Abstract
Background
Delayed renal graft function (DGF) contributes to length of hospitalization, risk of acute rejection, and graft loss. Existing tools aid the diagnosis of specific DGF etiologies such as antibody-mediated rejection, but markers of recovery have been elusive. The peroxisome proliferator gamma co-activator-1-alpha (PGC1α) is highly expressed in the renal tubule, regulates mitochondrial biogenesis, and promotes recovery from experimental acute kidney injury.
Objectives
We aimed to determine the association between renal allograft PGC1α expression and recovery from delayed graft function.
Methods
We retrospectively analyzed patients undergoing renal transplantation at a single center from January 1, 2008 to June 30, 2014. PGC1α expression was assessed by immunostaining and ultrastructural characteristics by transmission electron microscopy. Of 34 patients who underwent renal biopsy for DGF within 30 days of transplant, 21 were included for analysis.
Results
Low PGC1α expression was associated with a significantly longer time on dialysis after transplant (median of 35.5 versus 16 days, p < 0.05) and a significantly higher serum creatinine at 4 weeks after transplantation among those who discontinued dialysis (5 versus 1.65 mg/dl, p < 0.0001). Low PGC1α expression was not associated with higher serum creatinine at 12 weeks after transplantation. Ultrastructural characteristics including apical membrane blebbing and necrotic luminal debris were not informative regarding clinical outcomes.
Conclusions
These data suggest that higher PGC1α expression is associated with faster and more complete recovery from DGF. Mitochondrial biogenesis may be a therapeutic target for DGF. Larger studies are needed to validate these findings.
Keywords: delayed graft function, kidney transplantation, acute kidney injury, mitochondria
INTRODUCTION
The incidence rate of end stage renal disease in the United States continues to rise, from 278 per million/year in 1996 to 370 per million/year in 2014. Similarly, the annual number of kidney transplants in the United States has risen from over 12,000 in 1996 to almost 19,000 in 2014. However, the average waiting time for first listing has increased from 2.3 years in 1997 to 3.4 years in 2009 [1]. To accommodate the increasing numbers of patients on the waiting list, the donor pool now includes expanded criteria and donation after cardiac death. As a consequence, complications from transplantation have risen, including delayed graft function (DGF) [2]. DGF negatively impacts allograft and patient survival, increasing the risk of acute rejection [3] and independently predicting 5-year graft loss [4].
Numerous mechanisms during the peri-transplant period contribute to the cascade of events underlying DGF including vasospasm, epithelial and endothelial cell injury, and the innate and adaptive immune responses [5–9]. Apoptosis related to mitochondrial injury may be critical for tubular cell death in DGF [10]. Mitochondrial disruption in human renal ischemia precedes clinical AKI [11]. A growing body of experimental literature has implicated defects in mitochondria and oxidative metabolism in diverse forms of AKI [12–16]. Finally, mechanisms that restore mitochondrial health and abundance, such as mitochondrial biogenesis, ameliorate experimental AKI [12,17–21]. The peroxisome proliferator gamma co-activator-1-alpha (PGC1α) is highly expressed in the cortical tubular epithelium where it regulates mitochondrial biogenesis. In both mouse AKI models and human AKI biopsies, renal PGC1α expression is suppressed [17,18]. Intriguingly, the severity of experimental AKI is inversely proportional to renal PGC1α expression [17].
In view of this we hypothesized that the expression status of renal PGC1α may be associated with recovery from DGF. We assessed ultrastructural characteristics of mitochondrial injury and PGC1α expression renal allograft biopsies from patients with DGF at a single center and correlated these with DGF outcomes.
RESULTS
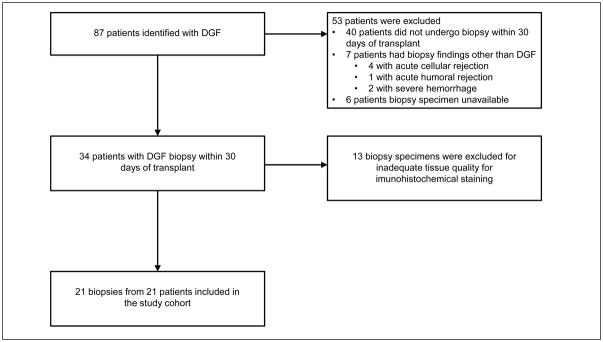
We included 21 patients who underwent renal transplant at Beth Israel Deaconess Medical Center from January 1, 2008 through June 30, 2014 and had an allograft biopsy for DGF within 30 days of transplant (Figure 1). Baseline characteristics of the study population are shown in Table 1. Of 87 patients who underwent renal transplant and had a charted diagnosis of DGF, 21 patients underwent biopsy within 30 days of transplantation and had histological findings supportive of DGF. There were 13 patients with DGF on biopsy but the biopsy specimen quality was insufficient for analysis. There were 53 patients who did not undergo biopsy within 30 days of transplant, the biopsy showed findings other than DGF, or the biopsy specimen was unavailable. Among the 21 subjects included in our cohort, the average age was 54.6 years, 52% were male, 29% were African American, 62% Caucasian, and there was 1 Asian and 1 Hispanic individual. Average cold ischemia time was 18.7 hours. These aggregate data are presented according to low versus intact PGC1α groups in Table 1. These features mirror larger and nationally representative DGF cohorts [22].
Figure 1.
Patient population and sample selection.
Table 1.
Patient, donor and recipient characteristics at baseline
| Low PGC1α (n=8) | Intact PGC1α (n=13) | Excluded (n=66) | P value | |
|---|---|---|---|---|
| Recipient | ||||
| Age (y) | 50.9 ± 7.8 | 56.9 ± 14.0 | 55.2 ± 9.3 | 0.290 |
|
| ||||
| Male sex | 5 (62.5) | 6 (46.2) | 47 (71.2) | 0.203 |
|
| ||||
| African American race | 3 (37.5) | 3 (23.1) | 20 (30.3) | 0.562 |
|
| ||||
| BMI > 30 kg/m2 | 3 (37.5) | 5 (38.5) | 23 (34.8) | 1.000 |
|
| ||||
| Cause of ESRD | 0.140 | |||
|
| ||||
| Diabetes | 1 (12.5) | 5 (38.5) | 25 (37.9) | |
|
| ||||
| Hypertension | 1 (12.5) | 2 915.4) | 14 (21.2) | |
|
| ||||
| Glomerular | 1 (12.5) | 1 (7.7) | 7 (10.6) | |
|
| ||||
| Tubulointerstitial | 0 | 4 (30.8) | 4 (6.1) | |
|
| ||||
| Allograft failure | 3 (37.5) | 0 | 5 (7.7) | |
|
| ||||
| Polycystic kidneys | 1 (12.5) | 1 (7.7) | 6 (9.1) | |
|
| ||||
| Other | 1 (12.5) | 0 | 5 (7.7) | |
|
| ||||
| Donor | ||||
| Source | 0.875 | |||
|
| ||||
| SCD | 4 (50.0) | 5 (38.5) | 31 (47.0) | |
|
| ||||
| ECD | 1 (12.5) | 2 (15.4) | 12 (18.2) | |
|
| ||||
| DCD | 2 (25.0) | 6 (46.2) | 18 (27.3) | |
|
| ||||
| LRRT | 0 | 0 | 2 (3.0) | |
|
| ||||
| LURT | 1 (12.5) | 0 | 3 (4.5) | |
|
| ||||
| Transplant | ||||
| HLA mismatches | 4 ± 1.9 | 4.5 ± 1.7 | 4.4 ± 1.8 | 0.950 |
|
| ||||
| Cold ischemia time (hr) | 16.5 ± 2.6 | 20.1 ± 3.1 | 14.3 ± 7.7a | 0.277 |
|
| ||||
| Induction therapy | 0.183 | |||
|
| ||||
| ATG | 8 (100.0) | 10 (76.9) | 62 (93.9) | |
|
| ||||
| Basiliximab | 0 | 2 (15.4)b | 3 (4.5)b | |
BMI, body mass index; SCD, standard criteria donor; ECD, extended criteria donor; DCD, donation after cardiac death; LRRT, living related renal transplant; LURT, living unrelated renal transplant; ATG, anti-thymocyte globulin
Values are mean ± SD or n (%) unless otherwise indicated
One subject without documented cold ischemia time
One subject received neither ATG nor basiliximab
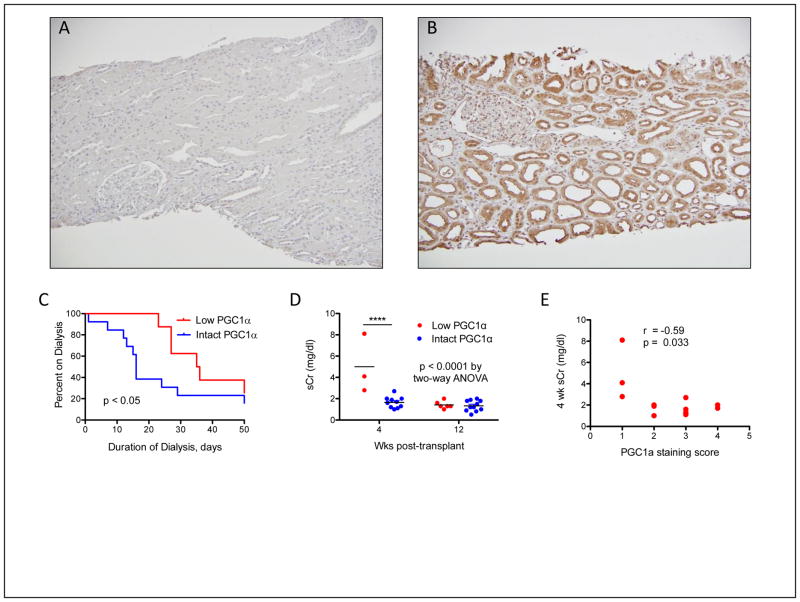
Figure 2 demonstrates the association between renal PGC1α expression and DGF outcomes. We used a validated anti-PGC1α antibody (Figure 2A,B) and a previously published scoring system to characterize biopsies based on PGC1α expression levels [18]. Biopsies were characterized as low PGC1α expression (score of 1) or intact PGC1α expression (score of 2–4). There was no difference in average cold ischemia time (18.32 versus 18.36 hours respectively), time from transplant to biopsy (14.38 versus 12.54 days), or proportion of allografts on mechanical perfusion (37.5% versus 61.5%) between the low and intact PGC1α expression groups (p > 0.05 for all comparisons). Low PGC1α expression was associated with a median post-transplant dialysis requirement of 35.5 days versus 16 days in the intact group (p < 0.05, Figure 2C). Among patients able to discontinue dialysis early, low PGC1α expression was still associated with a significantly higher serum creatinine at 4 weeks post-transplantation (5 versus 1.65 mg/dl, 0.0001 by Bonferroni-corrected p < 0.0001 for 4-wk comparison, Figure 2D). Serum creatinine at 4 weeks post-transplantation among subjects off dialysis was significantly correlated with PGC1α expression (Figure 2E, r =−0.59, p = 0.033). PGC1α expression was not associated with serum creatinine by 12 weeks post-transplantation. There was a non-significant trend toward more rapid recovery of renal function with intact PGC1α expression, defined as a serum Cr of < 2.0 mg/dl off dialysis (6 versus 11 weeks, p = 0.08).
Figure 2. Renal PGC1α expression and early DGF outcomes.
(A, B) Representative low- power photomicrographs of PGC1α immunostaining indicative of low or intact expression, respectively. Original magnification 10x. (C) Duration of dialysis after transplantation in the low and intact PGC1α expression groups, median of 35.5 days in the low and 16 days in the intact PGC1α group. (D) Serum creatinine (sCr, mg/dL) in patients no longer on dialysis 4 or 12 weeks after transplant, stratified by PGC1α expression. P value by two-way ANOVA. ****p < 0.0001 after Bonferroni post-hoc correction for 4-wk comparison. (E) Correlation of PGC1α immunostaining score with serum creatinine (sCr, mg/dL) at 4 weeks after transplantation among those subjects no longer on dialysis.
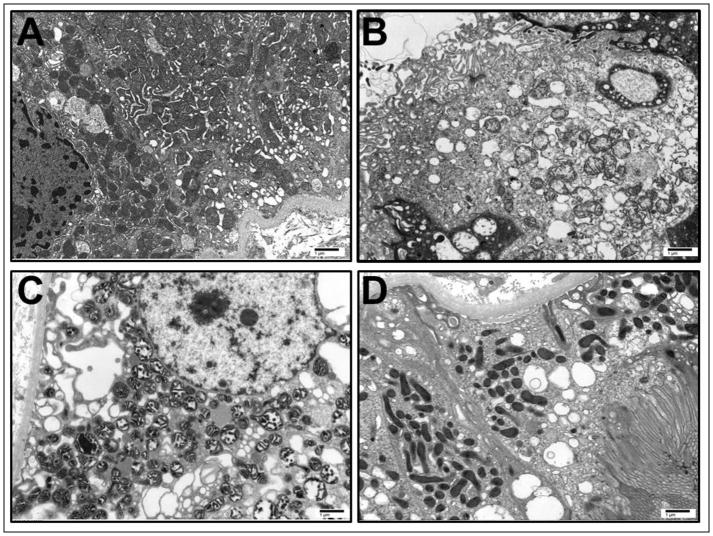
Ultrastructural mitochondrial injury has been reported in kidney explants and DGF biopsies [23,24]. We examined 5 random biopsies by electron microscopy. We found varying degrees of mitochondrial and proximal tubular injury consistent with patterns observed in ischemia-reperfusion injury (Figure 3) [11,25]. The most notable findings included mitochondrial swelling, apical membrane blebbing, and necrotic luminal debris. While there was no significant association between ultrastructural injury scores and time to renal recovery (r = 0.4) or serum creatinine at 4 (r = 0.3) or 12 weeks (r = 0.2) post-transplantation, this examination was limited in its statistical power by sample availability.
Figure 3. Representative ultrastructural characteristics of delayed graft function.
All images are original magnification, 10,000X. (A) Minimal mitochondrial swelling. (B) Moderate mitochondrial swelling with disruption of the brush border. (C) Severe mitochondrial swelling and condensation. (D) Normal mitochondrial morphology and position, with intact brush border. Scale bar, 1μm.
DISCUSSION
To our knowledge, this is the first study to evaluate the expression of PGC1α in renal DGF. The results suggest that PGC1α expression may be associated with higher rates and more rapid recovery from DGF. As such, these data echo the robust, graded association between renal PGC1α expression and AKI severity previously reported in mice [17]. The inability to restore normal PGC1α expression is detrimental following experimental ischemia or inflammation, leading to more severe, prolonged AKI. Unsurprisingly, knockout animals are more vulnerable to ischemic or inflammatory AKI whereas mice with forced renal PGC1α expression in the tubule exhibit remarkable protection [18]. Excess PGC1α expressed in proximal tubular cells accelerates mitochondrial biogenesis and restores oxidative function after oxidant injury [26]. The link between reduced intra-graft PGC1α expression and prolonged DGF may therefore reflect active noxious stimuli that continue to suppress PGC1α, chronic reduction in PGC1α—as has been suggested by biopsies of chronically injured kidneys [18]—or some combination of both.
It is not clear from the present study how PGC1α expression in DGF kidneys differs from that of functioning allografts or those with rejection. However, there is a similar reduction of PGC1α expression in other AKI models including ischemia-reperfusion injury and acute interstitial nephritis that is distinct from several models of CKD in which PGC1α expression remains intact [18]. In this context, our findings parallel data reported in other forms of AKI.
Both molecular and clinical results implicate mechanisms related to ischemic AKI in the development of DGF. For example, systematic mRNA profiling has identified a distinct AKI molecular phenotype within early kidney transplants [27,28]. Recent observational and clinical trial data also illustrate the promise of anti-ischemic strategies to improve DGF outcomes when applied to standard donors, not just marginal kidneys—e.g., ex vivo machine perfusion and therapeutic donor hypothermia [29.30]. Conversely, experts in AKI have highlighted the unique opportunity to study human AKI by focusing on DGF [31]. DGF is therefore increasingly recognized as an important nexus for unraveling the mechanistic consequences of ischemia in human allografts and native kidneys alike.
Important limitations of this study should be noted. First, immunosuppression after transplant was guided by clinician preference and may have affected outcomes. For example, sirolimus prolongs DGF [32], and its effects on renal PGC1α expression are unknown. However, only one subject was on sirolimus before DGF recovery. Second, this is a small single-center study whose core findings should be validated in larger samples. However, baseline characteristics of our study population largely mirrored nationally representative cohorts of deceased donor kidney transplants [22]. We lacked sufficient power to explore secondary questions—e.g., is PGC1α expression reduced in expanded criteria kidneys? Is low PGC1α expression at the time of biopsy for DGF associated with long-term graft function? Interestingly, analysis of gene expression profiles of kidney biopsies taken one year after transplantation have shown that grafts with interstitial fibrosis and inflammation had significantly lower expression of genes involved in the regulation of mitochondrial biogenesis, maintenance, and removal by mitophagy [33]. Finally, our study was not designed to determine if renal PGC1α immunostaining could aid the prediction of recovery from DGF. A formal determination of receiver-operator characteristics in a large cross-sectional study would be needed to understand the predictive performance of such a test.
In summary, the present pilot study suggests that the intensity of intragraft PGC1α expression at the time of biopsy for DGF is associated with subsequent clinical renal recovery. This finding affirms and extends the body of preclinical results linking PGC1α to AKI recovery, suggests the potential utility of markers for renal recovery from AKI, advances the concept of utilizing transplantation to study AKI mechanisms in humans, and supports future investigation of the PGC1α pathway for the diagnosis and treatment of DGF.
METHODS
Study population
Our study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center in Boston, Massachusetts, USA and conduced in accordance with the declaration of Helsinki. We were granted a waiver for patient consent. We included all patients who underwent renal transplantation at Beth Israel Deaconess Medical Center between January 1, 2008 and June 30, 2014 and had a diagnosis code for DGF (n = 87). The online transplant management system at Beth Israel Deaconess Medical Center was used to identify these patients. We excluded patients who did not undergo biopsy within 30 days of renal transplant (n = 40), whose biopsy showed findings other than DGF (n = 7), or for whom a biopsy specimen was unavailable (n = 6). Of the remaining patient (n = 34), we excluded 13 biopsies for inadequate tissue quality. Our study cohort consisted of 21 total biopsies from 21 unique patients. The cohort included one simultaneous liver-kidney transplant and two kidneys that were subsequently determined to have primary non-function.
Data collection
Both the online medical record and the online transplant management system at our institution were used to obtain data. Demographic and clinical variables included age at the time of transplant, sex, race, BMI, cause of ESRD, donor type, HLA (-A, -B, -DR) mismatch, cold ischemia time, use of machine perfusion, induction therapy, and serum creatinine. Duration of dialysis was verified in both the medical chart and inpatient dialysis unit records.
Electron microscopy
Ultrathin sections (0.5 μm) were examined by transmission electron microscopy (JEOL 1011, JEOL Corp.) with Orca-HR Digital Camera (Hamamatsu Corp.) and Advanced Microscopy Technique Corporation image capture system. Ten randomly selected proximal tubules were analyzed at low and high power. Mitochondrial swelling, mitochondrial condensation, brush border membrane disruption, apical membrane blebbing, loss of basolateral infoldings, pale cytosol, and necrotic luminal debris were individually scored by a single observer blinded to clinical outcomes (ERD) on a 4-point Likert scale (0 = pristine, 4 = severe injury) adapted from schema previously reported [11,24]. Parameter scores were averaged to generate a mean score for each biopsy.
Immunohistochemical analysis
Anti-PGC1α antibody (Abcam ab54481) was used at a dilution of 1:100 as previously described [18]. This antibody was validated by testing in PGC1α knockout mouse kidney sections versus wildtype littermate kidney sections and by peptide competition as previously described [18]. A semi-quantitative scoring system that has been reported previously [18] was used to characterize PGC1α expression in renal allograft biopsy specimens. Briefly, ten randomly selected high-powered fields were viewed per specimen, with each field scored on a 4-point scale based on the intensity of staining (1 = weakest, 4 = strongest). To avoid confounding from cell death or dropout, areas of necrosis and scarring were excluded. All scoring was performed by a single operator blinded to clinical status (IES). We confirmed the robustness of the scoring method by repeating the scoring in a blinding fashion and determining the correlation between the scoring results (r > 0.9). As part of the training set published previously [18] we also determined immunostaining on control nephrectomy specimens. In our study cohort, specimens with a score of 1 were classified as “low” (n=8) and those scoring 2–4 as “intact” PGC1α expression (n=13).
Statistical analysis
Comparisons between groups were analyzed by Mann-Whitney U test for two groups or Kruskal-Wallis test for three or more groups. Proportions were compared by Fisher’s exact test and correlations by the Spearman rank method. Two-way analysis of variance (ANOVA) was used for stratified analysis with post-hoc pairwise Bonferroni corrections. Time-to-event comparisons were analyzed using Gehan-Breslow-Wilcoxon. Results and statistical analysis were prepared in GraphPad Prism. Two-tailed P values of < 0.05 were considered significant.
Acknowledgments
Sources of Support: This research was supported by the National Institutes of Health (R01-DK095072 awarded to SMP and K08-DK101560 awarded to EVK) and institutional funds (SMP).
We thank the members of the Transplant Institute at Beth Israel Deaconess Medical Center for data gathering support and Terry Strom for critical input and feedback.
Footnotes
DISCLOSURES: The authors declare no conflicts of interest.
References
- 1.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 2.Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27:19–27. doi: 10.1111/tri.12217. [DOI] [PubMed] [Google Scholar]
- 3.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilling M, Holzinger F, Friess H, Seiler C, Buchler MW. Pathogenesis of delayed kidney graft function: role of endothelin-1, thromboxane B2, and leukotriene B4. Transplant Proc. 1996;28:304–305. [PubMed] [Google Scholar]
- 7.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 10.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation. 2003;76:50–54. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 11.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, Kinsey GR, Stevens BK, Jobin K, Kenwood BM, Hoehn KL, Lynch KR, Okusa MD. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol. 2015;26:908–925. doi: 10.1681/ASN.2013121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273:345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013;83:72–83. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA. Mitochondrial Pathology and Glycolytic Shift during Proximal Tubule Atrophy after Ischemic AKI. J Am Soc Nephrol. 2016;27:3356–3367. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 2009;76:1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014;25:1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, Kim SJ. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall-Craggs M, Little JR, Sadler JH, Trump BF. Structural changes following hypothermic preservation of human cadaveric kidneys. Hum Pathol. 1980;11:23–36. doi: 10.1016/s0046-8177(80)80102-x. [DOI] [PubMed] [Google Scholar]
- 24.Olsen S, Burdick JF, Keown PA, Wallace AC, Racusen LC, Solez K. Primary acute renal failure (“acute tubular necrosis”) in the transplanted kidney: morphology and pathogenesis. Medicine (Baltimore) 1989;68:173–187. doi: 10.1097/00005792-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Cekauskas A, Bruns H, Manikas M, Herr I, Gross ML, Zorn M, Jankevicius F, Strupas K, Schemmer P. Sulforaphane decreases kidney injury after transplantation in rats: role of mitochondrial damage. Ann Transplant. 2013;18:488–496. doi: 10.12659/AOT.884013. [DOI] [PubMed] [Google Scholar]
- 26.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12:534–548. doi: 10.1038/nrneph.2016.85. [DOI] [PubMed] [Google Scholar]
- 28.Famulski KS, de Freitas DG, Kreepala C, Chang J, Sellares J, Sis B, Einecke G, Mengel M, Reeve J, Halloran PF. Molecular phenotypes of acute kidney injury in kidney transplants. J Am Soc Nephrol. 2012;23:948–958. doi: 10.1681/ASN.2011090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon RM, Brock GN, Garrison RN, Marvin MR, Franklin GA, Davis EG. Machine perfusion: not just for marginal kidney donors. Am Surg. 2015;81:550–556. [PubMed] [Google Scholar]
- 30.Niemann CU, Malinoski D. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 2015;373:2687. doi: 10.1056/NEJMc1511744. [DOI] [PubMed] [Google Scholar]
- 31.Silver SA, Cardinal H, Colwell K, Burger D, Dickhout JG. Acute kidney injury: preclinical innovations, challenges, and opportunities for translation. Can J Kidney Health Dis. 2015;2:30. doi: 10.1186/s40697-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stallone G, Di Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, Gesualdo L, Grandalino G, Schena FP. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1-year graft function. J Am Soc Nephrol. 2004;15:228–233. doi: 10.1097/01.asn.0000102469.32182.8c. [DOI] [PubMed] [Google Scholar]
- 33.Zepeda-Orozco D, Kong M, Scheuermann RH. Molecular Profile of Mitochondrial Dysfunction in Kidney Transplant Biopsies Is Associated With Poor Allograft Outcome. Transplant Proc. 2015;47:1675–1682. doi: 10.1016/j.transproceed.2015.04.086. [DOI] [PubMed] [Google Scholar]