Abstract
Epoxides of free fatty acids (FFAs), especially epoxyeicosatrienoic acids (EETs), are lipid mediators with beneficial effects in metabolic and cardiovascular (CV) health. FFA epoxides are quickly metabolized to biologically less active diols by soluble epoxide hydrolase (sEH). Inhibition of sEH, which increases EET levels, improves glucose homeostasis and CV health and is proposed as an effective strategy for the treatment of diabetes and CV diseases. Here, we show evidence that sEH activity is profoundly reduced in postprandial states in rats; plasma levels of 17 sEH products (i.e., FFA diols), detected by targeted oxylipin analysis, all decreased after a meal. In addition, the ratios of sEH product to substrate (sEH P/S ratios), which may reflect sEH activity, decreased ~70% on average 2.5 h after a meal in rats (P < 0.01). To examine whether this effect was mediated by insulin action, a hyperinsulinemic euglycemic clamp was performed for 2.5 h, and sEH P/S ratios were assessed before and after the clamp. The clamp resulted in small increases rather than decreases in sEH P/S ratios (P < 0.05), indicating that insulin cannot account for the postprandial decrease in sEH P/S ratios. Interestingly, in rats treated with antibiotics to deplete gut bacteria, the postprandial effect to decrease sEH P/S ratios was completely abolished, suggesting that a gut bacteria-derived factor(s) may be responsible for the effect. Further studies are warranted to identify such a factor(s) and elucidate the mechanism by which sEH activity (or sEH P/S ratio) is reduced in postprandial states.
Keywords: FFA epoxides, cardiovascular disease, oxylipin analysis, dietary potassium
1. INTRODUCTION
Oxylipins, produced by oxidation of essential free fatty acids (FFAs), are signaling molecules involved in various biological functions, including pro- and anti-inflammatory responses [1–3]. The biosynthesis of a variety of oxylipins involves three families of enzymes, that is, cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP). Epoxy FFAs (or FFA epoxides), produced by CYP from several precursor FFAs, are known to exert beneficial effects on insulin secretion, glucose homeostasis, blood pressure, and CV health [4–6]. FFA epoxides (e.g., EETs [or EpETrE] derived from arachidonic acid) are metabolized to biologically less active diols (e.g., DHETs [or DiHETrE]) by sEH. Inhibition of sEH, which increases FFA epoxides, improves glucose homeostasis, blood pressure, and CV health in insulin resistant or diabetic animals [4–6] and has been proposed as an effective strategy for the treatment of diabetes and CV diseases [7]. Understanding mechanisms by which sEH is regulated in vivo would provide new therapeutic targets or strategies in the treatment of diabetes or CV diseases.
In our study originally aimed at examining the effect of dietary potassium (K+) on oxidative stress (see Discussion for rationale), we performed targeted oxylipin analysis of plasma samples from rats acutely fed a K+-rich or a K+-deficient diet. In this study, we found no significant effects of dietary K+ on postprandial levels of plasma oxylipins. However, we observed significant effects of meals on plasma oxylipin levels. In particular, we found profound effects of meals on sEH products (diols); 17 diols detected all decreased in postprandial states, compared to basal (i.e., pre-meal) states, despite no significant changes in many of their corresponding FFA epoxides. The ratios of sEH product to substrate (sEH P/S ratios), which may reflect in vivo sEH activity [29, 30], profoundly decreased 2.5 h after a meal, suggesting a strong postprandial effect to decrease sEH activity (or increase diol metabolism, see Discussion). A follow-up study showed evidence suggesting that the postprandial effect to decrease sEH P/S ratios is not mediated by insulin, a hormone that is secreted after a meal and strongly influences fuel metabolism. Interestingly, the postprandial effect on sEH P/S ratios was completely abolished in rats treated with antibiotics to deplete gut bacteria, suggesting that a gut bacteria-derived factor(s) may be responsible for the apparent reduction of sEH activity in postprandial states. Thus, the present study suggests the existence of a novel mechanism of in vivo regulation of sEH activity (or epoxide-to-diol ratios) involving gut bacteria.
2. MATERIALS AND METHODS
2.1 Animals and catheterization
Male Wistar rats weighing 280–300 g (approximately 9 weeks old) were obtained from Envigo Laboratories, and studied at least 5 days after arrival. Animals were housed under controlled temperature (22 ± 2°C) and lighting (12-h light, 6 AM–6 PM; 12-h dark, 6 PM–6 AM) with free access to water and standard rat chow. At least 4 days before the experiment, the animals were placed in individual cages with tail restraints, as previously described [8–9], which was required to protect tail blood-vessel catheters during the experiments. The animals were free to move about and were allowed unrestricted access to food and water. A tail-vein catheter for an insulin infusion and/or a tail-artery catheter for blood sampling were placed in the morning of the experiment (~7 AM). All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
2.2 Acute meal feeding and blood sampling
Animals were given either a K+-rich (2% vs. normal 1%) or a K+-deficient (0%) diet (n = 6 each) immediately before 6 PM when lights are off and feeding begins. The diets were prepared from K+-deficient powdered rat diet (TD.88239.PWD; Envigo Teklad) with or without supplementation with KCl. The diets were gelled by heating and dissolving 30 g agarose in 500 ml of deionized water and adding to 500 g of powdered diet [10]. Gelled diets were cut in small blocks and stored at −4°C until use. Animals were acclimatized to gel diets by feeding gel diets containing normal 1% K+ for 3–4 days before the acute feeding experiment. Blood samples were collected using the tail artery catheter before (basal) and 2.5 h after the initiation of feeding (i.e., 8:30 PM; postprandial). Blood samples were rapidly spun, and plasmas were isolated and mixed with triphenylphosphine (TPP; 4 µg/ml), butylated hydroxytoluene (BHT; 4 µg/ml), and EDTA (20 µg/ml). TPP was used to reduce peroxides to their monohydroxy equivalent, and BHT was used to quench radical catalyzed reactions [11]. Plasma samples were isolated and frozen immediately in liquid N2, and stored at −80°C until analysis. Diet consumption was matched between the groups by giving slightly less than the amount of ad libitum consumption for the first 2.5 hours of feeding. This resulted in identical postprandial conditions between the different K+ diet groups in terms of glucose, FFAs, insulin etc. (Oh et al., unpublished data).
2.3 Antibiotic treatment
The acute meal feeding experiment was also conducted in animals treated with antibiotics to remove gut bacteria. Animals were maintained on drinking water (autoclaved tap water) containing vancomycin, metronidazole, neomycin, and ampicillin (0.5 mg/ml for vancomycin and 1 mg/ml for the others) for a week. After this treatment, fecal DNA content decreased to 3% of control (data not shown), indicating that the antibiotic treatment was effective to remove most gut bacteria. After the antibiotic treatment, the animals were acutely fed the 0% or 2% K+ diet (n = 6 each), with drinking water containing antibiotics, and blood samples were collected before and after the 2.5-h feeding as described above.
2.4 Hyperinsulinemic euglycemic clamp
Blood samples were collected immediately before 6 PM through the tail-artery catheter for determination of basal glucose and later analysis of oxylipins. At 6 PM, a 2.5-h hyperinsulinemic-euglycemic clamp (n = 6) was begun by infusing human insulin (5 mU/kg/min; Novolin, Novo Nordisk, Princeton, NJ) to raise plasma insulin in physiological ranges [8, 9]. Blood samples were collected, and plasma glucose was monitored at 10–20 minute intervals, and 20% dextrose solution was infused to maintain plasma glucose at basal levels (~140 mg/dl). In addition, Intralipid (20%; 0.5 ml/h) and heparin (40 U/h with a bolus injection of 10 U) were infused to prevent depletion of plasma FFAs [12], arising from insulin inhibition of lipolysis in adipocytes, which may deplete plasma oxylipins. At the end of the clamp (i.e., 8:30 PM), additional blood samples were collected for oxylipin analysis. The timing of blood samples (basal and post treatment) were the same as the acute feeding experiments to avoid confounding effects of possible diurnal variations. Blood samples for oxylipin analysis were handled as described above, and plasma samples were frozen immediately in liquid N2, and stored at −80°C.
2.5 Oxylipin Analysis
Oxylipins were analyzed as previously described [11, 13]. Briefly, plasma samples underwent solid phase extraction (SPE) on 60 mg Waters Oasis-HLB cartridges (Milford, MA). The elutions from the SPE cartridges were evaporated using a Speedvac (Jouan, St-Herblain, France) and reconstituted in a 200 nM of 1-cyclohexyl ureido, 3-dodecanoic acid (CUDA) in a methanol solution. The LC system used for analysis was an Agilent 1200 SL (Agilent Corporation, Palo Alto, CA) equipped with a 2.1 × 150 mm Eclipse Plus C18 column with a 1.8 µm particle size (Agilent Corporation, Palo Alto, CA). The autosampler was kept at 4 °C. Mobile phase A was water with 0.1 % glacial acetic acid. Mobile phase B consisted of acetonitrile/methanol (84:16) with 0.1 % glacial acetic acid. Gradient elution was performed at a flow rate of 250 µL/min. Chromatography was optimized to separate all analytes in 21.5 min according to their polarity with the most polar analytes, prostaglandins and leukotrienes eluting first, followed by the dihydroxy, hydroxy and epoxy fatty acids. The column was connected to a 4000 QTrap tandem mass spectrometer (AB Sciex, Foster City, CA) equipped with an electrospray ion source (Turbo V). The instrument was operated in negative multiple reaction monitor (MRM) mode. The optimized conditions and the MRM transitions, as well as extraction efficiencies were reported previously [11]. Quality control samples were analyzed at a minimum frequency of 10 h to ensure stability of the analytical calibration throughout the analysis. Analyst software 1.4.2 was used to quantify the peaks according to the standard curves.
2.6 Statistical analysis
All data are expressed as means ± S.E.M. The significance of differences in the mean values were assessed by student’s t-tests or one-way ANOVA followed by ad hoc analysis using the Bonferroni method for multiple comparisons. A P value less than 0.05 was considered to be statistically significant.
3. RESULTS
3.1 Effects of a K+-rich or a K+-deficient meal on plasma oxylipins
Oxylipin analysis detected a total of 59 oxylipins in rat plasma samples, derived from 5 precursor FFAs, that is, linoleic acid (LA; 18:2n6), α-linolenic acid (ALA; 18:3n3), arachidonic acid (ARA; 20:4n6), eicosapentaenoic acid (EPA; 20:5n3), and docosahexaenoic acid (DHA 22:6n3). Table 1 shows the effects of a single meal with a K+-deficient (0% K+) or a K+-rich (2% K+) diet on plasma oxylipin levels. No significant effects of dietary K+ content on postprandial oxylipin levels were observed. However, significant postprandial changes (0% K+ or 2% K+ diet vs. basal) were observed in numerous oxylipins. First, 20-COOH-LTB4 derived from ARA in the 5-LOX pathway increased 3-fold after a meal independent of dietary K+ content (P < 0.05). This postprandial increase was specific for 20-COOH-LTB4, as other oxylipins in the 5-LOX pathway derived from ARA or LA were not significantly altered. In addition, some oxylipins derived from ARA (e.g., 8- and 11-HETE) or EPA (5-, 8-, and 15-HEPE) through 12/15-LOX decreased after a meal independent of dietary K+ content (P < 0.05). However, the most dramatic and consistent changes were observed with sEH products (i.e., diols) in the CYP pathway; 17 diols derived from different precursor FFAs (i.e., DiHOME from LA, DiHODE from ALA, DiHETrE from ARA, DiHETE from EPA, and DiHDPE from DHA) all decreased 40–90 % in the postprandial states (P <0.01 for all). In contrast, their corresponding epoxides (i.e., sEH substrates; EpOME, EpODE, EpETrE, EpETE, and EpDPE, respectively) were not significantly altered except for those derived from EPA (i.e., EpETE).
Table 1.
Plasma oxylipin levels in the basal and the postprandial states after a K+-deficient (0% K) or a K+-rich (2% K) meal in rats.
| Oxylipin | Enzyme | Precursor | Basal | 0% K Meal | 2% K Meal |
|---|---|---|---|---|---|
| 9-HODE | 5-LOX | LA | 29 ± 4 | 48 ± 8 | 34 ± 6 |
| 9-oxo-ODE | 5-LOX | LA | 48 ± 6 | 85 ± 17 | 89 ± 20 |
| 9,10,13-TriHOME | 5-LOX | LA | 4.5 ± 1.1 | 4.7 ± 1.2 | 5.6 ± 2.3 |
| 9,12,13-TriHOME | 5-LOX | LA | 7.5 ± 1.9 | 8.4 ± 2.1 | 9.6 ± 3.9 |
| 20-COOH-LTB4 | 5-LOX | ARA | 0.8 ± 0.2 | 2.0 ± 0.4 * | 2.5 ± 0.4** |
| 5-HETE | 5-LOX | ARA | 7.1 ± 0.7 | 5.5 ± 0.7 | 5.6 ± 0.9 |
| 5-oxo-ETE | 5-LOX | ARA | 4.0 ± 1.7 | 3.0 ± 0.7 | 3.8 ± 1.5 |
| 13-HODE | 12/15-LOX | LA | 78 ± 14 | 122 ± 21 | 88 ± 15 |
| 13-oxo-ODE | 12/15-LOX | LA | 1.4 ± 0.4 | 2.1 ± 0.6 | 2.0 ± 0.6 |
| 9-HOTrE | 12/15-LOX | ALA | 1.4 ± 0.2 | 1.6 ± 0.3 | 1.0 ± 0.2 |
| 13-HOTrE | 12/15-LOX | ALA | 1.5 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.5 |
| 8-HETE | 12/15-LOX | ARA | 3.4 ± 0.4 | 2.1 ± 0.2* | 2.2 ± 0.4* |
| 9-HETE | 12/15-LOX | ARA | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 11-HETE | 12/15-LOX | ARA | 3.1 ± 0.3 | 1.6 ± 0.2** | 1.8 ± 0.3** |
| 12-HETE | 12/15-LOX | ARA | 6.7 ± 1.3 | 4.6 ± 0.6 | 5.3 ± 1.0 |
| 15-HETE | 12/15-LOX | ARA | 6.3 ± 1.2 | 3.7 ± 0.4 | 3.8 ± 1.0 |
| 12-oxo-ETE | 12/15-LOX | ARA | 7.4 ± 2.0 | 8.0 ± 1.2 | 9.6 ± 1.9 |
| 15-oxo-ETE | 12/15-LOX | ARA | 3.3 ± 1.3 | 2.3 ± 0.5 | 3.2 ± 1.0 |
| LXA4 | 12/15-LOX | ARA | 0.5 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 |
| 5-HEPE | 12/15-LOX | EPA | 2.2 ± 0.4 | 0.9 ± 0.1 ** | 0.9 ± 0.1** |
| 8-HEPE | 12/15-LOX | EPA | 6.5 ± 2.2 | 1.5 ± 0.4 * | 2.4 ± 0.5* |
| 12-HEPE | 12/15-LOX | EPA | 11.0 ± 2.2 | 4.3 ± 1.0* | 8.9 ± 2.2 |
| 15-HEPE | 12/15-LOX | EPA | 1.0 ± 0.2 | 0.4 ± 0.1 ** | 0.3 ± 0.1** |
| 9(10)-EpOME | CYP | LA | 20 ± 3 | 33 ± 6 | 34 ± 6 |
| 12(13)-EpOME | CYP | LA | 22 ± 4 | 37 ± 6 | 35 ± 6 |
| 9,10-DiHOME | CYP | LA | 5.3 ± 0.5 | 2.7 ± 0.4*** | 1.9 ± 0.3*** |
| 12,13-DiHOME | CYP | LA | 12.3 ± 1.6 | 2.9 ± 0.8*** | 2.2 ± 0.4*** |
| 9(10)-EpODE | CYP | ALA | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| 12(13)-EpODE | CYP | ALA | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| 15(16)-EpODE | CYP | ALA | 7.5 ± 1.6 | 4.0 ± 0.7* | 3.3 ± 0.4** |
| 9,10-DiHODE | CYP | ALA | 0.29 ± 0.07 | 0.12 ± 0.02** | 0.04 ± 0.02*** |
| 12,13-DiHODE | CYP | ALA | 0.23 ± 0.05 | 0.05 ± 0.02*** | 0.03 ± 0.01*** |
| 15,16-DiHODE | CYP | ALA | 1.5 ± 0.2 | 0.3 ± 0.1 *** | 0.3 ± 0.0*** |
| 8(9)-EpETrE | CYP | ARA | 3.6 ± 0.7 | 3.2 ± 0.6 | 3.6 ± 1.0 |
| 11(12)-EpETrE | CYP | ARA | 7.8 ± 1.5 | 6.8 ± 1.3 | 8.4 ± 2.0 |
| 14(15)-EpETrE | CYP | ARA | 4.3 ± 0.8 | 3.3 ± 0.5 | 4.2 ± 0.9 |
| 5,6-DiHETrE | CYP | ARA | 0.32 ± 0.01 | 0.18 ± 0.01*** | 0.19 ± 0.01*** |
| 8,9-DiHETrE | CYP | ARA | 0.53 ± 0.06 | 0.14 ± 0.01*** | 0.12 ± 0.01*** |
| 11,12-DiHETrE | CYP | ARA | 2.5 ± 0.2 | 0.6 ± 0.1*** | 0.5 ± 0.0*** |
| 14,15-DiHETrE | CYP | ARA | 1.7 ± 0.1 | 0.5 ± 0.1 *** | 0.5 ± 0.0*** |
| 20-HETE | CYP | ARA | 3.8 ± 0.3 | 0.9 ± 0.1*** | 0.7 ± 0.1*** |
| 11(12)-EpETE | CYP | EPA | 0.9 ± 0.2 | 0.3 ± 0.1 * | 0.5 ± 0.1 |
| 14(15)-EpETE | CYP | EPA | 0.7 ± 0.2 | 0.2 ± 0.1** | 0.3 ± 0.1* |
| 17(18)-EpETE | CYP | EPA | 1.0 ± 0.2 | 0.3 ± 0.1** | 0.4 ± 0.1** |
| 11,12-DiHETE | CYP | EPA | 0.24 ± 0.05 | 0.04 ± 0.0*** | 0.03 ± 0.00*** |
| 14,15-DiHETE | CYP | EPA | 0.26 ± 0.06 | 0.04 ± 0.01*** | 0.04 ± 0.01*** |
| 17,18-DiHETE | CYP | EPA | 1.4 ± 0.3 | 0.2 ± 0.0*** | 0.2 ± 0.0*** |
| 10(11)-EpDPE | CYP | DHA | 1.6 ± 0.3 | 1.5 ± 0.5 | 1.4 ± 0.4 |
| 13(14)-EpDPE | CYP | DHA | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| 16(17)-EpDPE | CYP | DHA | 1.1 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.2 |
| 19(20)-EpDPE | CYP | DHA | 3.8 ± 0.7 | 2.8 ± 0.6 | 2.8 ± 0.5 |
| 4,5-DiHDPE | CYP | DHA | 5.6 ± 0.6 | 1.5 ± 0.1 *** | 1.6 ±0.3*** |
| 10,11-DiHDPE | CYP | DHA | 0.39 ± 0.05 | 0.07 ± 0.00*** | 0.07 ± 0.01*** |
| 13,14-DiHDPE | CYP | DHA | 0.15 ± 0.01 | 0.05 ± 0.00*** | 0.04 ± 0.00*** |
| 16,17-DiHDPE | CYP | DHA | 0.33 ± 0.04 | 0.08 ± 0.01*** | 0.07 ± 0.01*** |
| 19,20-DiHDPE | CYP | DHA | 1.9 ± 0.4 | 0.4 ± 0.0 *** | 0.4 ± 0.0*** |
| 7(8)-EpDPE | CYP | DHA | 30 ± 7 | 32 ± 11 | 29 ± 8 |
| PGD2 | COX | ARA | 0.13 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0.03 |
| EKODE | None | LA | 23 ± 14 | 46 ± 20 | 32 ± 10 |
P < 0.05;
P < 0.01;
P < 0.001 vs. basal.
3.2 Effects of a meal and dietary K+ on sEH activity
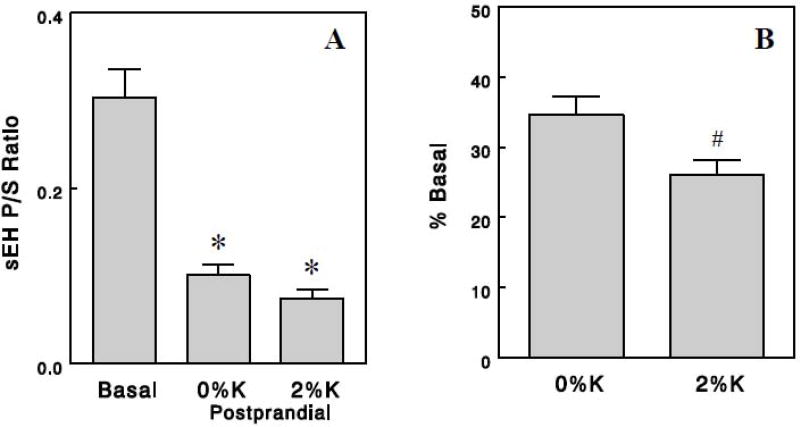
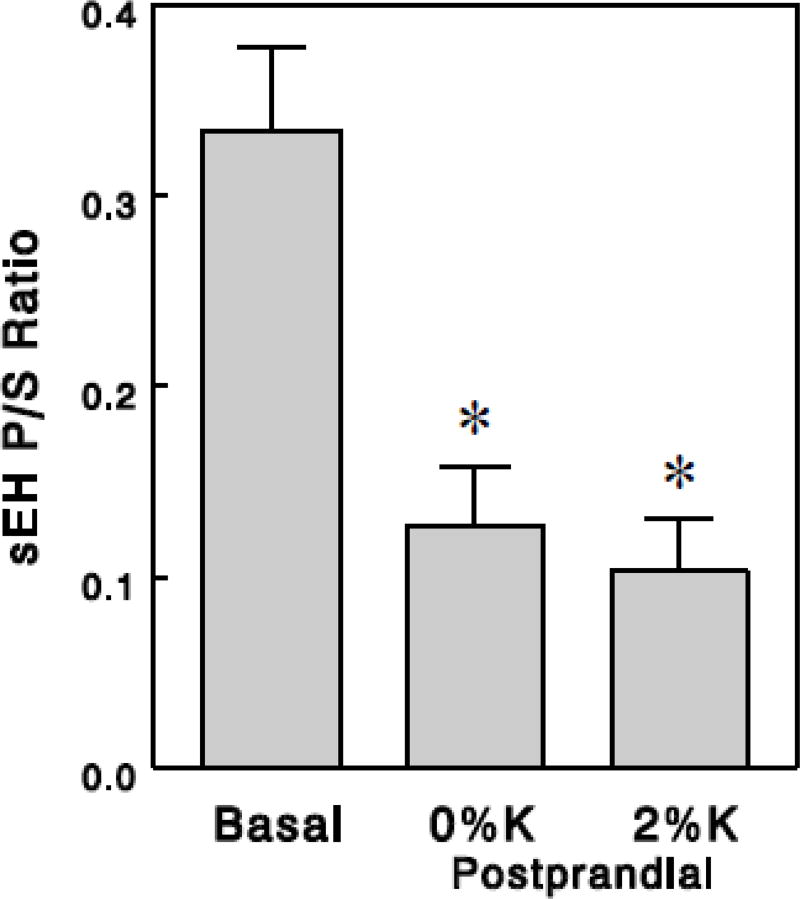
The ratios of sEH product to substrate (sEH P/S ratios), which may reflect in vivo sEH activity ([29, 30]; also see Discussion), were calculated for 15 pairs of sEH substrates (FFA epoxides) and products (diols) (Table 2). First, we calculated these ratios from group averages of oxylipins, which resulted in similar values among different epoxide-diol pairs with standard errors of 10–13% in all (i.e., basal and postprandial) groups, excluding one outlier determined by 2 standard deviations from the mean (Table 2). Thus, sEH P/S ratios produced reasonably consistent values among epoxide-diol pairs within each experimental condition. We found profound (~70%) effects of meals to decrease sEH P/S ratios (P < 0.001; Figure 1A), suggesting that sEH activity may be reduced in the postprandial states. In addition, dietary K+ showed a tendency to increase the postprandial effect to decrease sEH P/S ratios; when this effect was quantified for each epoxide-diol pair as decrease from basal, the dietary K+ effect gained statistical significance (P < 0.05; Table 2 & Figure 1B). We also compared sEH P/S ratios among the basal and postprandial groups by estimating sEH P/S ratios in each rat based on each rat's oxylipin levels (rather than group averages). This showed similar postprandial or dietary K+ effects although variations were somewhat greater (Figure 2) probably because of a small number of animals for each group (5 or 6). Thus, this analysis suggests that sEH activity may be markedly reduced in postprandial states, and this effect may be enhanced by dietary K+ content.
Table 2.
sEH P/S ratios calculated based on group averages of oxylipin levels (Table 1).
| Precursor FFA |
Oxylipin | sEH P/S ratio | Postprandial Effect | ||||
|---|---|---|---|---|---|---|---|
| sEH Substrate | sEH Product | Basal (B) | 0%K | 2%K | 0%K / B | 2%K / B | |
| LA (18:2n6) | 9(10)-EpOME | 9,10-DiHOME | 0.26 | 0.08 | 0.06 | 0.32 | 0.22 |
| 12(13)-EpOME | 12,13-DiHOME | 0.55 | 0.08 | 0.06 | 0.14 | 0.12 | |
| ALA (18:3n3) | 9(10)-EpODE | 9,10-DiHODE | 0.24 | 0.11 | 0.04 | 0.45 | 0.16 |
| 12(13)-EpODE | 12,13-DiHODE | 0.31 | 0.11 | 0.06 | 0.35 | 0.20 | |
| 15(16)-EpODE | 15,16-DiHODE | 0.20 | 0.08 | 0.09 | 0.41 | 0.44 | |
| ARA (20:4n6) | 8(9)-EpETrE | 8,9-DiHETrE | 0.15 | 0.04 | 0.03 | 0.30 | 0.23 |
| 11(12)-EpETrE | 11,12-DiHETrE | 0.32 | 0.09 | 0.06 | 0.29 | 0.19 | |
| 14(15)-EpETrE | 14,15-DiHETrE | 0.40 | 0.17 | 0.12 | 0.42 | 0.31 | |
| EPA (20:5n3) | 11(12)-EpETE | 11,12-DiHETE | 0.26 | 0.11 | 0.06 | 0.42 | 0.24 |
| 14(15)-EpETE | 14,15-DiHETE | 0.37 | 0.20 | 0.13 | 0.54 | 0.35 | |
| 17(18)-EpETE | 17,18-DiHETE | 1.46^ | 0.53^ | 0.49^ | 0.36 | 0.34 | |
| DHA (22:6n3) | 10(11)-EpDPE | 10,11-DiHDPE | 0.24 | 0.05 | 0.05 | 0.20 | 0.20 |
| 13(14)-EpDPE | 13,14-DiHDPE | 0.15 | 0.06 | 0.05 | 0.41 | 0.32 | |
| 16(17)-EpDPE | 16,17-DiHDPE | 0.30 | 0.08 | 0.08 | 0.28 | 0.26 | |
| 19(20)-EpDPE | 19,20-DiHDPE | 0.50 | 0.15 | 0.16 | 0.30 | 0.31 | |
|
| |||||||
| mean ± SE | 0.30 ± 0.03 | 0.10 ± 0.01** | 0.08 ± 0.01** | 0.35 ± 0.03 | 0.26 ± 0.02# | ||
P < 0.01 vs. basal;
P < 0.05 vs. 0%K / B;
outlier.
Figure 1.
sEH P/S ratios, expressed as absolute values (A) and as % changes from basal (B). These values were calculated based on group averages of epoxides and diols. Values are means ± SEM for 14 epoxide-diol pairs. *, P < 0.001 vs. basal; #, P < 0.05 vs. 0% K.
Abbreviations used: HODE, hydroxyoctadienoic acid; oxo-ODE, oxo-octadecadienoic acid; TriHOME, trihydroxyoctamonoenoic acid; 20-COOH-LTB4, 20-carboxy-leukotriene B4; HETE, hydroxyeicosatetraenoic acid; oxo-ETE, oxo-eicosatetraenoic acid; HOTrE, hydroxyoctadecadienoic acid; LXA4, lipoxin A4; HEPE, hydroxyeicosaptenaenoic acid; EpOME, epoxyoctamonoenoic acid; DiHOME, dihydroxyoctamonoenoic acid; EpODE, epoxyoctadecadienoic acid; DiHODE, dihydroxyoctadecadienoic acid; EpETrE (or EET), epoxyeicosatrienoic acid; DiHETrE (or DHET), dihydroxyeicosatrienoic acid; EpETE, epoxyeicosatetreaenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; EpDPE, epoxydocosapentaenoic acid; DiHDPE, dihydroxydocosapentaenoic acid; PGD2, prostaglandin D2; EKODE, epoxy-keto-octadecenoic acid; COX, cyclooxygenase; LOX, lipoxygenase; CYP, cytochrome P 450; LA, linoleic acid; ALA, α-linolenic acid; ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Figure 2.
sEH P/S ratios, estimated from individual rats based on each rat's oxylipin levels (rather than group averages), in the basal and the postprandial states. Values are means ± SEM for 5 (basal) or 6 (postprandial) rats. *, P < 0.01 vs. basal.
3.3 Effects of insulin on sEH activity
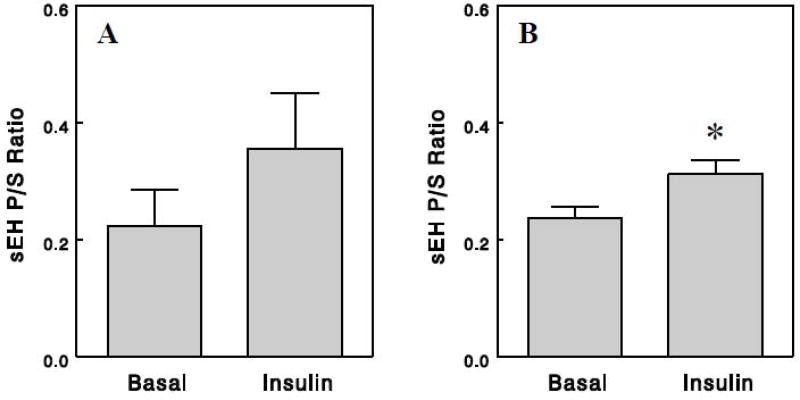
Insulin is a hormone that is secreted in response to meal intake. Insulin has strong influences on glucose and lipid metabolism and cellular signaling in postprandial states. To test whether insulin plays a role in the postprandial reduction of sEH activity, we performed a hyperinsulinemic euglycemic clamp (instead of feeding) for 2.5 h, and oxylipin analysis was performed on plasma samples collected before and after the clamp. Numerous oxylipins derived from LA or ALA were significantly increased after the clamp, whereas many oxylipins derived from DHA or EPA decreased (Table 3). In particular, we noted that the clamp marked increased 9-HODE and 13-HODE, oxidative stress markers derived from LA [14, 15], and 9-HOTrE and 13- HOTrE, derived from ALA and known to exert anti-inflammatory effects [18, 19] (P < 0.001; see Discussion). These precursor FFA-dependent changes in oxylipins might be related to differential changes in precursor FFA levels during the clamp; plasma levels of EPA or DHA levels might decrease as a result of inhibition of lipolysis in adipocytes by insulin, whereas plasma levels of LA or ALA might increase as a result of lipolysis of Intralipid infused during the clamp that provides LA and ALA (see Discussion). FFA epoxides also increased (EpOME & EpODE) or decreased (EpETE & EpDPE) after the clamp, depending largely on their precursor FFAs. However, their corresponding diols changed similarly, resulting in sEH P/S ratios generally unaltered by insulin (Figure 3A). In fact, when sEH P/S ratios were estimated for each rat and compared among groups, a small but statistically significant increase in sEH P/S ratios was observed after the clamp (Figure 3B). These data suggest that insulin increases rather than decreases sEH activity and cannot account for the postprandial reduction of sEH activity.
Table 3.
Effects of insulin on plasma oxylipin levels.
| Oxylipin | Oxylipin Level (nM) | Fold Effect |
P Value | Precursor FFA |
|
|---|---|---|---|---|---|
| Basal | Insulin | ||||
| 9-HODE | 51 ± 12 | 678 ± 77 | 13.3 | <0.001 | LA |
| 13-HODE | 104 ± 19 | 1193 ± 121 | 11.5 | <0.001 | LA |
| EKODE | 8.5 ± 1.5 | 31 ± 8 | 3.6 | 0.025 | LA |
| 13-oxo-ODE | 11 ± 1 | 19 ± 2 | 1.8 | 0.011 | LA |
| 9(10)-EpOME | 81 ± 7 | 134 ± 12 | 1.6 | 0.003 | LA |
| 12(13)-EpOME | 88 ± 8 | 129 ± 11 | 1.5 | 0.014 | LA |
| 9,10-DiHOME | 32 ± 2 | 77 ± 6 | 2.4 | <0.001 | LA |
| 9,12,13-TriHOME | 14 ± 1 | 18 ± 1 | 1.3 | 0.013 | LA |
|
| |||||
| 9-HOTrE | 5.2 ± 1.4 | 119 ± 16 | 22.8 | <0.001 | ALA |
| 13-HOTrE | 5.7 ± 1.4 | 104 ± 14 | 18.3 | <0.001 | ALA |
| 9(10)-EpODE | 5.3 ± 0.5 | 16 ± 1 | 3.0 | <0.001 | ALA |
| 12(13)-EpODE | 4.0 ± 0.4 | 8.6 ± 0.9 | 2.2 | 0.001 | ALA |
| 15(16)-EpODE | 65 ± 8 | 42 ± 5 | 0.7 | 0.033 | ALA |
| 9,10-DiHODE | 2.9 ± 0.4 | 27 ± 3 | 9.4 | <0.001 | ALA |
| 12,13-DiHODE | 1.3 ± 0.1 | 2.5 ± 0.3 | 1.9 | 0.002 | ALA |
| 15,16-DiHODE | 7.9 ± 0.6 | 42 ± 5 | 5.4 | <0.001 | ALA |
|
| |||||
| 12-oxo-ETE | 17 ± 2 | 27 ± 3 | 1.6 | 0.024 | ARA |
| 14(15)-EpETrE | 16 ± 2 | 11 ± 1 | 0.7 | 0.018 | ARA |
|
| |||||
| 5-HEPE | 9.8 ± 1.6 | 5.4 ± 1.0 | 0.6 | 0.043 | EPA |
| 12-HEPE | 34 ± 4 | 20 ± 4 | 0.6 | 0.031 | EPA |
| 14(15)-EpETE | 7.6 ± 0.8 | 3.1 ± 0.4 | 0.4 | 0.001 | EPA |
| 17(18)-EpETE | 12.7 ± 0.8 | 6.3 ± 0.7 | 0.5 | <0.001 | EPA |
| 11,12-DiHETE | 0.35 ± 0.02 | 0.27 ± 0.02 | 0.8 | 0.024 | EPA |
| 14,15-DiHETE | 0.60 ± 0.05 | 0.38 ± 0.06 | 0.6 | 0.016 | EPA |
|
| |||||
| 10(11)-EpDPE | 7.2 ± 0.5 | 4.9 ± 0.7 | 0.7 | 0.028 | DHA |
| 13(14)-EpDPE | 4.7 ± 0.4 | 3.0 ± 0.3 | 0.6 | 0.011 | DHA |
| 16(17)-EpDPE | 6.1 ± 0.5 | 3.5 ± 0.3 | 0.6 | 0.002 | DHA |
| 19(20)-EpDPE | 10.8 ± 0.7 | 7.3 ± 0.8 | 0.7 | 0.011 | DHA |
| 16,17-DiHDPE | 0.58 ± 0.03 | 0.40 ± 0.07 | 0.7 | 0.035 | DHA |
| 19,20-DiHDPE | 2.7 ± 0.1 | 1.6 ± 0.1 | 0.6 | <0.001 | DHA |
Figure 3.
Effects of insulin on sEH P/S ratios estimated from group averages of epoxides and diols as in Figure 1 (A) or from individual rats as in Figure 2 (B). Values are means ± SEM for 12 epoxide-diol pairs (A) or for 6 rats (B). *, P < 0.05 vs. basal.
3.4 Role of gut bacteria in postprandial reduction of sEH activity
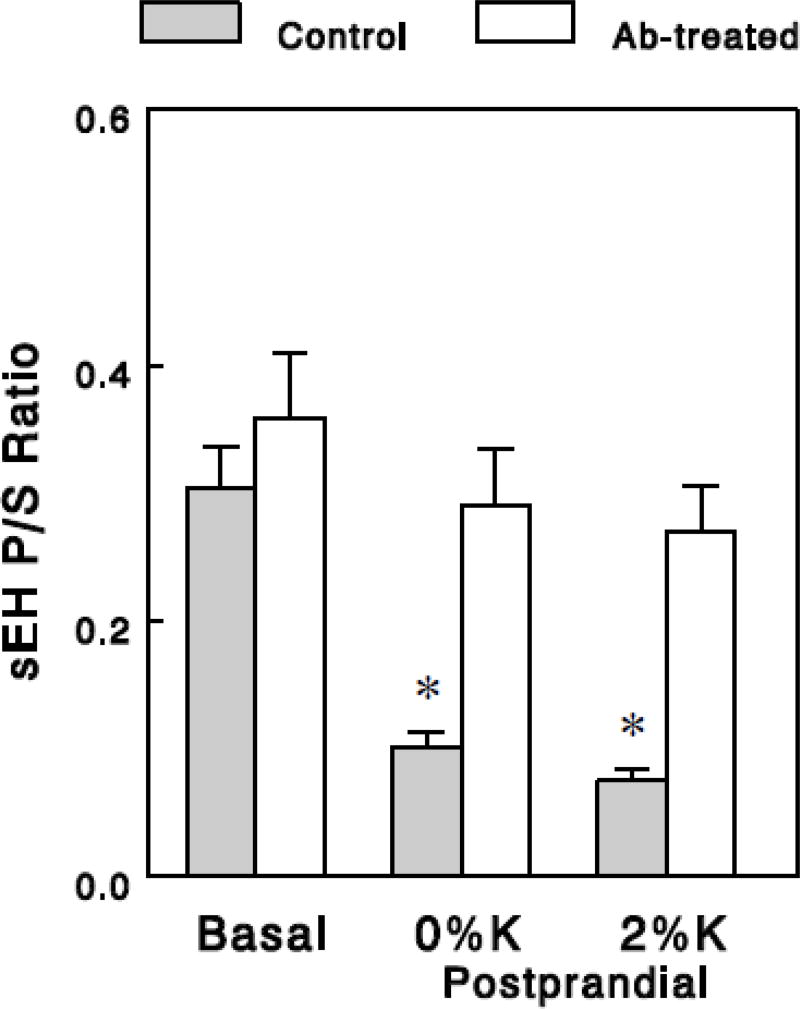
To test whether gut bacteria play a role in the postprandial reduction of sEH activity, we repeated the acute feeding experiments in rats treated with antibiotics for one week to deplete gut bacteria (see Methods). Plasma oxylipin levels in the basal state were not vastly altered by the antibiotic treatment (data not shown), although a few oxylipins generated in the 5-LOX (9,10,13- and 9,12,13-TriHOME) or the 12/15-LOX (8-, 11-, and 15-HETE) pathway showed 3 to 4-fold decreases, suggesting possible inhibition of LOX after the depletion of gut bacteria. Interestingly, in these antibiotic-treated animals, we saw no significant effects of meals on sEH P/S ratios, in sharp contrast with the profound postprandial effects in control animals (Figure 4), suggesting that gut bacteria may be required for the postprandial effects to reduce sEH activity.
Figure 4.
sEH P/S ratios in the basal and the postprandial states in control and antibiotic (Ab)-treated rats. sEH P/S ratios were estimated from group averages of epoxides and diols as in Figure 1, and the data for control rats are the same as those in Figure 1. Values are means ± SEM for 14 (A) or 7 (B) epoxide-diol pairs. *, P < 0.001 vs. basal.
4. DISCUSSION
Numerous studies have demonstrated the importance of sEH to the regulation of glucose homeostasis, blood pressure, and cardiac health [4–6]. However, mechanisms by which sEH is regulated in vivo are largely unknown. The present study demonstrates that sEH P/S ratios, calculated from 14–15 different sEH substrate-product pairs detected in rat plasma, showed very similar values. In addition, these ratios were all altered in postprandial states in a similar fashion, suggesting that they may reflect changes in sEH activity as noted below. Using this indicator of sEH activity [29, 30], the present study demonstrates profound decreases in sEH activity in postprandial states, which were enhanced by dietary K+. We found that insulin increases rather than decreases sEH P/S ratios, suggesting that insulin action cannot account for the postprandial reduction of sEH activity. Interestingly, the postprandial effects were completely abolished in antibiotic-treated animals, suggesting that a gut flora-derived factor(s) may be responsible for the reduction of sEH activity in postprandial states. Thus, the present study suggests the existence of a novel mechanism for in vivo regulation of sEH activity (or oxylipin metabolism) involving gut bacteria.
sEH P/S ratios have been used as indirect measures of in vivo sEH activity [29, 30]. However, sEH substrate (epoxides) or product (diols) levels may be affected by many factors besides sEH activity. For example, epoxide levels may be increased by their biosynthesis or release from phospholipid stores or decreased by degradation through pathways independent of sEH [1]. Also, diol levels may be altered by their metabolism through sulfation or glucuronidation for elimination from the body. Therefore, sEH P/S ratios (i.e., diol to epoxide ratios) may be affected by factors other than sEH and, as such, may not always reflect sEH activity. However, when 14–15 different diol-epoxide pairs show identical changes in diol-to-epoxide ratios, these changes are best explained by changes in sEH activity, which is the common denominator of factors affecting these ratios. Therefore, similar changes in all sEH P/S ratios may faithfully reflect changes in sEH activity, although we cannot exclude the possibility that all diol levels are altered similarly by a factor other than sEH (e.g., excretion through urine or feces). On the other hand, when these ratios change individually, other factors associated with specific epoxide-diol pairs may be involved.
Our analysis was based on plasma oxylipins. Plasma oxylipin levels may be determined by exchanges of oxylipins between plasma and body tissues. Oxylipin metabolism in individual tissues may be quite different, depending on tissue availability of precursor FFAs as well as activities of enzymes involved in oxylipin metabolism. The sEH activity reflected in plasma oxylipins (i.e., sEH P/S ratios) may represent an average of individual tissue sEH activities. Alternatively, it may represent sEH activities in specific tissues that predominantly contribute to plasma oxylipin levels. sEH is highly expressed in the liver and kidneys [7], and it is possible that the sEH activity estimated from plasma oxylipins reflects sEH activities in these metabolically active organs. This issue can be tested by examining whether changes in sEH activity predicted from plasma oxylipins (e.g., in postprandial states) are similarly observed in all or specific tissues, as estimated from tissue oxylipin levels or measured directly by enzymatic assays.
Regulation of sEH activity has been studied under various conditions, including diabetes, obesity, and treatment with hormones or drugs [16]. These studies have been carried out mainly in animal tissues or incubated cells by measuring sEH expression or its enzyme activities. Limited access to human tissues makes it difficult to study the regulation of sEH activity in humans. The approach of estimating sEH activity from plasma oxylipins can be applied to human studies, allowing studies of in vivo regulation of this important enzyme and identification of major regulatory factors, although, as discussed above, tissues represented by this estimation remain to be determined. In fact, recent human studies have used epoxide-diol ratios, estimated from select epoxide-diol pairs, as proxy markers of in vivo sEH activity [29, 30]. The present study demonstrates that sEH P/S ratios are estimated quite similarly from oxylipins derived from all major FFA precursors (i.e., LA, ALA, ARA, EPA, and DHA; Table 2). Increasing the number of epoxide-diol pairs in estimating sEH P/S ratios would increase the power of detecting changes in sEH activity, and, in the present study, a small (26%) effect of dietary K+ on postprandial sEH activity gained statistical significance (Figure 1B). Another advantage of this approach is that it may detect changes in sEH activity independent of gene expression. The profound effect of meals to decrease sEH P/S ratios was observed only after 2.5 hours, and this acute control may not involve altered gene expression. If so, understanding mechanisms underlying such regulations would provide important insights into the physiological or pathological role of sEH.
Insulin increased LA- and ALA- derived oxylipins but decreased DHA- and EPA-derived oxylipins. These changes may be related to precursor FFA availability; plasma EPA and DHA levels might decrease during insulin infusion (or the clamp), as a result of insulin inhibition of lipolysis in adipocytes, whereas LA and ALA levels might increase, as a result of Intralipid and heparin infusion during the clamp. Intralipid and heparin were infused during insulin infusion in order to prevent a depletion of plasma FFAs and their oxylipin products. Intralipid contains substantial amounts of LA (53%) or ALA (7.3%), but not ARA (0.2%), DHA (0.2%), or EPA (0%) [17]. Increased or decreased precursor FFAs might have caused similar changes in their oxylipin products by mass action, i.e., in the absence of changes in oxidative enzymes. However, the increases in LA-derived 9- and 13-HODE (> 10-fold), known as oxidative stress markers [14, 15], were much greater than those in other LA-derived oxylipins (e.g., EpOME, ~1.5-fold; EKODE, 3.6-fold; Table 3). Although this may indicate differential sensitivity of different biochemical pathways to substrate availability, these data may suggest that the effect of insulin on 9- and 13-HODE may indicate more than mass action effects, and insulin may increase oxidative stress by stimulating fuel oxidation and thus generation of reactive oxygen species. In addition, insulin dramatically increased 9- and 13-HOTrE (23- and 18-fold, respectively) derived from ALA. Again, these increases may indicate more than mass action effects arising from increases in plasma ALA levels, as other ALA-derived oxylipins also increased but not as much as 9- or 13-HOTrE (Table 3). HOTrEs are known to exert anti-inflammatory effects [18, 19], and our data suggest that these effects of insulin to increase 9- or 13-HOTrE may contribute to its well-known anti-inflammatory effects [20].
It is well known that dietary K+ intake improves blood pressure and CV health [21, 22]. Although beneficial effects of dietary K+ intake have been well documented, underlying molecular mechanisms have been elusive. Young and colleagues have suggested that beneficial effects of increased dietary K+ intake are brought about by increased plasma K+ concentration ([K+]), based on in vitro observations that high extracellular [K+] exerts cellular effects to inhibit free-radical formation, smooth-muscle proliferation, and thrombus formation [23, 24]. However, beneficial effects of K+ supplementation have often been observed in clinical studies without an increase in plasma [K+] [25, 26], suggesting they may be mediated by a mechanism independent of plasma [K+]. Our preliminary metabolomic study in rats showed that dietary K+ had profound effects to decrease plasma levels of oxidized lipids, including 9-HODE and 13-HODE (Oh et al, unpublished data), suggesting that dietary K+ may decrease postprandial oxidative stress. Because oxidative stress is known to cause endothelial dysfunction [27, 28], these data suggest the intriguing possibility that dietary K+ brings about beneficial CV effects by suppressing postprandial oxidative stress. The present study was designed to follow up the metabolomic study and examine dietary K+ effects on plasma oxylipins. Although we observed that 9-HODE and 13-HODE increased by 60–70% after a K+-deficient diet, and these increases were almost completely suppressed by a K+-rich diet, these changes were only marginally significant. Unexpectedly, we found a significant effect of dietary K+ to increase postprandial effect to reduce sEH activity, which may help improve blood pressure and CV health. Although this effect was small (~25%), this was observed after a single meal, and a long-term feeding with a K+-rich (vs. K+-deficient) diet may produce more profound effects. The idea of a potential link between dietary K+ and CV health through sEH is novel, and future studies are warranted to directly test this intriguing idea.
Acknowledgments
J.Y. and Y.T.O designed and performed the experiments. J.Y., D.W., and Y.T.O. analyzed most of the data and wrote a draft manuscript. R.M.W. and B.D.H. participated in discussing the data and editing the manuscript. J.H.Y. was involved in all stages of the project and edited the manuscript.
Funding: This work was supported by ADA Basic Science Award 1-16-IBS-332 and Pilot Project grant from the West Coast Metabolomics Center at UC Davis (to JHY), NIEHS grant R01 ES002710 and NIEHS Superfund Research Program P42 ES004699 (to BDH), and the West Coast Metabolomics Center at UC Davis (NIH/NIDDK U24 DK097154) (to BDH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
We have no conflicts of interest to disclose in connection with the manuscript.
References
- 1.Taha AY, Hennebelle M, Yang J, Zamora D, Rapoport SI, Hammock BD, Ramsden CE. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot Essent Fatty Acids. 2016 doi: 10.1016/j.plefa.2016.05.004. pii: S0952-3278(16)30017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Weng J, Wang MH. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat. 2016;125:80–9. doi: 10.1016/j.prostaglandins.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Mustafa S, Sharma V, McNeill JH. Insulin resistance and endothelial dysfunction: Are epoxyeicosatrienoic acids the link? Exp Clin Cardiol. 2009;14:e41–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–43. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012:758614. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–8. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–37. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 8.Youn JH, Buchanan TA. Fasting does not impair insulin-stimulated glucose uptake but alters intracellular glucose metabolism in conscious rats. Diabetes. 1993;42:757–63. doi: 10.2337/diab.42.5.757. [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, 1, Wi JK, Youn JH. Metabolic impairment precedes insulin resistance in skeletal muscle during high-fat feeding in rats. Diabetes. 1996;45:651–8. doi: 10.2337/diab.45.5.651. [DOI] [PubMed] [Google Scholar]
- 10.Oh YT, Kim J, Youn JH. Role of pituitary in K+ homeostasis: impaired renal responses to altered K+ intake in hypophysectomized rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1166–74. doi: 10.1152/ajpregu.00495.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–93. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh YT, Kim J, Kang I, Youn JH. Regulation of hypothalamic-pituitary-adrenal axis by circulating free fatty acids in male Wistar rats: role of individual free fatty acids. Endocrinology. 2014;155:923–31. doi: 10.1210/en.2013-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zivkovic AM, 1, Yang J, Georgi K, Hegedus C, Nording ML, O'Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C, Hammock BD. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida Y, Niki E. Bio-markers of lipid peroxidation in vivo: hydroxyoctadecadienoic acid and hydroxycholesterol. Biofactors. 2006;27(1–4):195–202. doi: 10.1002/biof.5520270117. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida Y, Itoh N, Hayakawa M, Piga R, Cynshi O, Jishage K, Niki E. Lipid peroxidation induced by carbon tetrachloride and its inhibition by antioxidant as evaluated by an oxidative stress marker, HODE. Toxicol Appl Pharmacol. 2005;208:87–97. doi: 10.1016/j.taap.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Harris TR, Hammock BD. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris S, 1, Simmer K, Gibson R. Characterization of fatty acid clearance in premature neonates during intralipid infusion. Pediatr Res. 1998;43:245–9. doi: 10.1203/00006450-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Gupta G, Anilkumar K, Fatima N, Karnati R, Reddy GV, Giri PV, Reddanna P. 15-Lipoxygenase metabolites of α-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci Rep. 2016;6:31649. doi: 10.1038/srep31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze-Tanzil G, 1, de SP, Behnke B, Klingelhoefer S, Scheid A, Shakibaei M. Effects of the antirheumatic remedy hox alpha--a new stinging nettle leaf extract--on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol. 2002;17:477–85. doi: 10.14670/HH-17.477. [DOI] [PubMed] [Google Scholar]
- 20.Dandona P, 1, Chaudhuri A, Ghanim H, Mohanty P. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol. 2009;53(5 Suppl):S14–20. doi: 10.1016/j.jacc.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 22.D'Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–9. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 23.Young DB, Lin H, McCabe RD. Potassium's cardiovascular protective mechanisms. Am J Physiol. 1995;268:R825–37. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Young DB. Interaction between plasma potassium and epinephrine in coronary thrombosis in dogs. Circulation. 1994;89:331–8. doi: 10.1161/01.cir.89.1.331. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, Lu X, Skurnick J, Awad G, Bogden J, Kemp F, et al. Potassium chloride supplementation diminishes platelet reactivity in humans. Hypertension. 2004;44:969–73. doi: 10.1161/01.HYP.0000147660.58694.6f. [DOI] [PubMed] [Google Scholar]
- 26.Braschi A, Naismith DJ. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br J Nutr. 2008;99:1284–92. doi: 10.1017/S0007114507864853. [DOI] [PubMed] [Google Scholar]
- 27.Heo KS, Fujiwara K, Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J. 2011;75:2722–30. doi: 10.1253/circj.cj-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, Palesty A, McFadden DW, Parinandi NL, Maulik N. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386:233–49. doi: 10.1007/s11010-013-1861-x. [DOI] [PubMed] [Google Scholar]
- 29.Lee JP, Yang SH, Kim DK, Lee H, Kim B, Cho JY, Yu KS, Paik JH, Kim M, Lim CS, Kim YS. In vivo activity of epoxide hydrolase according to sequence variation affects the progression of human IgA nephropathy. Am J Physiol Renal Physiol. 2011;300:F1283–90. doi: 10.1152/ajprenal.00733.2010. [DOI] [PubMed] [Google Scholar]
- 30.Shih PB, Yang J, Morisseau C, German JB, Zeeland AA, Armando AM, Quehenberger O, Bergen AW, Magistretti P, Berrettini W, Halmi KA, Schork N, Hammock BD, Kaye W. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol Psychiatry. 2016;21:537–46. doi: 10.1038/mp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]