Abstract
Background
Polybrominated diphenyl ethers (PBDEs) were widely used as flame retardants until the early 2000s, mainly in home furnishings and electronics. The persistence of PBDEs in the environment leads to continued ubiquitous exposure to low levels, with infants and children experiencing higher exposures than adults. Accumulating evidence suggest that low-level exposures during early life stages can affect brain development and lead to long-term behavioral impairments. We investigated the effects of zebrafish exposure to low doses of the two prominent PBDEs; 2,2',4,4',5,-Pentabromodiphenyl ether (BDE-99) and 2,2',4,4',-Tetrabromodiphenyl ether (BDE-47), during embryo-development on short- and long-term behavioral endpoints. We included the organophosphate pesticide chlorpyrifos (CPF) due to its well documented neurotoxicity across species from zebrafish to humans.
Methods
Zebrafish embryos were exposed to the following individual treatments; 0.1% DMSO (vehicle control); 0.3 µM CPF; 0.01, 0.03, 0.1, 0.3 µM BDE-47; 0.003, 0.03, 0.3, 1, 3, 10, 20 µM BDE-99 from 5 until 120 hours post fertilization (hpf). Low exposure levels were determined as those not causing immediate overt toxicity, and behavior assays were conducted in the low-level range. At 144 hpf the larvae were tested for locomotor activity. At approximately 6 months of age adult zebrafish were tested in a behavioral battery including assays for anxiety-related behavior, sensorimotor response and habituation, social interaction, and predator avoidance.
Results
In the short-term, larval locomotor activity was reduced in larvae treated with 0.3 µM CPF and 0.1 µM BDE-47. BDE-99 treatment caused non-monotonic dose effects, with 0.3 µM causing hyperactivity and 1 µM or higher causing hypoactivity. In the long-term, adult anxiety-related behavior was reduced in all treatments as measured in both the novel tank dive test and tap test.
Discussion
We show that exposure of zebrafish embryos to low concentrations of the brominated flame retardants BDE-47 and BDE-99, and the organophosphate pesticide CPF, caused both short- and long-term behavioral impairments. Interestingly, we also found that at very low exposure concentrations, where there were no visible effects on larval activity, adult behavior was still strongly affected.
Keywords: Zebrafish, chlorpyrifos, BDE 47, BDE 99, Brominated Flame Retardants, Development, Neurobehavioral Toxicology
Introduction
Polybrominated diphenyl ethers (PBDEs) are a group of chemicals used as flame retardants in many consumer products. PBDEs, similarly to other flame retardants, are not chemically bound to the material in which they are inserted, and thus gradually leach into the surrounding air and dust. PBDEs were heavily used, mainly in the United States, until the early 2000s when based on scientific indications for toxicity they were largely phased out in the US and around the world (Reviewed by Fromme et al., 2016; Hites, 2004; USEPA, 2010). Despite the reduction in use of PBDEs in the last decade, high levels are still detected in house dust and air, and in human blood, cord blood and breast milk samples, with 2,2'4,4'-tetrabromodiphenyl ether (BDE-47) and 2,2',4,4',5-pentabromodiphenyl ether (BDE-99) being two of the most dominant congeners (Besis and Samara, 2012; Dodson et al., 2012; Fromme et al., 2016; Stapleton et al., 2009; Stapleton et al., 2011). Exposure of toddlers and children to PBDEs, including BDE-47 and BDE-99, is significantly higher than that of adults (Fromme et al., 2016; Lunder et al., 2010; USEPA, 2010; Wu et al., 2015). In addition, it has been shown that PBDEs cross the placenta in a congener-dependent rate (Doucet et al., 2009; Fromme et al., 2016; Schecter et al., 2007; USEPA, 2010). Due to the persistence of PBDEs in the environment and the presence in households of older products that still contain these chemicals, exposure to PBDEs is likely to continue posing a toxic risk to many people.
Exposure to many environmental contaminants during early, developmental life stages has been associated with adverse health effects throughout the life span (Heindel and Vandenberg, 2015; Linares et al., 2015). The developing brain is especially sensitive to perturbation, and even very low levels of chemical exposure may cause persistent impairments in cognitive functions and other types of behavior (Grandjean and Landrigan, 2006, 2014). The potential for PBDEs to cause developmental neurotoxicity has been studied in various in vivo and in vitro model systems. Mechanistically, evidence is presented in support of a number of toxicity pathways including impairment of thyroid hormone homeostasis, cytotoxicity caused by oxidative stress and mitochondrial disruption, and interference with calcium signaling (Costa et al., 2014; Hendriks and Westerink, 2015). In epidemiological studies, prenatal as well as childhood exposures to BDE-47 and BDE-99 were associated with behavioral alterations such as impaired motor skills and cognition, and lower IQ (Herbstman and Mall, 2014; Linares et al., 2015). Behavioral studies conducted in rodent models revealed effects of pre- and postnatal exposures to BDE-47 and BDE-99 on behavioral functions including spontaneous locomotor activity, habituation to anxiety-promoting situations and for BDE-99 also learning (Costa and Giordano, 2007; Hendriks and Westerink, 2015).
The current study was conducted in zebrafish to provide an important extension from classic rodent studies to an intact organism, which is much less expensive and could more easily be used for broader screening studies. Zebrafish are increasingly used for medium and large-scale screenings of chemicals, such as environmental contaminants, for potential developmental neurotoxicity. In these screens zebrafish are exposed to different chemicals throughout the entire period of embryonic development or parts of it, and neurotoxicity is tested using larval activity analysis in various protocols of alternating light and dark conditions. In several such screening studies, exposure to either BDE-47 or BDE-99 during embryo-development caused significant alterations in activity of larvae at 5 and 6 days post fertilization (dpf) (Jarema et al., 2015; Noyes et al., 2015; Usenko et al., 2011; Zhao et al., 2014). While larval activity testing can provide important information on a large number of potential neurotoxins in a short period of time, it is also limited by the behavioral information it provides, which is limited to states of hyper- or hypoactivity and altered response to lights-on or lights-off. In addition, short-term experiments cannot provide information on persistence or delay in behavioral alterations throughout the life-time. To date, reliable and consistent behavioral assays have already been developed for direct testing of juvenile and adult zebrafish anxiety-related behavior, sensorimotor plasticity, social affiliation, learning and memory and other cognitive functions. Although extending the testing period to later life stages reduces the screening throughput, it remains significantly higher than that of rodent and other mammalian models, and maintains an advantage in providing data on a wide range of behavioral endpoints in shorter time scales and lower costs.
The current study is part of a larger project aimed at screening for potential persistent neurobehavioral effects of developmental exposure to low levels of several classes of flame retardants. Here we present results from exposure to the two dominant PBDE congeners, BDE-47 and BDE-99. We first exposed zebrafish embryos to a wide range of concentrations of each of the two PBDEs. We then tested for behavioral alterations in larvae and in adults exposed to levels that were below the threshold for adverse dysmorphogenesis or increased lethality. Larval testing consisted of activity analysis during alternating light/dark conditions. Adult testing included a battery or four assays for evaluation of anxiety-related behavior, sensorimotor response and habituation, social affiliation and predator recognition and escape. In addition to the BDE treatments and vehicle control, we included in our exposures a treatment with the organophosphate pesticide chlorpyrifos (CPF), that has been extensively shown to cause neurotoxicity and neurobehavioral alterations in both larval and adult zebrafish following developmental low-level exposure (Eddins et al., 2010; Levin et al., 2003; Levin et al., 2004; Oliveri et al., 2015; Richendrfer and Creton, 2015; Richendrfer et al., 2012).
Materials and Methods
Fish Housing and Husbandry
All the experiments were conducted using a local colony of AB* wild-type strain of zebrafish, maintained and bred at the Levin Lab. The experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee. Adult zebrafish were held in mixed (females and males) groups at a density of ≤5 fish/l in 3 or 10 l tanks in a recirculating flowing water systems (Aquatic Habitats, Inc., Apopka, FL, USA; Aquatic Enterprises, Inc., Bridgewater, MA, USA). System water was a mixture of sea salt (Instant Ocean, 0.5 parts per thousand) and buffer (Seachem Neutral Regulator, 125 mg/l) in de-ionized water. Water chemistry, salinity and temperature were monitored weekly. Illumination was set to 14:10 h light:dark cycle and water temperature was kept at 28 ± 1 °C. The fish were fed three times daily; morning and afternoon with brine shrimp (Artemia salina) hatched in-house over 24 h (eggs from Brine Shrimp Direct, Ogden, UT, USA); and noon feeding with a mixture of solid pellet food containing; TetraMIN Tropical Flakes (Blacksburg, VA, USA); GEMMA Micro 300 micro-pellets (Skretting USA, Tooele, Utah); Zebrafish Complete Diet (Ziegler Bros., Inc., Gardners, PA, USA).
Fertilized eggs were obtained by group breeding using in-tank inserts. The inserts were placed in the flow-through tanks at the end of the previous day. Embryos were collected 1–2 h after the lights turned on the next morning. Immediately after collection the embryos were rinsed with a 10,000× diluted solution of bleach for 1 min followed by two quick rinses with system water, and transferred to large plastic Petri dishes. At 0–5 days post fertilization (dpf) the embryos were kept in an incubator held at 28–29 °C, with a 14:10 h light:dark cycle. From 6 dpf until 11 dpf, larvae were kept in 3 l tanks with a small amount of system water that was increased daily, and fed three times a day with fine-particle solid food (Golden Pearl Reef & Larval Fish Diet, 5–50 micron size, Brine Shrimp Direct, Inc., Ogden, UT, USA). At 11 dpf the tanks were started on water flow-through, and the diet was changed to twice daily feeding with brine shrimp (Brine Shrimp Direct, Inc.) and once daily fine-particle solid food. At 3–4 weeks post fertilization, juvenile fish were transferred to the densities and diet as detailed above for adult fish.
Chemicals
Dimethyl Sulfoxide ReagentPlus®, ≥99.5% (DMSO; CAS# 67-68-5, Lot# SHBG9650V) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Chlorpyrifos (CPF), 2,2',4,4',5,-Pentabromodiphenyl ether (BDE-99), and 2,2',4,4',-Tetrabromodiphenyl ether (BDE-47) were provided by the National Toxicology Program (NTP), each at a volume of 5 ml and a concentration of 20 mM. Table S1 presents supplier specifications and purity analysis for the NTP-provided chemicals.
Developmental Exposure
Zebrafish embryos were exposed from approximately 5 h post fertilization (hpf) until 5 dpf to either 0.1% DMSO alone or one of the following individual chemicals and concentrations in 0.1% DMSO; CPF (0.3 µM); BDE-99 (0.003, 0.03, 0.3, 1, 3, 10, 20 µM); BDE-47 (0.01, 0.03, 0.1, 0.3 µM). Stock solutions were 20 mM, and working dilutions were prepared at 1000× concentration in 100% DMSO. Dose ranges were selected based on information from the published literature, as well as preliminary range finding exposures conducted in our lab. At 5 hpf embryos were sorted under a dissecting microscope, discarding unfertilized or abnormally developing embryos, then randomly and evenly distributed into glass Petri dishes filled with system water, and immediately exposed to the above detailed treatments. Every 24 h the embryos were examined and dead or malformed individuals were recorded and excluded, and the exposure solution was renewed. At 5 dpf the embryos were rinsed twice with non-dosed system water, transferred to clean glass Petri dishes with non-dosed system water, and placed in the incubator until larval activity testing at 6 dpf.
Larval Locomotor Activity Testing
At 6 dpf larvae were placed into 96-well plates with 0.5 ml glass well inserts filled with system water, and tested for locomotor activity in response to alternating light conditions. Table S2 summarizes the exposure cohorts and fish numbers used in larval behavior testing. Exposure conditions were all represented within each plate and across multiple plates. The plates were then returned to the incubator for an hour before being placed into a DanioVision™ lightbox controlled by the EthoVision XT® tracking software (version 11.5, Noldus, Wageningen, The Netherlands). Locomotor activity was tracked during a paradigm in which an initial 10-min habituation period in the dark (0% illumination) was followed by 2 cycles of 10 min at 100% illumination (5000 l×) and 10min at 0% illumination. An infrared camera tracked larval locomotion across the 50-min trial. All larval testing was conducted between 11 AM and 5 PM. Locomotor activity was recorded at a rate of 30 frames per second, and a track smoothing protocol was applied based on 10 samples before and after every sample point in order to exclude slight movements that might introduce noise to the calculations. Total distance moved is reported in cm per minute or cm per 10 min. The initial habituation phase is presented in the results, however it is not considered in our overall analysis of treatment effects, since it immediately follows handling of the plate and the recording of this phase starts 1–2 minutes after the larvae have been placed in the dark testing apparatus, thus it does not fully represent their activity during this period. After testing the larvae were placed in flow-through tanks and reared as described above (Fish housing and husbandry).
Adult Behavioral Test Battery
Developmentally-exposed adult zebrafish were tested at the age of 5–7 months in a series of 4 behavioral assays; novel tank dive test; tap test; shoaling test; and predator avoidance test, to evaluate several emotional, social and cognitive functions. Each assay was conducted on a separate day. All testing was conducted between 10 AM and 5 PM, and testing times were counterbalanced across all experimental groups. Each testing day began after the routine morning brine shrimp feeding. Fish tanks designated for testing were transferred to the behavior testing room, and let acclimate for 30–60 min. Freshly made system water was used in all testing apparatuses. An HD camcorder (VIXIA HFR700; Canon Inc., Tokyo, Japan) was used for video recording in all assays, and the videos were fed to the EthoVision XT® software (Noldus) for fish tracking and activity analysis. Table S3 summarizes the exposure cohorts and fish numbers used in adult behavior testing. Briefly, we tested 21 fish from two cohorts treated with 0.1% DMSO alone; 13 fish from two cohorts treated with 0.3 µM CPF; 11 fish from two cohorts treated with 0.01 µM BDE-47; 9 fish from one cohort treated with 0.03 µM BDE-47; 12 fish from one cohort treated with 0.1 µM BDE-47; 28 fish from two cohorts treated with 0.003 µM BDE-99; and 26 fish from three cohorts treated with 0.3 µM BDE-99. Differences in numbers of fish tested and exposure cohorts per treatment are a result of our range seeking process in which certain concentrations were present in most or all exposure cohorts while other, lower or higher concentrations, were added in subsequent exposures. See results section on Survival and dysmorphogenesis at 6 dpf (Table S4) for details on all exposure cohorts and concentrations.
Novel Tank Dive Test
Adult zebrafish were tested for novel environment response and recovery based on the method employed previously in our laboratory (Levin et al., 2007) with modifications. The experimental set-up consisted of two adjacent 1.5-l plastic tanks (Aquatic Habitats) filled with 10 cm of system water. Each tank was a trapezoid: 22.9 cm along the bottom, 27.9 cm at the top, 15.2 cm high and 15.9 along the diagonal side. It was 6.4 cm wide at the top, and tapered to 5.1 cm at the bottom. The tanks were video recorded from the side. At the beginning of each trial two fish were individually placed in the testing tanks and recorded for 5 min. Measurements extracted were total distance traveled in cm per minute and the mean distance from the tank floor in cm per min. A dive recovery value was calculated for each treatment group by subtracting the distance from the tank floor in the first minute of testing from the mean distance in the last 4 min of testing.
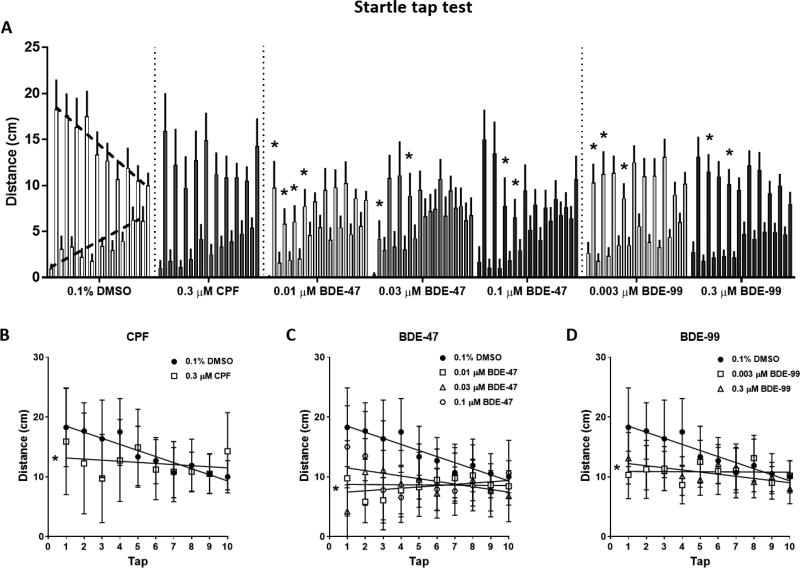
Startle Tap Test
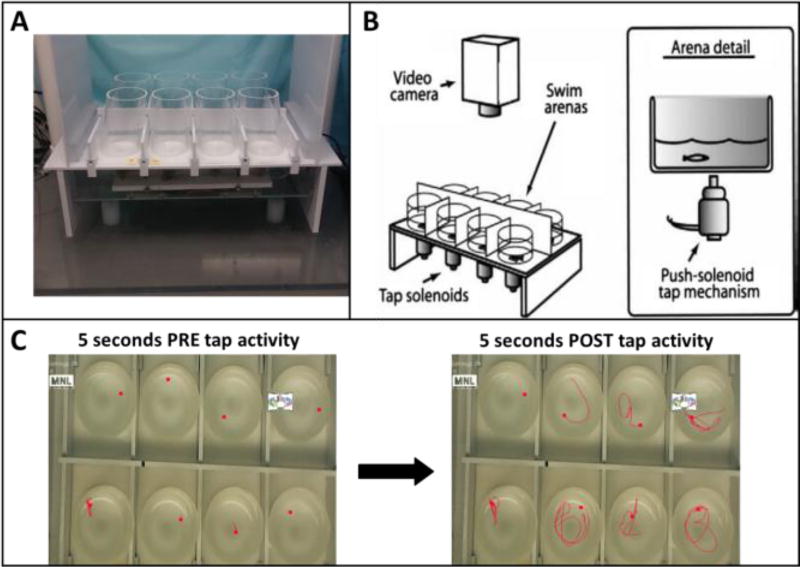
Sensorimotor startle response and habituation were tested using a custom-built apparatus and based on a protocol developed in our laboratory (Crosby et al., 2015; Eddins et al., 2010) with modifications. The testing apparatus (Fig. 1) consisted of flat white 23 × 39 cm surface with white 23 × 27 cm frontal and rear blocking barriers attached. On the flat surface were attached eight clear cylindrical arenas, 5.7 cm in diameter, which were made of Plexiglas and arranged in a 2 × 4 setup. Each arena was clear with horizontal bottoms and slightly angled sides to enable complete visibility to the camera fixed overhead. Opaque screens separated the arenas, isolating subjects from each other to eliminate shoaling behavior. Each arena contained 40 ml of system water (17 mm depth) that were replaced after each trial. The apparatus was video recorded from above. Below each arena was a centrally located 24-volt DC push solenoid that provided a sudden tap when activated by the EthoVision XT® software. At the beginning of each trial eight fish were individually placed in the testing arenas and the testing sequence was initiated. The testing sequence consisted of a 30-s acclimation period followed by 10 consecutive taps at 1 min intervals. Measurements extracted were total distance traveled in cm per the 5-s period before (pre) and after (post) each tap. The choice of pre- and post-tap 5-sec time segments was based on pilot tests conducted in our lab during method development (Eddins et al, 2010), and were found to provide consistent and sensitive measures of baseline and tap-response activity, respectively. Additional calculations were done to extract the percent of fish in each treatment group that were active in each treatment group during each tap, and the percent of individuals that were responsive to the tap. An individual fish was considered active if it moved more than 3 cm during the 5-sec post-tap period, and a tap-responsive fish showed an increase in movement of more than 3 cm compared to the corresponding pre-tap period.
Figure 1.
The zebrafish startle tap testing apparatus and method. A) Top view showing the testing apparatus. Fish were tested in eight cylindrical chambers arranged in a 2 × 4 array. B) Schematic diagram illustrating the testing chambers above the tap solenoids which are computer controlled to deliver one vibrational startle per minute (ten total trials). Modified from Eddins et al. (2010). C) Representative tracking of eight zebrafish during the startle task. Left image-5 sec before the tap (pre-tap); right image-5 sec after the tap (post-tap).
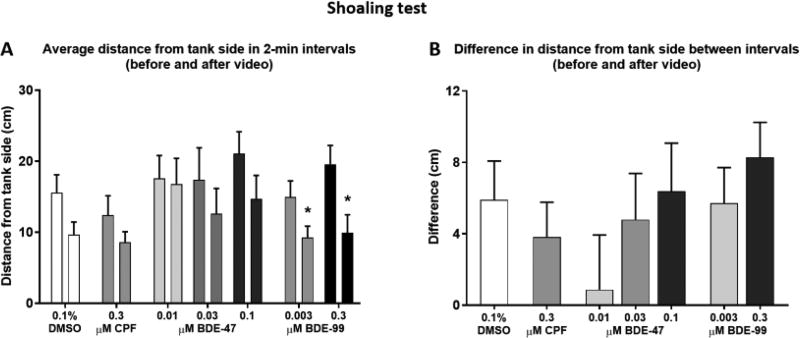
Shoaling Test
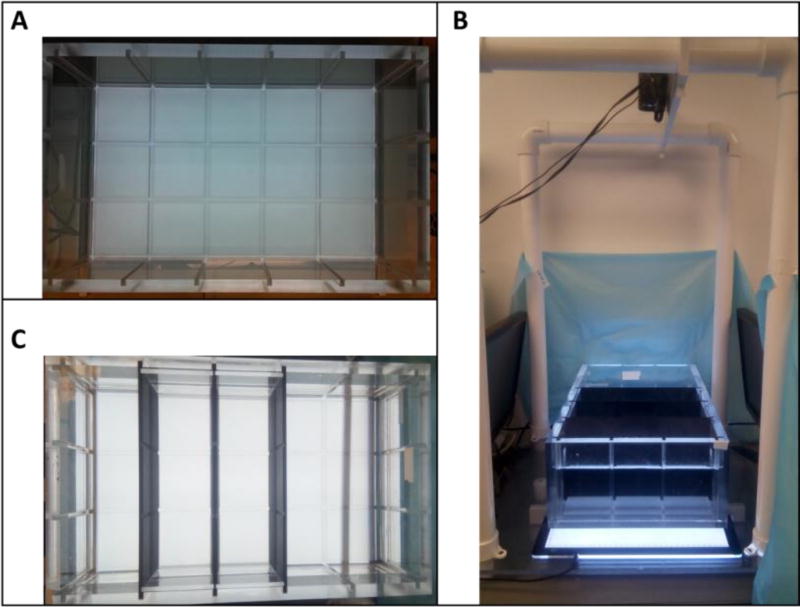
Individual social interaction was tested using a custom-built adult behavior testing tank called Multiple Use Partitioned Experimental Tank (MUPET) that was constructed at the Woods Hole Oceanographic Institution and completed at Duke University (Fig. 2). The tank is a 519.85 × 327.15 mm (L×W, outer measurements) rectangle. The sides and bottom are made of 12.7 mm thick transparent acrylic sheets. The bottom sheet is sandblasted to reduce glare, and divided into a 5 × 3 grid by a network of slots that are 6.35 mm wide and 10 mm deep. The grid slots continue up the walls of the tank, and along the walls on the inner bottom perimeter. Three 16 mm×31.4 mm black partitions were inserted to create two adjacent lanes across the tank width. The MUPET was situated on two metal bars and an A2 60 × 40 cm light box (Huion Technology, Shenzhen, China) was placed underneath the tank bottom providing even light throughout the tank. Two 19.5 in LCD monitors flanked the narrow ends of the two lanes. A digital video camcorder was placed above the tank.
Figure 2.
The MUPET apparatus. A) Top view showing the tank and bottom 3 × 5 grid. B) Top view showing the experimental set-up for the shoaling and predator avoidance tests, using partitions to create two adjacent lanes across the tank width. C) Side view of the experimental set-up. The MUPET is situated on top of a light box with a computer screen on each side of the tank and a video camcorder placed above the tank.
The test procedure was based on a protocol developed in our laboratory (Oliveri et al., 2015), with modifications. Adult fish were singly isolated in 1.5-L tanks surrounded by opaque dividers for 30 min before being netted into the MUPET lanes described above and recorded for 7 min. During the first 2 min of the test, each monitor screen displayed a background of static ovals approximately the size of an adult zebrafish and displaying the pattern and colors of a typical zebrafish. At the end of the first two min one of the monitors began to display a video recording of a zebrafish shoal for the remaining 5 min. Measurements extracted were total distance traveled in cm per min, and the mean distance in cm per min to the side of the tank on which the video was displayed. A pre-post video difference value was calculated for each treatment group by subtracting the average distance from the tank side in the two minutes after the video started playing from the average distance in the two minutes before the video began.
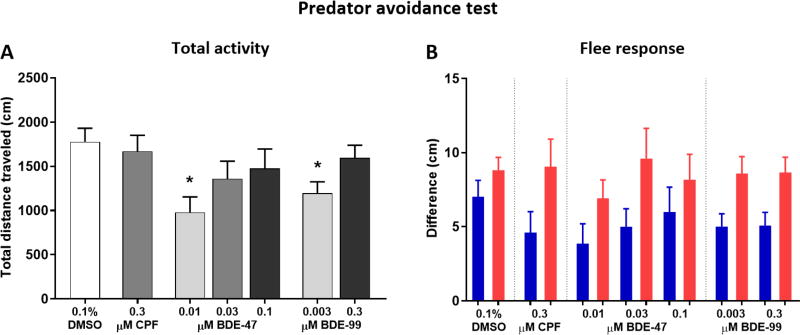
Predator Avoidance Test
Threat recognition and evasion behavior were tested using a testing apparatus and set-up as described in the previous section (Shoaling test). The test procedure was based on a protocol developed in our laboratory (Crosby et al, 2015; Oliveri et al., 2015), with modifications. Individual fish were placed in the MUPET lanes and recorded for 9 min consisting of one min acclimation followed by 8 min of alternating minute-long stimulus/no stimulus (NS) events. The stimulus was a PowerPoint™ presentation showing either a blue slow-growing dot (4 s) or a red fast-growing dot (1 s) appearing repeatedly on one of the screens. The blue dot appeared in the first two stimulus events and the red dot appeared in the last two stimulus events. Measurements extracted were total distance traveled in cm per min, and the mean distance in cm per min to the side of the tank on which the stimulus was displayed. A flee response value was calculated separately for the blue and red stimuli, and for each treatment group, as the difference in average distance from the tank side between trial minutes in which the dot stimulus was presented and minutes in which there was no stimulus (NS).
Statistical Analysis
Supplementary tables S2 and S3 list the cohorts and fish numbers in all treatments used for larval and adult behavioral testing, respectively. All statistical analyses were performed in GraphPad Prism (GraphPad Software, Inc., version 7.01). Post hoc comparisons were done using Dunnett’s test, unless stated otherwise. Significance was set at p < 0.05 for all analysis of variance (ANOVA) and post hoc comparisons, and for linear regression comparisons.
Larval locomotor activity at 6 dpf was analyzed with two-way ANOVA, with treatment as the between-subjects factor, and either illumination phase as the within-subjects factor, or minute as the within-group repeated measure. Distance moved (cm per 1-min increment or 10-min illumination phase) was the dependent variable.
Two-way was used to analyze novel tank total distance traveled and mean distance from the tank floor; tap test total distance traveled pre- and post-tap; shoaling test and predator avoidance test total distance traveled and mean distance from tank side. Treatment was defined as the between subjects factor, time (minute) or tap as the repeated measure, and distance (cm per minute) as the dependent variable. Two-way ANOVA was also used to analyze shoaling test before and after video 2-min intervals. Treatment was defined as the between subjects factor, the 2-min intervals as the repeated measure, and average distance from tank side as the dependent variable. One-way ANOVA was used to analyze shoaling test before and after video difference; predator avoidance test blue or red flee response. Treatment was defined as the between subjects factor, and the above described calculated values were defined as dependent variables.
Linear regression was used to analyze pre- and post-tap patterns of total distance traveled, and patterns of percent post-tap activity and responsiveness. Distance traveled was the dependent variable, and treatment and tap were independent variables, and the significance was calculated for the difference in slopes and intercepts between treatment groups. For total distance traveled analysis, each replicate Y value (distance traveled per tap per fish) was considered as an individual point. A ‘runs test’ was conducted for all pre- and post-tap slopes, and the slopes were not found to deviate from linearity.
All data are presented as mean ± standard error of mean (S.E.M.).
Results
Survival and dysmorphogenesis at 6 dpf
Dead or deformed (spinal curvature, short-body, craniofacial malformations, edema) individuals were noted and removed each day from 1 until 6 dpf, and summed across the entire period. Table S4 summarizes the percent of survival (including dead and deformed individuals) and highlights incidents of increased dysmorphogenesis at 6 dpf in each cohort and treatment. Control fish exposed to 0.1% DMSO had an average survival percentage of 82.8% (range across cohorts 75–92.5%). Exposure to 0.3 µM CPF resulted in an average of 75% survival (range 55–85%). In both treatments, the number of dead embryos or individuals with any specific malformation did not exceed 10% overall. Treatment with 0.3 µM BDE-47 caused spinal curvature by 6 dpf in almost 100% of the larvae in two of three cohorts. Therefore, this group was not included in the behavior testing, and the range of exposure concentrations was adjusted in subsequent cohorts. Increased dysmorphogenesis was not observed at lower BDE-47 exposure concentrations, however average survival appeared lower than in the control. Exposure to BDE-99 was not seen to increase mortality. BDE-99 exposure resulted in average survival of more than 75% of embryos in all exposure concentrations.
Larval Locomotor Activity
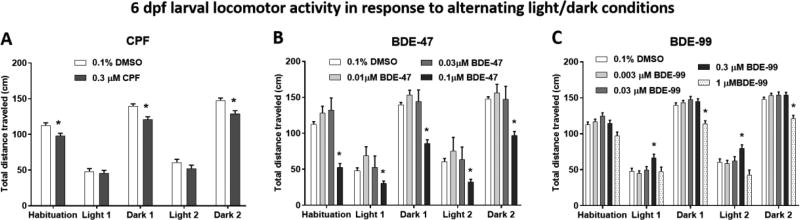
Developmental exposure to 0.3 µM CPF caused a significant reduction in larval locomotor activity (hypo-locomotion) at 6 dpf during all dark phases (Fig. 3A and Fig. S1A). Two-way ANOVA analysis revealed overall effects of both illumination phase (F(4, 900) = 248.8, P < 0.0001) and of treatment (F(1, 900) = 28.83, P < 0.0001) on larval activity, but no interaction between the two parameters.
Figure 3.
6 dpf larval locomotor activity in response to alternating light/dark conditions following developmental exposure to CPF (A), BDE-47 (B), and BDE-99 (C). The fish were recorded for 50 min, starting with 10 min in the dark (Habituation), followed by two cycles of 10 min in the light and 10 min in the dark. Asterisks indicate significant difference from the 0.1% DMSO control group.
Exposure to 0.1 µM BDE-47, but not 0.03 µM or 0.01 µM caused hypo-locomotion in all illumination phases (Fig. 3B and Fig. S1B). Two-way ANOVA analysis revealed overall effects of both illumination phase (F(4, 644) = 129.8, P < 0.0001) and of treatment (F(3, 161) = 38.63, P < 0.0001) on larval activity, as well as an interaction between the two parameters (F(12, 644) = 5.963, P < 0.0001). Post-hoc analyses comparing activity levels between the DMSO control and BDE-47 treatments found significant differences in both illumination phases.
BDE-99 exposure caused an overall main effect of illumination phase (F(4, 1464) = 819.8, P < 0.0001) and treatment (F(4, 366) = 6.715, P < 0.0001) as well as an interaction between the two factors (F(16, 1464) = 4.038, P < 0.05) (Fig. 3C and Fig. S1C). There was a biphasic dose-effect function of BDE-99 exposure on larval activity. Exposure to 0.3 µM BDE-99, but not lower doses of 0.03 µM or 0.003 µM caused a significant (P < 0.01) increase in locomotor activity during both light phases. In contrast, at the higher exposure concentration of 1 µM there was a reduction in larval activity in ‘Dark 1’ and ‘Dark 2’. Higher BDE-99 concentrations were tested, including 3, 10 and 20 µM, in order to identify an acute level of exposure that causes increased lethality or dysmorphogenesis in the embryonic stages. We did not observe an acute effect even at the highest exposure concentration, however all the higher concentrations of 1–20 µM caused a decrease in larval activity, with 20 µM exposure leading to decreased activity in all illumination phases (data not shown).
Adult Novel Tank Dive Test
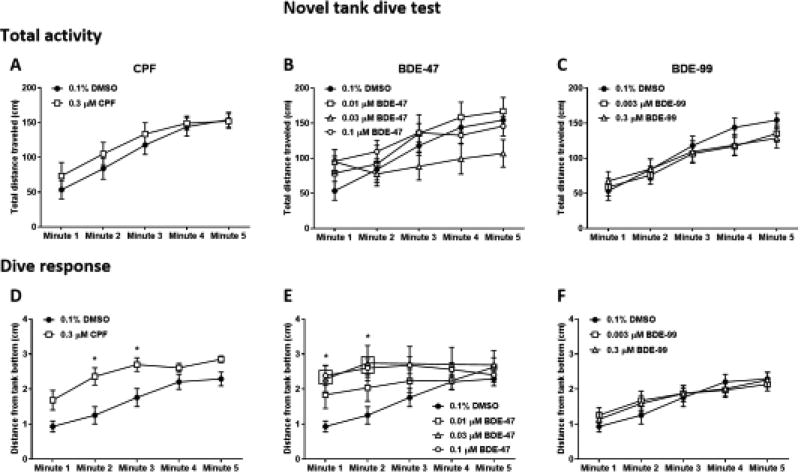
To evaluate response to the novel tank dive test, two parameters were analyzed– total activity of the fish measured as distance traveled per each minute of the trial (Fig. 4A–C), and dive response measured as the average distance from the tank bottom per each minute (Fig. 4D–F). Total activity of zebrafish in the novel tank test is commonly reported to be low at the beginning of the trial and gradually increase over time. Accordingly, an overall ANOVA analysis of the total activity across all treatments found a main effect of time (increase in activity) (F(4, 400) = 48.76, P < 0.0001). However, there was no effect of treatment nor a time × treatment interaction.
Figure 4.
Novel tank dive test. Adult zebrafish that were developmentally exposed to CPF (A, D), BDE-47 (B, E), and BDE-99 (C, F) were individually placed in the testing tank (novel environment) and recorded for 5 minutes. Total activity (A–C) was calculated as the total distance traveled by the fish in each minute of the trial. Dive response (D–F) was calculated as the average distance of the fish from the bottom of the tank in each minute of the trial. Asterisks indicate significant difference from the 0.1% DMSO control group.
A similar overall ANOVA analysis of the average distance from the tank bottom across all treatments found a main effect of time (F(4, 400) = 24.8, P < 0.0001), as well as treatment (F(6, 100) = 3.178, P < 0.01), and a time × treatment interaction (F(24, 400) = 1.624, P < 0.05). CPF-exposed fish exhibited a reduction in dive response (F(1, 28) = 11.2, P < 0.01), with a significantly increased distance from the bottom of the tank in minutes 2 and 3 of the trial (Fig. 4D). Regression analysis of the linear slope revealed no difference from the control in the pattern of gradual increase in distance from bottom throughout the 5-min trial, however there was a significant difference in slope elevation (Fig. S4). Exposure to BDE-47 also resulted in a significant reduction in dive response (F(3, 44) = 3.435, P < 0.05), with increased distance from the bottom in the first two minutes at the two higher concentrations of 0.03 and 0.1 µM (Fig. 4E; Table S5). Regression slopes were different from control in the 0.03 and 0.1 µM doses, but not the 0.01 µM dose, while in the later there was a significant difference in slope elevation (Table S5). Exposure to BDE-99 did not cause a change in dive response (Fig. 4F).
Adult Startle Tap Test
Behavioral patterns in the tap test were analyzed by measuring the distance that the fish traveled in the 5 seconds before (pre) and after (post) each tap. The pre-tap activity of the fish is regarded as the baseline level of activity, and in the 0.1% DMSO-exposed control group it gradually increases during testing (Fig. 5A, bottom dashed line). The post-tap activity is the tap response, and in the control group it gradually decreases during testing (Fig. 5A, top dashed line). Overall, pre-tap activity is lower than post-tap activity throughout the assay. Two statistical tests were used to compare behavioral patterns between the control group and the treated groups. The first test was an ANOVA analysis comparing the pre- and post-tap activity levels across the ten taps followed by post-hoc comparisons of each tap response. The second test was a linear regression analysis of pre- and post-tap slopes. Pre-tap activity levels and slopes were not altered by any of the treatments (data not shown).
Figure 5.
Startle tap test. Adult zebrafish were individually placed in cylindrical arenas, allowed a short 30 sec acclimation period and subjected to a sequence of 10 taps, one tap per minute. A) Average activity of the fish during 5-sec before and after each tap. The lower, gradually rising bars (upward dashed line on 0.1% DMSO) show the pre-tap activity, and the higher, gradually decreasing bars (downward dashed line on 0.1% DMSO) show the post-tap activity. B–D) Post-tap linear regression slopes of 0.1% DMSO compared to CPF (B), BDE-47 (C) and BDE-99 (D). Asterisks indicate significant difference from the 0.1% DMSO control group.
CPF exposure did not alter post-tap activity in any of the taps (Fig. 5A). However, linear regression analysis of post-tap slopes revealed that CPF exposure caused a significant reduction in the downward trend of post-tap activity that was due to lower tap response at the beginning of the trial (Fig. 5B; statistics in Table S6). Exposure to 0.01, 0.03 and 0.1 µM BDE-47 resulted in decreases in post-tap activity that were identified as a main effect in the ANOVA analysis (F(3, 47) = 5.748, P < 0.01). Post-hoc comparisons found that in all the BDE-47 exposures the activity level reductions occurred within the first four taps (Fig. 5A). In the two lower concentrations of BDE-47 the decreases in tap response also resulted a reduction in post-tap slopes (Fig. 5C; statistics in Table S6). BDE-99 exposure caused similar decreases in post-tap activity levels at both 0.003 and 0.3 µM. The decreases were identified in the post-hoc comparisons within the first four taps (Fig. 5A). Although the ANOVA analysis did not identify an overall main effect of treatment, there was an effect of tap (F(9, 630) = 2.398, P < 0.05), although no treatment×tap interaction. The slopes in both BDE-99 concentrations were reduced (Fig. 5D; statistics in Table S6).
We further calculated the percent of fish that were active in each treatment group during each tap, and the percent of individuals that were responsive to the tap (Fig. S2). Overall, the percent of active fish in all treatment groups was not altered or slightly increased from the first to the tenth tap. In all treatment groups except the CPF-exposed fish, the percent of tap-responsive individuals decreased significantly during the test. In the CPF-exposed group, the percent of tap-responsive fish was not different than the percent of active fish throughout the test.
Adult Shoaling Test
Analysis of social affiliation in the shoaling test was done by comparing the average distance of the fish from the side of the tank on which the shoal video was played, in the 2-min time interval before and after the video started playing (Fig. 6A). Fish in all treatment groups swam closer to the video side of the tank after the shoaling video started playing, as confirmed by the ANOVA analysis revealing an overall effect of time interval (F(1, 100) = 29.34, P < 0.0001). Post-hoc analyses, however, found the difference between intervals to be statistically significant only in the two BDE-99 treatments (0.003 µM, P < 0.05; 0.3 µM, P < 0.001). Differences in distance from the tank side between the first and second intervals were calculated to be used as a measure of the extent of shoaling, however they were not found to be significantly altered by any of the treatments (Fig. 6B). Total activity of the fish (Fig. S3A–C) and distance from the video-screening side of the tank (Fig. S3D–F) were compared between the control and treated groups over time (minute), and not found to be significantly different.
Figure 6.
Shoaling test. Adult zebrafish that were developmentally exposed to CPF (A, D), BDE-47 (B, E), and BDE-99 (C, F) were individually placed in the testing tank (MUPET) and recorded for 7 minutes. After the first two minutes a video of a zebrafish shoal was played on one of the two flanking monitors for the remaining 5 minutes of the trial. A) Average distance from the tank side on which the video was played in the 2-min before and the 2-min after the video began playing for each treatment. Asterisk indicates significant difference between the two time-intervals. B) Difference calculated between the two intervals described in A in each treatment.
Adult Predator Avoidance Test
Total activity during the predator avoidance test was lower in the 0.01 µM BDE-47 (P < 0.05) and 0.003 µM BDE-99 (P < 0.05) groups (Fig. 7A). Flee response was overall greater during the red predator-simulating stimulus compared to the blue stimulus (Fig. 7B), as identified in the repeated measures ANOVA analysis (F(1, 107) = 30.83, P < 0.0001), however there was no treatment × stimulus interaction. Further, we did not detect an effect of treatment on the response to either the blue or red stimulus. Total activity of the fish (Fig. S4A–C) and distance from the video-screening side of the tank (Fig. S4D–F) were compared between the control and treated groups over time (minute). Significant reductions in distance traveled were found in the 0.01 µM BDE-47 and 0.003 µM BDE-99 throughout the testing period.
Figure 7.
Predator avoidance test. Developmentally exposed adult zebrafish were individually placed in the testing tank (MUPET) and recorded for 9 minutes consisting of one min acclimation followed by alternating minute-long stimulus/no stimulus events. The stimulus was either a blue slow-growing dot or a red fast-growing dot appearing repeatedly on one of the screens. A) Total activity of the fish calculated as the total distance traveled throughout the trial. B) Flee response was calculated as the difference in average distance from the tank side between trial minutes in which the dot stimulus was presented and minutes in which there was no stimulus. Blue bars represent blue dot flee response and red bars represent red dot flee response.
Discussion
We found that exposure of zebrafish embryos to low concentrations of the brominated flame retardants BDE-47 and BDE-99, and the organophosphate pesticide CPF, caused both short- and long-term behavioral alterations. Overall BDE-47 had the higher potency to cause overt toxicity at the embryonic stage as well as behavioral alterations in adults. In the short-term, larval locomotor activity was affected in a chemical specific manner, with BDE-99 treatment causing a non-monotonic dose effect in the larval motility measure, with lower dose range causing hyperactivity and the higher dose range causing hypoactivity. In the long-term, adult behaviors in the novel tank dive test and tap test were the most sensitive to effects caused by all treatments, with both brominated FRs causing impairments that were measured at doses below those causing altered larval activity.
We defined the concentration range for low-level exposure as doses below those causing overt toxicity at the time of exposure or shortly after (by 6 dpf). Exposure to 0.3 µM BDE-47 caused widespread spinal curvature, which led us to adjust the dose range to 0.01–0.1 µM for behavioral testing. In contrast, based on endpoints of lethality and dysmorphogenesis, we did not see a level of overt toxicity for BDE-99, up to a maximal dose of 20 µM. Overt toxicity levels for BDE-47 or BDE-99 were identified in four other recent studies in which zebrafish developmental exposures were conducted from approximately 6 hpf until 5 or 6 dpf (Behl et al., 2015; Jarema et al., 2015; Noyes et al., 2015; Usenko et al., 2011). Behl et al. (2015) assessed developmental toxicity of BDE-47 at 6 dpf and observed increases in mortality and abnormality starting at 28.2 µM. Jarema et al. (2015) observed an increase in mortality and malformations at 40 µM BDE-47, but not at 12.6 µM or lower. Usenko et al. (2011) exposed zebrafish embryos to 6 different BDE congeners, including BDE-47 and BDE-99. Exposure to 4.6 µM (2.25 mg/L) BDE-47 induced a significant increase in spinal curvature initially observed at 5 dpf, while the median lethal concentration (LC50) was at 8.6 µM (4.2 mg/L) and occurred at 6 dpf. In the same study, the LC50 for BDE-99 was 9.2 µM (5.2 mg/L), but there was no significant increase in malformations. Noyes et al. (2015) also conducted developmental exposures to both BDE-47 and BDE-99, and found significant increases in mortality at 5 dpf at levels as low as 0.0064 µM for BDE-47 and 0.064 µM for BDE-99. Thus, it seems that the levels at which overt toxicity is determined can vary widely, at least in part likely influenced by study-specific alterations in exposure methodology such as embryo dechorionation prior to exposure, vehicle concentration, the material from which the exposure dishes are made of etc. In this study we did not measure the real-time chemical levels in the exposure dishes nor the amount of chemicals taken up by the embryos, however this might be a more accurate way to compare exposure across studies in the future. Despite the discrepancies in toxicity levels across studies, it appears that BDE-47 is consistently more potent than BDE-99 in causing over toxicity at lower concentrations.
All three chemicals tested caused alterations in 6 dpf larval locomotor activity in response to alternating illumination conditions. Exposure to 0.3 µM CPF caused a decrease in larval activity during all dark phases of the assay. Hypoactivity in larvae following developmental exposure to CPF has been previously reported by our group as well as others (Dishaw et al., 2014; Jarema et al., 2015; Levin et al., 2004; Richendrfer and Creton, 2015; Richendrfer et al., 2012), with the exception of one study that found an increase in larval activity during the first, habituation, dark phase in 0.03 µM but not 0.3 µM exposed fish (Oliveri et al., 2015). Exposure to BDE-47 at the highest concentration of 0.1 µM caused a decrease in activity in all illumination phases, while the lower concentrations of 0.01 and 0.03 µM had no effect on larval swimming. This result is in accordance with previous reports of larval hypoactivity caused by BDE-47 exposure (Jarema et al., 2015; Noyes et al., 2015; Usenko et al., 2011; Zhao et al., 2014). Although there were no visible findings indicating effects on general health, we cannot rule out systemic toxicity at the higher concentration of 0.1 µM as the cause for reduced activity. Unlike CPF and BDE-47, BDE-99 was found to affect larval activity in a non-linear pattern, with an increase in activity during light phases following 0.3 µM exposure and a decrease in activity during dark phases at exposure concentrations of 1 µM or higher. Since the highest dose of 20 µM caused a hypoactivity in both light and dark, we regarded 1 µM or above as potential overt toxicity levels and conducted adult behavior testing on lower dose treatments. Doses lower than 0.3 µM did not cause changes in larval activity. To our knowledge, changes in larval activity following developmental exposure to BDE-99 were reported in only one other study (Noyes et al., 2015), in which exposure to concentrations of 0.0064 µM or higher caused hypoactivity in the dark. As seen above with overt toxicity levels, the concentrations at which larval activity was affected were highly variable across studies making it difficult to determine specific effective doses, however most studies seem to agree on the characteristics of the behavioral effects caused by each chemical.
In adults, developmental exposure to all three chemicals caused lower anxiety-related responses in the novel tank and tap tests. In contrast, social interaction and predator recognition did not appear to be affected by any of the treatments. CPF exposure had only a mild effect in the novel tank test, with treated fish maintaining an overall larger distance than controls from the bottom of the tank throughout the 5-min testing period. However, they also maintained the same dive pattern of gradual increase in distance from the bottom over time. In the tap test, exposure to CPF resulted in loss of the descending post-tap slope, however there were no significant differences from control in any specific tap. Together, these results indicate that zebrafish exposure to CPF during development has not only a short-term effect on larval swimming activity, but also a long-term impact on processing of anxiety and fear. Two other studies investigated the effects of developmental exposure to 0.3 µM CPF on adult zebrafish using the novel tank and/or sensorimotor tap test, following very similar exposure protocols (Eddins et al., 2010; Oliveri et al., 2015). While no significant long-term effect was found in the Oliveri et al. (2015) study, Eddins et al. (2010) found an effect in the tap test that was opposite to our observation, i.e. pronounced increase in startle-response throughout the assay. Two differences in the experimental set-up and procedure may account for the discrepancy between the results presented here and those presented by Eddins et al, include changes of the surroundings of the testing apparatus (see Fig. 1 here and in Eddins et al for comparison), and a reduction in the habituation time before initiation of the taps from 10 minutes to 30 seconds. In rodent models, a number of studies show decreases in anxiety-related responses in several assays following exposure to CPF or other organophosphate pesticides. Female mice exposed to a subtoxic dose of CPF during postnatal days (PND) 11–14 were quicker to enter the light compartment in the Dark-light test, indicating reduced anxiety (Venerosi et al., 2008). Mice of both sexes showed reduced anxiety in the plus-maze when exposed perinatally to low doses of CPF (Ricceri et al., 2006). In rats, males exposed to CPF on postnatal days 1–4 spent more time than controls in the open arms of the elevated plus maze (Aldridge et al., 2005). Interestingly, exposure to the organophosphate parathion during PND 1–4 resulted in increases in both percent open arm choice and center crossings in the elevated plus maze, as well as a decrease in tactile startle response in the prepulse inhibition test (Timofeeva et al., 2008). The effects observed in the current study strongly align with results from the rodent models, suggesting a potentially common mechanism of action for these chemicals in mammals and fish.
Both BDEs caused impairments in anxiety-related behavior. In the novel tank test exposure to BDE-47 caused both lack of the initial dive response and loss of the overall dive pattern. This effect was highly significant in the two highest doses, while only trending towards significance in the lowest dose. The behavior of BDE-99-exposed fish in the novel tank test was not different from controls. In the tap test, both BDE-47 and BDE-99 caused reduction in post-tap activity within the first four taps, that led to loss of normal post-tap activity slopes. Our further analysis of the percent of individuals in the control and BDE-treated groups that were active and that were responsive to each tap likely indicates two things; that habituation to the tap is a result of decrease in the numbers of tap-responsive fish; and that the reduced initial post-tap activity in the BDE-treated groups is due to lower response amplitude rather than lower numbers of responsive fish. To our knowledge, this is the first report on the effects of either BDE-47 or BDE-99 on adult zebrafish anxiety-related behaviors, however several rodent studies indicate that both BDEs cause alterations in this domain. A set of studies carried out by a group from Sweden showed that exposure of mice to sub-acute levels of either BDE-47 or BDE-99 on PND 10 resulted in inversed novelty habituation behavior that can be interpreted as an alteration of the basic anxiety level of the animals or as impairment in their ability to habituate to novel situations (Eriksson et al., 2001; Eriksson et al., 2002; Viberg et al., 2002, 2004b). Decreased thigmotaxis, suggesting a decrease in anxiety-response, was also observed in two studies; one was conducted in rats in which gestational exposure was carried out by a single oral dose of BDE-47 (Kuriyama et al., 2004); the other conducted in mice that were perinatally exposed to BDE-99 (Branchi et al., 2002).
BDE-47 was more potent than BDE-99, not only in causing overt toxicity, but also in its behavioral effects, since it caused the strongest effects in both the novel tank and tap tests at the lowest exposure levels. In the 2008 update of the integrated risk information system (IRIS) summaries on the toxicity levels of BDE-47 and BDE-99, the U.S. environmental protection agency (EPA) assigned benchmark doses for neurobehavioral effects at 0.35 mg/kg for BDE-47 and 0.29 mg/kg BDE-99. These levels were based on the available literature from mammalian studies, namely in mice and rats, following developmental exposure (Eriksson et al., 2001; Viberg et al., 2004a). Our current study provides supporting evidence that there is indeed an effect on adult behavior following developmental exposure, however currently we do not know how the zebrafish doses compare with rodents. Since our results point to a large difference in neurotoxic potency between the two chemicals that is not observed in rodents, it is possible that the zebrafish is a more sensitive species and can be used to capture effects at lower doses. Model extrapolation studies are required in this field to determine the dose correlation between zebrafish and rodents.
An integration of the larval and adult behavior results in this study shows that larval locomotor activity testing was not always predictive of adult behavior. With the lower doses of exposure, larval activity analysis was negative for effects by both BDE treatments, while these same doses did cause significant impairments in the behavioral tests of adults. Zebrafish embryos and larvae are often used in toxicity screens as a high throughput in vivo model providing integrated whole animal information on multiple toxicity end-points, including neurotoxicity, while maintaining the ability to test a large number of chemicals and concentrations in a robust manner and at reasonable costs. The most common behavioral neurotoxicity end-point in zebrafish screens is the larval locomotor activity assay usually conducted at 5 or 6 dpf in 96-well plates. Most screening studies conclude their behavioral testing at this stage, and do not go further to the juvenile or adult stages that require longer experimental time scales and lower throughput, as well as additional testing apparatuses and added housing and maintenance resources. Our results strongly suggest that testing larval activity is not sufficient for predicting potential neurobehavioral toxicity, and may result in a large proportion of false negative data, especially in the lower levels of exposure that are an order of magnitude or more below the threshold for overt toxicity. This discrepancy may be due to lack of neurobehavioral maturity in larvae, or to the limitations of the locomotor activity assay in the behavioral domains that it is testing.
In conclusion, the evidence presented here strongly support the role of three ubiquitous persistent organic pollutants; CPF, BDE-47 and BDE-99 in causing developmental neurotoxicity resulting in long-term behavioral impairments. Despite the accumulation of over a decade worth of data on these chemicals, there is still very little known about the mechanisms underlying the long-term neurobehavioral effects. Our results on altered responses to anxiety-promoting situations in adult fish are well aligned with results from studies conducted in rodent models, thus suggesting the existence of a common mechanism across vertebrate species that drives emotional processing, and further establishing the utility of the zebrafish model in investigating these mechanisms. Furthermore, we were able to detect behavioral alterations in adults following exposure to doses that did not cause changes in larval activity, and to identify a large difference in neurotoxic potency between the two BDE congeners in causing those effects, thus showing that zebrafish are not only a robust model in terms of animal numbers and costs, but it is also sensitive to even subtle effects. We propose that the zebrafish may be considered a suitable model organism to be included in the EPA risk assessment process for determining benchmark doses for neurobehavioral developmental effects.
Supplementary Material
Highlights.
In the short-term, larval locomotor activity was reduced in larvae treated with 0.3 µM CPF and 0.1 µM BDE-47. BDE-99 treatment caused non-monotonic dose effects, with 0.3 µM causing hyperactivity and 1 µM or higher causing hypoactivity.
In the long-term, adult anxiety-related behavior was reduced in all treatments as measured in both the novel tank dive test and tap test.
Exposure of zebrafish embryos to low concentrations of the brominated flame retardants BDE-47 and BDE-99, and the organophosphate pesticide CPF, caused both short- and long-term behavioral impairments.
At very low exposure concentrations, where there were no visible effects on larval activity, adult behavior was still strongly affected.
Acknowledgments
This work was supported by National Toxicology Program grant HHSN273201500214P and the Leon Golberg Postdoctoral Fellowship. The MUPET was funded by NIH grant P01ES021923 and NSF grant OCE-1314642 (Woods Hole Center for Oceans and Human Health). We would like to thank Drs. Mark Hahn and Neel Aluru from the Woods Hole Oceanographic Institution (WHOI) for providing the funding and support for transforming the MUPET idea into reality. We would also like to thank James Brown, lead mechanist at the WHOI Mechanical Shop, for his patient and skilled design and fabrication of the MUPET, and to Richard Nappi from the Duke University Department of Physics for fabricating additional critical parts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 2005;113(5):527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, Jarema KA, Padilla S, Tice RR. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 2015;52:181–193. doi: 10.1016/j.ntt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments--a review on occurrence and human exposure. Environ. Pollut. 2012;169:217–229. doi: 10.1016/j.envpol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology. 2002;23(3):375–384. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014;230(2):282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28(6):1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby EB, Bailey JM, Oliveri AN, Levin ED. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 2015;49:81–90. doi: 10.1016/j.ntt.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio) Toxicol. Sci. 2014;142(2):445–454. doi: 10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012;46(24):13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from Greater Montreal, Quebec: a longitudinal study from 1998 through 2006. Environ. Health Perspect. 2009;117(4):605–610. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010;32(1):99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109(9):903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2',4,4',5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol. Sci. 2002;67(1):98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Fromme H, Becher G, Hilger B, Volkel W. Brominated flame retardants - exposure and risk assessment for the general population. Int. J. Hyg. Envir. Heal. 2016;219(1):1–23. doi: 10.1016/j.ijheh.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr. Opin. Pediatr. 2015;27(2):248–253. doi: 10.1097/MOP.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks HS, Westerink RHS. Neurotoxicity and risk assessment of brominated and alternative flame retardants. Neurotoxicol. Teratol. 2015;52:248–269. doi: 10.1016/j.ntt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep. 2014;1(2):101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 2015;52:194–209. doi: 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Chahoud I. Sex-dependent behavioral changes in rat offspring after in utero administration of a single low dose of PBDE 47. Organohalogen Compd. 2004;66:3893–3900. [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007;90(1):54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003;25(1):51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 2004;26(6):719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Linares V, Belles M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015;89(3):335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young US children than in their mothers. Environ. Sci. Technol. 2010;44(13):5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015;145(1):177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri AN, Bailey JM, Levin ED. Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicol. Teratol. 2015;52:220–227. doi: 10.1016/j.ntt.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol. Sci. 2006;93(1):105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Richendrfer H, Creton R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology. 2015;49:50–58. doi: 10.1016/j.neuro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer H, Pelkowski SD, Colwill RM, Creton R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol. Teratol. 2012;34(4):458–465. doi: 10.1016/j.ntt.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of US human fetuses and newborns. J. Toxicol. Env. Heal. A. 2007;70(1):1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and US house dust. Environ. Sci. Technol. 2009;43(19):7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008;77(6):404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Robinson EM, Usenko S, Brooks BW, Bruce ED. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 2011;30(8):1865–1872. doi: 10.1002/etc.570. [DOI] [PubMed] [Google Scholar]
- USEPA. An exposure assessment of polybrominated diphenyl ethers. USEPA; 2010. p. 378. [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L, Calamandrei G. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol. Teratol. 2008;30(6):468–474. doi: 10.1016/j.ntt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2',4,4',5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol. Sci. 2002;67(1):104–107. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Investigations of strain and/or gender differences in developmental neurotoxic effects of polybrominated diphenyl ethers in mice. Toxicol. Sci. 2004a;81(2):344–353. doi: 10.1093/toxsci/kfh215. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame-retardant, 2,2',4,4',5-pentabromodiphenyl ether, decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behaviour in the adult mouse. Environ. Toxicol. Pharmacol. 2004b;17(2):61–65. doi: 10.1016/j.etap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Moran RE, Sjodin A, Jones RS, Tancredi DJ, Tulve NS, Clifton MS, Colon M, Weathers W, Hertz-Picciotto I. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ. Health. 2015;14(23):1–11. doi: 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Xu T, Yin DQ. Locomotor activity changes on zebrafish larvae with different 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere. 2014;94:53–61. doi: 10.1016/j.chemosphere.2013.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.