (128–260ms), N2 (200–344ms), and P3 (296–500ms) time windows are displayed to the right. Time windows are demarcated with vertical lines to aid visualization.
To measure N2 and P3 amplitude, we intended to compute mean amplitude over a pre-defined time window based upon prior literature, but inspection of the data suggested this approach was inadequate for the following reasons: 1) The present study sample was large and diagnostically heterogeneous, which was ideal for testing relations between DI, a transdiagnostic variable, and neural activity, but made a priori time window selection difficult given that prior studies utilized simple rather than complex Go/No-go tasks (e.g., Kamarajan et al., 2005) and/or non-comparable samples (i.e., healthy or diagnostically homogenous; Kaiser et al., 2003; Rietdijk, Franken, & Thurik, 2014); 2) Time window selection for the N2 was particularly difficult given that it was surrounded by two larger, positive-going deflections (i.e., P2 and P3), enhancing the likelihood that a predefined N2 time window would partially capture adjacent components; 3) There was considerable N2 and P3 latency variability across subjects, which also enhanced the likelihood that predefined time windows would inadequately capture the intended components. To address these issues, computation of signed area amplitudes using difference waves was considered given that this approach would have allowed specification of wide time windows to accommodate latency variability without capturing adjacent components (see Luck, 2014 for more detail); however, the N2 area was not negative across all subjects and thus would not be distinct from adjacent, positive components for some subjects. Thus, the local peak (as defined by Luck, 2014) was chosen to measure component amplitude from the raw waveforms to accommodate observed latency variability and temporospatial principal components analysis (PCA) (Dien & Frishkoff, 2005) was used to extract latent ERP components. Temporospatial PCA allows for measurement of component amplitude while minimizing the influence of overlapping components, mitigating the disproportionate influence of larger components (e.g., P3) on the observed waveforms.
Local Peak Amplitudes
N2 and P3 local peak amplitudes were measured using time windows (i.e., N2: 200 – 344 ms; P3: 296 – 500 ms) derived from visual inspection of the grand-averaged (i.e., collapsed across all subjects; N = 256) No-Go and Go waveforms at channels selected based on prior studies (N2 at Fz; P3 at Cz) (Folstein & Van Petten, 2008; Groom & Cragg, 2015; Huster et al., 2013; Watson & Garvey, 2013). Topographical maps of N2 and P3 components for the No-go, Go, and No-go/Go difference were generated by plotting mean amplitudes across the component’s time window at each channel (see Figure 1). In contrast with prior studies using complex go/no-go tasks (Harper, Malone, Bachman, & Bernat, 2016; Roche, Garavan, Foxe, & O’Mara, 2005) but consistent with some other studies using a complex go/no-go paradigm (Littel et al., 2012; Maij, van de Wetering, & Franken, 2017; Rietdijk, Franken, & Thurik, 2014), a frontal negativity distribution typical of the N2 was not observed, which may be attributable to large adjacent positivities (i.e., P2,P3) partially captured by the 200–344ms window or the actual absence of a typical latent N2 component in the complex go/no-go task used in the present study (see PCA section below for further detail). In line with prior literature (Bokura et al., 2001; Fallgatter et al., 2004), the No-go P3 was maximal at central midline channels, the Go P3 was maximal at parietal midline channels, and the trial type effect on the P3 was maximal at frontal-central/central midline sites.
To visualize differences between subjects with high vs. low DI, No-go and Go waveforms averaged across subjects scoring above (n = 120) and below (n = 122) the median DII score (20) are presented in Figure 2. To test hypothesized relations between DI and the N2, the frontal midline site (i.e., Fz) was selected based on prior studies (Folstein & Van Petten, 2008; Groom & Cragg, 2015; Watson & Garvey, 2013). To test hypothesized relations between DI and the P3a component, the central midline site (i.e., Cz) was selected based on prior studies (Groom & Cragg, 2015; Huster et al., 2013). Finally, given the apparent high vs. low DI difference in P2 amplitudes across No-go and Go trial types (see Figure 2), exploratory analyses were conducted to test the relations between DI and the P2. P2 peak amplitudes and latencies were extracted using a time window (i.e., 128 – 260 msec) derived from visual inspection of the sample grand-averaged No-go and Go waveforms at Cz. This channel was selected for statistical analyses based upon prior Go/No-go studies that found the P2 to be maximal at central midline sites (Kirmizi-Alsan et al., 2006; Steele et al., 2014). Topographical maps of the P2 for the No-go, Go, and No-go/Go difference were generated by plotting mean amplitudes across the 128–260ms window at each channel (see Figure 1).
Figure 2.
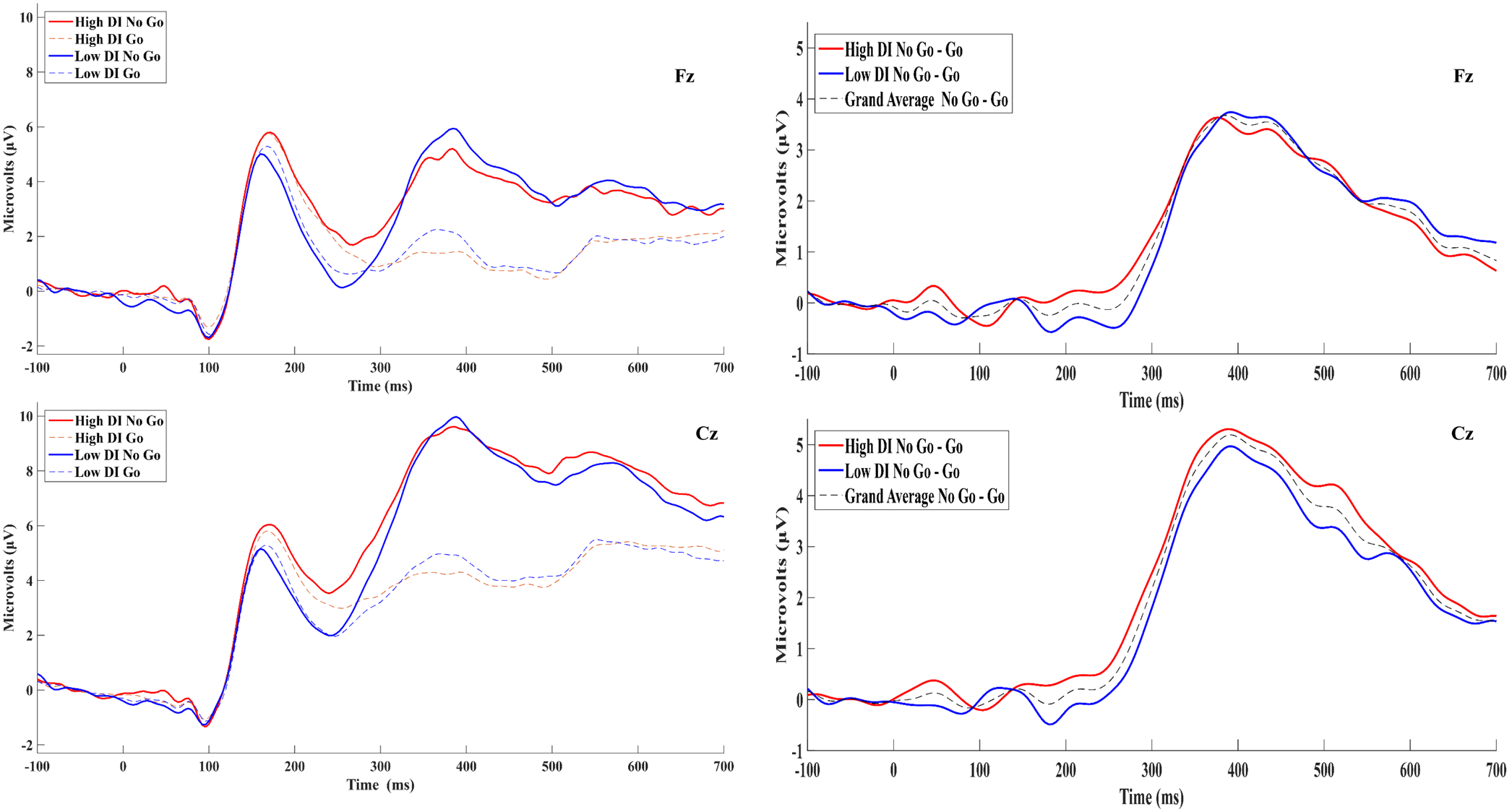
Grand-averaged No-go and Go ERPs for participants scoring above (high DI; n=120) and below (low DI; n=122) the median DII score are displayed adjacent to their Corresponding differences waves at Fz and Cz. The difference waveform for the entire sample (N=256) is also presented.
Latent Component Amplitudes using Temporospatial PCA
To isolate the latent P2, N2, and P3 components from the scalp-recorded waveforms, a temporospatial PCA was employed, a technique which extracts linear combinations of data points that meet particular criteria that tend to distinguish between consistent patterns of neurophysiological activity (Dien & Frishkoff, 2005). The temporospatial PCA was conducted using the ERP PCA toolkit, version 2.63 (Dien, 2010a). In line with guidelines for conducting PCA on ERP data (Dien 2010b; Dien, Khoe, & Mangun, 2007; Dien, Spencer, & Donchin, 2003), a temporal PCA was performed first to isolate consistent and dissociable temporal patterns of electrocortical activity occurring from −100 to 1150ms (i.e., start of pre-stimulus baseline period to the end of the response window). The temporal PCA uses the time points from each participant’s average ERP as the variables, and participants, trial types (i.e., No-go vs. go), and recording sites are utilized as observations. Promax rotation was used, and 12 temporal factors were extracted after examination of the Scree plot (Cattell, 1966). For each temporal factor, factor scores were generated for every observation (i.e., observed voltages across each combination of participant, trial type, and recording site), reflecting the variance in the original data captured by that temporal factor. A subsequent spatial PCA was conducted for each of the 12 temporal factors to distinguish among ERP components with comparable time courses but distinct spatial distributions. For each spatial PCA, recording sites were used as the variables, and participants, trial types, and temporal factor scores were utilized as observations. Infomax rotation was used, and five spatial factors were extracted for all 12 temporal factors after examination of the averaged Scree plot, resulting in 60 unique factor combinations. The covariance matrix and Kaiser normalization were used for each PCA.
Of the 60 unique factor combinations extracted by the temporospatial PCA, 17 accounted for at least 1% of the total variance in the data. These 17 factors were converted into microvolt-scaled waveforms representing the portion of the original data accounted for by that factor by multiplying the factor loadings, scores, and their standard deviations (Dien et al., 2003). In line with established guidelines (Dien, Beal, & Berg, 2005), the time course and spatial distribution of each of the 17 factor waveforms was assessed for resemblance to the P2, N2, and P3 components expected in go/no-go and related cognitive control tasks. Six factors fit this criteria and thus were retained for analyses (see Figure 3).1 Of the six retained factors, a central P150 factor emerged, peaking at 164 ms at Cz (TF4SF1; 3.12% variance). Interestingly, the only factor to occur in the N2 time range appeared to be a frontal P2 factor (TF6SF1), accounting for 1.73% of the variance and peaking at 228ms at Fz. As expected, P3a (TF1SF1; 14.60% variance) and P3b (TF1SF3; 4.03% variance) factors emerged, peaking at 336ms at Cz and Pz, respectively. Unexpectedly, a frontal-polar positivity (TF1SF2; 5.17% variance) and negativity (TF1SF4; 2.76% variance) were also observed in the P3 time range, peaking at 336ms at Fpz. Amplitudes for each of these factors were generated for each participant using the peak values on Go and No-go trials.
Figure 3.
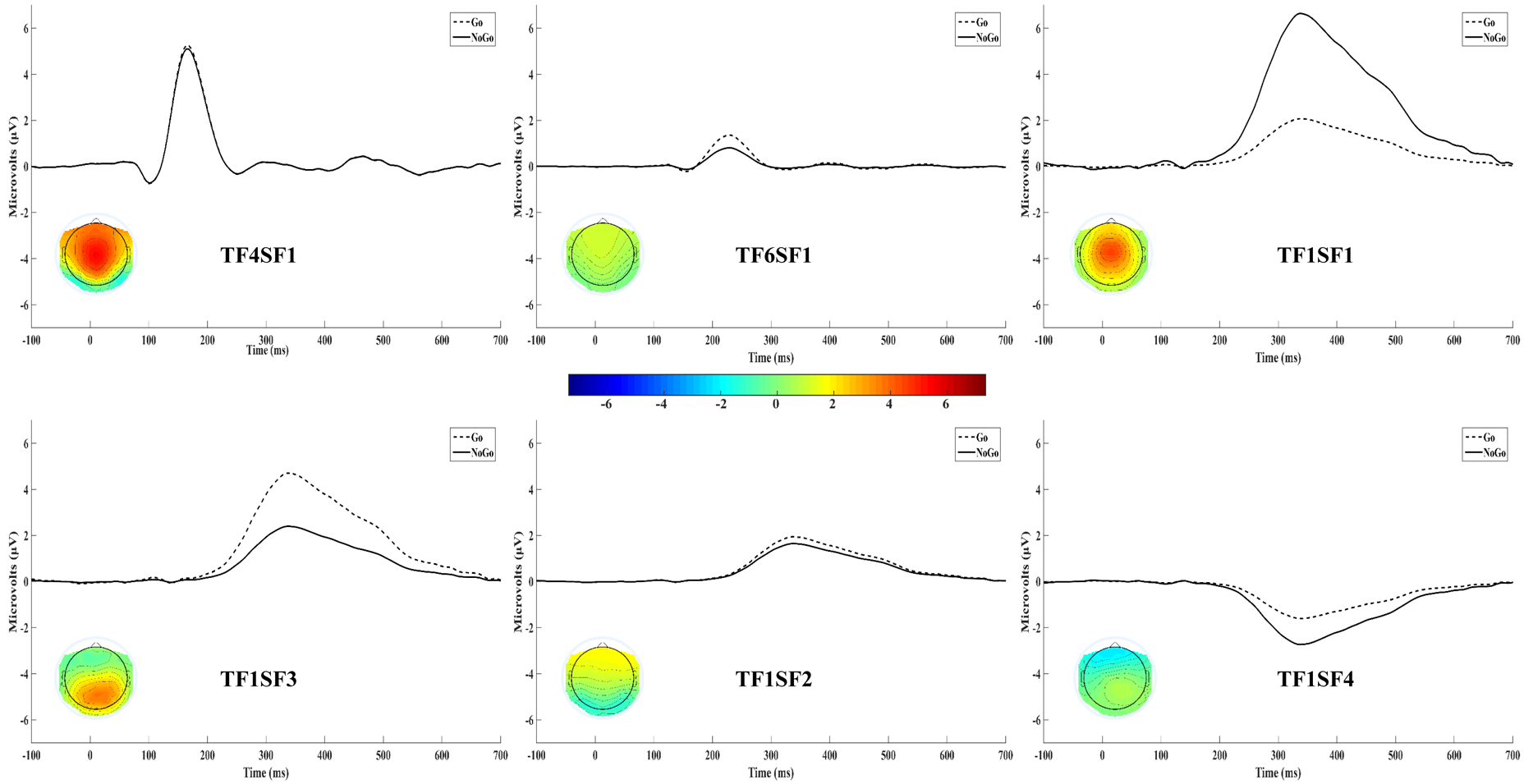
Temporospatial PCA extracted six latent ERP components that occurred in the typical time windows of the P2, N3, and P3. These factor waveforms in the No-go and Go conditions are displayed above. A topographical map of each component collapsed across trial types is also presented in the corresponding component waveform.
To evaluate the relative influence of each factor on the local peak amplitudes scored from the observed waveforms, bivariate correlations between local peak amplitudes and each of the six factors were conducted (see supplemental material for complete correlation table). The central P150 factor for No-go (r = .87, p < .001) and Go (r = .89, p < .001) trials was highly correlated with corresponding local P2 peak amplitude, whereas correlations with the later frontal P2 (rs < .36), P3a (rs < .52), P3b (rs = −.03, .15), and frontal-polar (rs = −.24 to .34) factors were substantially smaller. The frontal P2 factor for No-go (r = .47, p < .001) and Go (r = .54, p < .001) trials was moderately correlated with corresponding local N2 peak amplitude, but correlations with the subsequent P3a (rs < .65), P3b (rs = −.42), and frontal-polar positivity (rs < .56) factors were of comparable magnitude, whereas relations with the central P150 factor (rs < .26) and subsequent frontal-polar negativity (rs < .31) were more modest; these associations suggest that the peak of the apparent negativity in the N2 time window in the raw waveforms is largely the result of concurrent frontal and subsequent parietal to frontal positive components. Finally, the P3a factor for No-go (r = .90, p < .001) and Go (r = .84, p < .001) trials was highly correlated with corresponding local P3 peak amplitude, whereas correlations with the central P150 (rs < .38), frontal P2 (rs < .14), P3b (rs = −.17, .07), and frontal-polar (rs = −.44 to .42) factors were substantially smaller.
Overall, these data support the use of PCA-derived factor amplitudes to test hypotheses, particularly for the N2 given that the local N2 peak amplitude was comparably correlated with multiple latent factors (i.e., frontal P2, P3a, P3b, frontal-polar positivity). Given that the frontal P2 factor (i.e., TF6SF1) most resembles the typical N2 component in terms of spatial distribution, time course, and sensitivity to trial type (see N2 sub-heading of Statistical Analyses section below), it was used along with the local N2 peak to test the a priori N2 hypothesis; further, the frontal-polar factors (i.e., TF1SF2 and TF1SF4) were also assessed given their frontal distribution and comparable sensitivity to trial type as the frontal P2 factor. The a priori P3 hypothesis was tested using the P3a factor (i.e., TF1SF1) as well as the local P3 peak. Finally, the exploratory P2 analyses were conducted using the central P150 factor (i.e., TF4SF1) as well as the local P2 peak.
Data Analytic Strategy
Mixed repeated measures ANOVA analyses were conducted to test hypotheses. Condition was entered as a within-subject factor to reflect trial type (i.e., No-go vs. Go). For all a priori and exploratory ANOVAs, peak amplitudes and latent factor scores were entered as dependent variables. First, an ANOVA with only the Condition within-subject factor was run to evaluate the main effect of trial type. A second ANOVA was then run in which the DII was entered as a continuous, between-subjects predictor to test the hypothesized association between DI and inhibition-related neural activity. Finally, a third ANOVA was conducted in which negative affect was also entered as a continuous, between-subjects predictor to test DI’s hypothesized specificity to inhibition-related neural activity. Negative affect was operationalized by z-scoring the BDI and BAI, and then computing the average of the two z-scores; this approach was taken rather than entering both measures simultaneously in the third ANOVA given concerns that the BDI/BAI may not reflect the same construct when variance from the other is removed due to their moderately high correlation (r=.57,p<.001) (e.g., see Miller & Chapman, 2001). Significant interactions and between-subjects main effects were probed using follow-up regression analyses. Because one participant did not complete the BDI and two participants did not turn in any self-report measures, 254 participants were available for the analyses in which only the DII was entered as a between-subjects predictor and 253 participants were available for analyses including negative affect as a covariate.
Results
Descriptives and Relations between Behavioral Performance and Self-Report/ERP measures
Descriptives for all study variables are presented in Table 2. Due to the high skewness of the Go and No-go error percentage variables, spearman correlations were used to assess relations among these task performance indices and self-report/ERP measures. Given that mean RT and RT variability on correct Go trials approached normality, pearson correlations were used for these variables (see Tables S1 and S2 in Supplemental material for all bivariate correlations among ERP and task performance variables).
Table 2.
Descriptives
| Measure | Mean | SD |
|---|---|---|
| Self-Report | ||
| Distress Intolerance Index | 19.75 | 10.66 |
| Beck Depression Inventory-II | 22.51 | 12.13 |
| Beck Anxiety Inventory | 18.51 | 13.10 |
| Behavioral | ||
| No-Go Error Percentage | 0.15 | 0.10 |
| Go Error Percentage | 0.04 | 0.05 |
| Correct Reaction Time (ms) | 521.75 | 97.09 |
| RT Variability | 0.28 | 0.06 |
| ERPs | ||
| P3 (Cz) | ||
| No-go | 12.42 | 6.89 |
| Go | 6.51 | 4.78 |
| P3a factor (TF1SF1) | ||
| No-go | 6.65 | 6.72 |
| Go | 2.09 | 4.46 |
| N2 (Fz) | ||
| No-go | −1.48 | 3.91 |
| Go | −0.45 | 3.76 |
| Frontal P2 factor (TF6SF1) | ||
| No-go | 0.78 | 3.37 |
| Go | 1.35 | 3.04 |
| P2 (Cz) | ||
| No-go | 7.71 | 4.53 |
| Go | 7.19 | 3.98 |
| Central P150 factor (TF4SF1) | ||
| No-go | 5.10 | 4.15 |
| Go | 5.27 | 3.88 |
Note. Amplitudes are in microvolts. Latencies are in milliseconds. Descriptives reflect the subsample with all self-report measures available (n=253).
The DII was non-significantly related to No-go errors, r = .06, p = .32, as well as all other task performance indices, rs = −.07 to .08, ps > .23. Relations between the local P3 peak and P3a factor with behavioral performance revealed that larger amplitudes across trial types were generally associated with improved task performance across RT and error rate indices. A more negative N2 peak was generally related to more No-go errors, though associations with task performance variables were generally modest and non-significant. In contrast, the frontal P2 factor was unrelated to error rates, though more positive frontal P2 factor amplitude was significantly correlated with slower mean RT. Greater local P2 peak amplitude was generally related to decreased error rates, though only one correlation reached significance, with most associations with task performance variables weak and non-significant; a comparable pattern was observed for the central P150 factor.
N2
RM-ANOVA results for the N2 peak amplitude and frontal P2 factor analyses are presented in Table 3. As expected, the main effect of Condition on peak amplitude was significant, F(1,255) = 41.86, p < .001, pη2 = .141, 90% CI [.081, .206]2, with local negative peaks in the N2 time window significantly more negative on No-go, M = −1.45, SD = 3.93, relative to Go, M = −0.44, p = 3.78, trials. Contrary to hypotheses, the Condition*DII interaction was non-significant, F(1,252) = 3.57, p = .06, pη2 = .014, 90% CI [.000, .047] though the between-subjects main effect of the DII was significant, F(1,252) = 4.62, p = .033, pη2 = .018, 90% CI [.001, .054] which revealed a positive relationship between the DII and No-go, β = .16, t(252) = 2.62, p = .009, and Go, β = .09, t(252) = 1.43, p = .15, amplitudes. When negative affect was added to the model, the DII main effect remained significant, F(1,250) = 4.94, p = .027, pη2 = .019, 90% CI [.001, .056]. Consistent with predictions, the Condition*DII interaction was significant, F(1,250) = 8.65, p = .004, pη2 = .033, 90% CI [.006, .077], after negative affect was included in the model; interestingly, the Condition*negative affect interaction was also significant, F(1,250) = 5.23, p = .023, pη2 = .021, 90% CI [.002, .058]. Subsequent regressions revealed the interaction effects were largely driven by relations with the No-go N2 peak in opposite directions, such that greater DI was associated with more positive amplitude (No-go: β = .24, t(250) = 3.02, p = .003; Go: β = .09, t(250) = 1.16, p = .25) and greater negative affect was associated with more negative amplitude (No-go: β = −.13, t(250) = −1.60, p = .11; Go: β = −.01, t(252) = −0.13, p = .90). Thus, covariation among DI and negative affect in conjunction with the opposing effects of these variables on No-go peak N2 amplitude reduced the size of the Condition*DII interaction effect when negative affect was left out of the model. In contrast, the main effect of DII on N2 peak amplitudes was consistent regardless of inclusion of negative affect in the model.
Table 3.
Relations between DI, NA, and the N2 peak and Frontal P2 factor (TF6SF1)
| N2 Peak | Frontal P2 factor (TF6SF1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | df | MS | F | p | pη2 | SS | df | MS | F | p | pη2 | |
| Condition | Condition | |||||||||||
| Condition | 86.74 | 1 | 86.74 | 28.22 | <.001 | 0.101 | 15.02 | 1 | 15.02 | 8.95 | 0.003 | 0.035 |
| Condition*DII | 26.6 | 1 | 26.6 | 8.65 | 0.004 | 0.033 | 2.23 | 1 | 2.23 | 1.33 | 0.25 | 0.005 |
| Condition*NA | 16.09 | 1 | 16.09 | 5.23 | 0.023 | 0.021 | 0.38 | 1 | 0.38 | 0.23 | 0.636 | 0.001 |
| Error(Condition) | 768.5 | 250 | 3.07 | 419.38 | 250 | 1.68 | ||||||
| Between-subjects effects | Between-subjects effects | |||||||||||
| DII | 128.18 | 1 | 128.18 | 4.94 | 0.027 | 0.019 | 174.2 | 1 | 174.2 | 9.59 | 0.002 | 0.037 |
| NA | 22.12 | 1 | 22.12 | 0.85 | 0.357 | 0.003 | 3.27 | 1 | 3.27 | 0.18 | 0.672 | 0.001 |
| Error | 6492.38 | 250 | 25.97 | 4539.88 | 250 | 18.16 | ||||||
Note. SS = Type III Sum of Squares. MS = Mean Square. DII = Distress Intolerance Index; NA = Negative affect. Bolded values are significant at p < .05.
Consistent with the peak amplitude results, the main effect of Condition on the frontal P2 factor was significant, F(1,255) = 23.44, p < .001, pη2 = .084, 90% CI [.037, .142], with smaller amplitudes on No-go, M = 0.81, SD = 3.38, relative to Go, M = 1.36, SD = 3.03, trials. Contrary to hypotheses, the Condition*DII interaction was non-significant, F(1,252) = 1.18, p = .28, pη2 = .005, 90% CI [.000, .029] though, as with the local peak analyses, the between-subjects main effect of the DII was significant, F(1,252) = 13.06, p < .001, pη2 = .049, 90% CI [.015, .099], which revealed a positive association between the DII and No-go, β = .22, t(252) = 3.60, p < .001, and Go, β = .20, t(252) = 3.31, p = .001 amplitude (see Figure 4). When negative affect was added to the model, the DII main effect remained significant, F(1,250) = 9.59, p = .002, pη2 = .037, 90% CI [.008, .082], and the Condition*DII interaction remained non-significant.
Figure 4.
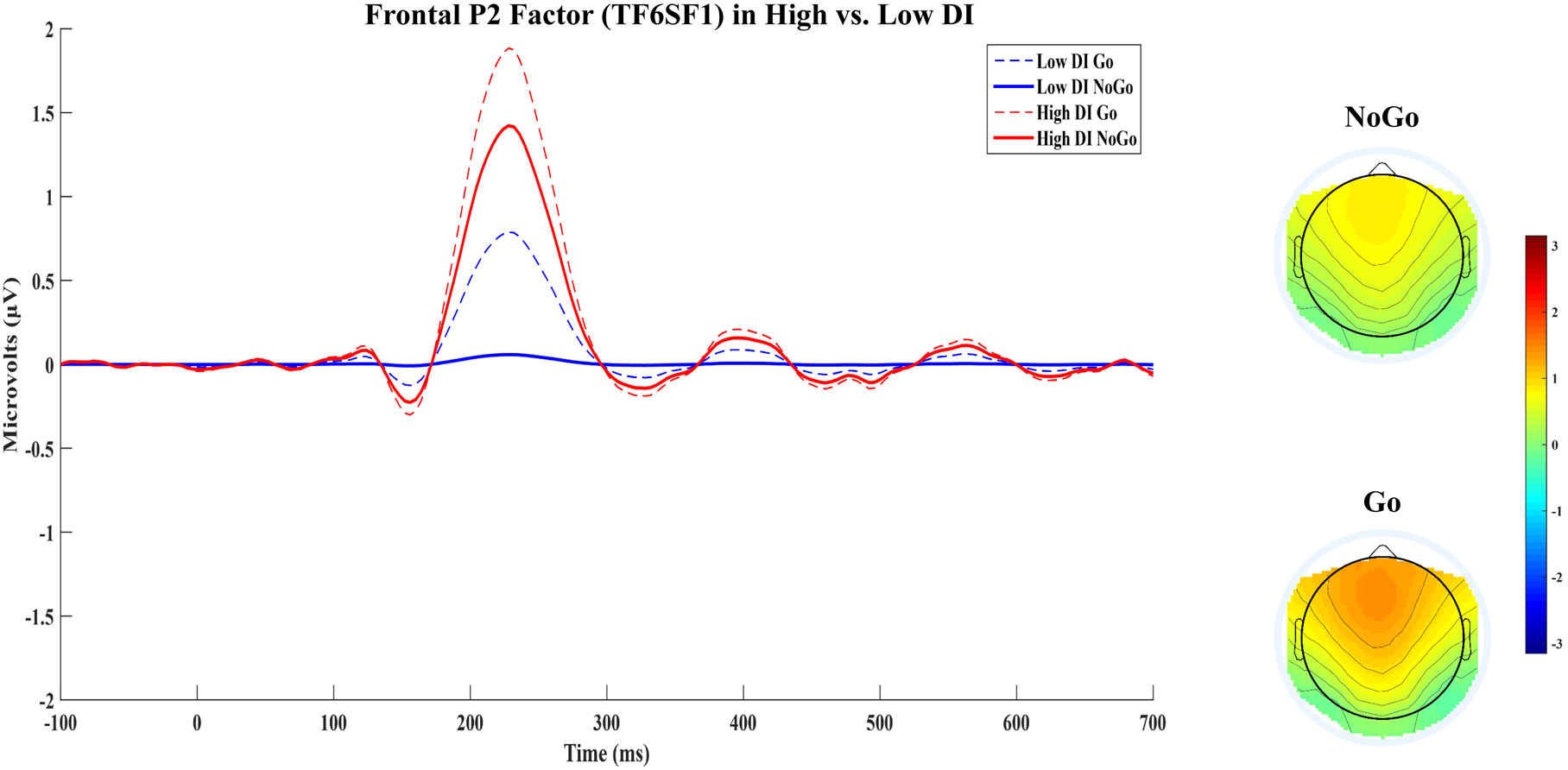
Frontal P2 factor (TF6SF1) waveforms in No-go and Go conditions are displayed for participants scoring above (high DI; n=120) and below (low DI; n=122) the median DII score. Topographical maps of the frontal P2 factor (TF6SF1) in No-go and Go conditions are also presented.
Exploratory analyses on the frontal-polar positivity factor were conducted to test DI’s specificity to the frontal P2 factor given that the frontal-polar positivity was also a frontally-distributed positivity and demonstrated comparable sensitivity to the effect of Condition, F(1,255) = 9.26, p = .003, pη2 = .035, 90% CI [.007, .079], such that amplitudes were lower on No-go, M = 1.65, SD = 3.15, relative to Go, M = 1.94, SD = 3.05, trials. To test specificity, Component was entered as an additional within-subject factor. The Component*DII interaction was significant with, F(1,250) = 10.65, p=.001, pη2 = .041, 90% CI [.010,.088], and without negative affect in the model, F(1,252) = 13.57, p < .001, pη2 =.051, 90% CI [.016,.101]; a follow-up analysis on frontal-polar positivity factor amplitude revealed a non-significant Condition*DII interaction, F(1,252)=0.02, p = .89, pη2 = .000, 90% CI [.000, .003], and DII main effect, F(1,252) = 0.57, p = .45, pη2 = .002, 90% CI[.000, .022], which also emerged when negative affect was added to the model (ps > .44). The same analyses were run on the frontal-polar negativity factor given its resemblance to a later N2 with respect to its spatial distribution and sensitivity to the effect of Condition, F(1,255) = 84.81, p < .001, pη2 = .250, 90% CI [.177, .319], such that amplitudes were lower on No-go, M = −2.73, SD = 3.35, relative to Go, M = − 1.60, SD = 3.09, trials. As with the results for the frontal-polar positivity factor, the Component*DII interaction was significant with, F(1,250) = 6.13, p=.014, pη2 = .024, 90% CI [.003,.063], and without negative affect in the model, F(1,252) = 6.75, p = .010, pη2 = .026, 90% CI [.003,.066]; a follow-up analysis on frontal-polar negativity factor amplitude revealed a non-significant Condition*DII interaction, F(1,252) = 1.96, p = .16, pη2 = .008, 90% CI [.000, .035], and DII main effect, F(1,252) = 0.18, p = .67, pη2 = .001, 90% CI [.000, .016], with comparable results when negative affect was added to the model (ps > .24). Thus, among the factors resembling the typical N2 component, the effect of DI was specific to the frontal P2 factor.
To determine if DI’s main effect on the frontal P2 component was consistent across subsamples with a primary anxiety/anxiety-related disorder (i.e., obsessive-compulsive disorder; post-traumatic stress disorder) (n=143) vs. a primary depressive disorder (n=59), exploratory analyses were conducted. In the primary depressive disorder subsample, the main effect of DI was significant with, F(1,56) = 5.90, p = .018, pη2 = .095, 90% CI [.009, .225], and without, F(1,57) = 9.57, p = .003, pη2 = .144, 90% CI [.031, .280], negative affect included in the model. Likewise, in the primary anxiety disorder subsample the main effect of DI was significant with, F(1,139) = 7.90, p = .006, pη2 = .054, 90% CI [.009, .124], and without, F(1,141) = 7.87, p = .006, pη2 = .053, 90% CI [.009, .123], negative affect included in the model. Because of accumulating data on DI’s particularly robust relations with distress relative to fear disorders, the primary anxiety disorder subsample was split into subsamples with a primary distress (i.e., generalized anxiety disorder, post-traumatic stress disorder; n=70) vs. fear (i.e., specific phobia; social anxiety disorder; panic disorder; n=55) diagnosis. Consistent with existing literature, in the primary distress disorder subsample the main effect of DI was significant with, F(1,66) = 14.95, p < .001, pη2 = .185, 90% CI [.062, .315], and without, F(1,68) = 16.66, p < .001, pη2 = .197, 90% CI [.072, .325], negative affect in the model, whereas in the primary fear disorder subsample the main effect of DI was non-significant with, F(1,52) = 0.31, p = .583, pη2 = .006, 90% CI [.000, .080], and without, F(1,53) = 0.49, p = .489, pη2 = .009, 90% CI [.000, .090], negative affect in the model.
P3
RM-ANOVA results for the peak amplitude and P3a factor are presented in Table 4. As expected, the main effect of Condition on peak amplitude was significant, F(1,255) = 471.86, p < .001, pη2 = .649, 90% CI [.595, .691], with local positive peaks in the P3 time window significantly more positive on No-go, M = 12.40, SD = 6.87, relative to Go, M = 6.51, p = 4.79, trials. Contrary to hypotheses, the Condition*DII interaction was non-significant, F(1,252) = 0.05, p = .83, pη2 = .000, 90% CI [.000, .008], as was the between-subjects main effect of the DII, F(1,252) = 1.05, p = .31, pη2 = .004, 90% CI [.000, .027]. When negative affect was added to the model, the DII main effect remained non-significant, F(1,250) = 1.56, p = .21, pη2 = .006, 90% CI [.000, .032], as did the Condition*DII interaction, F(1,250) = 0.18, p = .67, pη2 = .001, 90% CI [.000, .016].
Table 4.
Relations between DI, NA, and the P3 peak and P3a factor (TF1SF1)
| P3 Peak | P3a factor (TF1SF1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | df | MS | F | p | pη2 | SS | df | MS | F | p | pη2 | |
| Condition | Condition | |||||||||||
| Condition | 744.88 | 1 | 744.88 | 78.02 | <.001 | 0.248 | 345.58 | 1 | 345.58 | 39.26 | <.001 | 0.136 |
| Condition*DII | 1.75 | 1 | 1.75 | 0.18 | 0.669 | 0.001 | 2.84 | 1 | 2.84 | 0.32 | 0.57 | 0.001 |
| Condition*NA | 1.69 | 1 | 1.69 | 0.18 | 0.675 | 0.001 | 0.02 | 1 | 0.02 | 0 | 0.965 | 0.000 |
| Error(Condition) | 2386.8 | 250 | 9.55 | 2200.67 | 250 | 8.8 | ||||||
| Between-subjects effects | Between-subjects effects | |||||||||||
| DII | 94.82 | 1 | 94.82 | 1.56 | 0.21 | 0.006 | 0.27 | 1 | 0.27 | 0.01 | 0.945 | 0.000 |
| NA | 26.33 | 1 | 26.33 | 0.43 | 0.51 | 0.002 | 17.7 | 1 | 17.7 | 0.31 | 0.577 | 0.001 |
| Error | 15242.06 | 250 | 60.97 | 14161.34 | 250 | 56.65 | ||||||
Note. SS = Type III Sum of Squares. MS = Mean Square. DII = Distress Intolerance Index; NA = Negative affect. Bolded values are significant at p < .05.
Consistent with the amplitude results, the main effect of Condition on the P3a factor was significant, F(1,255) = 308.12, p < .001, pη2 = .547, 90% CI [.482, .600], with more positive amplitudes on No-go, M = 6.64, SD = 6.71, relative to Go, M = 2.07, SD = 4.51, trials. As with the peak amplitudes and contrary to hypotheses, the Condition*DII interaction was non-significant, F(1,252) = 0.43, p = .51, pη2 = .002, 90% CI [.000, .020], as was the between-subjects main effect of the DII, F(1,252) = 0.07, p = .80, pη2 = .000, 90% CI [.000, .011]. When negative affect was added to the model, both the DII main effect and Condition*DII interaction remained non-significant (ps > .56).
Given prior findings on DI and parietal No-go P3 amplitude (Zhou et al., 2015), an exploratory analysis on the P3b factor was conducted. The main effect of Condition on P3b amplitude was significant, F(1,255) = 216.83, p < .001, pη2 = .460, 90% CI [.388, .520], with greater amplitude on Go, M = 4.71, SD = 3.24, relative to No-go, M = 2.40, SD = 3.32, trials; however, as with the P3a analyses, the DII main effect and Condition*DII interaction were non-significant both without (ps > .83) and with negative affect (ps > .33) included in the model.
P2
RM-ANOVA results for the P2 peak amplitude and central P150 factor are presented in Table 4. As with the P3 analyses, the main effect of Condition on peak amplitude was significant, F(1,255) = 14.19, p < .001, pη2 = .053, 90% CI [.017, .103], such that amplitudes were more positive on No-go, M = 7.72, SD = 4.51, relative to Go, M = 7.18, SD = 3.96, trials. The Condition*DII interaction was non-significant, F(1,252) = 2.56, p = .11, pη2 = .010, 90% CI [.000, .040], though the DII main effect was significant, F(1,252) = 7.29, p = .007, pη2 = .028, 90% CI [.004, .070]; however, after inclusion of negative affect in the model the DII main effect became non-significant, F(1,250) = 0.75, p = .39, pη2 = .003, 90% CI [.000, .024], whereas the negative affect main effect was significant, F(1,250) = 4.42, p = .037, pη2 = .017, 90% CI [.001, .053]. Interestingly, when negative affect was included in the model, both the Condition*DII, F(1,250) = 6.58, p = .011, pη2 = .026, 90% CI [.003, .066], and Condition*negative affect, F(1,250) = 3.94, p = .048, pη2 = .016, 90% CI [.0001, .050], interactions were significant. Follow-up regression analyses revealed a positive association between negative affect and peak amplitude on No-go, β = .11, t(250) = 1.40, p = .16, and Go, β = .21, t(250) = 2.75, p = .006, trials, suggesting that the apparent P2 amplitude difference between high and low DI groups in Figure 2 is attributable to co-occurring negative affect. In contrast, there was some indication of a positive association between the DII and peak amplitude on NoGo, β = .11, t(250) = 1.43, p = .15, but not Go, β = .01, t(250) = 0.15, p = .88, trials.
In contrast with the peak amplitude results, the main effect of Condition on the central P150 factor was non-significant, F(1,255) = 1.90, p = .17, pη2 = .007, 90% CI [.000, .034], such that amplitudes on No-go, M = 5.10, SD = 4.13, and Go, M = 5.25, SD = 3.86, trials were non-significantly different. However, consistent with the results for peak amplitude, the Condition*DII interaction was non-significant, F(1,252) = 2.03, p = .16, pη2 = .008, 90% CI [.000, .036], whereas the between-subjects main effect of the DII, F(1,252) = 6.76, p = .010, pη2 = .026, 90% CI [.004, .067], was significant, though again the DII main effect became non-significant, F(1,250) = 0.15, p = .70, pη2 = .001, 90% CI [.000, .015], after negative affect was included in the model. As with the peak amplitude results, the main effect of negative affect was significant, F(1,250) = 7.74, p = .006, pη2 = .030, 90% CI [.005, .073], such that greater negative affect was related to more positive central P150 factor amplitude on No-go, β = .19, t(250) = 2.40, p = .017, and Go, β = .24, t(250) = 3.05, p = .003, trials. In contrast to the peak amplitude results, neither the Condition*DII, F(1,250) = 3.16, p = .077, pη2 = .012, 90% CI [.000, .045], or Condition*negative affect, F(1,250) = 1.04, p = .31, pη2 = .004, 90% CI [.000, .027], interactions were significant.
Discussion
The results of the present study were somewhat supportive of hypotheses. In line with predictions, greater DI was associated with a more positive N2 peak amplitude on No-go relative to Go trials independent of negative affect severity, though this effect was partially driven by negative affect’s opposite association with No-go N2 peak amplitude. Further, PCA decomposition revealed that, in contrast to the P2 and P3 peaks, the N2 peak amplitude was comparably associated with multiple latent ERP components, complicating interpretation of N2 amplitudes measured from the raw waveforms. Analyses of DI’s link with the factor most reflective of the typical N2 with respect to spatial distribution, time course, and sensitivity to condition (i.e., frontal P2 factor; TF6SF1) revealed a significant main effect only such that greater DI was linked with more positive frontal P2 factor amplitude across No-go and Go trials independent of negative affect severity, suggesting that DI was specifically related to increased early, conflict-related processing of task stimuli regardless of inhibition demand. In contrast to hypotheses, DI was non-significantly associated with No-go peak P3 and P3a factor amplitude. Finally, exploratory analyses revealed that DI was non-significantly associated with the P3b factor, and DI’s apparent relationship with the P2 peak and central P150 factor was attributable to co-occurring NA. Overall, results are inconsistent with response inhibition impairment as an underlying mechanism of individual differences in DI, but rather suggest that DI is linked with a more basic alteration in early cognitive control functioning, possibly reflective of inefficient controlled attention/conflict-related processing.
The present results are inconsistent with prior findings on behaviorally-indexed DI and response inhibition-related alterations in the N2 and P3 (Zhou et al., 2015). Zhou and colleagues (2015) found a significant inverse correlation between DI and parietal P3 amplitude on No-go trials but no relation with central No-go P3 or frontal N2 amplitudes, whereas DI was only independently associated with N2 amplitude in the present study. These discrepant results may be attributable to differences between behavioral and self-reported DI. Indeed, modest to non-significant associations between behavioral and self-report DI measures suggests that they may be measuring largely different constructs (Kiselica et al., 2015; McHugh et al., 2011; McHugh & Otto, 2011), though the self-report DI measure used in the present study was explicitly chosen to better capture cross-method variance in the construct (McHugh & Otto, 2011; McHugh et al., 2016; Seo & Kwon, 2016; Szuhany & Otto, 2015; but see Williams, Vik, & Wong, 2015).
Further, in contrast to two prior studies using behavioral DI measures (Bagge et al., 2013; Ledgerwood et al., 2009), self-reported DI was unrelated to commission errors in the present study, which may be attributable to method variance or different underlying constructs assessed by self-report vs. behavioral DI measures. It is also possible that discrepant results were observed because of differences in sample (i.e., presence of psychopathology, age range) or go/no-go task parameters (i.e., No-go trial probability, complex vs. simple). Nevertheless, despite the discrepant findings with respect to N2 and parietal P3 amplitude, both the present study and Zhou and colleagues’ (2015) study’s findings are conceptually consistent in that both behavioral and perceived DI were related to altered neural activity during a response inhibition task, providing support to the notion that cognitive control functioning is relevant to individual differences in DI.
In the present study, though DI was independently related to N2 peak and frontal P2 factor amplitude, is it important to note that a typical N2 latent component did not emerge after PCA. Instead, a frontal positivity with a similar time course (i.e., peak within 200–300ms), spatial distribution (i.e., maximal at frontal midline sites), and sensitivity to condition (i.e., more negative amplitude on No-go relative to Go trials) was extracted. In conjunction with the larger, adjacent ERP components, the absence of an N2 trial effect in the grand-average raw waveforms (see Figure 1) may be attributable to the absence of a typical latent N2 component, possibly due to the greater complexity of the go/no-go task used in the present study. The complex go/no-go task used in the present study simultaneously functioned as a response inhibition paradigm (i.e., stopping a practiced motor response on relatively infrequent inhibition trials) and a 1-back task (i.e., evaluating if the current stimulus is identical to the stimulus presented on the previous trial); further, long-term memory was also required because participants needed to learn novel S-R mappings (e.g., X - left button, Y - right button) every 24 trials. With regard to the latter point specifically, though trial type effects on the N2 have been found with complex go/no-go tasks in prior work (e.g., Roche et al., 2005), studies using a greater variety of stimuli have found null results (Littel et al., 2012; Maij, van de Wetering, & Franken, 2017; Rietdijk, Franken, & Thurik, 2014), suggesting that the typical latent N2 component may be more likely to emerge on simpler go/no-go tasks that minimize the range of possible S-R associations. Nevertheless, the frontal P2 factor appears to be functionally similar to the N2 in that it was the earliest component sensitive to trial type, which may reflect a high-order, controlled attention/conflict-related process linked to working memory given that the necessary first post-sensory stage of stimulus processing involves determining if the current stimulus is identical to the stimulus held in working memory. Indeed, ERP studies of working memory have offered similar interpretations of the cognitive function reflected by the frontal P2 component (McEvoy, Pellouchoud, Smith, & Gevins, 2001; Zhao, Zhou, & Fu, 2013). Thus, DI’s unique and independent positive association with frontal P2 factor amplitude may indicate that individuals with high DI recruited greater attentional resources during working memory-based stimulus evaluation, possibly reflective of inefficient controlled attention/conflict-related processing.
Interpretation of enhanced frontal P2 factor amplitude as indicative of inefficient controlled attention/conflict-related processing is supported by data from the present study as well as prior investigations. First, greater frontal P2 factor amplitude was significantly linked with slower RT on Go trials, suggesting that more neural activation during working memory-based stimulus evaluation was related to prolonged decision latency. Second, the frontal P2 factor was positively associated with the subsequent frontal-polar negativity factor (i.e., more positive frontal P2 related to less negative frontal-polar negativity factor), a component which has been suggested to be related to memory retrieval processes (i.e., N300; Keage et al., 2008). Indeed, the robust relationship between a more positive frontal-polar negativity factor and worse Go trial performance specifically (see Supplemental material) suggests that greater inefficiency of the early controlled attention process (i.e., enhanced frontal P2 amplitude) may have deleterious downstream effects on subsequent retrieval of accurate S-R associations necessary for successful Go trial performance. Third, greater frontal P2 amplitude during working memory updating has been associated with advanced age (McEvoy et al., 2001), an association also observed in the present study.3 Fourth, frontal P2 amplitude has been found to decrease after training on computerized working memory updating tasks, whereas an increase in P3 amplitude has been observed (Zhao, Zhou, & Fu, 2013). Taken together, these data suggest that DI’s hypothesized relationship to underlying alterations in cognitive control may be specific to working memory-related controlled attention processes rather than response inhibition, S-R association retrieval/activation, or context updating.
Although exploratory, a positive relationship between DI and the central P150 factor also emerged, but, in contrast to the frontal P2 factor, this association was attributable to current negative affect severity. The main effect of trial type on the central P150 factor was non-significant, indicating that this component may reflect relatively low-order stimulus processing, possibly related to an early sensory gating or attentional allocation function (Ferreira-Santos et al., 2012; Keage et al., 2008; Kemp et al., 2009; Lijffijt et al., 2009). A comparable central positivity with a similar time course was found to be larger in working memory relative to control tasks but, as in the current study, the positivity was non-significantly affected by match vs. non-match trials (Gevins et al., 1996), which may indicate that the component reflects allocation of attentional/working-memory resources. Thus, the observed link between negative affect severity and enhanced central P150 factor amplitude may reflect greater recruitment of attentional/working-memory resources during the initial stage of stimulus processing in more distressed individuals. Further, the central P150 factor was weakly to non-significantly related with improved task performance (see Supplemental material), suggesting that high negative affect individuals’ greater recruitment of attentional/working-memory resources was inefficient and possibly reflective of compensatory activity. Overall, these results suggest that individuals experiencing high levels of distress recruit greater attentional/working-memory resources during initial stimulus processing, whereas individuals with greater intolerance of distress demonstrate inefficient attentional functioning during a later, more controlled stage of processing related to conflict.
The results of the present study have theoretical implications. High DI is theorized to be a transdiagnostic individual difference variable with relevance to multiple forms of psychopathology (Leyro et al., 2010), but few studies have examined possible underlying mechanisms. The findings of the present study suggest that perceived intolerability of distress is associated with the functioning of controlled attention/conflict-related neural systems in a sample characterized by high levels of emotional psychopathology and co-morbidity. Further, exploratory analyses revealed that the association was robust in subsamples with primary distress disorders (i.e., depressive disorders, generalized anxiety disorder, post-traumatic stress disorder) but absent in the primary fear disorder subsample (i.e., specific phobia, social phobia, panic disorder), indicating that high DI may be driven by distinct neural mechanisms in different forms of psychopathology. For instance, whereas individuals with a primary distress disorder may experience difficulty tolerating distress due to attentional control deficits resulting in dysregulated intrusive cognition and associated negative affect (e.g., Ruscio, Seitchik, Gentes, Jones, & Hallion, 2011), individuals with a primary fear disorder may struggle with tolerating distress due to exaggerated threat appraisals of the consequences of experiencing negative affect (e.g., heart attack or humiliation from signs of anxious arousal). Both of these mechanisms could plausibly lead to the generalized avoidance responding characteristic of high DI, underscoring the importance of testing hypothesized mechanisms of dysfunction in different groups given that the same individual difference (e.g., perceived intolerability of distress) may arise from multiple sources.
The present study has a number of limitations. First, only a self-report measure of DI was administered; although accumulating evidence suggests that, in contrast to other measures of perceived DI, the DII is correlated with behavioral measures (McHugh & Otto, 2011; McHugh et al., 2016; Seo & Kwon, 2016; Szuhany & Otto, 2015; but see Williams, Vik, & Wong, 2015), associations are small to medium (rs < .50), indicating that each measure captures unique variance. Further, though the present results conceptually replicated Zhou and colleagues’ (2015) findings of altered inhibition-related neural activity, it is unclear if the discrepant relations with specific ERPs are attributable to DI measurement (i.e., behavioral vs. self-report) or other study differences (i.e., healthy older adult vs. clinical sample; simple vs. complex Go/No-go task; equiprobable Go/No-go stimuli vs. 25% No-go stimuli), underscoring the need to include self-report and behavioral DI measures in future studies. Second, the present study utilized a complex Go/No-go, which likely engaged additional cognitive control brain regions relative to simple Go/No-go tasks (e.g., right dlPFC; Simmonds et al., 2008). Relatedly, the particular variant of the complex Go/No-go used in the present study introduced novel S-R mappings every 24 trials, which may be related to the lack of an observable N2 effect in the grand-averaged waveforms (Littel et al., 2012; Maij, van de Wetering, & Franken, 2017; Rietdijk, Franken, & Thurik, 2014). Thus, the relations between DI and ERPs observed in the present study may differ in simple go/no-go tasks and/or go/no-go paradigms with a more restricted range of S-R pairings. Third, it is not clear if the observed DI-linked alteration in neurophysiological activity (i.e., enhanced frontal P2 factor amplitude) reflects working memory-specific controlled attention/conflict-related processing or domain-general controlled attention/conflict-related processing; future studies should utilize a variety of cognitive control tasks differing in working memory demand to better characterize the functional meaning of the DI-linked neural activity observed in the present study. Fourth, the response inhibition task used in the present study employed neutral stimuli without a negative mood induction. Although an appropriate preliminary test of cognitive control impairment as an underlying mechanism of DI, future studies should examine DI’s association with cognitive control-related neural activity in contexts theoretically relevant to DI (e.g., acute negative mood, negative reinforcement cues).
The present study provides empirical support for the hypothesized relevance of cognitive control-linked neural activity to individual differences in DI. However, DI was not specifically related to response inhibition-elicited neural activity, but rather was associated with greater early controlled attention/conflict-related processing on Go and No-go trials, possibly reflecting inefficient functioning of a more basic attentional control system. Importantly, DI’s association with altered controlled attention/conflict-related neural activity was independent of current negative affect severity, indicating that variability in perceived tolerance of negative affect is distinct from current distress with respect to neural activity during a cognitive control task.
Overall, these data contribute to our understanding of the neurobiological correlates of DI, an individual difference with transdiagnostic relevance to psychopathology, and encourage ongoing efforts to identify alterations in neurobiologically-based functional domains (e.g., working memory, conflict-monitoring, response inhibition) that may underlie transdiagnostic risk factors for psychopathology (Cuthbert & Insel, 2013).
Supplementary Material
Table 5.
Relations between DI, NA, and the P2 peak and central P150 factor (TF4SF1)
| P2 Peak | Central P150 factor (TF4SF1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | df | MS | F | p | pη2 | SS | df | MS | F | p | pη2 | |
| Condition | Condition | |||||||||||
| Condition | 2.28 | 1 | 2.28 | 0.89 | 0.346 | 0.004 | 7.72 | 1 | 7.72 | 4.9 | 0.028 | 0.019 |
| Condition*DII | 16.83 | 1 | 16.83 | 6.58 | 0.011 | 0.026 | 4.98 | 1 | 4.98 | 3.16 | 0.077 | 0.012 |
| Condition*NA | 10.08 | 1 | 10.08 | 3.94 | 0.048 | 0.016 | 1.63 | 1 | 1.63 | 1.04 | 0.31 | 0.004 |
| Error(Condition) | 639.82 | 250 | 2.56 | 394.15 | 250 | 1.58 | ||||||
| Between-subjects effects | Between-subjects effects | |||||||||||
| DII | 24.34 | 1 | 24.34 | 0.75 | 0.387 | 0.003 | 4.33 | 1 | 4.33 | 0.15 | 0.701 | 0.001 |
| NA | 143.21 | 1 | 143.21 | 4.42 | 0.037 | 0.017 | 226.13 | 1 | 226.13 | 7.74 | 0.006 | 0.030 |
| Error | 8109.31 | 250 | 32.44 | 7302.27 | 250 | 29.21 | ||||||
Note. SS = Type III Sum of Squares. MS = Mean Square. DII = Distress Intolerance Index; NA = Negative affect. Bolded values are significant at p < .05.
General Scientific Summary.
Difficulty tolerating distress is a key feature of multiple mental illnesses. This study supports the idea that distress intolerance is associated with altered neural activity during controlled attention/conflict-related processing on a cognitive control task. Variability in the neural substrates of cognitive control may contribute to individual differences in distress intolerance.
Footnotes
Two frontal-central factors (TF5SF1; 2.23% variance; TF7SF1; 1.44% variance) also emerged but were not presented in the manuscript due to having time courses outside of the typical P3 response. However, these factors had peaks before 600ms (TF5SF1: 476ms; TF7SF1: 552ms) and spatial distributions (i.e., TF5SF1: FCz; TF7SF1: FFC1h) as well as sensitivity to trial type (i.e., more positive amplitude on No-go relative to Go trials) comparable to the P3a. Thus, these factors are presented in the supplemental material. Importantly, no relations with DI were observed with (ps > .13) or without (ps > .14) NA included in the models.
A 90% CI is used for F tests because an F test is always a one-sided test and thus a 90% CI ensures that the CI will exclude zero when the test is statistically significant (for more information see Lakens, 2013; Smithson, 2001).
Age was significantly positively correlated with frontal P2 amplitude, rs = .20, ps = .001. Importantly, when age was included in the RM-ANOVA as a covariate, the effect of DI remained significant with, F(1,249) = 10.24, p = .002, pη2 = .039, and without, F(1,251) = 12.88, p < .001, pη2 = .049, negative affect also entered as a covariate.
References
- Allan NP, Macatee RJ, Norr AM, & Schmidt NB (2014). Direct and interactive effects of distress tolerance and anxiety sensitivity on generalized anxiety and depression. Cognitive Therapy and Research, 38(5), 530–540. [Google Scholar]
- Albanese BJ, Macatee RJ, Schmidt NB, Leeson B, Clemans TA, Mintz J, … & Bryan CJ (in press). Interactive Effects of Traumatic Brain Injury and Anxiety Sensitivity Cognitive Concerns on Post-traumatic Stress Among Active Duty Soldiers. Cognitive Therapy and Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameral V, Palm Reed KM, Cameron A, & Armstrong JL (2014). What are measures of distress tolerance really capturing? A mixed methods analysis. Psychology of Consciousness: Theory, Research, and Practice, 1(4), 357. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, & Robbins TW (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature neuroscience, 6(2), 115. [DOI] [PubMed] [Google Scholar]
- Aviyente S, Tootell A, & Bernat EM (2017). Time-frequency phase-synchrony approaches with ERPs. International Journal of Psychophysiology, 111, 88–97. [DOI] [PubMed] [Google Scholar]
- Bagge CL, Littlefield AK, Rosellini AJ, & Coffey SF (2013). Relations among behavioral and questionnaire measures of impulsivity in a sample of suicide attempters. Suicide and Life-Threatening Behavior, 43(4), 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banducci AN, Bujarski SJ, Bonn-Miller MO, Patel A, & Connolly KM (2016). The impact of intolerance of emotional distress and uncertainty on veterans with co-occurring PTSD and substance use disorders. Journal of anxiety disorders, 41, 73–81. [DOI] [PubMed] [Google Scholar]
- Banducci AN, Connolly KM, Vujanovic AA, Alvarez J, & Bonn-Miller MO (2017). The impact of changes in distress tolerance on PTSD symptom severity post-treatment among veterans in residential trauma treatment. Journal of anxiety disorders, 47, 99–105. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck Depression Inventory-II. San Antonio, TX, 78204–2498. [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, & Nopoulos P (2008). Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social cognitive and affective neuroscience, 4(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, & Kobayashi S (2001). Electrophysiological correlates for response inhibition in a go/nogo task. Clinical Neurophysiology, 112(12), 2224–2232. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Nick B, Delany-Brumsey A, Lynch TR, … & Lejuez CW (2008). A multimodal assessment of the relationship between emotion dysregulation and borderline personality disorder among inner-city substance users in residential treatment. Journal of Psychiatric Research, 42(9), 717–726. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, … & Price LH (2009). A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research, 11(5), 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakır Z (2016). Examining psychometric properties of distress intolerance index and cognitive-behavioral avoidance scale. Anatolian Journal of Psychiatry, 17, 24–32. [Google Scholar]
- Carver CS, Johnson SL, & Timpano KR (2017). Toward a Functional View of the p Factor in Psychopathology. Clinical Psychological Science, 2167702617710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB (1966). The scree test for the number of factors. Multivariate behavioral research, 1(2), 245–276. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Timpano KR, Fitch KE, & Hawkins KA (2011). Distress tolerance and obsessions: an integrative analysis. Depression and anxiety, 28(10), 906–914. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Bornovalova MA, Ojanen T, Hunt E, MacPherson L, & Lejuez C (2013). Time doesn’t change everything: The longitudinal course of distress tolerance and its relationship with externalizing and internalizing symptoms during early adolescence. Journal of abnormal child psychology, 41(5), 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine, 11(1), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, & Brown RA (2005). Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. Journal of Abnormal Psychology, 114(4), 729. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Ross TJ, Bell RP, Yi JY, Ryan J, & Stein EA (2016). Distress tolerance among substance users is associated with functional connectivity between prefrontal regions during a distress tolerance task. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Sargeant MN, Bornovalova MA, Gratz KL, & Lejuez CW (2008). The relationship between distress tolerance and antisocial personality disorder among male inner-city treatment seeking substance users. Journal of Personality Disorders, 22(5), 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J (2010a). The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of neuroscience methods, 187(1), 138–145. [DOI] [PubMed] [Google Scholar]
- Dien J (2010b). Evaluating two‐step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology, 47(1), 170–183. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, & Berg P (2005). Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clinical neurophysiology, 116(8), 1808–1825. [DOI] [PubMed] [Google Scholar]
- Dien J, & Frishkoff G (2005). Principal components analysis of eventrelated potential datasets In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: The MIT Press; pp 189–208. [Google Scholar]
- Dien J, Khoe W, & Mangun GR (2007). Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human brain mapping, 28(8), 742–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Spencer KM, & Donchin E (2003). Localization of the event-related potential novelty response as defined by principal components analysis. Cognitive Brain Research, 17(3), 637–650. [DOI] [PubMed] [Google Scholar]
- Donkers FC, & Van Boxtel GJ (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and cognition, 56(2), 165–176. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, & Mathias CW (2002). Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behavior Research Methods, Instruments, & Computers, 34(3), 391–398. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, & Marsh DM (2003). GoStop Impulsivity Paradigm (Version 1.0)[Manual]. Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston, Houston, Texas. [Google Scholar]
- Ellis AJ, Vanderlind WM, & Beevers CG (2013). Enhanced anger reactivity and reduced distress tolerance in major depressive disorder. Cognitive Therapy and Research, 37(3), 498–509. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: attentional control theory. Emotion, 7(2), 336. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, & Hohnsbein J (1999). ERP components in go/nogo tasks and their relation to inhibition. Acta Psychologica, 101(2), 267–291. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, … & Warnke A (2004). Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clinical Neurophysiology, 115(4), 973–981. [DOI] [PubMed] [Google Scholar]
- Fan J, Liu W, Lei H, Cai L, Zhong M, Dong J, … & Zhu X (2016). Components of inhibition in autogenous-and reactive-type obsessive-compulsive disorder: Dissociation of interference control. Biological psychology, 117, 117–130. [DOI] [PubMed] [Google Scholar]
- Ferreira-Santos F, Silveira C, Almeida PR, Palha A, Barbosa F, & Marques-Teixeira J (2012). The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clinical Neurophysiology, 123(7), 1300–1308. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer R (2015). Structured Clinical Interview for DMS-5, Research Version. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology, 45(1), 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D, & Chambless DL (1992). Reliability and validity of the Beck Anxiety Inventory. Journal of Anxiety Disorders, 6(1), 55–61. [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, & Stein EA (2003). A midline dissociation between error-processing and response-conflict monitoring. Neuroimage, 20(2), 1132–1139. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, & Stein EA (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage, 17(4), 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Le J, Leong H, Bennett J, Martin N, … & Whitfield S (1996). High resolution evoked potential imaging of the cortical dynamics of human working memory. Electroencephalography and clinical neurophysiology, 98(4), 327–348. [DOI] [PubMed] [Google Scholar]
- Glassman LH, Martin LM, Bradley LE, Ibrahim A, Goldstein SP, Forman EM, & Herbert JD (2016). A Brief Report on the Assessment of Distress Tolerance: Are We Measuring the Same Construct?. Journal Of Rational-Emotive & Cognitive-Behavior Therapy, 34(2), 87–99. [Google Scholar]
- Glischinski M, Teismann T, Prinz S, Gebauer JE, & Hirschfeld G (2016). Depressive Symptom Inventory Suicidality Subscale: Optimal Cut Points for Clinical and Non‐ Clinical Samples. Clinical psychology & psychotherapy, 23(6), 543–549. [DOI] [PubMed] [Google Scholar]
- Groom MJ, & Cragg L (2015). Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain and Cognition, 97, 1–9. [DOI] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bachman MD, & Bernat EM (2016). Stimulus sequence context differentially modulates inhibition‐related theta and delta band activity in a go/no‐go task. Psychophysiology, 53(5), 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington N (2005). It’s too difficult! Frustration intolerance beliefs and procrastination. Personality and Individual Differences, 39(5), 873–883. [Google Scholar]
- Hasan NS, Babson KA, Banducci AN, & Bonn-Miller MO (2015). The prospective effects of perceived and laboratory indices of distress tolerance on cannabis use following a self-guided quit attempt. Psychology of Addictive Behaviors, 29(4), 933. [DOI] [PubMed] [Google Scholar]
- Hatz F, Hardmeier M, Bousleiman H, Rüegg S, Schindler C, & Fuhr P (2015). Reliability of fully automated versus visually controlled pre-and post-processing of resting-state EEG. Clinical Neurophysiology, 126(2), 268–274. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, & Garavan H (2004). Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex, 14(9), 986–994. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, & Herrmann CS (2013). Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. International Journal of Psychophysiology, 87(3), 217–233. [DOI] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, & Ungerleider LG (2015). A role of right middle frontal gyrus in reorienting of attention: a case study. Frontiers in systems neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, & Weisbrod M (2003). Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Research: Neuroimaging, 122(3), 169–184. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, … & Begleiter H (2005). Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological psychology, 69(3), 353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage HA, Clark CR, Hermens DF, Williams LM, Kohn MR, Clarke S, … & Gordon E (2008). ERP indices of working memory updating in AD/HD: differential aspects of development, subtype, and medication. Journal of Clinical Neurophysiology, 25(1), 32–41. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, & Garavan H (2004). Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience, 19(11), 3105–3112. [DOI] [PubMed] [Google Scholar]
- Kemp A, Hopkinson PJ, Hermens DF, Rowe DL, Sumich AL, Clark CR, … & Boyce P (2009). Fronto-temporal alterations within the first 200 ms during an attentional task distinguish major depression, non-clinical participants with depressed mood and healthy controls: A potential biomarker?. Human brain mapping, 30(2), 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough ME, Riccardi CJ, Timpano KR, Mitchell MA, & Schmidt NB (2010). Anxiety symptomatology: The association with distress tolerance and anxiety sensitivity. Behavior Therapy, 41(4), 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Kim YY, Yoo SY, & Kwon JS (2007). Electrophysiological correlates of behavioral response inhibition in patients with obsessive–compulsive disorder. Depression and anxiety, 24(1), 22–31. [DOI] [PubMed] [Google Scholar]
- Kirmizi-Alsan E, Bayraktaroglu Z, Gurvit H, Keskin YH, Emre M, & Demiralp T (2006). Comparative analysis of event-related potentials during go/nogo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Research, 1104(1), 114–128. [DOI] [PubMed] [Google Scholar]
- Kiselica AM, Rojas E, Bornovalova MA, & Dube C (2015). The nomological network of self-reported distress tolerance. Assessment, 22(6), 715–729. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposa JM, Collimore KC, Hawley LL, & Rector NA (2015). Distress tolerance in OCD and anxiety disorders, and its relationship with anxiety sensitivity and intolerance of uncertainty. Journal of anxiety disorders, 33, 8–14. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Phoenix N, & Petry NM (2009). Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls. Drug and Alcohol Dependence, 105(1), 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, & Brown RA (2003). A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. The Behavior Therapist, 26(4), 290–293. [Google Scholar]
- Leyro TM, Zvolensky MJ, & Bernstein A (2010). Distress tolerance and psychopathological symptoms and disorders: a review of the empirical literature among adults. Psychological Bulletin, 136(4), 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, … & Swann AC (2009). P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology, 46(5), 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel M, Berg I, Luijten M, Rooij AJ, Keemink L, & Franken IH (2012). Error processing and response inhibition in excessive computer game players: an event‐related potential study. Addiction biology, 17(5), 934–947. [DOI] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique. MIT Press. [Google Scholar]
- Luijten M, Littel M, & Franken IH (2011). Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials. PloS one, 6(4), e18898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Albanese BJ, Allan NP, Schmidt NB, & Cougle JR (2016). Distress intolerance as a moderator of the relationship between daily stressors and affective symptoms: tests of incremental and prospective relationships. Journal of Affective Disorders, 206, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Capron DW, Guthrie W, Schmidt NB, & Cougle JR (2015). Distress tolerance and pathological worry: tests of incremental and prospective relationships. Behavior Therapy, 46(4), 449–462. [DOI] [PubMed] [Google Scholar]
- Macatee RJ, Capron DW, Schmidt NB, & Cougle JR (2013). An examination of low distress tolerance and life stressors as factors underlying obsessions. Journal of Psychiatric Research, 47(10), 1462–1468. [DOI] [PubMed] [Google Scholar]
- Maij DL, van de Wetering BJ, & Franken IH (in press). Cognitive control in young adults with cannabis use disorder: An event-related brain potential study. Journal of Psychopharmacology, 0269881117719262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Pellouchoud E, Smith ME, & Gevins A (2001). Neurophysiological signals of working memory in normal aging. Cognitive Brain Research, 11(3), 363–376. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Daughters SB, Lejuez CW, Murray HW, Hearon BA, Gorka SM, & Otto MW (2011). Shared variance among self-report and behavioral measures of distress intolerance. Cognitive therapy and research, 35(3), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Kertz SJ, Weiss RB, Baskin-Sommers AR, Hearon BA, & Björgvinsson T (2014). Changes in distress intolerance and treatment outcome in a partial hospital setting. Behavior therapy, 45(2), 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, & Otto MW (2011). Domain-general and domain-specific strategies for the assessment of distress intolerance. Psychology of Addictive Behaviors, 25(4), 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, & Otto MW (2012). Refining the measurement of distress intolerance. Behavior Therapy, 43(3), 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Weiss RD, Cornelius M, Martel MO, Jamison RN, & Edwards RR (2016). Distress intolerance and prescription opioid misuse among patients with chronic pain. The Journal of Pain, 17(7), 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel NM, Rowa K, Young L, & McCabe RE (2016). Emotional distress tolerance across anxiety disorders. Journal of anxiety disorders, 40, 94–103. [DOI] [PubMed] [Google Scholar]
- Miller GA, & Chapman JP (2001). Misunderstanding analysis of covariance. Journal of abnormal psychology, 110(1), 40. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, & Kakigi R (2012). The relationship between reaction time and response variability and somatosensory no-go potentials. European Journal of Applied Physiology, 112(1), 207–214. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Strickland C, Krueger RF, Arbisi PA, & Patrick CJ (2016). Neurobehavioral traits as transdiagnostic predictors of clinical problems. Assessment, 23(1), 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H, Whelan R, & Reilly RB (2010). FASTER: fully automated statistical thresholding for EEG artifact rejection. Journal of Neuroscience Methods, 192(1), 152–162. [DOI] [PubMed] [Google Scholar]
- Norr AM, Oglesby ME, Capron DW, Raines AM, Korte KJ, & Schmidt NB (2013). Evaluating the unique contribution of intolerance of uncertainty relative to other cognitive vulnerability factors in anxiety psychopathology. Journal of affective disorders, 151(1), 136–142. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, & Woldorff MG (2000). Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry, 48(3), 238–246. [DOI] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology, 118(10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, & Comerchero MD (2003). P3a from visual stimuli: typicality, task, and topography. Brain topography, 15(3), 141–152. [DOI] [PubMed] [Google Scholar]
- Renna ME, Chin S, Seeley SH, Fresco DM, Heimberg RG, & Mennin DS (in press). The Use of the Mirror Tracing Persistence Task as a Measure of Distress Tolerance in Generalized Anxiety Disorder. Journal of Rational-Emotive & Cognitive-Behavior Therapy, 1–9. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, & McNally RJ (1986). Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy, 24(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Rietdijk WJ, Franken IH, & Thurik AR (2014). Internal consistency of event-related potentials associated with cognitive control: N2/P3 and ERN/Pe. PLOS ONE, 9(7), e102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi S, Mecacci L, & Viggiano MP (2009). Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders, 23(8), 1132–1138. [DOI] [PubMed] [Google Scholar]
- Roche RA, Garavan H, Foxe JJ, & O’Mara SM (2005). Individual differences discriminate event-related potentials but not performance during response inhibition. Experimental Brain Research, 160(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Beschoner P, Hermle L, Ebert D, & Falkenstein M (2008a). Electrophysiological evidence for reduced inhibitory control in depressed patients in partial remission: a Go/Nogo study. International Journal of Psychophysiology, 68(3), 209–218. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Buchheim A, Walter H, Martius P, … & Falkenstein M (2008b). Response inhibition in borderline personality disorder: event-related potentials in a Go/Nogo task. Journal of neural transmission, 115(1), 127–133. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Seitchik AE, Gentes EL, Jones JD, & Hallion LS (2011). Perseverative thought: A robust predictor of response to emotional challenge in generalized anxiety disorder and major depressive disorder. Behaviour research and therapy, 49(12), 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant MN, Daughters SB, Curtin JJ, Schuster R, & Lejuez CW (2011). Unique roles of antisocial personality disorder and psychopathic traits in distress tolerance. Journal of Abnormal Psychology, 120(4), 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapkin SA, Falkenstein M, Marks A, & Griefahn B (2007). Practice-related effects in a go-nogo task. Perceptual and Motor Skills, 105(3 suppl), 1275–1288. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Norr AM, Allan NP, Raines AM, & Capron DW (2017). Randomized clinical trial evaluating the efficacy of an automated intervention targeting anxiety sensitivity cognitive concerns for patients with suicidal ideation. Manuscript submitted for publication. [Google Scholar]
- Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, & Beste C (2010). ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia, 48(9), 2488–2495. [DOI] [PubMed] [Google Scholar]
- Seo JW, & Kwon SM (2016). Testing an affective judgment model of distress tolerance in college heavy drinkers. Addictive Behaviors, 58, 100–103. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Macatee R, & Chronis-Tuscano A (2016). Frustration Tolerance in Youth With ADHD. Journal of attention disorders, 1087054716653216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain, and cognitive control in the cingulate cortex. Nature reviews. Neuroscience, 12(3), 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, & Mostofsky SH (2008). Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46(1), 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, & Gaher RM (2005). The distress tolerance scale: development and validation of a self-report measure. Motivation and Emotion, 29(2), 83–102. [Google Scholar]
- Singh SM, & Basu D (2009). CLINICAL STUDY: the P300 event‐related potential and its possible role as an endophenotype for studying substance use disorders: a review. Addiction biology, 14(3), 298–309. [DOI] [PubMed] [Google Scholar]
- Smithson M (2001). Correct Confidence Intervals for Various Regression Effect Sizes and Parameters: The Importance of Noncentral Distributions in Computing Intervals. Educational and Psychological Measurement, 61(4), 605–632. [Google Scholar]
- Snyder HR, Hankin BL, Sandman CA, Head K, & Davis EP (2017). Distinct Patterns of Reduced Prefrontal and Limbic Gray Matter Volume in Childhood General and Internalizing Psychopathology. Clinical Psychological Science, 2167702617714563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Fink BC, Maurer JM, Arbabshirani MR, Wilber CH, Jaffe AJ, … & Kiehl KA (2014). Brain potentials measured during a Go/NoGo task predict completion of substance abuse treatment. Biological psychiatry, 76(1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Sims M, Herman DS, Anderson BJ, & Stein MD (2012). Persistence during stress-challenge associated with lapse to opioid use during buprenorphine treatment. Journal of addiction medicine, 6(3), 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuhany KL, & Otto MW (2015). Contextual influences on distress intolerance: priming effects on behavioral persistence. Cognitive Therapy and Research, 39(4), 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, & Gifford EV (2011). Biological bases of distress tolerance. Distress Tolerance: Theory, Research, and Clinical Applications, 80–102. [Google Scholar]
- Van Orden KA, Cukrowicz KC, Witte TK, & Joiner TE Jr (2012). Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychological assessment, 24(1), 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Hicks BM, Yancey JR, Kramer MD, Nelson LD, Strickland CM, … & Patrick CJ (2017). Evidence of a prominent genetic basis for associations between psychoneurometric traits and common mental disorders. International Journal of Psychophysiology, 115, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz AR, Dennis PA, Dennis MF, Calhoun PS, Wilson SM, & Beckham JC (2014). The role of daily hassles and distress tolerance in predicting cigarette craving during a quit attempt. Nicotine & Tobacco Research, 16(6), 872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Kotov R, Ruggero C, Gamez W, & Watson D (2017). Hierarchical Structure of Emotional Disorders: From Individual Symptoms to the Spectrum. Journal of Abnormal Psychology, 126(5), 613–34. [DOI] [PubMed] [Google Scholar]
- Watson D (2005). Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of abnormal psychology, 114(4), 522. [DOI] [PubMed] [Google Scholar]
- Watson TD, & Garvey KT (2013). Neurocognitive correlates of processing food-related stimuli in a go/no-go paradigm. Appetite, 71, 40–47. [DOI] [PubMed] [Google Scholar]
- Wessel JR (in press). Prepotent motor activity and inhibitory control demands in different variants of the go/no‐go paradigm. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Williams AD, Thompson J, & Andrews G (2013). The impact of psychological distress tolerance in the treatment of depression. Behaviour research and therapy, 51(8), 469–475. [DOI] [PubMed] [Google Scholar]
- Williams CL, Vik PW, & Wong MM (2015). Distress tolerance in social versus solitary college student drinkers. Addictive Behaviors, 50, 89–95. [DOI] [PubMed] [Google Scholar]
- Zhou S, Kemp J, Despres O, Pebayle T, & Dufour A (2015). The association between inhibition and pain tolerance in the elderly: evidence from event‐related potentials. European Journal of Pain, 19(5), 669–676. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhou R, & Fu L (2013). Working memory updating function training influenced brain activity. PloS one, 8(8), e71063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


