Abstract
Migraine is the third most common disease worldwide; however, the mechanisms underlying migraine headache are still not fully understood. Previous studies have demonstrated that α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor phosphorylation plays an important role in central sensitization of pain transmission. In the present study, we observed that AMPA receptor GluA1 Ser831 phosphorylation was enhanced in the spinal trigeminal nucleus caudalis (Sp5C) after intraperitoneal injection of nitroglycerin (NTG). The NTG injection induced acute migraine-like pain including photophobia and mechanical hypersensitivity as reported previously. Interestingly, targeted mutation of GluA1 Ser831 site to prevent phosphorylation significantly inhibited NTG-induced migraine-like pain. Moreover, NTG incubation caused a robust Ca2+ influx in cultured brainstem neurons, which was dramatically inhibited by GluA1 S831A (serine at the 831 site of GluA1 is mutated to alanine) phospho-deficient mutation, and treatment with 1-naphthyl acetyl spermine (NASPM), a selective Ca2+-permeable AMPA receptor channel blocker, dose-dependently blocked the NTG-evoked increase of Ca2+ influx in the cultured neurons. We further found that intra-Sp5C injection of NASPM significantly inhibited NTG-produced mechanical hypersensitivity. These results suggest that AMPA receptor phosphorylation at the Ser831 site in the Sp5C is critical for NTG-induced migraine-like pain.
Keywords: migraine-like pain, AMPA receptor phosphorylation, spinal trigeminal nucleus caudalis, nitroglycerin
1. Introduction
Migraine is a common neurological disorder featuring recurrent headache accompanied with nausea, vomiting, photophobia, and phonophobia (Society, 2013). Currently available therapies for migraine are inadequate (Bigal and Lipton, 2008; Stovner et al., 2007). Understanding pathogenic mechanisms of migraine headache will enable us to develop a new therapy for such pain, which will benefit hundreds of thousands of migraineurs. In the present study, we investigated the mechanisms underlying migraine using a published nitroglycerin (NTG)-induced acute migraine-like pain mouse model (Bates et al., 2010; Farkas et al., 2016; Markovics et al., 2012; Pradhan et al., 2014). Previous studies have shown that NTG not only triggers migraine attacks in migraine-susceptible patients, but also causes headache in healthy people (Afridi et al., 2005; Christiansen et al., 1999; Iversen et al., 1989; Olesen, 2008). The NTG-induced migraine-like pain model has been employed in both clinical and preclinical research (Bates et al., 2010; Iversen, 2001; Markovics et al., 2012; Olesen, 2008, 2010). The administration of NTG in rodents causes sensory hypersensitivity associated with migraine and produces light-aversive behaviors (Bates et al., 2010; Markovics et al., 2012). Thus, the NTG model we used in this study can effectively mimic symptoms in migraineurs.
It has been demonstrated that α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor phosphorylation is critically involved in activity-dependent changes in synaptic processing of nociceptive inputs and central sensitization of pain transmission (Choi et al., 2010; Fang et al., 2003; Lu et al., 2008; Park et al., 2009). The C-terminal domains of different AMPA receptor subunits have different phosphorylation sites (Bredt and Nicoll, 2003; Malinow and Malenka, 2002; Shepherd and Huganir, 2007; Song and Huganir, 2002). Nociceptive stimulation can induce phosphorylation of AMPA receptors in the central nervous system (CNS) and in turn promote their synaptic targeting and trafficking (Choi et al., 2010; Park et al., 2009), which will lead to a switch of AMPA receptors from Ca2+-impermeable to Ca2+-permeable (Li et al., 2014). It has been reported that AMPA receptors are involved in the cortical mechanisms of neuropathic pain in a spared nerve injury model (Giordano et al., 2012). Our previous study indicates that AMPA receptor GluA1 Ser831 phosphorylation is required for the development of stress-enhanced persistent postsurgical pain (Li et al., 2014). These results suggest that AMPA receptors and their phosphorylation contribute to central sensitization underlying chronic pain. In this study, we found that GluA1 Ser831 phosphorylation was significantly increased in the spinal trigeminal nucleus caudalis (Sp5C) after intraperitoneal (i.p.) injection of NTG. By using GluA1 S831A phospho-deficient mutant mice, we further investigated the role of AMPA receptor phosphorylation in the pathogenesis of NTG-induced migraine-like pain.
2. Materials and Methods
2.1. Animals
Male C57BL/6 mice (8–10 weeks) and AMPA receptor GluA1 S831A phospho-deficient mutant mice with C57BL/6 genetic background (8–10 weeks) were used in this study. Animals were housed under standard conditions with a 12 h light-dark cycle, with water and food pellets available ad libitum. In all behavioral experiments, the animals were acclimated in our animal facility for a minimum of 1 week before use in experiments and acclimated in the laboratory for at least 30–60 min before testing. All animal procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All experiments were approved by the Animal Research and the Animal Care Committee at Texas A&M University.
2.2. Drug administration
NTG (American Regent, Shirley, NY) in a stock solution (5 mg/ml) containing 30% alcohol and 30% propylene glycol was freshly diluted with 0.9% saline to a dose of 10 mg/kg. Thus, we used a vehicle solution including 30% alcohol and 30% propylene glycol as the control for NTG. Mice received i.p. injections of NTG or the vehicle solution at a 10 ml/kg volume. We conducted microinjection of 1-naphthyl acetyl spermine (NASPM; 0.5 μl, 3 mM in 0.9% saline; Sigma-Aldrich, St. Louis, MO) into unilateral/bilateral Sp5C using a Hamilton syringe at 30 min prior to NTG injection. For the microinjection, mice were anesthetized with 2% isoflurane and placed onto the stereotaxic instrument. After skin cut and appropriate hemostasis using sterile technic, a hole on the skull was drilled and 0.5 μl of NASPM was injected into the Sp5C according to predetermined coordinates (AP, −8.0 mm; ML, 1.5 mm; DV, 4.5 mm) (Romero-Reyes et al., 2013). Intra-Sp5C injection of NASPM or saline control was done within one min and the needle was remained in place for additional one min. At the end of experiments, the microinjection site was confirmed histologically. The dose of NASPM we used was based on previous studies (Conrad et al., 2008; Li et al., 2015; Lu et al., 2007; Takazawa et al., 1996).
2.3. Light-aversive behavior test
We carried out light-aversive behavior test immediately following NTG injection. A custom-made light/dark box (30 cm length × 30 cm width × 30 cm height) with two equally-sized compartments was used for this test as described in previous studies (Farkas et al., 2016; Markovics et al., 2012; Recober et al., 2010). In the light/dark box, one compartment is wrapped with white paper without a lid under a LEDs illuminator (1000 lx, Fisher Scientific, USA), giving a cool, shadow-free illumination without heating, and the other compartment is wrapped with black paper and covered with a lid. There is a gate (7 × 7 cm) connecting the two compartments. Mice were individually tested for 30 min in the light/dark box and they were allowed to move freely during the 30-min test period. We recorded the mouse behaviors using a video camera. The percentage of time the mice spent in dark compartment was calculated. All behavioral tests in this study were carried out by an investigator blinded to the treatment groups.
2.4. Paw withdrawal threshold test
Paw withdrawal threshold was measured with von Frey filaments at 2 h after NTG injection as described previously (Li et al., 2014; Pogatzki and Raja, 2003). In brief, mice were placed on an elevated wire mesh floor and were covered with a clear Plexiglas chamber. After a habituation period of 30 min, the plantar surface of the mouse hind paw was stimulated with a series of calibrated von Frey filaments (0.08, 0.15, 0.25, 0.41, 0.7, 1.2, and 2.0 g). A positive response was defined as a licking, lifting or shaking of the paw upon stimulation. Each filament was applied five times to the plantar side of the hind paws for 1–2 s with a 10 s interval, starting from the lowest force of filament (0.08 g) continuing in ascending order. The paw withdrawal threshold was calculated as the force at which the positive response occurred in three of five stimuli.
2.5. Orofacial mechanical hypersensitivity test
The calibrated von Frey filaments were also used to test orofacial mechanical hypersensitivity at 2 h after NTG injection. The mice were placed into a 10-cm long restraining glass cylinder and allowed to poke out their heads and forepaws, but the restrainer prevented them from turning around (Farkas et al., 2016). After acclimation for 5 min, the filament was applied to the midline of the forehead at the level of the eyes (innervated by trigeminal nerve V1 branch). A positive response was defined as a sharp withdrawal of the head upon stimulation. Each filament was applied five times to the V1-innervated skin area for 1–2 s with a 10 s interval, starting from the lowest force of filament (0.08 g) continuing in ascending order. The head withdrawal threshold was calculated as the force at which the positive response occurred in three of five stimuli.
2.6. Western blotting
The mice were sacrificed at 2 h after NTG injection under isoflurane anesthesia and the Sp5C tissues were harvested. The expression of total AMPA receptor GluA1 and phosphorylated GluA1 at the Ser831 site was analyzed with quantitative Western blotting. The affinity-purified antibodies against GluA1 (1:1000, Cat. # AB1504, Millipore, Burlington, MA, USA), phospho-GluA1-Ser831 (1:2000, Cat. # ab109464, Abcam, Cambridge, MA, USA) and phospho-GluA1-Ser845 (1:1000, Cat. # 8084S, Cell Signaling, Beverly, MA, USA) were used to assess the expression levels of total GluA1 and phosphorylated GluA1 at the Ser831/845 site, respectively. β-actin served as a loading control in all Western blot experiments. The intensities of bands in the Western blotting were quantified with densitometry. The values of phospho-GluA1-Ser831 bands were normalized to total GluA1 and expressed as a ratio of phospho-GluA1-Ser831/total GluA1.
2.7. Calcium imaging in cultured neurons
Changes in intracellular Ca2+ concentration were measured using Fura-2-AM as described previously (El Karim et al., 2015; He et al., 2012; Jara et al., 2007; Madji Hounoum et al., 2016). Cultured embryonic Sp5C-containing brainstem neurons were used for calcium imaging on day 7 after plating. The neurons were incubated with 6 μM Fura-2-AM in 37°C for 45 min (Chiu et al., 2013). The ratio of F340/380 (the emission at 500 nm induced by 340 and 380 nm excitation) was used to indicate Ca2+ influx. After basal Ca2+ recording, the cultured neurons were incubated with 0.25 μM NTG and/or 5 μM NASPM to examine the mechanism for the effect of NTG on Ca2+ influx.
2.8. Locomotor function test
A rotarod apparatus was used to measure locomotor function of mice. The rods in the apparatus were accelerated from 4 to 40 rpm over 5 min. The falling speed and the time to fall off the rod were recorded.
2.9. Statistical analysis
Data are expressed as mean ± the standard error of the mean (S.E.M.). All statistical analyses were performed by SigmaStat software. A Student’s t-test was used for analyzing Western blotting data, one-way analyses of variance (ANOVA) was performed for calcium imaging data, and two-way ANOVA was performed for behavioral testing data. The Student-Newman-Keuls method was used for post-hoc test of ANOVA. P < 0.05 was considered statistically significant.
3. Results
3.1. NTG induces photophobia and migraine-like pain
NTG-induced migraine-like pain was confirmed in the present study. In C57BL/6 wild-type (WT) mice, we observed that NTG injection (i.p.) induced photophobia and mechanical hypersensitivity. Our results showed that NTG not only increased the time WT mice spent in dark compartment in the light-aversive behavior test (Fig. 1A), but also significantly decreased both paw withdrawal threshold and head withdrawal threshold (Fig. 1B and C). The NTG-induced light-aversive behavior only lasted for 30 min. And the NTG-induced mechanical pain in both paw and head started from 2 h post-injection and lasted about 6 h.
Figure 1.
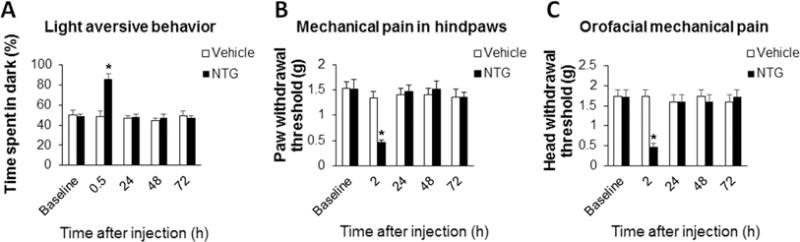
NTG induces photophobia and migraine-like pain in C57BL/6 WT mice. (A) NTG injection (10 ml/kg, i.p.) increased the time the mice spent in dark compartment in the light-aversive behavior test. (B) The NTG administration significantly decreased paw withdrawal threshold. (C) The NTG administration significantly decreased head withdrawal threshold. *P < 0.05 vs the vehicle group. n = 6 for each group; two-way ANOVA with the post-hoc Student-Newman-Keuls test was performed. NTG, nitroglycerin.
3.2. NTG enhances AMPA receptor GluA1 Ser831 phosphorylation in the Sp5C
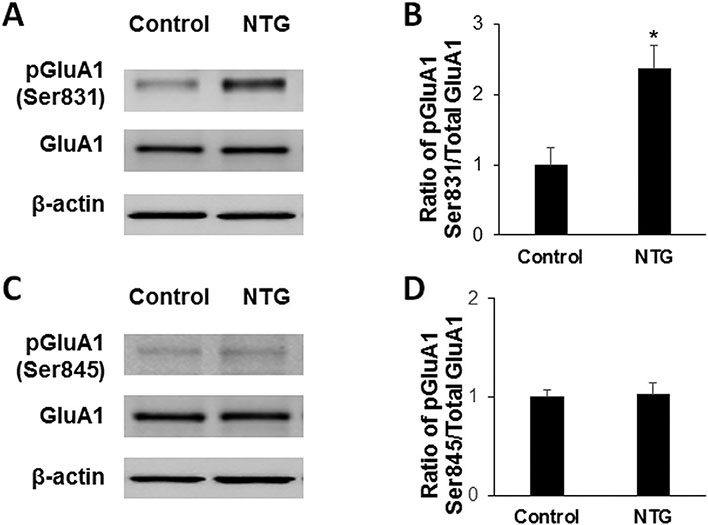
To reveal whether AMPA receptor GluA1 phosphorylation is involved in NTG-induced migraine-like pain, we harvested Sp5C tissues at 2 h after NTG injection and assessed the expression of total GluA1 and GluA1 phosphorylation at the Ser831 and Ser845 sites in the Sp5C by quantitative Western blotting. We found that NTG injection enhanced Sp5C GluA1 phosphorylation at the Ser831 site compared to the vehicle control group, but had no effect on the expression of total GluA1 in the Sp5C (Fig. 2A). Statistical analysis showed that ratio of pGluA1-Ser831/total GluA1 significantly increased following NTG injection (Fig. 2B). We also observed that the administration of NTG did not alter Sp5C GluA1 phosphorylation at the Ser845 site (Fig. 2C and D).
Figure 2.
NTG enhances AMPA receptor GluA1 Ser831 phosphorylation in the Sp5C. (A) NTG injection enhanced Sp5C GluA1 phosphorylation at the Ser831 site compared to the vehicle control group, but had no effect on the expression of total GluA1 in the Sp5C. (B) Statistical analysis showed that ratio of pGluA1-Ser831/total GluA1 significantly increased following NTG injection. (C and D) NTG injection did not alter GluA1 phosphorylation at the Ser845 site in the Sp5C. *P < 0.05 vs the vehicle control group. n = 6 for each group; The Student’s t-test was performed. NTG, nitroglycerin; pGluA1, phosphorylated GluA1.
3.3. Targeted mutation of AMPA receptor GluA1 Ser831 phosphorylation site inhibits NTG-induced migraine-like pain
To further investigate the potential role of GluA1 Ser831 phosphorylation in NTG-induced migraine-like pain, we employed AMPA receptor GluA1 S831A phospho-deficient mutant mice (Lee et al., 2003; Li et al., 2014). In the mutant mice, GluA1 phosphorylation at the Ser831 site is mutated using a gene knock-in technique, therefore the GluA1 Ser831 in the mice cannot be phosphorylated anymore. The mutant mice showed no overt behavioral phenotype and bred normally (Lee et al., 2003; Li et al., 2014). We also observed that the mutant mice had similar baseline values to WT mice in the light-aversive behavior test and the von Frey filament test for paw and head. In the WT mice, we confirmed that NTG injection (i.p.) induced migraine-like pain, including increased time spent in dark compartment in the light-aversive behavior test (Fig. 3A) and decreased withdrawal thresholds in the mechanical pain test (Fig. 3B and C). Compared to the WT mice, the GluA1 S831A mutant mice displayed marked inhibition in NTG-produced photophobia and mechanical hypersensitivity. The time spent in dark compartment following NTG injection significantly decreased in the mutant mice compared to that in the WT mice (Fig. 3A). Both paw withdrawal threshold and head withdrawal threshold at 2 h post-NTG significantly increased in the mutant mice compared to those in the WT mice (Fig. 3B and C). These results indicate that GluA1 phosphorylation at the Ser831 site is critical for NTG-induced migraine-like pain.
Figure 3.
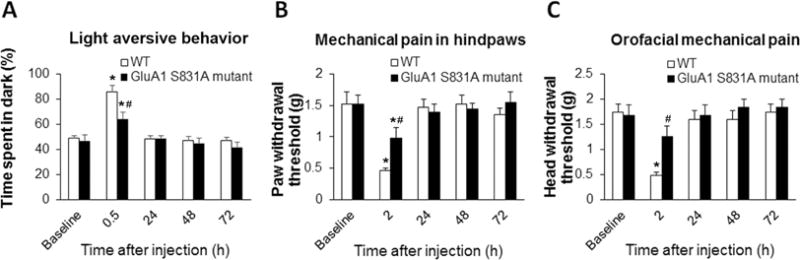
Targeted mutation of AMPA receptor GluA1 Ser831 phosphorylation inhibits NTG-induced migraine-like pain. Compared to the WT mice, the GluA1 S831A mutant mice displayed marked inhibition in NTG-produced photophobia and mechanical hypersensitivity. (A) In the light-aversive behavior test, the time spent in dark compartment following NTG injection significantly decreased in the mutant mice compared to that in the WT mice. (B) In the paw withdrawal threshold test, the mutant mice showed increased paw withdrawal thresholds at 2 h post-NTG compared to those in the WT mice. (C) In the orofacial mechanical hypersensitivity test, the mutant mice showed increased head withdrawal thresholds at 2 h post-NTG compared to those in the WT mice. *P < 0.05 vs baseline, #P < 0.05 vs the WT mice. n = 6 for the WT mice and n = 5 for the mutant mice; two-way ANOVA with the post-hoc Student-Newman-Keuls test was performed. WT, wild-type.
3.4. NTG enhances Ca2+ influx via Ca2+-permeable AMPA receptors in the cultured brainstem neurons
To examine the effect of NTG administration on neuronal Ca2+ activities, we cultured Sp5C-containing brainstem neurons and incubated the cultured neurons with NTG (0.25 μM) on day 7 after plating. By calcium imaging, we observed that NTG incubation evoked a sharp increase of Ca2+ influx (Fig. 4A). Interestingly, the NTG-enhanced Ca2+ influx was dramatically inhibited by AMPA receptor GluA1 S831A phospho-deficient mutation (Fig. 4A). Statistical analysis showed that average maximum Fura-2 fluorescence intensity indicated by ratio of F340/380 significantly increased after NTG incubation and that the GluA1 S831A mutation markedly diminished the NTG-enhanced Ca2+ influx (Fig. 4B). We also observed that co-incubation with NASPM, a selective Ca2+-permeable AMPA receptor channel blocker (Gangadharan et al., 2011; Yin et al., 2007), dose-dependently blocked the NTG-enhanced Ca2+ influx, though NASPM alone had no effect on Ca2+ influx in the cultured neurons (Fig. 4C). Statistical analysis showed that NASPM co-incubation dose-dependently counteracted the effect of NTG on Ca2+ influx in the cultured neurons (Fig. 4D).
Figure 4.
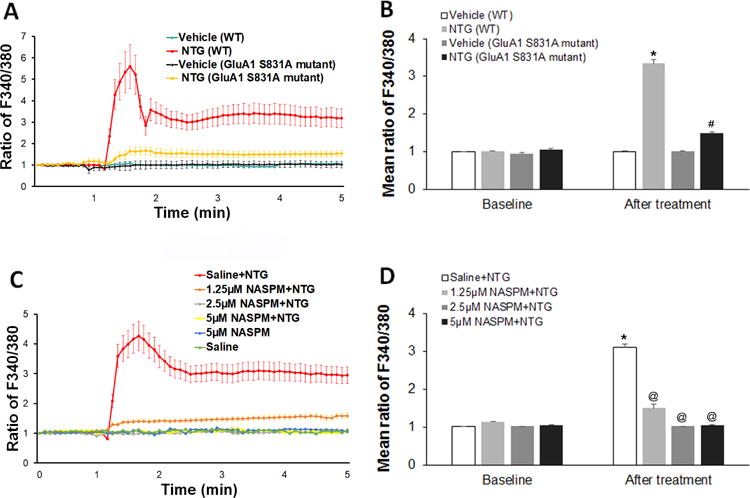
NTG enhances Ca2+ influx via Ca2+-permeable AMPA receptors in the cultured brainstem neurons. (A) In the cultured Sp5C-containing brainstem neurons from WT mice, NTG incubation (0.25 μM) evoked a sharp increase of Ca2+ influx. And the NTG-enhanced Ca2+ influx was dramatically inhibited in the cultured Sp5C-containing brainstem neurons from AMPA receptor GluA1 S831A phospho-deficient mutant mice. (B) Statistical analysis showed that average maximum Fura-2 fluorescence intensity indicated by ratio of F340/380 significantly increased after NTG incubation and that the GluA1 S831A mutation markedly diminished the NTG-enhanced Ca2+ influx. (C) Co-incubation with NASPM, a selective Ca2+-permeable AMPA receptor channel blocker, dose-dependently blocked the NTG-enhanced Ca2+ influx, though NASPM alone had no effect on Ca2+ influx in the cultured neurons. (D) Statistical analysis showed that NASPM co-incubation dose-dependently counteracted the effect of NTG on Ca2+ influx in the cultured neurons. *P < 0.05 vs baseline, #P < 0.05 vs the NTG (WT) group, @P < 0.05 vs the “Saline+NTG” group. n = 24–26 for each group; one-way ANOVA with the post-hoc Student-Newman-Keuls test was performed. WT, wild-type; NTG, nitroglycerin; NASPM, 1-naphthyl acetyl spermine.
3.5. Blockade of Ca2+-permeable AMPA receptors in the Sp5C diminishes NTG-induced migraine-like pain
To further investigate the role of Ca2+-permeable AMPA receptors in NTG-induced migraine-like pain, we injected NASPM into the Sp5C and examined the effect of blockage of Ca2+-permeable AMPA receptors on migraine-like pain in the NTG model. In the paw withdrawal threshold test, we found that unilateral intra-Sp5C injection of NASPM (0.5 μl, 3 mM) partially inhibited NTG-induced mechanical pain in ipsilateral hindpaw (Fig. 5A), but not contralateral hindpaw (Fig. 5B). In the orofacial mechanical hypersensitivity test, we found that bilateral intra-Sp5C NASPM also partially inhibited NTG-induced trigeminal nerve V1-mediated orofacial mechanical hypersensitivity (Fig. 5C). However, we did not observe the influence of intra-Sp5C NASPM on NTG-induced light-aversive behavior (Fig. 5D). As a control, intra-Sp5C injection of NASPM alone had no significant effect on baseline values of all behaviors (Fig. 5A–D). Taken together, these results suggest that AMPA receptor phosphorylation-produced switch from Ca2+-impermeable to Ca2+-permeable receptors in the Sp5C contributes to NTG-induced mechanical hypersensitivity.
Figure 5.
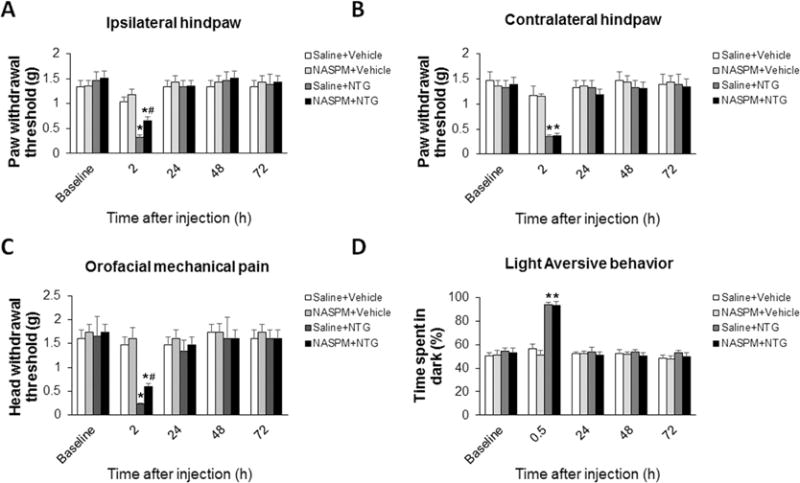
Blockade of Ca2+-permeable AMPA receptors in the Sp5C diminishes NTG-induced migraine-like pain. (A and B) In the paw withdrawal threshold test, unilateral intra-Sp5C injection of NASPM (0.5 μl, 3 mM) significantly inhibited NTG-induced mechanical hypersensitivity in ipsilateral hindpaw (A), but not contralateral hindpaw (B). (C) In the orofacial mechanical hypersensitivity test, bilateral intra-Sp5C NASPM markedly inhibited NTG-induced trigeminal nerve V1-mediated orofacial mechanical hypersensitivity. (D) Intra-Sp5C NASPM had no effect on NTG-induced light-aversive behavior. As a control, intra-Sp5C injection of NASPM alone had no significant effect on baseline values of all behaviors. *P < 0.05 vs baseline, #P < 0.05 vs the “Saline+NTG” group. n = 6–10 for each group; two-way ANOVA with the post-hoc Student-Newman-Keuls test was performed. NTG, nitroglycerin; NASPM, 1-naphthyl acetyl spermine.
3.6. Both NTG and NASPM have no side effects on locomotor function of mice
To exclude potential toxic effects produced by NTG (10 mg/kg, i.p.) and/or NASPM (0.5 μl, 3 mM, intra-Sp5C) administration, we performed locomotor function testing using a rotarod apparatus. We recorded falling speed and time to fall off the rod before and after treatment with the two drugs. Our data showed that both NTG and NASPM used in this study had no effect on the falling speed (Fig. 6A) and the time to fall (Fig. 6B) in the rotarod test, suggesting that the mice administered with the two drugs have normal locomotor function.
Figure 6.
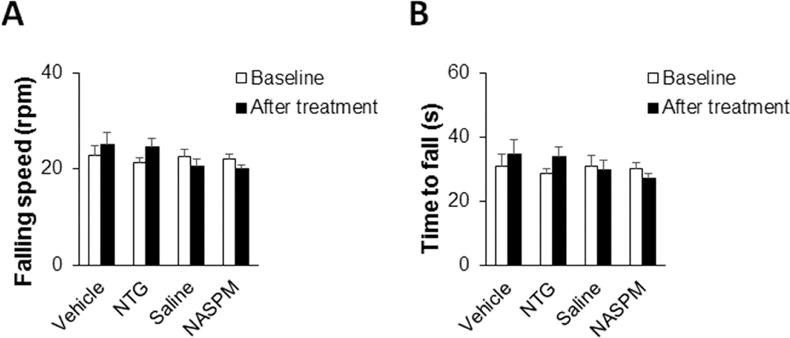
Both NTG and NASPM have no side effects on locomotor function of mice. A rotarod test was performed to assess locomotor function of mice. We recorded falling speed and time to fall off the rod before and after treatment with NTG or NASPM. (A and B) Both NTG and NASPM in the doses used in this study had no effect on the falling speed (A) and the time to fall (B) in the rotarod test. n = 6 for each group; two-way ANOVA with the post-hoc Student-Newman-Keuls test was performed. NTG, nitroglycerin; NASPM, 1-naphthyl acetyl spermine.
4. Discussion
Migraine headache substantially impairs quality of life in migraineurs. Despite its high prevalence, the underlying mechanisms are not fully understood and thereby its treatment remains inadequate. NTG, a donor of nitric oxide (Li et al., 2016; Neeb and Reuter, 2007; Pardutz et al., 2007; Varga et al., 2009), or mimicking the effect of nitric oxide intracellularly by activating guanylate cyclase at low concentrations (Kleschyov et al., 2003), has been widely used as an experimental agent to induce migraine-like pain in biomedical research. Previous studies demonstrate that NTG administration causes a delayed migraine-like pain in migraineurs and an immediate headache in both migraine sufferers and healthy subjects (Ashina et al., 2013; Olesen, 2008; Thomsen et al., 1994). Because NTG-produced nitric oxide can modulate cortical spreading depression in humans and rodents (Petzold et al., 2008) and NTG can also enhance the propagation of cortical spreading depression (Knapp et al., 2017), the NTG-induced migraine model might display the cortical spreading depression-mediated aura. The administration of NTG not only triggers migraine in humans, but also produces cutaneous hyperalgesia in rodents (Ramachandran et al., 2012; Tassorelli et al., 2003). In the present study, we used the NTG-induced migraine-like pain model to investigate the molecular mechanisms by which migraine headache develops. We measured mechanical hypersensitivity induced by NTG injection in both paw and head. Recently, we used Mouse Grimace Scale to test spontaneous pain following NTG administration and observed that NTG caused immediate spontaneous pain indicated by facial expression alteration (supplemental figure 1), suggesting that the NTG model can be used to study migraine-like pain. Typical migraine headache is unilateral and localized pain in the frontotemporal and ocular area, but the pain may be felt anywhere around the head or neck. The unilateral head pain has been reported in a genetic mouse model of migraine (Chanda et al., 2013). In the systemically injected NTG model, we did not observe the difference of pain behaviors between left and right sides of the mouse head, which is consistent with other studies (Farkas et al., 2016; Pradhan et al., 2014) using this model.
We revealed for the first time that AMPA receptor GluA1 Ser831 phosphorylation in the Sp5C is critical for the development of NTG-induced migraine-like pain, and that the phosphorylation-mediated switch from Ca2+-impermeable to Ca2+-permeable AMPA receptors in the Sp5C contributes to the pathogenesis of such pain. Our results suggest that by enhancing GluA1 Ser831 phosphorylation, systemic administration of NTG can increase the expression of Ca2+-permeable AMPA receptors and in turn promote Ca2+ influx and activate Ca2+-dependent kinases, thereby further enhancing AMPA receptor phosphorylation. And this positive feedback loop may play an important role in the development of NTG-induced migraine-like pain. In the NTG model, we observed that both paw withdrawal threshold and head withdrawal threshold were similarly influenced by targeted mutation of GluA1 Ser831 phosphorylation site and blockade of Ca2+-permeable AMPA receptors, indicating that the NTG-induced migraine-like pain is not just trigeminal pain. Migraine-like pain induced by dural afferent sensitization in rats also includes behavioral alterations in both paw and head withdrawal (Yan et al., 2011; Yan et al., 2012).
AMPA receptors are composed of four subunits including GluA1, GluA2, GluA3, and GluA4 (Ozawa et al., 1998; Parsons et al., 2005; Traynelis et al., 2010). Among the four AMPA receptor subunits, GluA1 is the most abundant and highly concentrated in the Sp5C (McCulloch et al., 2013). In this study, we found that systemic injection of NTG time-dependently enhances GluA1 phosphorylation at the Ser831 site in the Sp5C, but does not alter total GluA1 of Sp5C, suggesting that Sp5C GluA1 phosphorylation may be involved in NTG-induced migraine-like pain. By using GluA1 S831A phospho-deficient mutant mice, we further found that targeted mutation of GluA1 Ser831 phosphorylation markedly inhibits NTG-produced photophobia and mechanical hypersensitivity. Photophobia is a diagnostic feature of migraine, and it is an abnormal discomfort to non-noxious levels of light (Farkas et al., 2016; Mason et al., 2017). Together, our data indicate that Sp5C GluA1 phosphorylation plays a key role in the development of NTG-induced migraine-like pain.
In the C-terminus of GluA1, both Ser831 and Ser845 sites can be phosphorylated by different protein kinases (Barria et al., 1997; Mammen et al., 1997; Roche et al., 1996). GluA1 Ser831 is phosphorylated by calcium/calmodulin dependent protein kinase II and protein kinase C, whereas GluA1 Ser845 is phosphorylated by protein kinase A (Barria et al., 1997; Mammen et al., 1997; Roche et al., 1996). Genetically modified mice with knock-in mutations that block phosphorylation at both Ser831 and Ser845 sites of GluA1 show disturbances in synaptic plasticity and learning (Lee et al., 2003). However, GluA1 phosphorylation at the Ser831 and Ser845 sites affects AMPA receptors in different ways. The Ser831 phosphorylation increases GluA1 single channel conductance and open probability (Banke et al., 2001; Derkach et al., 1999) whereas the Ser845 phosphorylation stabilizes GluA1 homomers (He et al., 2009). In our study, the NTG-enhanced GluA1 phosphorylation occurs specifically at the Ser831 site, but not Ser845 site, suggesting that GluA1 phosphorylation at the two sites may play distinct roles in central sensitization of nociceptive transmission. Based on our data, GluA1 Ser831 phosphorylation could be used as a potential target to develop a new therapy for migraine headache.
It has been demonstrated that AMPA receptor phosphorylation activities promote the receptor trafficking (Choi et al., 2010; Fang et al., 2003; Lu et al., 2008; Park et al., 2009) thereby leading to a switch from Ca2+-impermeable to Ca2+-permeable receptors (Li et al., 2014). The phosphorylation-induced increase in Ca2+-permeable AMPA receptors can enhance Ca2+ influx and further activate Ca2+-dependent protein kinases. A reduction in spinal Ca2+-permeable AMPA receptors causes a loss of nociceptive plasticity; conversely, an increase in spinal Ca2+-permeable AMPA receptors promotes nociceptive plasticity and increases long-term potentiation and long-lasting inflammatory hyperalgesia (Hartmann et al., 2004; Youn et al., 2008). In the present study, we measured Ca2+ activities in cultured Sp5C-containing brainstem neurons using Ca2+ imaging. We observed that NTG incubation robustly enhances Ca2+ influx in the cultured neurons and that co-incubation with NASPM, a selective Ca2+-permeable AMPA receptor channel blocker, completely blocks the NTG-evoked increase of Ca2+ influx. The embryonic brainstem neurons we cultured may include different types of neurons, but the blockade of NTG-enhanced Ca2+ influx by NASPM indicates that the activation of Ca2+-permeable AMPA receptors in the brainstem primarily contribute to the NTG-enhanced Ca2+ influx. Combining these results with NTG-enhanced GluA1 phosphorylation in the Sp5C, our data indicate that NTG administration can increase Ca2+ influx through GluA1 phosphorylation-mediated AMPA receptor switch from Ca2+-impermeable to Ca2+-permeable. Furthermore, we injected NASPM into the Sp5C and found that the selective Ca2+-permeable AMPA receptor channel blocker significantly diminishes NTG-induced mechanical hypersensitivity, suggesting that GluA1 phosphorylation-mediated AMPA receptor switch from Ca2+-impermeable to Ca2+-permeable in the Sp5C contributes to the pathogenesis of NTG-induced migraine-like pain.
In conclusion, our study demonstrates that regulation of AMPA receptor GluA1 phosphorylation in the Sp5C is an important molecular mechanism for NTG-induced migraine-like pain. The phosphorylation-triggered AMPA receptor switch can enhance Ca2+ influx, activate intracellular protein kinases, and further upregulate AMPA receptor phosphorylation. The positive feedback loop could underlie the pathophysiology of migraine-like pain.
Supplementary Material
Highlights.
Nitroglycerin enhances AMPA receptor GluA1 Ser831 phosphorylation in the Sp5C.
Targeted mutation of GluA1 Ser831 reduces nitroglycerin-induced migraine-like pain.
GluA1 Ser831 phosphorylation in the Sp5C contributes to migraine-like pain.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers R01 DE022880, K02 DE023551]. The authors thank Dr. Richard Huganir (Johns Hopkins University School of Medicine) for providing AMPA receptor GluA1 S831A phospho-deficient mutant mice.
Abbreviation List
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- NTG
nitroglycerin
- CNS
central nervous system
- Sp5C
spinal trigeminal nucleus caudalis
- NASPM
1-naphthyl acetyl spermine
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128:932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia. 2013;33:540–553. doi: 10.1177/0333102412475234. [DOI] [PubMed] [Google Scholar]
- Banke TG, Greenwood JR, Christensen JK, Liljefors T, Traynelis SF, Schousboe A, Pickering DS. Identification of amino acid residues in GluR1 responsible for ligand binding and desensitization. J Neurosci. 2001;21:3052–3062. doi: 10.1523/JNEUROSCI.21-09-03052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–1828. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O’Shea L, Silver A, Zendegui E, Mariette Lenselink A, Spijker S, Ferrari MD, van den Maagdenberg AM, Mogil JS. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154:1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. discussion 626. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Karim I, McCrudden MT, Linden GJ, Abdullah H, Curtis TM, McGahon M, About I, Irwin C, Lundy FT. TNF-alpha-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol. 2015;185:2994–3002. doi: 10.1016/j.ajpath.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Farkas S, Bolcskei K, Markovics A, Varga A, Kis-Varga A, Kormos V, Gaszner B, Horvath C, Tuka B, Tajti J, Helyes Z. Utility of different outcome measures for the nitroglycerin model of migraine in mice. J Pharmacol Toxicol Methods. 2016;77:33–44. doi: 10.1016/j.vascn.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Invest. 2011;121:1608–1623. doi: 10.1172/JCI44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Cristino L, Luongo L, Siniscalco D, Petrosino S, Piscitelli F, Marabese I, Gatta L, Rossi F, Imperatore R, Palazzo E, de Novellis V, Di Marzo V, Maione S. TRPV1-dependent and -independent alterations in the limbic cortex of neuropathic mice: impact on glial caspases and pain perception. Cereb Cortex. 2012;22:2495–2518. doi: 10.1093/cercor/bhr328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Jia C, Feng S, Zhou K, Tai Y, Bai X, Wang Y. TRPC5 channel is the mediator of neurotrophin-3 in regulating dendritic growth via CaMKIIalpha in rat hippocampal neurons. J Neurosci. 2012;32:9383–9395. doi: 10.1523/JNEUROSCI.6363-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen HK. Human migraine models. Cephalalgia. 2001;21:781–785. doi: 10.1111/j.1468-2982.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem. 2007;100:1407–1420. doi: 10.1111/j.1471-4159.2006.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, Sydow K, Fichtlscherer B, Mulsch A, Munzel T. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ Res. 2003;93:e104–112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- Knapp L, Szita B, Kocsis K, Vecsei L, Toldi J. Nitroglycerin enhances the propagation of cortical spreading depression: comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des Devel Ther. 2017;11:27–34. doi: 10.2147/DDDT.S117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, Tao F. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci. 2014;34:13737–13746. doi: 10.1523/JNEUROSCI.2130-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang MM, Tu K, Wang J, Feng B, Zhang ZN, Lei J, Li YQ, Du JQ, Chen T. The excitatory synaptic transmission of the nucleus of solitary tract was potentiated by chronic myocardial infarction in rats. PLoS One. 2015;10:e0118827. doi: 10.1371/journal.pone.0118827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Qi D, Zhang L, Yi L, Li Q, Zhang Z. Valproate ameliorates nitroglycerin-induced migraine in trigeminal nucleus caudalis in rats through inhibition of NF-small ka, CyrillicB. J Headache Pain. 2016;17:49. doi: 10.1186/s10194-016-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun YN, Wu X, Sun Q, Liu FY, Xing GG, Wan Y. Role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunit GluR1 in spinal dorsal horn in inflammatory nociception and neuropathic nociception in rat. Brain Res. 2008;1200:19–26. doi: 10.1016/j.brainres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Madji Hounoum B, Vourc’h P, Felix R, Corcia P, Patin F, Gueguinou M, Potier-Cartereau M, Vandier C, Raoul C, Andres CR, Mavel S, Blasco H. NSC-34 Motor Neuron-Like Cells Are Unsuitable as Experimental Model for Glutamate-Mediated Excitotoxicity. Front Cell Neurosci. 2016;10:118. doi: 10.3389/fncel.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, Russo AF. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci. 2017;37:204–216. doi: 10.1523/JNEUROSCI.2967-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch PF, DiNovo KM, Westerhaus DJ, Vizinas TA, Peevey JF, Lach MA, Czarnocki P. Trigeminal Medullary Dorsal Horn Neurons Activated by Nasal Stimulation Coexpress AMPA, NMDA, and NK1 Receptors. ISRN Neurosci. 2013;2013:152567. doi: 10.1155/2013/152567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets. 2007;6:258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics. 2010;7:183–190. doi: 10.1016/j.nurt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pardutz A, Hoyk Z, Varga H, Vecsei L, Schoenen J. Oestrogen-modulated increase of calmodulin-dependent protein kinase II (CamKII) in rat spinal trigeminal nucleus after systemic nitroglycerin. Cephalalgia. 2007;27:46–53. doi: 10.1111/j.1468-2982.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Zieglgansberger W. Excitatory amino acid neurotransmission. Handb Exp Pharmacol. 2005:249–303. doi: 10.1007/3-540-28082-0_10. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Haack S, von Bohlen Und Halbach O, Priller J, Lehmann TN, Heinemann U, Dirnagl U, Dreier JP. Nitric oxide modulates spreading depolarization threshold in the human and rodent cortex. Stroke. 2008;39:1292–1299. doi: 10.1161/STROKEAHA.107.500710. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Bhatt DK, Ploug KB, Olesen J, Jansen-Olesen I, Hay-Schmidt A, Gupta S. A naturalistic glyceryl trinitrate infusion migraine model in the rat. Cephalalgia. 2012;32:73–84. doi: 10.1177/0333102411430855. [DOI] [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Romero-Reyes M, Akerman S, Nguyen E, Vijjeswarapu A, Hom B, Dong HW, Charles AC. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013;53:137–151. doi: 10.1111/j.1526-4610.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Society, H. C. C. o. t. I. H. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Takazawa A, Yamazaki O, Kanai H, Ishida N, Kato N, Yamauchi T. Potent and long-lasting anticonvulsant effects of 1-naphthylacetyl spermine, an analogue of Joro spider toxin, against amygdaloid kindled seizures in rats. Brain Res. 1996;706:173–176. doi: 10.1016/0006-8993(95)01334-2. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats–a time-course study. Eur J Pharmacol. 2003;464:159–162. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol. 1994;1:73–80. doi: 10.1111/j.1468-1331.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga H, Pardutz A, Vamos E, Bohar Z, Bago F, Tajti J, Bari F, Vecsei L. Selective inhibition of cyclooxygenase-2 attenuates nitroglycerin-induced calmodulin-dependent protein kinase II alpha in rat trigeminal nucleus caudalis. Neurosci Lett. 2009;451:170–173. doi: 10.1016/j.neulet.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HZ, Tang DT, Weiss JH. Intrathecal infusion of a Ca(2+)-permeable AMPA channel blocker slows loss of both motor neurons and of the astrocyte glutamate transporter, GLT-1 in a mutant SOD1 rat model of ALS. Exp Neurol. 2007;207:177–185. doi: 10.1016/j.expneurol.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn DH, Royle G, Kolaj M, Vissel B, Randic M. Enhanced LTP of primary afferent neurotransmission in AMPA receptor GluR2-deficient mice. Pain. 2008;136:158–167. doi: 10.1016/j.pain.2007.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.