Abstract
The high comorbidity among neuropsychiatric disorders suggests a possible common neurobiological phenotype. Resting-state regional cerebral blood flow (CBF) can be measured noninvasively with MRI and abnormalities in regional CBF are present in many neuropsychiatric disorders. Regional CBF may also provide a useful biological marker across different types of psychopathology. To investigate CBF changes common across psychiatric disorders, we capitalized upon a sample of 1,042 youths (ages 11 to 23 years) who completed cross-sectional imaging as part of the Philadelphia Neurodevelopmental Cohort. CBF during a resting state was quantified on a voxelwise basis using arterial spin labeled perfusion MRI at 3T. A dimensional measure of psychopathology was constructed using a bifactor model of item-level data from a psychiatric screening interview, which delineated four factors (fear, anxious-misery, psychosis, and behavioral symptoms) plus a general factor: overall psychopathology. Overall psychopathology was associated with elevated perfusion in several regions including the right dorsal anterior cingulate cortex (ACC) and left rostral ACC. Furthermore, several clusters were associated with specific dimensions of psychopathology. Psychosis symptoms were related to reduced perfusion in the left frontal operculum and insula, whereas fear symptoms were associated with less perfusion in the right occipital/fusiform gyrus and left subgenual ACC. Follow-up functional connectivity analyses using resting-state fMRI collected in the same participants revealed that overall psychopathology was associated with decreased connectivity between the dorsal ACC and bilateral caudate. Together, the results of this study demonstrate common and dissociable CBF abnormalities across neuropsychiatric disorders in youth.
Keywords: cerebral blood flow, perfusion, psychopathology, development, anterior cingulate
INTRODUCTION
Co-morbidity among psychiatric disorders is highly common. Epidemiological research confirms that of the 26.2% of adults with a mental health diagnosis in the U.S., 55% have one diagnosis, while 22% carry two mental health diagnoses, and 23% carry three or more.1 Comorbidity and a lack of clear diagnostic boundaries may be particularly challenging in children, where clinical phenotypes are often less distinct.2 For example, a meta-analysis of community sample based studies on comorbidity between childhood psychiatric disorders showed that diagnoses such as attention-deficit/hyperactivity disorder (ADHD), conduct disorder, depression, and anxiety were all highly comorbid.3
The comorbidity among neuropsychiatric disorders suggests common neurobiological origins, which may confer vulnerability to a range of symptoms. Prior neuroimaging studies have typically focused on adults in a case control design that compares a specific disorder to a control group. Given the high comorbidity among disorders, studies limited to those with “pure” singular diagnoses may not be representative of the actual presentation of psychiatric symptoms. Therefore, the search for common neural abnormalities that are present across disorders has recently accelerated.4–13 One possible common neuroanatomical substrate was reported by Goodkind and colleagues,10 who showed that gray matter loss in the anterior insula and dorsal anterior cingulate cortex (ACC) were common across psychotic and nonpsychotic disorders in adults. Thus, abnormalities in the ACC may represent a risk factor shared across diagnoses.
While most prior across-disorder studies have focused on brain structure or signals from fMRI, regional cerebral blood flow (CBF) has been much less studied. Regional CBF, which can be quantified noninvasively using arterial spin labeled (ASL) perfusion MRI, is tightly coupled to regional brain metabolism.14 Alterations in regional CBF have been demonstrated in neuropsychiatric disorders, such as generalized anxiety disorder and posttraumatic stress disorder,15,16 and during stress provocation paradigms.17 However, these case-control studies did not measure psychopathology symptoms across multiple disorders.
There is a growing recognition that psychiatric symptoms exist on a continuum in contrast to a categorical approach to diagnosis. Thus, dimensional measures of psychopathology that cut across categorical clinical diagnoses are needed.18,19 Such measures may enhance the power to detect differences compared to healthy controls and may improve interpretation of these differences.19 A number of models have been proposed to identify broad traits shared across psychiatric disorders, such as measures of neuroticism and negative emotionality.20,21 In particular, Lahey and colleagues used a bifactor model to quantify psychopathology dimensionally across psychiatric disorders.22 They identified a general factor associated with psychopathology as well as dimensions such as externalizing, distress, and fear. Other studies have shown a similar bifactor structure, with factors for anxious-misery, psychosis, behavioral (externalizing), and fear as well as a general factor representing overall psychopathology.12 Bifactor analysis has the advantage of identifying a general factor and orthogonal factors among highly correlated symptoms. Previously, we have used this approach to document that overall psychopathology was associated with reduced activation within executive regions including the ACC on a dimensional basis across psychiatric symptoms in youth.12
In contrast to existing studies of brain structure10 and executive function,12 few studies have examined associations between CBF and dimensional measures of psychopathology across clinical diagnoses, and fewer still have done so in youth. In one previous study,23 we examined the association between CBF and a dimensional measure of mood and anxiety using the State Trait Anxiety Inventory. We found that greater mood/anxiety traits were associated with elevated CBF in the left amygdala, bilateral insula, and left fusiform gyrus in adolescence.23 This suggests that CBF may be a useful biological marker of dimensions of emotion. However, we only examined anxiety and mood traits in that study and did not directly examine overall symptoms of psychopathology.23
In this study, we investigated a potential common neurobiological phenotype related to regional resting-state CBF using a dimensional measure of psychopathology symptoms across a broad range of neuropsychiatric disorders in a large sample of children, adolescents, and young adults imaged as part of the Philadelphia Neurodevelopmental Cohort (PNC). We applied a bifactor analysis to dimensionally quantify psychopathology.12 As suggested by both our prior work on executive function12 and the results of Goodkind et al.,10 we hypothesized that greater overall psychopathology would be associated with CBF changes within previously-implicated regions such as the ACC. In order to further understand the functional implications of such differences, we conducted follow-up analyses examining changes in functional connectivity in cingulate regions where alterations in CBF were found. Finally, while the focus of this study was on regional CBF changes associated with overall psychopathology, we also explored dissociable relationships between regional CBF and specific dimensions of psychopathology.
METHODS AND MATERIALS
Participants
Of the total sample of 1,601 youths imaged in the Philadelphia Neurodevelopmental Cohort (PNC),24,25 we included participants age 11 years and older who completed a psychiatric assessment interview (n=1,288); participants under 11 who only received a collateral assessment were not included. An additional 127 participants were excluded for: medical disorders that could affect brain functioning (n=68), non-psychiatric medication use that could impact CNS functioning (n=53), or substantial structural brain abnormalities (n=16); several subjects were excluded for multiple criteria. Of the remaining participants, 119 participants were excluded for missing clinical data (n=2), failing to complete perfusion imaging (n=5), or failing to meet ASL and structural image quality assurance protocols (n=117); 5 subjects were excluded for multiple reasons. This yielded a final sample of 1,042 participants (mean age = 16.12 years; range = 11–23 years; SD = 2.82 years; 468 males). Among this final sample, 120 participants (12%) were taking psychiatric medications at the time of imaging and were evaluated in sensitivity analyses, as described below.
Clinical assessment
The institutional review boards of both the University of Pennsylvania and the Children’s Hospital of Philadelphia approved all study procedures. Informed consent was obtained from all participants. As described previously,24,25 psychiatric symptoms were assessed using a structured screening interview (GOASSESS) based on a modified version of the Kiddie-Schedule for Affective Disorders and Schizophrenia (see Supplementary Methods).26 Demographic data and lifetime prevalence are summarized in Table 1.
Table 1.
Summary of demographic data.
| M | SD | |
|---|---|---|
| Age (years) | 16.12 | 2.82 |
|
| ||
| N | Percent | |
|
| ||
| Gender | ||
| Male | 468 | 45% |
| Female | 574 | 55% |
| Race | ||
| Caucasian | 473 | 45% |
| Non-Caucasian | 569 | 55% |
| Maternal Level of Education | ||
| 12 years or less | 395 | 38% |
| Greater than 12 years | 634 | 61% |
| Missing | 13 | 1% |
| Lifetime Prevalence* | ||
| Typically Developing | 284 | 27% |
| ADHD | 168 | 16% |
| Agoraphobia | 72 | 7% |
| Anorexia | 14 | 1% |
| Bulimia | 4 | .3% |
| Conduct Disorder | 101 | 10% |
| Generalized Anxiety Disorder | 22 | 2% |
| Major Depression | 171 | 16% |
| Mania | 11 | 1% |
| Obsessive-Compulsive Disorder | 36 | 3% |
| Oppositional Defiant Disorder | 386 | 37% |
| Panic | 13 | 1% |
| Psychosis-spectrum | 351 | 34% |
| PTSD | 147 | 14% |
| Separation Anxiety | 42 | 4% |
| Social Phobia | 275 | 26% |
| Specific Phobia | 322 | 31% |
Note.
Due to comorbidity, individual participants may be present in more than one category.
Factor analysis to create a dimensional measure of psychopathology
As described in detail elsewhere,27 (see Supplementary Methods) we used a confirmatory bifactor analysis28,29 implemented in Mplus30 to quantify a dimensional measure of psychopathology. This analysis yielded four orthogonal dimensions of psychopathology (anxious-misery, psychosis, behavioral (externalizing), and fear), plus a fifth factor that was common across all psychiatric disorders, which we termed overall psychopathology (Figure 1).
Figure 1. Bifactor model of psychopathology reveals common and divergent dimensions of psychopathology across categorical screening diagnoses.
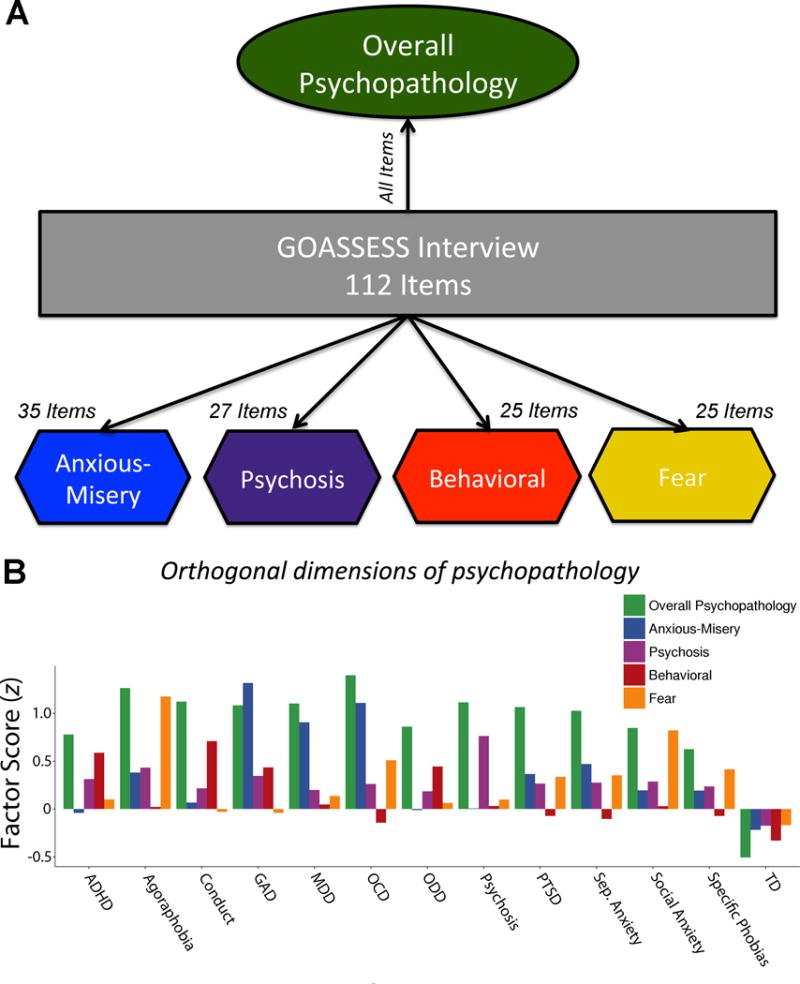
A) Confirmatory bifactor analysis of 112 items from the GOASSESS screening interview revealed four orthogonal dimensions of psychopathology (anxious-misery, psychosis, behavioral, and fear), plus a general factor shared across disorders (overall psychopathology). B) Mean factor scores for each orthogonal dimension of psychopathology are presented by screening categories with at least 20 subjects. Overall psychopathology was common across diagnostic screening categories. ADHD = attention deficit hyperactivity disorder; GAD = generalized anxiety disorder; MDD = major depressive disorder; OCD = obsessive-compulsive disorder; ODD = oppositional defiant disorder; PTSD = posttraumatic stress disorder; TD = typically developing.
Image acquisition, preprocessing, and perfusion quantification
Image acquisition and processing are reported in detail elsewhere;24,31 see Supplementary Methods for details. Perfusion was measured using a pseudo-continuous arterial spin labeling (pCASL) sequence sampled with spin-echo echoplanar imaging. These data were then pre-processed using standard tools included with FSL.32 Following distortion correction using the B0 map with FUGUE, the first four image pairs were removed, the timeseries were realigned in MCFLIRT,33 the skull was removed with BET,34 and the images were smoothed at 6mm FWHM using SUSAN.35 Resting-state CBF was quantified from control-label pairs using ASL Toolbox.36 As prior,37 the T1 relaxation parameter was modeled on an age- and sex-specific basis.38 This model accounts for the fact that T1 relaxation time differs according to age and sex, and has been shown to enhance the accuracy and reliability of results in developmental samples.39 Participant-level CBF images from each subject were co-registered to the T1 structural image using boundary-based registration,40 and normalized to the PNC adolescent template using the top-performing deformable registration included in Advanced Normalization Tools.41–43 Images were down-sampled to 2-mm resolution prior to group-level analysis. All transformations were concatenated so that only one interpolation was performed. After statistical testing, images were registered to the Montreal Neurologic Institute (MNI) 152 1-mm template space for reporting of standard coordinates and display.
Group-level analyses
We conducted voxelwise analyses that modeled overall psychopathology as well as each of the four psychopathology sub-factors. We included model covariates to control for subject demographics as well as data quality. Specifically, due to known nonlinear age-by-sex interactions in the development of CBF during adolescence,37 the model included age, age squared, sex, an interaction between sex and age, and an interaction between age squared and sex. Furthermore, to control for the known confounding effects of motion in developmental populations,31,44 motion during the ASL acquisition (mean frame displacement) was also included as a covariate. Thus, our group level model was as follows:
We also examined age by sex effects in regions found to be significantly associated with overall psychopathology, as well as interactions between overall psychopathology and both age and sex. Based on recent evidence that lower thresholds provide inadequate Type I error control,45 we used AFNI 3dClustSim (version 17.0.13) with a cluster defining threshold of p = 0.001 (z = 3.09) and a corrected cluster significance of p < 0.01. Data were normally distributed. For additional details, see the Supplementary Methods section.
Sensitivity analyses
Sensitivity analyses were conducted after excluding the minority (12%) of participants who were treated with psychiatric medication at the time of imaging and adding both race and maternal level of education as additional covariates. Lastly, due to the known relationship between brain structure and CBF, we re-evaluated associations between psychopathology and CBF while including gray matter density within each cluster as an additional model covariate (see Supplementary Methods).
Resting state functional connectivity
As a final step, in order to further understand the functional implications of our results, we conducted seed-based connectivity analyses that evaluated the functional connectivity of the dorsal ACC seed, which showed a significant association between CBF and overall psychopathology and has been implicated in prior studies.10 Functional connectivity was available for 833 participants who received both ASL and resting-state imaging as part of the PNC; these data were processed using a previously-described pipeline that minimizes the problematic influence of motion artifact on resting-state functional connectivity MRI31,44,46–48 (see Supplementary Methods). Functional connectivity group level analyses used similar models and Type I error correction as for ASL data.
RESULTS
Elevated CBF in the ACC is associated with overall psychopathology
We began by testing the hypothesis that greater overall psychopathology would be associated with regional resting-state CBF differences. Voxelwise analyses revealed that greater overall psychopathology was associated with elevated perfusion in several regions including the dorsal anterior cingulate cortex (ACC) and rostral ACC (Figure 2). Other regions that also showed higher CBF included the right postcentral gyrus, parahippocampal cortex, and midbrain (Table S1). We found significant associations between age and perfusion in many of these regions, with CBF declining with age in the right postcentral gyrus, dorsal ACC, and rostral ACC. Furthermore, consistent with our prior work,37 linear age by sex interactions were found in all clusters: perfusion declined in males, while perfusion either declined more slowly or increased in females over the age range sampled (Figure S1). However, there were no significant two-way or three-way interactions between overall psychopathology, age, and sex in regions where a main effect of overall psychopathology was present. Sensitivity analyses showed that these results were similar when participants taking psychiatric medications were excluded from the analysis, and when participant race and maternal education were included in the model (Table S2). Notably, results were unchanged when cluster gray matter density was added as a model covariate (Table S3).
Figure 2. Overall psychopathology is linked to elevated perfusion in the anterior cingulate circuit.
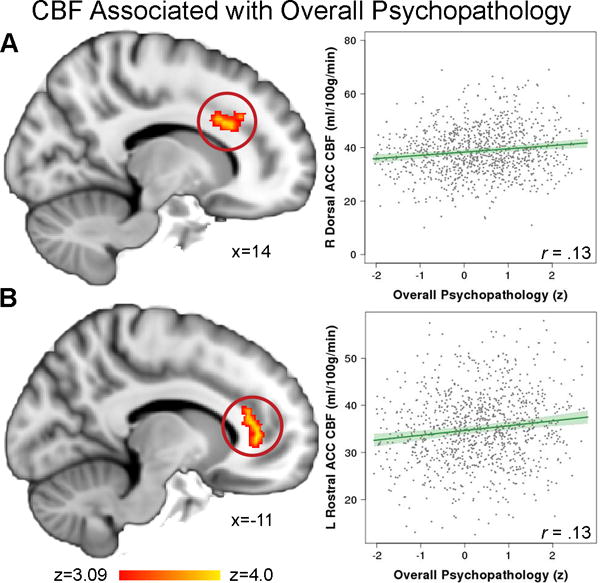
Greater overall psychopathology across categorical clinical diagnoses was associated with elevated perfusion in A) right dorsal anterior cingulate cortex (ACC) and B) left rostral ACC. Images thresholded at z > 3.09, cluster corrected p < 0.01.
Regional cerebral blood flow differences associated with the symptom-specific sub-factors
Next, we examined each of the orthogonal sub-factors representing distinct dimensions of psychopathology (anxious-misery, psychosis, behavioral, and fear) to investigate dissociable associations with resting-state CBF. We found that psychosis-spectrum symptoms were related to reduced perfusion in the left frontal operculum/left insula, whereas fear symptoms were associated with reduced perfusion in the right occipital/fusiform gyrus and left subgenual ACC (Figure 3 and Table S1). No significant results were found for the anxious-misery or behavioral factors. As expected, age by sex interactions were also found in these regions, with perfusion declining more steeply in males than in females (Figure S2). No significant interactions between sub-factors and age or sex were found in these regions. Results remained similar after a sensitivity analysis that excluded participants taking psychiatric medications and after including participant race and maternal education as covariates (Table S2), or when cluster gray matter density was added as a covariate (Table S3).
Figure 3. Dissociable dimensions of psychopathology are associated with cerebral perfusion.
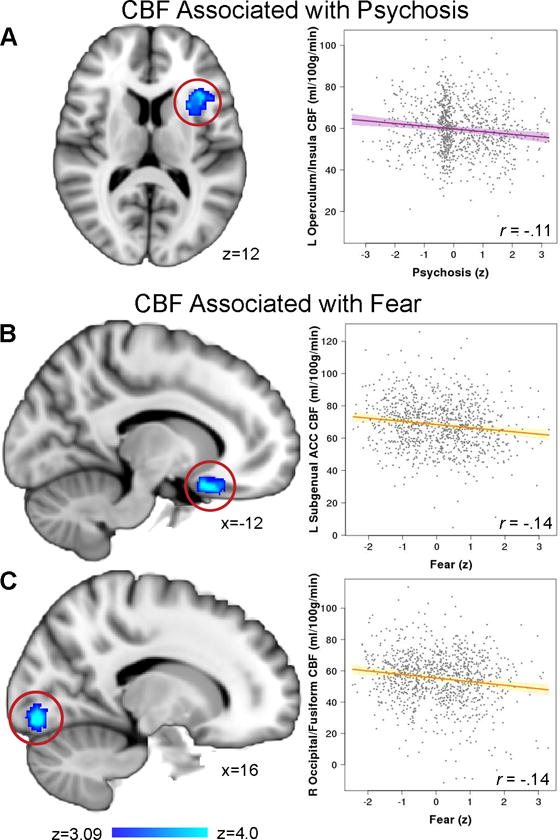
Greater psychosis-spectrum symptoms were related to reduced perfusion in A) left frontal operculum/left insula, whereas fear symptoms were associated with less perfusion in B) left subgenual ACC and C) right occipital/fusiform gyrus. Images thresholded at z > 3.09, cluster corrected p < 0.01.
Dimensional psychopathology is associated with reduced connectivity between dorsal ACC and bilateral caudate
Finally, based on prior work implicating the dorsal ACC in psychopathology,10 we conducted seed-based connectivity analyses to further understand the functional implications of CBF abnormalities in the dorsal ACC. These seed-based analyses revealed that higher levels of overall psychopathology were associated with diminished connectivity between dorsal ACC and bilateral caudate (Figure 4). Overall psychopathology was also associated with diminished connectivity between the dorsal ACC and other regions including the right thalamus, supramarginal gyrus, and right putamen, as well as increased connectivity with the dorso-medial frontal cortex (Table S4).
Figure 4. Overall psychopathology is associated with diminished functional connectivity between the dorsal ACC and bilateral caudate.
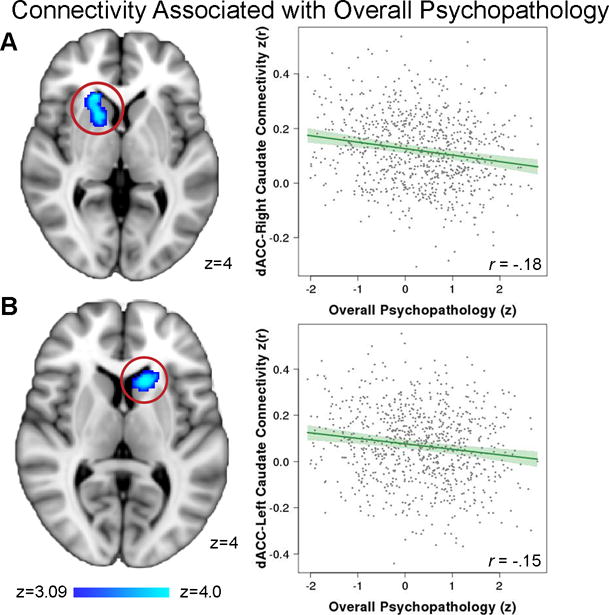
Resting-state functional connectivity data was collected in the same subjects (n=833), and a seed analysis from the dorsal ACC (see Figure 2A) was conducted. This data revealed diminished functional connectivity between the dorsal ACC and the head of the both A) the right caudate and B) the left caudate. Images thresholded at z > 3.09, cluster corrected p < 0.01.
DISCUSSION
In this study, we provide novel evidence that higher resting-state CBF in multiple regions including the ACC is associated with overall psychopathology. This dimensional measure of overall psychopathology was also associated with reduced dorsal ACC functional connectivity with regions such as the caudate. Finally, specific dimensions of psychopathology such as fear and psychosis were associated with diminished CBF in several regions. Taken together, the results suggest the existence of both common and dissociable perfusion abnormalities across traditional categorical psychiatric diagnoses.
Advantages of a dimensional approach to psychopathology
To dimensionally quantify overall psychopathology, we used a bifactor analysis28,29 of the item-level data from a clinician-administered screening interview. As we have shown previously,12 this identifies orthogonal latent dimensions of psychopathology (anxious-misery, psychosis, behavioral, and fear) as well as a dimensional measure of general psychopathology. This approach is advantageous for several reasons. First, the bifactor model allows us to estimate variance that is shared across highly comorbid disorders.29 Studies comparing psychopathology across more than one disorder typically measure comorbidity as shared variance, which is controlled for but not directly evaluated. Second, this approach yields continuous dimensions of psychopathology, rather than “all or none” diagnostic labels. These dimensions allow evaluation of the degree to which individual differences in perfusion are associated with both sub- and supra-threshold symptoms. Third, traditional designs that compare multiple categorical diagnoses are often hindered by varying frequency of, and co-morbidity among, disorders. A bifactor model accounts for such structured covariance and yields orthogonal dimensions that can be included as predictors in a single model.29
Overall psychopathology is associated with common perfusion abnormalities
Using this bifactor approach, the present study demonstrated that common perfusion abnormalities are present across psychiatric diagnoses. Overall psychopathology was associated with elevated resting-state CBF in a network of regions including the anterior cingulate cortex (ACC). These ACC perfusion abnormalities are consistent with prior meta-analytic investigations of case-control structural and functional imaging studies in major depressive disorder,49,50 bipolar disorder,5,51 anxiety disorders,4,52 ADHD,53,54 and psychosis.5,55 This also accords with several across-disorder studies that have provided evidence for structural,10 task-based fMRI,12 and resting state functional connectivity13 abnormalities in the ACC. Given that the ACC is central to numerous processes including cognitive control,56 reward-related decision-making,57 and affect regulation,58 these results suggest that dysfunction in the ACC may impact multiple cognitive processes, and confer vulnerability to a wide variety of psychiatric symptoms.
The traditional conceptualization of the role of the ACC suggests that the dorsal anterior cingulate is involved in effortful regulation of emotional responses, thereby modulating affective limbic regions.59 Thus, activation of the dorsal ACC might be anticipated to potentially reduce a broad range of psychiatric symptoms, which would result in a negative correlation between ACC activation and symptomatology. However, meta-analyses of case-control studies implicate increased activation in the ACC during task-based fMRI studies in anxiety60 and depression,49 symptoms that are common across many disorders.1 The results of these meta-analyses are consistent with the findings of the current study and suggest that higher ACC perfusion may be shared across psychopathology.
Beyond the ACC, we also found elevated perfusion in the postcentral gyrus, parahippocampal cortex, and midbrain. Prior studies document sensorimotor abnormalities in regions such as the postcentral gyrus in incarcerated juveniles with high impulsivity,61 attention-deficit/hyperactivity disorder, autism spectrum disorders,62 and schizophrenia.63 Furthermore, hyperactivation of the parahippocampal cortex has been hypothesized to be a signature of stress in studies of childhood maltreatment.64
In addition to associations between overall psychopathology and CBF, functional connectivity analyses revealed reduced connectivity between the dorsal ACC and the caudate. Abnormalities of the dorsal ACC within a cortico-striatal circuit have been implicated in the pathogenesis of depression,65,66 anxiety,67 ADHD,68,69 and psychosis.70 No interactions between overall psychopathology and age or sex were found. This suggests that ACC abnormalities are established early in development and remain stable over time. Dysfunctional ACC perfusion and connectivity between the ACC and caudate may represent important risk factors for many types of psychopathology in youth of both sexes.
Orthogonal dimensions of psychopathology have dissociable relations with cerebral perfusion
The current study extends prior work on neurobiological phenotypes associated with psychopathology by showing that several clusters of reduced perfusion were associated with specific dimensions of psychopathology. We found that psychosis symptoms were related to reduced perfusion in the left frontal operculum and insula, consistent with prior findings linking schizophrenia to structural and functional abnormalities in the insular cortex.71 Although there is a large body of work demonstrating a connection between psychosis and abnormalities in the cingulo-opercular network, which includes the anterior insula/operculum, dorsal anterior cingulate cortex, and thalamus,70 we found an association between psychosis and only one region in this network.
We also found that fear symptoms were associated with less perfusion in the left subgenual ACC and right occipital/fusiform gyrus. Prior work has implicated subgenual ACC hypofunction in anxiety and fear. The subgenual ACC is thought to regulate limbic regions (such as the amygdala) that are important for generating fear responses.72 Therefore, we speculate that reduced activity of the subgenual ACC, as manifested by reduced subgenual ACC perfusion, may result in decreased regulation of these affective regions, leading to greater fear symptoms. Additionally, we found perfusion abnormalities in the occipital fusiform cortex, a region that is important for processing socially-relevant visual information such as faces, and is implicated in fear and anxiety.73 Taken together, these results suggest that in addition to common perfusion abnormalities associated with overall psychopathology, there are also dissociable perfusion abnormalities specific to psychosis and fear symptoms.
Limitations
Several limitations of the present work should be noted. First, the cross-sectional nature of the current study limits the ability to study developmental changes over time. Future work would benefit from longitudinal designs that allow investigation of when abnormalities in perfusion arise during development. Second, although the use of a community sample enhances the generalizability of these results, it would also be useful to examine these perfusion abnormalities in samples with more severe clinical presentations. Third, it should be noted that this study did not find specific significant associations between CBF and anxious-misery symptoms. In our earlier work,23 we found associations between a dimensional measure of anxious-misery (the State-Trait Anxiety Inventory, STAI) and higher perfusion of regions such as the insula and amygdala. Notably, these analyses of the STAI did not adjust for overall psychopathology as in the current bifactor model, and thus constitute a mix of overall and domain-specific anxious-misery symptoms. However, at lower thresholds both overall psychopathology and anxious-misery showed non-significant relationships with CBF in similar regions. This underlines the strengths and also limitations of the bifactor approach. Of note, the effect sizes in the current study were low (partial correlations ranging between .11 and .18), which may be due to the use of a community-based sample of non-help seeking youth, rather than a clinical population with more severe symptoms. However, small sample sizes with low statistical power are prone to overestimated effect sizes with low reproducibility.74 As the effect sizes reported here were derived from a much larger sample than most prior studies, they may provide a better estimate of the underlying magnitude of each association. It should also be acknowledged that the present results were found in a single sample, and should be replicated in future independent samples. Finally, ASL MRI technology has improved since these data were collected, and future studies will benefit from sequences which provide greater sensitivity and spatial resolution.75
Conclusions
The current study provides novel evidence that elevated perfusion in regions including the ACC is associated with dimensional burden of overall psychopathology across psychiatric disorders in youth. This suggests a shared mechanism for psychopathology symptoms that cuts across clinical diagnostic categories, supporting the pathophysiology-based conceptualization of neuropsychiatric disorders proposed by the National Institute of Mental Health’s Research Domain Criteria.19 Perfusion abnormalities in the ACC may also suggest possible candidate biomarkers for future work on pharmacological and clinical interventions. Importantly, interventions that target these shared circuits may be beneficial to a broad range of psychiatric disorders and not limited to a specific categorical diagnosis. Finally, longitudinal data could identify perfusion abnormalities in youth at risk for psychopathology and allow for biomarkers to guide the development of targeted early interventions during the vulnerable period of adolescence.
Supplementary Material
Acknowledgments
Thanks to the acquisition and recruitment team: Karthik Prabhakaran, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, and Rosetta Chiavacci. Thanks to Chad Jackson and Larry Macy for data management and systems support. Thanks to Kathleen Merikangas and Marcy Burstein at the NIMH. Supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924 and P50MH096891. Additional support was provided by R01MH107703 to TDS, R01MH101111 to DHW, K01MH102609 to DRR, K08MH079364 to MEC, R01NS085211 to RTS, and the Dowshen Program for Neuroscience. Support for developing statistical analyses (RTS & TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Data deposition: The data reported in this paper have been deposited in database of Genotypes and Phenotypes (dbGaP), www.ncbi.nlm.nih.gov/gap (accession no. phs000607.v1.p1).
Footnotes
FINANCIAL DISCLOSURES: Dr. Edna B. Foa receives royalties from the sale of the books, “Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide,” and “Reclaiming your Life from a Traumatic Experience Workbook” by Oxford University Press. Dr. Foa also receives payment for training workshops she conducts on prolonged exposure therapy. Dr. Shinohara has received legal consulting and advisory board income from Genentech/Roche. All other authors (Dr. Kaczkurkin, Dr. Moore, Dr. Calkins, Mr. Ciric, Dr. Detre, Dr. Elliott, Mr. de La Garza, Dr. Roalf, Mr. Rosen, Ms. Ruparel, Mr. Xia, Dr. Wolf, Dr. R.E. Gur, Dr. R.C. Gur, and Dr. Satterthwaite), reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flamez B, Sheperis C. Diagnosing and treating children and adolescents: A guide for mental health professionals. New Jersey: John Wiley and Son’s; 2016. [Google Scholar]
- 3.Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 4.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 8.Baker JT, Holmes AJ, Masters GA, Yeo BTT, Krienen F, Buckner RL, Öngür D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–18. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, et al. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: A replicated cross-diagnostic finding. Frontiers in Psychology. 2015;6:1–12. doi: 10.3389/fpsyg.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hägele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, et al. Dimensional psychiatry: Reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232:331–41. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. American Journal of Psychiatry. 2016;173:517–26. doi: 10.1176/appi.ajp.2015.15060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, et al. Common dimensional reward deficits across mood and psychotic disorders: A connectome-wide association study. American Journal of Psychiatry. 2017 doi: 10.1176/appi.ajp.2016.16070774. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Current Opinion in Neurology. 2009;22:348–55. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 15.Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, Aizenstein H. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depression and Anxiety. 2011;28:202–9. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage. 2011;54:S62–8. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17804–9. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: The NIMH research domain criteria project. Schizophrenia Bulletin. 2010;36:1061–2. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (rdoc): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 20.Lahey BB, Moffitt TE, Caspi A. Causes of conduct disorder and juvenile delinquency. New York: Guilford Press; 2003. [Google Scholar]
- 21.Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annu Rev Psychol. 2005;56:453–84. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- 22.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121:971–77. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, et al. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry. 2016;80:775–85. doi: 10.1016/j.biopsych.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–53. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The philadelphia neurodevelopmental cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124:1115–9. doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The philadelphia neurodevelopmental cohort: Constructing a deep phenotyping collaborative. Journal of Child Psychology and Psychiatry. 2015;56:1356–69. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzinger KJ, Swineford F. The bi-factor method. Psychometrika. 1937;2:41–54. [Google Scholar]
- 29.Reise SP, Moore TM, Haviland MG. Bifactor models and rotations: Exploring the extent to which multidimensional data yield univocal scale scores. Journal of Personality Assessment. 2010;92:544–59. doi: 10.1080/00223891.2010.496477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus The Comprehensive Modelling Program for Applied Researchers: Users Guide. Los Angeles, CA: Muthén and Muthén; 2012. [Google Scholar]
- 31.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SM, Brady JM. SUSANa new approach to low level image processing. International Journal of Computer Vision. 1997;23:45–78. [Google Scholar]
- 36.Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging. 2008;26:261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proceedings of the National Academy of Sciences. 2014;111:8643–8. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu W-C, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, Wang DJ. In vivo venous blood T1 measurement using inversion recovery true-fisp in children and adults. Magnetic Resonance in Medicine. 2010;64:1140–7. doi: 10.1002/mrm.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain V, Duda J, Avants B, Giannetta M, Xie SX, Roberts T, et al. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin–labeled perfusion MR imaging in typically developing children. Radiology. 2012;263:527–36. doi: 10.1148/radiol.12111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein A, Ghosh SS, Avants B, Yeo BTT, Fischl B, Ardekani B, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51:214–20. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fmri inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;80:775–85. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, et al. Linked sex differences in cognition and functional connectivity in youth. Cerebral Cortex. 2014;25:2383–94. doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciric R, Wolf DH, Power JD, Roalf DR, Baum G, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 50.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: A meta-analysis of voxel based morphometry studies. Journal of Affective Disorders. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 51.Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biological Psychiatry. 2010;67:1097–105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Radua J, Van Den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Archives of General Psychiatry. 2010;67:701–11. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 53.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: A meta-analysis of 55 fmri studies. American Journal of Psychiatry. 2012;169:1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 55.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008;64:774–81. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: Who’s in control? Nature Neuroscience. 2000;3:421–3. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- 57.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fmri experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–27. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 59.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 60.Rotge J-Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocation of obsessive–compulsive symptoms: A quantitative voxel-based meta-analysis of functional neuroimaging studies. Journal of Psychiatry & Neuroscience. 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 61.Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proceedings of the National Academy of Sciences. 2011;108:11241–5. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piek JP, Dyck MJ. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Human Movement Science. 2004;23:475–88. doi: 10.1016/j.humov.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 63.Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 64.Hein TC, Monk CS. Research review: Neural response to threat in children, adolescents, and adults after child maltreatment–a quantitative meta-analysis. Journal of Child Psychology and Psychiatry. 2016;58:222–30. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- 65.Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biology of Mood and Anxiety Disorders. 2011;1:1–10. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davey CG, Harrison BJ, Yücel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine. 2012;42:2071–81. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 67.Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:938–48. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–39. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch K-P, et al. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping. 2009;30:2252–66. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tu P-C, Hsieh J-C, Li C-T, Bai Y-M, Su T-P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fmri study. Neuroimage. 2012;59:238–47. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 71.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry and Neuroscience. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: A focused review. Therapeutics & Clinical Risk Management. 2015;11:115–26. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 75.Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the european consortium for ASL in dementia. Magnetic Resonance in Medicine. 2015;73:102–16. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


