Abstract
Background
In total knee arthroplasty (TKA) periprosthetic joint infection (PJI), irrigation debridement (I&D) with component retention is a treatment option with a wide variation in reported failure rates. The purpose of this study was to determine failure rates, outcomes, and factors that predict failure in I&D for TKA PJI.
Methods
A multicenter observational study of patients with a TKA PJI and subsequently undergoing an I&D with retention of components was conducted. The primary outcome was failure rate of I&D, where failure was defined as any subsequent surgical procedures.
Results
216 cases of I&D with retention of components performed on 206 patients met inclusion criteria. The estimated long-term failure rate at 4 years was 57.4%. Time-to-event analyses revealed that the median survival time was 14.32 months. Five-year mortality was 19.9%. Multivariable modeling revealed that time symptomatic and organism were independent predictors of I&D failure. Culture negative status had a higher hazard for failure than culture positive patients. When primary organism and time symptomatic were selected to produce an optimized scenario for an I&D, the estimated failure rate was 39.6%.
Conclusions
I&D with retention of components has a high failure rate, and there is a high incidence of more complex procedures after this option is chosen. The patient comorbidities we investigated did not predict I&D success. Our results suggest that I&D has a limited ability to control infection in TKA and should be used selectively under optimum conditions.
Introduction
Total knee arthroplasty (TKA) is becoming more prevalent in the United States. By 2030, approximately 4 million total knee and hip arthroplasty procedures are anticipated to be performed yearly [1, 2]. Among these patients, prosthetic joint infection (PJI) remains a prevalent cause of TKA failure [3]. For acute PJI, debridement and implant retention of the joint prosthesis or irrigation and debridement (I&D) with exchange of the polyethylene liner and component retention remains a treatment option [2]. Such a procedure avoids the need for hardware removal, which can be associated with increased costs as well as presumed potentially increased morbidity [4]. The disadvantage of this approach is an associated high failure rate.
A wide range of treatment failure rates associated with patients who undergo debridement with implant retention has been reported in the literature. Failure rates have been reported as high as 84% in a 2-year multi-center retrospective study of 19 patients who underwent debridement with implant retention [5]. Inclusion criteria included acute PJI cases with symptoms of less than 4 weeks that were culture positive with Methicillin Resistant Staphylococcus aureus (MRSA) and follow up of at least 2 years. Conversely, Kupier et al. reported a mere 34% failure rate in a 3-year multicenter observational study involving 91 patients [6]. This study had similar inclusion criteria of at least two year follow up, but included any culture positive or culture negative PJI and defined infection by a previously established criteria [7]. Such vast differences in failure rates underscore the wide variability in outcomes associated with I&D and component retention. This may in part be a consequence of various limitations in some of these studies, including small sample sizes, limited follow-up periods and the inability to reliably estimate time to treatment failure given short study durations [5, 6, 8–19]
We therefore asked in a large, multicenter study with extended follow-up: 1) What is the rate and time period to failure in I&D with retention of components in TKA PJI? (2) In failed cases of I&D with component retention, what are the subsequent outcomes in terms of future revision surgery and mortality? (3) What patient factors predict treatment failure?
Methods
Study Design
A retrospective, multicenter observational study of patients diagnosed with a TKA PJI and subsequently undergoing a debridement with retention of components between 2005 and 2015 was performed. Data were acquired from electronic medical records, and institutional review board approval was obtained. The study was completed at 16 hospitals in a regional health system compromising a variety of academic, hospital-employed, and private practice environments in both urban and rural settings.
Participants
The initial cohort was obtained using the International Classification of Disease-9 code (ICD-9 code) for periprosthetic joint infection (996.66). As this diagnosis code can be used for different anatomic arthroplasty implants, TKA PJI was identified as the intersection of all patients in the medical system with the diagnosis code and a concurrent surgical procedure on the knee, as determined by the medical record. Further inclusion criteria included any patient who received a debridement with component retention and exchange of the polyethylene liner. Exclusion criteria included any patient that had a previous surgical procedure after receiving a TKA or who had a musculoskeletal oncologic disease[20]. Diagnosis of TKA PJI was determined by the surgeon and identified using the electronic medical record. Initially, a total of 798 cases of debridement with component retention in 1,344 patients diagnosed with TKA PJI were identified in the electronic medical records. Laterality of the procedure was matched between surgical procedures. After applying the exclusion criteria of no previous TKA salvage procedures (e.g., two-stage revisions) or musculoskeletal oncologic diagnosis, there were 271 cases of debridement with component retention performed on 216 TKAs in 206 patients (10 bilateral). Musculoskeletal Infection Society (MSIS) Criteria [21] was used with modified minor criteria including synovial nucleated white blood cell count threshold of 2,500 cells/μL and a synovial polymorphonuclear percentage greater than 65% as a secondary analysis of infection status [11, 22, 23]. Any patient that did not meet this modified MSIS criteria was further excluded. Duration of follow-up was defined as the length of time between the initial debridement procedure and the last visit recorded in the medical system.
Description of Treatment
The treating surgeon directed diagnosis, surgery, and treatment; as a result, the characteristics of treatment varied across institutions. The initial infection was diagnosed with a variety of tools including erythrocyte sedimentation rate, C-reactive protein, synovial fluid analysis, cultures, intraoperative histologic sections and observation, in addition to experiential judgment of the surgeon. Although the observational period of our study began prior to the establishment of MSIS criteria, similar concepts were the basis for diagnosis in the majority of cases. Given the retrospective design of our study, there was no standard surgical technique for irrigation and debridement or postoperative antibiotic regimen.
Outcome Measures, Data Sources, and Bias
Failure of debridement and component retention was identified as any further surgical procedure on the operative knee excluding manipulation under anesthesia, periprosthetic fracture, and extensor mechanism disruption. Time to failure was measured as the time period between the irrigation and debridement and next surgical procedure. Mortality was determined as reported inside the electronic medical record. Follow-up was measured as any outpatient visit registered inside the medical system.
Statistical Analysis
Demographic characteristics of our sample participants were analyzed using univariate descriptive statistics. Means and standard deviations were calculated for approximately normally distributed variables; medians and inter-quartile ranges (IQRs) were computed for continuous variables with skewed distributions. Frequencies and percentages were determined for categorical variables. Similar univariate techniques were used to characterize secondary outcomes such as follow-up, mortality and secondary surgical procedures.
Comorbid conditions of patients were identified using ICD-9 codes obtained from electronic medical billing records. The Charlson Comorbidity Index (CCMI) was calculated for each patient using the conventional method [24]. Weighted counts of a patient’s comorbid conditions were used in conjunction with the patient’s age to compute the widely-used combined comorbidity index.
To obtain the estimated treatment failure probability as a function of time and the median time to failure, a survival curve for I&Ds with a corresponding TKA PJI diagnosis was constructed. Failure and survival rates were estimated using the nonparametric Kaplan-Meier product-limit method. As previously mentioned, survival times used in estimation were defined as the time lapse between the I&D procedure and the next surgical procedure performed on the knee. I&D cases in which patients continued in the study without any subsequent knee procedures, cases in which patients were lost to follow-up, and cases in which death occurred were right-censored. The complementary log-log transformation method was employed to obtain 95% point-wise confidence intervals for survival/failure rates.
Cox proportional hazards models were then constructed to identify significant predictors of treatment failure. Candidate variables included age, BMI, gender, CCMI, diabetes mellitus, rheumatoid arthritis, ASA score, host score, time period symptomatic and primary organism. First, unadjusted models were fit separately for each variable to determine significant univariate predictors of failure. Subsequently, a multivariable Cox model was constructed via forward selection of covariates with a p-value<0.10 criterion to enter the model; the model was then pruned such that only significant predictors with p-values<0.05 remained. Based on the model fit, a “best-case” scenario was identified by selecting values for these predictors that minimized the hazard of treatment failure, and an accompanying adjusted survival curve was produced. All management of electronic medical record data and statistical analyses were performed using Stata Version 14.1 and SAS Version 9.4
Results
Demographics and Description of the Study Participants
Demographics of the sample patients are presented in Table 1. A total of 216 TKAs (10 bilateral) in 206 patients were identified as meeting inclusion criteria. The mean age was 65.9±12.2. The mean body mass index was 34.0±9.0. The median Charlson Comorbidity Index (CCMI) was 3.0 [IQR 2.0, 5.0]. The median length of stay was 6.5 [IQR 4.9, 10.6]. All patients met modified MSIS criteria. The proportion of individuals with diabetes mellitus was 12.6%, and the proportion of individuals with rheumatoid arthritis was 8.7%. Females accounted for the 44.7% of the sample participants. The median follow up time was 31.5 months [IQR 14.4, 67.0]. Only 23 participants (11.2%) had a minimum follow-up of less than 6 months. Preoperative, surgical, and post-operative care was directed by and at the discretion of the patient’s medical team.
Table 1.
Patient demographic characteristics.
| Demographic | All patients (N=206) |
|---|---|
| Age, mean (SD) | 65.9 (12.2) |
| BMI, mean (SD) | 34.0 (9.0) |
| CCMI, median [IQR] | 3.0 [2.0, 5.0] |
| LOS, median [IQR] | 6.5 [4.9, 10.6] |
| DM, N (%) | 26 (12.6) |
| RA, N(%) | 18 (8.7) |
| Gender, N (%) | |
| Male | 114 (55.3) |
| Female | 92 (44.7) |
Treatment Failure
Failure of I&D with component retention was high and occurred primarily in the first year. The overall proportion of I&Ds that failed at any time point was 50.5%, and the long-run probability of treatment failure at 4 years was estimated to be 57.4% [95% CI: 50.0% to 65.2%]. Failure rates at 1 month, 3 months, 6 months, 1 year, and 2 years from initial I&D were estimated (Table 2), and the failure rate was 53.2% [95% CI: 46.2% to 60.6%] at 2 years. 89.9% of the failures occurred within the first year. The median survival time or time to failure was determined to be 13.32 months [95% CI: 7.59 to 49.28 months].
Table 2.
Cumulative failure rates for all cases of debridement with component retention.
| Time (months) | Failure Rate % (95% CI) |
|---|---|
| 1 | 15.6 (11.3, 21.2) |
| 3 | 27.6 (22.0, 34.3) |
| 6 | 40.9 (34.5, 48.0) |
| 12 | 49.3 (42.5, 56.5) |
| 24 | 53.2 (46.2, 60.6) |
Surgical Outcomes After Initial Failed I&D
An analysis of secondary surgical procedures in the sample of failed I&D was performed. Additional I&D were common. After the initial I&D, 45.9% of TKAs had no further I&D and instead had some other type of more complex procedure. The remainder had additional I&D where 32.1% had one additional I&D and 22.0% had two or more additional I&Ds (Table 3). Ultimately, I&D was the final procedure performed in only 28.4% of the TKAs that had a failed I&D. There was a variety of other types of procedures performed as the final procedure. The most common procedure performed was two stage revision (54.1%), then amputation (11.1%), and fusion (6.4%; Table 4). Overall mortality was 23.8% (Table 5).
Table 3.
Number of additional I&Ds after the initial failed debridement with component retention.
| Number of Additional I&Ds | N (%) |
|---|---|
| 0 | 50 (45.9) |
| 1 | 35 (32.1) |
| 2 | 13 (11.9) |
| 3 | 7 (6.4) |
| 4+ | 4 (3.7) |
Table 4.
Frequency of final procedure in the initial group of failed irrigation and debridements with component retention.
| Final Procedure: | N (%) |
|---|---|
| I&D | 31 (28.4) |
| Two-Stage Revision | 59 (54.1) |
| Amputation | 12 (11.1) |
| Fusion | 7 (6.4) |
Table 5.
Mortality in the initial group of failed irrigation and debridements with component retention.
| Time (year) | N (%) | Mean (SD) |
|---|---|---|
| 1 | 16 (7.8) | 0.3 (0.3) |
| 2 | 22 (10.7) | 0.6 (0.6) |
| 3 | 30 (14.6) | 1.1 (0.9) |
| 4 | 32 (15.5) | 1.2 (1.0) |
| 5 | 41 (19.9) | 2.0 (1.7) |
| Overall | 49 (23.8) | 2.8 (2.4) |
Factors Predicting Failure
Cox proportional hazard regression analyses demonstrated that a number of factors predicted failure. Unadjusted analyses revealed that BMI, CCMI, cultured organism, and the time period symptomatic were significant univariate predictors of treatment failure (Table 6). Other factors, such as DM, ASA score, and the systemic host grade for the McPherson staging system were not. Multivariable analysis revealed that only cultured organism and duration of symptoms independently predicted failure (Table 6). The variable with the greatest influence on failure hazard was the time period symptomatic. The hazard of failure for a patient with greater than 4 weeks of symptoms was 2.35 times the hazard for someone with less than one week of symptoms, after controlling for organism. In regards to organism, a culture negative infection had the largest hazard of failure, followed by an infection with S. aureus, after controlling for duration of symptoms. Other organisms had the lowest hazard of failure. The hazard of failure in a culture negative infection was 28% greater than the hazard in an S. aureus infection.
TABLE 6.
Predictors of treatment failure in univariable and multivariable Cox proportional hazards models
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
| ||||
| Covariate | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
|
| ||||
| Age | 0.987 (0.972, 1.001) | 0.0758 | ||
|
| ||||
| BMI | 1.024 (1.004, 1.046) | 0.0203 | ||
|
| ||||
| Gender | 0.3385 | |||
| Male | -reference level- | |||
| Female | 1.203 (0.824, 1.755) | |||
|
| ||||
| CCMI | 0.909 (0.837, 0.986) | 0.0220 | ||
|
| ||||
| DM | 0.942 (0.517, 1.718) | 0.8458 | ||
|
| ||||
| RA | 1.189 (0.637, 2.219) | 0.5861 | ||
|
| ||||
| ASA Score | 0.9255 | |||
| 1 or 2 | -reference level- | |||
| 3 | 1.028 (0.627, 1.684) | |||
| 4 | 1.116 (0.617, 2.017) | |||
|
| ||||
| Host Score | 0.4575 | |||
| A | -reference level- | |||
| B | 1.141 (0.656, 1.984) | |||
| C | 1.530 (0.756, 3.097) | |||
|
| ||||
| Days Symptomatic | 0.0041 | 0.0021 | ||
| <1 week | -reference level- | -reference level- | ||
| 2–4 weeks | 1.771 (1.151, 2.275) | 1.680 (1.067, 2.645) | ||
| >4 weeks | 2.093 (1.276, 3.434) | 2.345 (1.421, 3.871) | ||
|
| ||||
| Primary Organism | 0.0403 | 0.0207 | ||
| Staph Aureus | -reference level- | -reference level- | ||
| Culture Negative | 1.258 (0.570, 2.777) | 1.282 (0.561, 2.929) | ||
| Other | 0.636 (0.429, 0.943) | 0.595 (0.399, 0.889) | ||
A separate analysis was completed on the possible role of time between index procedure to initial I&D and probability of failure. There were 112 patients were the time of index TKA procedure was known. In the remainder of these patients, the time of index procedure was not known secondary to the procedure being completed at an outside institution or as it occurred before introduction of an electronic medical record. In the vast majority of these cases, the time of index procedure was not known as it occurred significantly beyond one year from the initial index TKA. A univariate analysis was performed with time between index procedure and initial I&D considered as a continuous variable (p=0.56) and as a categorical variable (more or less than one year from index procedure; p=0.71). The hazard ratio between the groups more or less than one year from the index procedure was 0.94 [95% CI: 0.77, 1.15]; (p=0.55). The time from the index procedure was not a significant variable in predicting I&D failure rates, both groups did equally poorly.
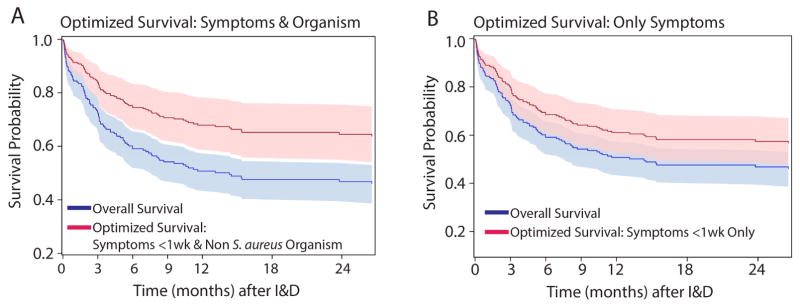
A survival curve was constructed to depict the probability of treatment failure as a function of time (Figure 1). Based on the results of multivariable analysis, a patient with less than one week of symptom duration and a non-S. aureus cultured organism is expected to have optimal treatment success. Defining the best-case, optimized scenario as less than one week of symptoms and a non-S. aureus organism, the probability of treatment failure decreased to 39.6% [95% CI: 27.4%, 49.7%] as compared to the overall failure rate for all individuals of 57.4% [95% CI: 50.0%, 65.2%] at 4 years (Figure 1A). The difference between the overall long term survival at 4 years in the best-case scenario as compared to all individuals was statistically significant as the confidence intervals did not overlap. In most cases, the organism is unknown at the time of surgery as multiple days are needed to obtain culture results. Defining the best-case, optimized scenario as only less than one week of symptoms and not including organism in the analysis, the probability of treatment failure decreased to 46.8% [95% CI: 35.6%, 56.0%] as compared to the overall failure rate for all individuals of 57.4% [95% CI: 50.0%, 65.2%] at 4 years (Figure 1B). When organism was excluded from the optimized analysis, there was no statistically significant difference between the overall and optimized scenario. Using established guidelines for comparing overlapping confidence intervals to determine statistically significant differences between outcomes [25], we conservatively compared 83% confidence intervals for the best-case scenario failure rate to the overall group failure rate and determined that the two failure probabilities are not statistically different at the Type I error rate of 0.05.
Figure 1.
The overall vs optimized estimated survival probability of I&D in TKA PJI. The overall failure rate was 57.4%. (A) When the optimized case is defined as less than one week of symptoms and a non S. aureus cultured organism, the failure rate was 39.6%. The 95% confidence intervals of overall vs optimized survival probabilities do not overlap indicating a significant difference. (B) Typically, the organism is not known at the time of surgery. When the optimized case is defined as only less than one week of symptoms and organism is excluded, the failure rate was 46.8%. Confidence intervals of overall vs optimized survival probabilities do not sufficiently overlap, suggesting no difference in outcomes.
Discussion
There is a wide variation in reported failure rates for irrigation and debridement with component retention. Previous studies had limitations in design including single center retrospective designs, small sample sizes, and limited follow-up durations. In this multicenter observational study design of 216 total knee arthroplasties with an extended follow up greater than 3 years, the overall proportion of failures as defined as additional surgical procedures after the initial debridement was 51%. The long-run probability of failure was estimated to be 61%, and 90% of the failures occurred in the first year. Multiple additional debridements were common, and 72% of all failures ultimately resulted in either a two-stage revision or a salvage procedure such as fusion or amputation.
As an observational study, there are a number of limitations. First, although a strength in most aspects, the multicenter study design also introduced a weakness. Dependent on the medical record, surgical indications, and timing between symptomatic presentation and debridement was determined by the surgeon. We attempted to account for this by excluding patients that did not meet MSIS criteria. As the study included a variety of practice types, although we had access to inpatient medical records, we did not have access to all outpatient notes that would likely include more complete documentation. We were unable to assess the length and type of antibiotic therapy. This included the inability to assess if patients were on long-term suppressive antibiotic therapy. The choice of antibiotic selection was made by the infectious disease physician on the treating team, and all patients were discharged on intravenous antibiotic therapy, the current standard of care. From this perspective, our results represent an intention to treat analysis. A second limitation included the definition of failure of debridement. Failure was defined as any additional procedures on the extremity. This definition does not include people who went on to chronic suppression with antibiotics, and our study did not collect patient reported outcome measures. Considering chronically suppressed patients or patients with a low functional outcome score would possibly increase the overall failure rate. Moreover, any type of revision for reasons unrelated to the infection including periprosthetic infection or aseptic loosening would be misclassified as a failure. However, the majority of failures occurred in the first year. Revision for aseptic loosening within one year from infection would be unexpected. We did exclude revisions for reasons unlikely to be related to treatment failure including periprosthetic fracture and manipulation under anesthesia. As a result, these had a low influence on our overall failure rate.
Our primary study question focused on the rate and timing of failure of debridement with component retention in TKA PJI. There has been a large number of studies that focused on this question in the past. The largest study was a single center retrospective design with a failure rate of 48% with a study size of 247 patients followed for a mean of 2.8 years [11].
A meta-analysis with a total study size of 530 patients quantified the failure rate using a number of variations based on the time to following revision as 68% [14]. The study was well designed but was completed a decade prior to the MSIS definition of PJI, which could possibly date results. Fehring et al. summarized a number of observational studies [9]. The total number of patients across all 13 studies was 1,403 patients, and the average failure rate was approximately 68%. Our results are comparable with an estimated probability of failure at 57%. Taken as a whole, our results are in agreement with previous literature that suggests the failure rate of debridement with component retention in TKA PJI is high. Using multivariable analysis to optimize patient selection, in a best-case scenario the estimated treatment failure probability was 40%, and statistically different from the overall rate of failure. If type of organism is excluded from the analysis, as this information is typically unknown at the time of surgery, the optimized estimated treatment failure is 47% and not statistically different from the overall failure rate.
We next questioned the ultimate outcome in these failures. Comparing PJI cases to aseptic revisions, two different studies noted mortality was at 18% at 4 years and 25% at 5 years [26, 27]. Overall mortality in our study was 24%. These previous studies measured mortality in all cases of PJI including more extensive two-stage revision procedures. We had a similar level of mortality with patients undergoing an I&D. A previous hypothesis of these studies included that mortality was increased from the morbidity of the procedure, especially with a two-stage. Even with I&D alone, mortality with PJI TKA remains surprisingly high suggesting the patient’s physiologic capacity may play a more substantial role than morbidity associated with a surgical procedure.
The failure group was followed to see the final outcomes of the secondary procedure. Multiple I&D procedures were common. Ultimately, the final procedure in 72% of cases was some type of salvage procedure such as a two-stage, amputation, of fusion. This would suggest that, in the setting of an initially failed debridement with component retention, continued I&D have questionable utility.
Factors that predicted treatment outcomes in a best-case scenario were identified. We anticipated that typical factors that could quantify overall individual health would predict I&D failure. When adjusted, these factors, such as DM, RA, ASA score, and McPherson host grade, did not predict failure. These results are in agreement with other studies demonstrating DM and ASA do not predict I&D failure rates [4, 11]. The time of the index TKA procedure was not a significant variable in predicting I&D failure rates. These results are not surprising. Fehring et al. completed a study focused entirely on early post-operative TKA PJI, and report an I&D failure rate of 63% [9]. In our study, we report a failure rate of 57%. These numbers are essentially identical. PJI diagnosed within one year from initial index procedure is likely related to the initial procedure and should be considered a chronic infection after more than 30 days from the index procedure. Interestingly, this group had a comparable high rate of failure as I&D after one year when the symptoms had been present for more than one week. Across multiple studies, the largest most consistent predictor of failure is duration of symptoms. Our results agree with a series of studies that note a large increase in failure rate in symptoms present for more than one week [4, 11]. This questions the utility of using the historical threshold of one month as a defining point of an acute verses chronic infection.
The host grade for the McPherson staging system did not predict I&D failure. The McPherson staging system has three components including infection type (acute vs chronic), systemic host grade, and local extremity grade [28]. Our results agreed with duration of symptoms. We did not assess local extremity grading as we did not have direct observation at the time. The lack of correlation on the second component of systemic host grade of the staging system does not negate the value of this system as it was originally designed to predict success from a two-stage revision scenario. It does highlight that a patient with a good host grade does not have an altered hazard of I&D failure as compared to a patient with a poor host grade.
A variety of results have been reported regarding the association between treatment success and involved organism. Multiple studies have demonstrated that outcomes with S. aureus infections are poor as compared to streptococci or coagulase-negative staphylococci PJI [4, 9, 11, 27]. Our results agree with these findings. Interestingly, we observed a higher hazard of failure when no organism could be identified. This is contrast to other studies that observed decreased I&D failure [11, 29] or no difference in infection control [30, 31]. An explanation for these observed differences include the possibility of sonication used for organism identification. Historically, culture negative results can be as high as 34% [32]. In our study, we observed a low incidence of culture negative cases likely as a result of implementing a sonication protocol. Our observed culture negative results are lower than these other studies and likely are not an equal comparison between groups.
In TKA PJI, I&D and component retention has a high failure rate. Although the literature has variations in reported failure rates, our results are in a similar range as a meta-analysis that combined multiple smaller studies at approximately 60%. When optimum conditions are selected, including less than one week of symptoms and a cultured organism that is not S. aureus, the predicted probability of failure improves to approximately 40%. Factors that would be suspected to predict I&D failure, such as BMI, CCMI, DM, or a poor host (McPherson host grade) did not predict I&D failure when adjusted. If the initial I&D fails, repeat I&D has a low likelihood of success. Together, these results suggest that I&D has a limited ability to control infection in TKA, the patient comorbidities we investigated did not predict I&D success, and I&D should be used selectively under optimum conditions.
Supplementary Material
Acknowledgments
Dr. Kenneth Urish is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS K08AR071494), the National Center for Advancing Translational Science (NCATS KL2TR0001856), the Orthopaedic Research and Education Foundation, and the Musculoskeletal Tissue Foundation.
Footnotes
Level of Evidence, Level III, Cohort Study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth L. Urish, Arthritis and Arthroplasty Design Group, The Bone and Joint Center, Magee Womens Hospital of the University of Pittsburgh Medical Center; Department of Orthopaedic Surgery, Department of Bioengineering, and Clinical and Translational Science Institute, University of Pittsburgh; Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA, 15219.
Andrew G. Bullock, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261.
Alex Kreger, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261.
Neel B. Shah, Division of Infectious Disease, Department of Internal Medicine; University of Pittsburgh Medical Center, Pittsburgh, PA 15219.
Kwonho Jeong, Clinical and Translation Science Institute; Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15213.
Scott D. Rothenberger, Clinical and Translation Science Institute; Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15213.
References
- 1.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 2.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42(4):471. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24(6 Suppl):101. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Kuiper JW, Vos SJ, Saouti R, Vergroesen DA, Graat HC, Debets-Ossenkopp YJ, Peters EJ, Nolte PA. Prosthetic joint-associated infections treated with DAIR (debridement, antibiotics, irrigation, and retention): analysis of risk factors and local antibiotic carriers in 91 patients. Acta Orthop. 2013;84(4):380. doi: 10.3109/17453674.2013.823589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80(9):1306. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Triantafyllopoulos GK, Poultsides LA, Zhang W, Sculco PK, Ma Y, Sculco TP. Periprosthetic knee infections treated with irrigation and debridement: outcomes and preoperative predictive factors. J Arthroplasty. 2015;30(4):649. doi: 10.1016/j.arth.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Fehring TK, Odum SM, Berend KR, Jiranek WA, Parvizi J, Bozic KJ, Della Valle CJ, Gioe TJ. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res. 2013;471(1):250. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop Relat Res. 1991;(273):113. [PubMed] [Google Scholar]
- 11.Buller LT, Sabry FY, Easton RW, Klika AK, Barsoum WK. The preoperative prediction of success following irrigation and debridement with polyethylene exchange for hip and knee prosthetic joint infections. J Arthroplasty. 2012;27(6):857. doi: 10.1016/j.arth.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Chiu FY, Chen CM. Surgical debridement and parenteral antibiotics in infected revision total knee arthroplasty. Clin Orthop Relat Res. 2007;461:130. doi: 10.1097/BLO.0b013e318063e7f3. [DOI] [PubMed] [Google Scholar]
- 13.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 14.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;(404):125. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24(5):914. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 16.Deirmengian C, Greenbaum J, Lotke PA, Booth RE, Jr, Lonner JH. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty. 2003;18(7 Suppl 1):22. doi: 10.1016/s0883-5403(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 17.Deirmengian C, Greenbaum J, Stern J, Braffman M, Lotke PA, Booth RE, Jr, Lonner JH. Open debridement of acute gram-positive infections after total knee arthroplasty. Clin Orthop Relat Res. 2003;(416):129. doi: 10.1097/01.blo.0000092996.90435.35. [DOI] [PubMed] [Google Scholar]
- 18.Ivey FM, Hicks CA, Calhoun JH, Mader JT. Treatment options for infected knee arthroplasties. Rev Infect Dis. 1990;12(3):468. doi: 10.1093/clinids/12.3.468. [DOI] [PubMed] [Google Scholar]
- 19.Odum SM, Fehring TK, Lombardi AV, Zmistowski BM, Brown NM, Luna JT, Fehring KA, Hansen EN. Irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplasty. 2011;26(6 Suppl):114. doi: 10.1016/j.arth.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83-A(11):1622. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J. 2013;95-B(11):1450. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 22.Betsch BY, Eggli S, Siebenrock KA, Tauber MG, Muhlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis. 2008;46(8):1221. doi: 10.1086/529436. [DOI] [PubMed] [Google Scholar]
- 23.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117(8):556. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 25.Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95(24):2177. doi: 10.2106/JBJS.L.00789. [DOI] [PubMed] [Google Scholar]
- 27.Choi HR, Bedair H. Mortality following revision total knee arthroplasty: a matched cohort study of septic versus aseptic revisions. J Arthroplasty. 2014;29(6):1216. doi: 10.1016/j.arth.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 28.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;(403):8. [PubMed] [Google Scholar]
- 29.Choi HR, Kwon YM, Freiberg AA, Nelson SB, Malchau H. Periprosthetic joint infection with negative culture results: clinical characteristics and treatment outcome. J Arthroplasty. 2013;28(6):899. doi: 10.1016/j.arth.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010;468(8):2039. doi: 10.1007/s11999-010-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang R, Hu CC, Adeli B, Mortazavi J, Parvizi J. Culture-negative periprosthetic joint infection does not preclude infection control. Clin Orthop Relat Res. 2012;470(10):2717. doi: 10.1007/s11999-012-2434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scorzolini L, Lichtner M, Iannetta M, Mengoni F, Russo G, Panni AS, Vasso M, Bove M, Villani C, Mastroianni CM, Vullo V. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol. 2014;37(3):321. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.