Abstract
This study aimed to address the mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA commonly reported in “Ecstasy” formulations: methylone, butylone, and pentylone. Whole-cell patch clamp techniques were used to assess the mechanism of each compound at the dopamine and serotonin transporters. Separate groups of rats were trained to discriminate methamphetamine, DOM, or MDMA from vehicle. Substitution studies were performed in each group and antagonism studies with SCH23390 were performed against each compound that produced substitution. Self-administration of each compound was evaluated under a progressive ratio schedule of reinforcement. Each compound produced an inward current at the serotonin transporter, but little or no current at the dopamine transporter. Each of the test compounds substituted fully for the discriminative stimulus effects of methamphetamine, methylone and butylone substituted partially for DOM and fully for MDMA, whereas pentylone failed to substitute for DOM and substituted only partially for MDMA. SCH23390 fully and dose-dependently attenuated methamphetamine-appropriate responding produced by each test compound, but was least potent against pentylone. MDMA-appropriate responding was minimally affected by SCH23390. Each test compound was robustly self-administered with pentylone producing the greatest self-administration at the doses tested. Given the prevalence of synthetic cathinones in “Ecstasy” formulations, these data indicate that adulterated “Ecstasy” formulations may drive more compulsive drug use than those containing only MDMA.
Keywords: MDMA, synthetic cathinones, drug discrimination, self-administration, patch clamp
1. Introduction1
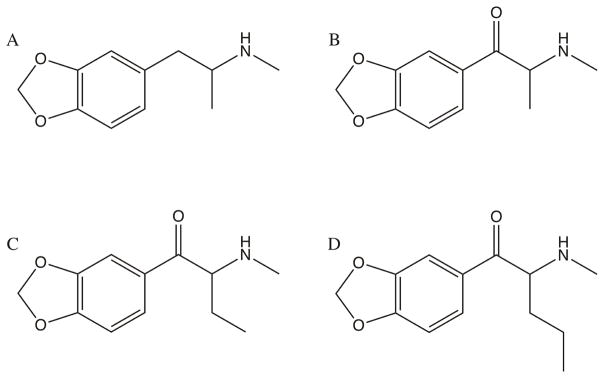
In recent years, the club drug “Molly”, which is typically sold in powdered form and popularly considered and branded as pure MDMA, has surged in popularity and is considered a staple in modern-day dance, rave, and festival culture (Palamar, 2017). However, much like the tablet-based “Ecstasy”, powdered “Molly” formulations are regularly adulterated with other psychoactive substances in addition to and in lieu of MDMA (UNODC, 2014; Ecstasydata.org, 2017; Palamar et al., 2016a; Saleemi et al., 2017). Synthetic cathinones are most commonly used as adulterants in these “Molly” formulations, which present with numerous adverse effects, including serotonin syndrome, psychiatric complications, and death (Palamar et al., 2016b; Elliott & Evans, 2014; German et al., 2014; Warrick et al., 2012). The current study aimed to determine the discriminative stimulus and reinforcing effects of three synthetic cathinone analogs of MDMA: methylone, butylone, and pentylone (fig. 1).
Fig. 1. Chemical Structures.
Chemical structures of MDMA (A) and its synthetic cathinone analogs: methylone (B), butylone (C), and pentylone (D).
MDMA is known and preferred for its unique subjective effects including improved sociability, an overall sense of trust or empathy, and enhanced appreciation of music in addition to the increased energy associated with other stimulant-type drugs (Pentney, 2001). Although MDMA operates through a similar mechanism as methamphetamine, MDMA is more selective for the serotonin transporter (SERT) than the dopamine transporter (DAT), whereas methamphetamine is predominately dopaminergic (Simmler et al., 2013; 2014). This DAT- and SERT-mediated mechanism is also apparent in MDMA’s discriminative stimulus effects. Studies using two- and three-choice drug discrimination procedures have indicated a complex discriminative stimulus of MDMA mediated by both dopamine and serotonin (Goodwin & Baker, 2000; Goodwin et al., 2003; Schechter, 1986), and the relative contributions of dopamine and serotonin appear to be dose- and time-dependent (Harper et al., 2014; Webster et al., 2017; Schechter, 1988). The complex nature of MDMA’s discriminative stimulus may provide the unique subjective effects that have made MDMA, “Ecstasy”, and “Molly” so popular amongst club-goers.
The synthetic cathinones are a structurally-diverse class of compounds that exist along a mechanistic spectrum from amphetamine-like monoamine releasing agents to cocaine-like uptake inhibitors, all with varying selectivity profiles (Simmler et al., 2013; 2014; Eshleman et al., 2013; 2017). In vitro evaluations of the molecular mechanisms of methylone, butylone, and pentylone, three popular synthetic cathinone analogs of MDMA, have provided some discrepant results as to whether these compounds operate as serotonin reuptake inhibitors or transporter substrates (Simmler et al., 2013, 2014; Eshleman et al., 2013; 2017). Of the three N-methyl cathinone derivatives of MDMA in the current study, methylone has been most thoroughly characterized. Previous reports have indicated substitution of methylone for the discriminative stimulus effects of cocaine and methamphetamine (Gatch et al., 2013) and MDMA, but not DOM (Dal Cason et al., 1997). Differing degrees of methylone self-administration have been reported throughout the literature (Watterson et al., 2012; Vandewater et al., 2015; Schindler et al., 2016). Butylone and pentylone both substitute for the discriminative stimulus effects of methamphetamine and cocaine (Gatch et al., 2013; 2015). Pentylone has been demonstrated to produce robust self-administration relative to methylone under a continuous schedule of reinforcement (Javadi-Paydar et al., 2017), but butylone has not been studied in a self-administration assay. These early data implicate dopamine in the discriminative stimulus effects of these cathinone analogs of MDMA, but provide limited, if any, information regarding serotonergic contributions. Furthermore, how the mechanisms translate into abuse liability is not fully understood.
The current study aims to clarify the in vitro mechanism of these compounds at DAT and SERT using electrophysiological techniques, evaluate the relative dopaminergic and serotonergic contributions to the discriminative stimulus effects of these compounds using rats trained to discriminate methamphetamine, DOM, or MDMA from vehicle, and to evaluate the reinforcing effects of these compounds using a self-administration assay under a progressive ratio schedule of reinforcement.
2. Materials and Methods
2.1 Cells and Transporters
Human embryonic kidney (HEK) 293 cells were used in electrophysiological experiments. Cells were transiently transfected using PolyJet™ transfection reagent (SignaGen, Rockville, MD) with 0.5 μg GFP-tagged human serotonin-transporter cDNA (hSERT, Cat# MG227448, Origene Technologies, Rockville, MD). We found that GFP-tagged human dopamine transporter cDNA (hDAT, Cat# RG219466, Origene) did not form functional expression as no dopamine-induced current could be recorded from GFP-staining cells. A tag-free form of hDAT cDNA (Cat# SC302961) was used for electrophysiological experiments. Expression was confirmed with fluorescent microscopy (Nikon Eclipse TS100 and Nikon Imaging Software, Nikon Instruments, Tokyo, Japan) for GFP-tagged hSERT or currents activated by DA for hDAT. Electrophysiological studies were performed 24–48 h following transfection.
2.2 Animals
Male Sprague-Dawley rats (Envigo, Indianapolis, IN) were housed individually and maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). All experiments were conducted during the light cycle. Body weights were maintained at 320–350 g by limiting food to 15 g/day. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.4 Drug Discrimination2.3 Electrophysiology
Whole-cell patch-clamp electrophysiology was used to assess neurotransmitter- (serotonin, 5-HT or dopamine, DA) and drug-induced currents via hSERT or hDAT. All experiments were conducted at room temperature (22–25°C) with membrane potential clamped at −70 mV. Patch pipettes of borosilicate glass (1B150F; World Precision Instruments, Inc., Sarasota, FL) were pulled (Flaming/Brown, P-87/PC; Sutter Instrument Company, Novato, CA) to a tip resistance of 4 to 6 MΩ. To record Na+-currents via hSERT, patch pipettes were filled with a solution consisting of 140 mM K-gluconate, 10 mM Na4-EGTA, 10 mM HEPES, 4 mM Na2-ATP, 4 mM MgCl, and 0.4 mM Na3-GTP, pH 7.3. To record Na+ and Cl− currents via hDAT, pipette solution contained (in mM) 140 CsCl, 10 EGTA, 10 HEPES, 4 Mg-ATP; pH 7.2. The cover slips containing cells were placed in a small chamber (~ 1.5 ml) and superfused continuously (7–10 ml/min) with an external solution consisting of 130 mM NaCl, 10 mM HEPES, 1.5 mM CaCl2, 1.3 mM KCl, 0.5 mM MgCl2, and 34 mM glucose, pH 7.3. The cells were visualized using an upright, fixed stage microscope (Nikon Optiphot-2UD) with a 12 V-100 W halogen lamp equipped with standard Hoffman modulation contrast optics and video camera system (Sony model XC-75 CCD video camera module and a video capture system). Agonist-induced currents were obtained with a patch clamp amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA) equipped with a CV203BU headstage. Currents were low-pass filtered at 5 kHz, monitored simultaneously on an oscilloscope and a chart recorder (Gould TA240; Gould Instrument Systems Inc., Cleveland, OH), sampled at 30 KHz using a Digidata 1440A and stored on a computer using an on-line data acquisition system (Clampex 10.6, Molecular Devices) for subsequent off-line analysis.
All drugs were directly dissolved into extracellular solution on the day of the experiment. 100 μM Na2S2O5 was added as an antioxidant to prevent DA from being oxidized. To qualitatively assess directionality of current, cocaine, MDMA, methylone, butylone or pentylone was applied for 5 sec at a concentration of 10 or 100 μM. 5-HT or DA, at a concentration of 10 or 100 μM was applied for 5 sec prior to and after drug application as a baseline control. Drugs were applied at 60 s intervals.
2.4.1 Apparatus
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for chamber operation and data collection.
2.4.2 Discrimination Procedures
Drug discrimination procedures occurred as previously described (Gatch et al., 2016). Briefly, a pool of rats trained to discriminate either methamphetamine (1 mg/kg), MDMA (1.5 mg/kg) or DOM (0.5 mg/kg) from saline was tested (n=8–9 rats/drug). Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serv, Frenchtown, NJ) was available under an FR10 schedule of reinforcement on a designated injection-appropriate lever. The pretreatment time was 10 min for methamphetamine, 15 min for MDMA, and 30 min for DOM. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. The training sessions occurred on separate days in a double alternating fashion until the training phase was complete, and rats received at least 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats continued training until they had achieved nine of ten sessions at 85% injection-appropriate responding, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test. The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
Test sessions lasted for a maximum of 20 min and both levers were active according to an FR10 schedule of reinforcement. Data were collected until 20 reinforcers were obtained, or until 20 min elapsed. The dose effect of each compound was tested from no effect to full effect or until rate suppression (<20% of vehicle control) or adverse effects were observed. For substitution experiments, MDMA, methylone, butylone, pentylone, or their vehicle were administered intraperitoneally (1 mL/kg) 15 minutes before the session started. For antagonism studies, SCH23390 was administered subcutaneously 30 min before the session started. SCH23390 was administered in conjunction with the lowest substituting dose of the test compound that fully substituted for the training drug. A repeated-measures design was used, such that each rat was tested at all doses of a given drug.
2.5 Self-Administration
2.5.1 Apparatus
All testing procedures occurred in standard operant chambers modified for self-administration experiments (Med Associates, St Albans, VT) containing a single lever, a cue light, and a house light at the rear of the chamber. The operant chambers were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the chamber operation and data collection.
2.5.2 Food Training Procedure
All rats were trained to respond for food reinforcers (45 mg food pellets; Bio-Serve, Frenchtown, NJ) under a FR5 schedule of reinforcement. Rats that completed at least 5 consecutive sessions in which 20 reinforcers were obtained then underwent surgery for intravenous self-administration testing.
2.5.3 Surgical Procedure
Rats received analgesia (Rimadyl 2 mg, p.o.; Bio-Serv, Frenchtown, NJ) 24 hrs before, immediately following, and 24 hrs after surgery. Rats were anesthetized with isofluorane (5%) and maintained under anesthesia throughout the surgery (2.5%). Polyurethane tubing (1.1 mm outer diameter, 0.6 mm inner diameter) was implanted in the right jugular vein, fixed in place with suture, passed subcutaneously, and affixed to an external guide cannula. Catheters were flushed daily before each session with 0.9% saline and locked after each session with heparinized (50 units/mL) saline containing timentin (310 mg/mL; 0.1 mL/rat). Rats were allowed 7 days for recovery prior to beginning testing.
2.5.4 Progressive Ratio Self-Administration Testing
Following recovery, rats (n=6) were trained to self-administer methamphetamine (0.05 mg/kg/inf). Responding according to a FR10 schedule resulted in a drug-solution infusion (0.1 mL/1sec) and dimming of the cue light for 20 sec. Sessions lasted two hours and rats were tested 7 days per week. Response-stability criteria were three consecutive sessions with <20% change in infusions among sessions. At least ten training sessions were completed prior to beginning substitution testing. Once stability criteria were reached, rats self-administered methamphetamine (0.05 mg/kg/inf) under a progressive ratio schedule of reinforcement. Ratio requirement was determined according to the equation: Ratio=5e(infusion number*0.3)-5 (Richardson and Roberts, 1996). The value of j=0.3 was chosen based on previous literature testing synthetic cathinones (Aarde et al., 2013) and preliminary data from our lab indicating this ratio array would produce a breakpoint in less than 4 hours. Sessions lasted until 60 min elapsed during which the rat failed to receive an infusion, which was considered the breakpoint. Stability criteria were defined as two consecutive sessions that varied by no more than ±2 infusions. Once stability criteria with methamphetamine were reached, rats began substitution testing under the same experimental conditions. Drug and dose order were assigned randomly using a Latin square. Each dose was tested at least twice until responding was stable. All doses were completed, along with saline and methamphetamine (0.05 mg/kg/inf) controls, before the next drug was tested. A repeated-measures design was used, such that each rat was tested at all doses of a given drug.
2.6 Data Analysis
Raw currents were normalized to the initial 5-HT or DA current (assigned as 100%), and analyzed with Clampfit 10.7 and Origin 5.0 software. The normalized currents were compared among drugs with a Student’s t-test or one-way ANOVA followed by Holm-Sidak post hoc test. Linear correlational analysis was conducted to determine the relation between the molecular weight of the α-alkyl side chain and SERT or DAT activation.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses. Full substitution was defined as >80% drug-appropriate responding and full antagonism was defined as <20% drug-appropriate responding.
Response rates were expressed as a function of the total responses divided by the total session time. Response rate data were analyzed by one-way repeated measures analysis of variance. Individual doses were compared to the vehicle control value in substitution tests and the antagonist vehicle + test drug control value in antagonism tests using a priori contrasts.
Self-administration data are expressed as the mean total infusions obtained and the mean total responses emitted. Data include two replications for each rat. Data were analyzed using a repeated-measures analysis of variance assessing effects of dose and drug. If a main effect of dose was present, individual doses were compared to the vehicle control value using a priori contrasts. If a main effect of drug was present, a one-way analysis of variance was utilized to assess differences between drugs at each dose.
2.7 Drugs
(+)-methamphetamine HCl, (±)-3,4-methylenedioxymethamphetamine HCl (MDMA), (−)-2,5-dimethyoxy-4-methylamphetamine HCl (DOM), (±)-methylone HCl, (±)-butylone HCl, and (±)-pentylone HCl were provided by the National Institute on Drug Abuse Drug Supply Program and were dissolved in 0.9% saline. SCH23390 HCl was purchased from Cayman Chemical (Ann Arbor, MI) and dissolved in 0.9% saline. 5-HT, dopamine HCl and Na2S2O5 were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were injected intraperitoneally at an injection volume of 1 mL/kg, except SCH23390, which was injected subcutaneously.
3. Results
3.1 Effect of synthetic cathinones MDMA analogs on hSERT and hDAT
In the HEK293 cells transiently transfected with GFP-SERT and clamped at a holding potential of −70 mV, 5-HT application (1 μM) induced an inward current. Co-application of 5-HT with cocaine (100 μM) reduced the 5-HT-induced currents to 8.6 ± 6.0% of control. The cocaine inhibitory effect was readily reversible after washout (n=5). Application of extracellular solution or 5-HT on GFP-negative cells did not evoke any response (data not shown). These data confirm functional hSERT expression in HEK cells.
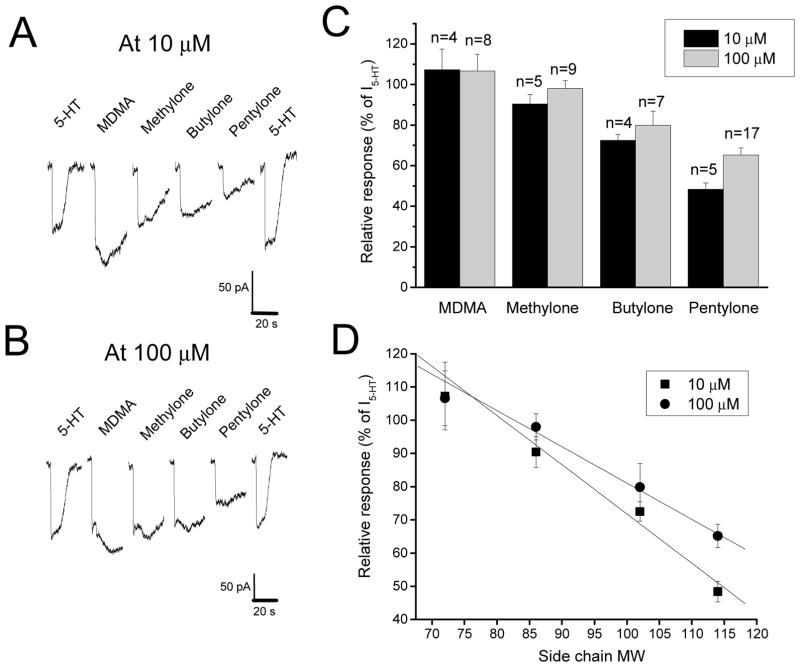
The cathinones were tested at 10 and 100 μM, and applied to GFP-positive cells for 5 sec. As shown in Fig. 1A&B, MDMA and its three cathinone analogs were able to activate hSERT as substrates. In contrast to 5-HT effect which had a fast dissociation rate, inward currents induced by MDMA and its analogs slowly returned to the baseline after drug washout, suggesting relatively slow dissociation from the transporter (Fig. 2A&B). The ability to activate hSERT was significantly different among MDMA and its analogs at 10 μM (F3,12=4.607, p<.01) 100 μM (F3,12=21.43, p<.001). As shown in Fig. 2C, hSERT was activated by cathinones in the following sequence: MDMA>methylone>butylone>pentylone. Furthermore, Fig. 2D shows that there was a negative linear correlation between hSERT activation and the α-alkyl side chain molecular weight (r=−0.98 for 10 μM and r=−0.99 for 100 μM, n=4, p<0.05). Taken together, these data indicate that the action of synthetic cathinones on hSERT decreases with increase in the side-chain length.
Fig. 2. Substrate Activity at the Serotonin Transporter.
Raw current tracings of 10 μM (A) or 100 μM (B) 5-HT, MDMA, methylone, butylone, and pentylone at the human serotonin transporter. Relative currents of each drug at 10 μM (black) and 100 μM (grey) are summarized in (C). Relative currents of 10 μM (squares) and 100 μM (circles) are summarized as a function of side-chain molecular weight (MW) in (D). Error bars represent ±SEM.
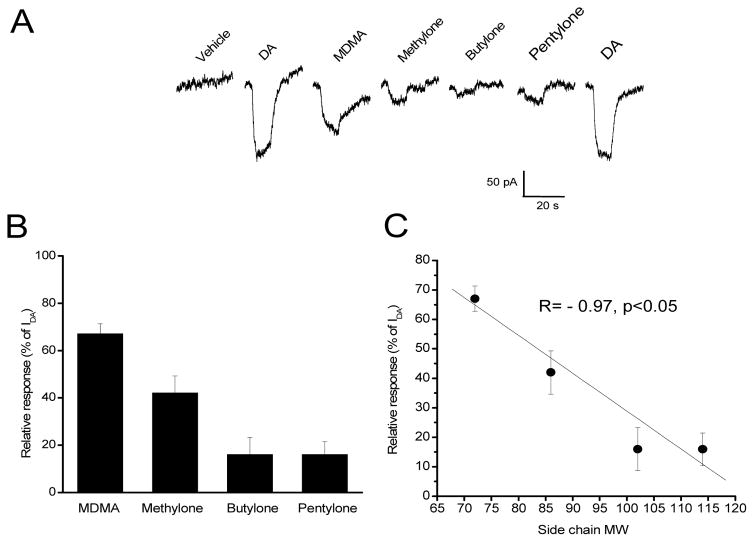
Effect of cathinones on DAT was examined in HEK293 cells transiently expressing hDAT. with no GFP tag as GFP-tagged DAT was not functionally expressed. The functional expression of hDAT was confirmed by an inward current activated induced by 100 μM DA and lack of response to vehicle control (Fig. 3A). MDMA and its three analogs were able activate hDAT at 100 μM. The drugs had relatively fast dissociation rates from the transporter as the current returned to the baseline 10–20 sec after washout. However, their ability to induce inward currents in hDAT was less than that in hSERT, ranging from 16% to 72% of DA-induced currents (n=13–15, Fig. 3B). Furthermore, the ability to activate hDAT was significantly hampered by introducing side-chain (p<0.001, three synthetic cathinones vs MDMA). Similar to their action on hSERT, cathinone-induced currents via hDAT are negatively correlated with the molecular weight of side chain (r=−0.97, n=4, p<0.05, Fig. 3C). These data suggest that synthetic cathinones activate hDAT in a similar pattern but with less efficacy than they do at hSERT.
Fig. 3. Substrate Activity at the Dopamine Transporter.
Raw current tracings of (A) vehicle, 100 μM DA, MDMA, methylone, butylone, and pentylone at the human dopamine transporter. Relative currents of each drug at 100 μM are summarized in (B). Relative currents of 100 μM are summarized as a function of side-chain molecular weight (MW) in (C). Error bars represent ±SEM.
3.2 Drug discrimination
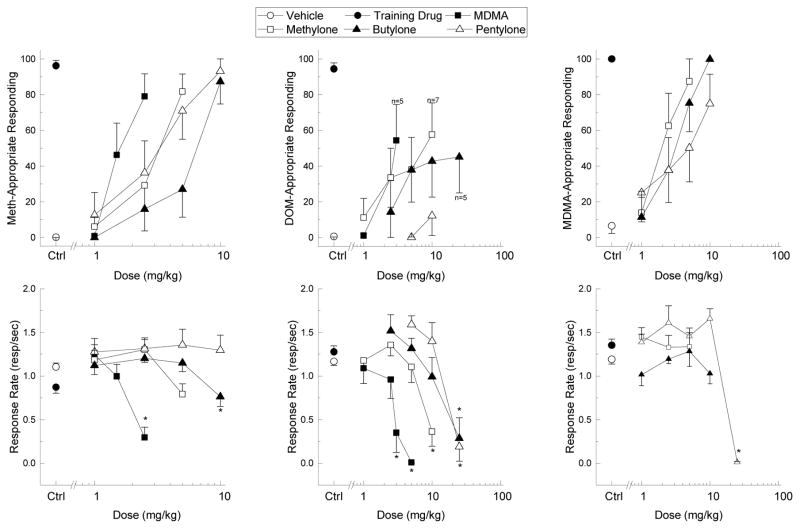
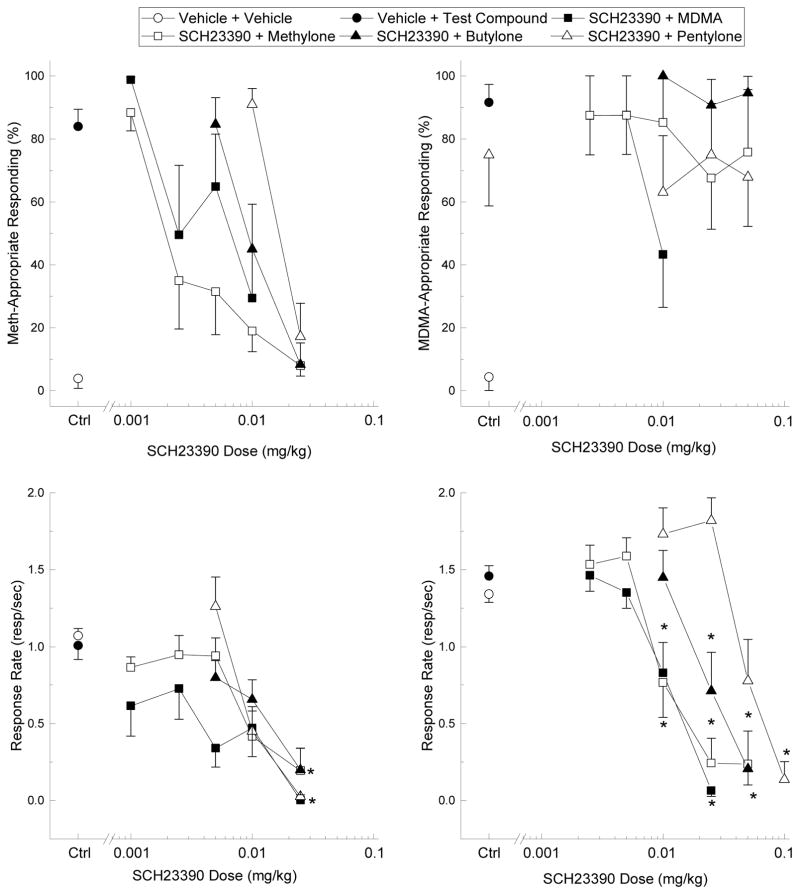
MDMA (ED50= 1.71 mg/kg, [95% CI= 0.71–4.15]), methylone (ED50= 2.99 mg/kg [95% CI= 0.66–13.53]), butylone (ED50= 5.53 mg/kg [95% CI= 0.69–44.45]), and pentylone (ED50= 3.07 mg/kg, [95% CI= 0.34–27.79]) each fully substituted for the discriminative stimulus effects of methamphetamine (Fig. 4, left panel). MDMA (F3,21= 17.841, p<.001) and butylone (F4,28= 6.176, p<.001) reduced response rate at the highest doses tested. SCH23390 dose-dependently and fully attenuated methamphetamine-appropriate responding (Fig. 5, left panel). SCH23390 was most potent at reducing methamphetamine-appropriate responding in MDMA- and methylone-treated rats, was slightly less potent in butylone-treated rats, and high doses of SCH23390 were needed to reduce the methamphetamine-appropriate responding or response rate of pentylone-treated rats. Response rate (Fig. 5, left panel) was decreased by 0.025 mg/kg SCH23390 in MDMA- (F5,35= 3.871, p<.01), methylone- (F5,35= 9.455, p<.001), butylone- (F3,21= 10.647, p<.001), and pentylone-treated rats (F3,21= 32.285, p<.001).
Fig. 4. Drug Discrimination Substitution Profiles.
Drug-appropriate responding (top panel) and response rate (bottom panel) produced by MDMA (filled squares), methylone (open squares), butylone (filled triangles), and pentylone (open triangles) in methamphetamine- (meth; left graph), DOM- (middle graph), and MDMA-trained (right graph) rats. The x-axis indicates test compound dose (mg/kg). Vehicle (open circles) and training drug (filled circles) controls are plotted to the left of the x-axis break. Error bars represent ±SEM. * p<.05 relative to vehicle control.
Fig. 5. Antagonism of Drug-Appropriate Responding.
Drug-appropriate responding (top panel) and response rate (bottom panel) produced by MDMA (2.5 mg/kg; filled squares), methylone (5 mg/kg; open squares), butylone (10 mg/kg; filled triangles), and pentylone (10 mg/kg; open triangles) in methamphetamine- (meth; left graph) and MDMA-trained (right graph) rats pretreated with the D1-selective antagonist SCH23390. The x-axis indicates pretreatment dose of SCH23390 (mg/kg). Vehicle + vehicle (open circles) and vehicle + test compound (filled circles) controls are plotted to the left of the x-axis break. Error bars represent ±SEM.* p<.05 relative to vehicle + test compound control.
MDMA, methylone, and butylone produced submaximal DOM-appropriate responding, whereas pentylone failed to produce any DOM-appropriate responding (Fig. 4, center panel). MDMA (F4,24= 16.15, p<.001), methylone (F4,32= 11.065, p<.001), butylone (F4,24= 7.25, p<.01), and pentylone (F3,24= 22.177, p<.001) reduced response rate in DOM-trained rats. Because each test compound failed to fully substitute for DOM, no antagonism studies were performed.
Methylone (ED50= 2.10 mg/kg, [95% CI= 0.33–13.35]) and butylone (ED50= 2.87 mg/kg, [95% CI= 0.4–20.54]) fully substituted for MDMA, whereas pentylone produced submaximal MDMA-appropriate responding (75%), which was just below the substitution threshold (Fig 4, right panel). Neither methylone nor butylone affected response rate, but testing of higher doses of pentylone was prevented by response-rate disruption at 25 mg/kg (F5,35= 45.775, p<.001). SCH23390 partially attenuated MDMA-appropriate responding by MDMA (43.3%) and methylone (67.6%), but had no effect against butylone or pentylone (Fig. 5, right panel). Like in the methamphetamine-trained rats, SCH23390 dose-dependently attenuated response rate against MDMA (F4,28= 31.537, p<.001), methylone (F5,35= 21.788, p<.001), butylone (F3,21= 18.142, p<.001), and pentylone F4,28= 12.929, p<.001). SCH23390 produced no substitution for either methamphetamine or MDMA (data not shown).
3.3 Self-administration
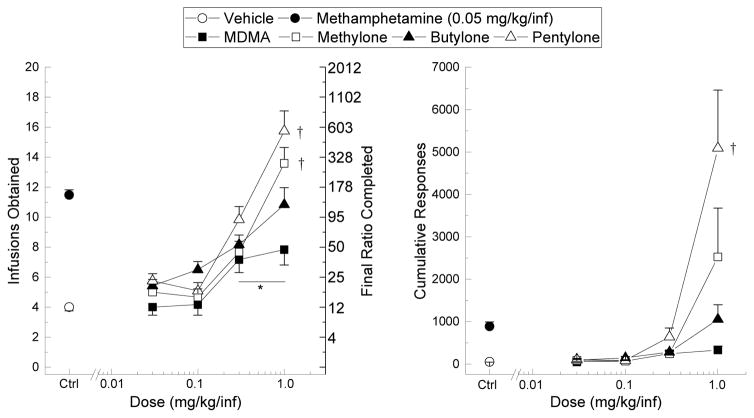
As shown in fig. 6, Each drug dose-dependently increased the total infusions self-administered relative to vehicle with main effects of drug (F3,44= 6.171, p=. 001), dose (F4,176= 116.238, p<. 001), and a significant dose by drug interaction (F12,176= 5.113, p< .001). A similar effect was demonstrated with cumulative responses emitted with main effects of drug (F3,44= 5.467, p= .003), dose (F4,176= 22.15, p< .001) and a significant dose by drug interaction (F12,176= 5.225, p<.001). Individual One-Way Analyses of Variance carried out against each compound determined that MDMA (F4,4= 18.703, p<.001), methylone (F4,44= 39.689, p<.001), butylone (F4,44= 20.75, p<.001), and pentylone (F4,44= 42.452, p<.001) produced significant increases in self-administration and responding relative to vehicle at the 0.3 and 1 mg/kg/inf doses. Secondary analyses with a One-Way Analysis of Variance (fig. 6) indicated significant between group differences at 1 mg/kg/inf in terms of infusions obtained (F3,44= 8.995, p< .001) and responses emitted (F3,44= 5.328, p= .003). According to a Tukey’s honestly-significant-difference test, pentylone and methylone, but not butylone, were self-administered to a greater degree than MDMA at 1 mg/kg/inf. Higher doses were not tested due to adverse effects (exophthalmos, drooling, stereotypy).
Fig. 6. Self-Administration.
Number of infusions obtained (left) and total responses emitted (right) by rats self-administering MDMA (filled squares), methylone (open squares), butylone (filled triangles), or pentylone (open triangles). Vehicle (open circles) and methamphetamine (filled circles) controls are plotted to the left of the x-axis break. Error bars represent ±SEM. * p<.05 relative to vehicle control. † p<.05 relative to MDMA at 1.0 mg/kg/inf.
4. Discussion
The electrophysiological approach utilized in the present study provides an alternative methodology for addressing the transporter mechanisms of these compounds. Each of the synthetic cathinones functioned as a transporter substrate, given the inward currents produced when applied to DAT and SERT similar to MDMA (DeFelice et al., 2014; Sitte et al., 1998). Furthermore, these data provide evidence for a structure-activity relation in which the α-alkyl-side-chain length is inversely associated with transporter-gating efficacy. At both 10 μM and 100 μM, which represent the mid- and upper-bounds of physiological brain concentrations, respectively, for each compound (Grecco et al., 2017), there is a significant decrease in activation with each additional hydrocarbon moiety, indicating that the side-chain length is an important determinant of transporter-gating activity.
Each compound tested demonstrated a slow dissociation from SERT and a prolonged inward current persisting long after drug-application cessation. Similar effects have been demonstrated previously with methcathinone and mephedrone at the dopamine transporter (Cameron et al., 2013a; 2013b). These data suggest that MDMA and the synthetic cathinones produce persistent leak currents at the serotonin transporter in a manner comparable to the effects seen with amphetamine derivatives at the dopamine transporter (Rodriguez-Menchaca et al., 2012). As the compounds tested here demonstrated rapid dissociation from DAT with no persistent current, it is possible that the addition of the methylenedioxy moiety alters the pharmacodynamics of cathinone derivatives at DAT relative to the unsubstituted or minimally-substituted analogs. Although a precedent exists for the robust, persistent leak current seen with the synthetic cathinones, previous electrophysiological evaluations of MDMA at SERT (Hilber et al., 2005) indicated a fairly-slow dissociation, but not the persistent leak current seen in the current data. Future evaluations of the electrophysiological effects of MDMA and the synthetic cathinones at SERT using longer recording times, different cell types, and intracellular drug application are necessary to confirm kinetic differences among these compounds and evaluate whether amphetamine analogs produce a comparable persistent leak current at SERT as at DAT.
Previous studies utilizing in vitro radiolabeled transmitter uptake and release assays to evaluate both substrate and uptake-inhibition properties of these compounds have yielded somewhat inconsistent results as to the exact mechanism by which these compounds increase synaptic monoamine concentrations. Methylone has been described as both a dopamine and serotonin reuptake inhibitor (Simmler et al., 2013) and a DAT and SERT substrate (Eshleman et al., 2013; Baumann et al., 2012), and pentylone has similarly been classified as a dopamine and serotonin reuptake inhibitor (Eshleman et al., 2017) and a hybrid compound producing dopamine uptake inhibition with weak SERT substrate activity (Simmler et al., 2014). The data from the current study indicate that methylone and pentylone both act as transporter substrates, and that pentylone is weaker-efficacy substrate at both transporters than methylone. Conversely, butylone consistently demonstrates hybrid dopamine uptake inhibition and SERT substrate activity in the previous literature (Simmler et al., 2013; Eshleman et al., 2013); however, the current data indicate butylone is a substrate at both transporters with very weak efficacy at DAT. Each cathinone compound was less efficacious at producing current relative to MDMA at either transporter, which my indicate that the transporter blocking effects of these compounds may be more important in determining their behavioral effects.
The drug discrimination data collected in this study complement and expand upon our in vitro data and indicate that methylone and butylone have a complex, MDMA-like, dopaminergically- and serotonergically-mediated discriminative stimulus, whereas pentylone has a primarily dopaminergic discriminative stimulus. Each of the synthetic cathinones tested had comparable potencies, but were slightly less potent than the training doses of MDMA or methamphetamine (ED50 values between 2–5 fold higher than the training doses). The substitution of each test compound for methamphetamine replicates previous findings from our laboratory (Gatch et al., 2013; 2015) and provides evidence for a dopaminergic component to the discriminative stimulus of each drug. The discriminative stimulus effects of methylone have previously been assessed elsewhere (Dal Cason et al., 1997), and our substitution data replicate those findings in amphetamine- and MDMA-trained rats; however, we demonstrated modest, submaximal substitution in DOM-trained rats, where they found none, an effect potentially resulting from their higher (1 mg/kg) DOM training dose. Furthermore, the differential substitution patterns in DOM- and MDMA-trained rats indicate divergent mechanisms among the compounds in terms of their serotonergic efficacy.
The methamphetamine data potentially indicate a stronger dopaminergic phenotype of methylone and pentylone relative to MDMA and butylone, given that methylone and pentylone substituted at doses that produced no rate disruption, whereas MDMA and butylone significantly attenuated response rate, an effect potentially mediated by the relatively greater efficacy of serotonin versus dopamine release by MDMA (Bauman et al., 2012). Although each compound substituted for methamphetamine with roughly similar potency, differences in dopaminergic efficacy among the compounds emerged after pretreatment with the D1-selective antagonist SCH23390, as higher SCH23390 doses were required to attenuate the methamphetamine-DAR of pentylone relative to other compounds. Given the D1-dependent nature of methamphetamine’s discriminative stimulus, the dose-dependent attenuation of methamphetamine-DAR in SCH23390-pretreated rats was anticipated. Because of the similarities in antagonism curves among MDMA, methylone, and butylone, we hypothesized that the increased sensitivity of methylone and butylone to SCH23390 relative to pentylone was mediated by unmasking of these compounds’ serotonergic effects, which is supported by the differential DAT and SERT activation from our electrophysiology data. D1-receptor blockade in rats treated with methylone or butylone may have shifted the balance of serotonin and dopamine towards a predominately serotonergic mechanism, thereby reducing the methamphetamine-like stimulus of these compounds. Similarly, a more prominent dopaminergic and limited serotonergic stimulus of pentylone would likely be able to surmount D1 blockade up to the point of saturation without unmasking additional transmitter systems that may contribute to its mechanism and discriminative stimulus.
The submaximal substitution for DOM and full substitution for MDMA by methylone and butylone provide further evidence for a potential serotonergic mechanism of these compounds relative to a predominately dopaminergic pentylone and are in line with the inverse relation between side-chain length and SERT activation. Although the synthetic cathinones have been reported, in some instances, to produce hallucinogenic effects (Vazirian et al., 2015), it is likely that the lack of full substitution in DOM resulted from indirect serotonin agonism, given that DOM is selective for 5HT2 receptors. Binding and functional assays have implicated 5HT2A receptors in the mechanism of MDMA, but similar reports for the synthetic cathinone derivatives indicate minimal affinity for and efficacy at any of the 5HT2 receptor subtypes relative to MDMA (Simmler et al., 2013; 2014; Eshleman et al., 2013). Thus, it seems likely that any DOM-like responding produced by methylone and butylone resulted from indirect 5HT2 receptor activation. Additionally, the MDMA (1.5 mg/kg) training dose used in these studies is particularly sensitive to serotonergic effects of novel test compounds, but can detect dopaminergic stimuli as well, typically evidenced as submaximal MDMA-appropriate responding (Webster et al., 2017). Given that methylone and butylone fully substituted for MDMA with no decrements in response rate, whereas pentylone substituted only partially, we can conclude that methylone and butylone produce complex discriminative stimulus effects mediated by both dopamine and serotonin whereas pentylone is predominately dopaminergic. Furthermore, butylone’s roughly two-fold higher potency when substituting for MDMA than methamphetamine suggests a greater serotonergic component to its discriminative stimulus, as more drug was necessary to elicit dopaminergic, methamphetamine-appropriate responding. However, given that SCH23390 failed to attenuate MDMA-appropriate responding by pentylone, it seems likely that D1-blockade may produce an opposite effect to what was seen in the methamphetamine substitution by unmasking the serotonergic effects of pentylone, which is supported by the modest SERT activation in the electrophysiological studies.
The currently-available data regarding the reinforcing nature of synthetic cathinones as a class demonstrate substantial variability among these compounds, illustrating the necessity for determining whether the differences in discriminative stimulus effects among the synthetic cathinone analogs of MDMA translate into differences in abuse potential. The current study demonstrated that butylone can serve as a reinforcer in rats and provided additional evidence for the reinforcing properties of methylone and pentylone. The dose-dependent increases in breakpoint under a progressive ratio schedule of reinforcement demonstrated by each compound further illustrate the reinforcing efficacy of these drugs by maintaining responding under conditions of progressively increasing effort requirements. At the highest dose tested, pentylone was administered to a greater degree than MDMA and butylone, and methylone was self-administered more than MDMA. These data indicate that, among the compounds tested, pentylone likely engenders the greatest potential for compulsive use, whereas the reinforcing effects and abuse liability of butylone are comparable to MDMA. The reinforcing effects of methylone lay between butylone and pentylone, but did not statistically differ from either cathinone, making strong conclusions about its relative reinforcing efficacy and abuse potential difficult to draw. Due to the onset of adverse effects with each compound tested (drooling, exophthalmos, stereotypy) at 1.0 mg/kg/inf, higher doses were not tested, preventing the establishment of both ascending and descending limbs of the dose-response curve. A previous study of methylone self-administration under comparable conditions (Gannon et al., 2017) demonstrated that peak responding occurs at 1.0 mg/kg/inf, with higher doses falling on the descending limb of the curve. Given the large differences in responding at 1.0 mg/kg/inf and the onset of adverse effects potentially representing overdose conditions, there appear to be true differences in reinforcing efficacy among drugs as opposed to simply differences in potency; however, the lack of a descending limb of the dose-response should be considered when interpreting the relative reinforcing effects among drugs.
Previous methylone self-administration studies have produced somewhat inconsistent results, with some data indicating limited reinforcing efficacy (Schindler et al., 2016; Vandewater et al., 2015), and others demonstrating fairly-robust self-administration (Gannon et al., 2017; Watterson et al., 2013). The current data suggest that methylone is a strong reinforcer that readily engenders self-administration. Given that our methodology most closely approximates the study by Gannon et al. (2017) in terms of sex, strain, ratio requirement, and dose-range, which demonstrated comparably high self-administration, it is possible that the differences among studies stem from differences in methodology such as strain (Vandewater et al., 2015), ratio requirement, and dose-range (Schindler et al., 2016). Another, recent investigation into the relative reinforcing effects of methylone and pentylone indicated significantly greater self-administration of pentylone relative to methylone (Javadi-Paydar et al., 2017). The previous study differed from ours in that the subjects were female Wistar rats, the monoamine uptake inhibitor pentedrone was used as the training drug, and self-administration occurred according to a continuous schedule of reinforcement. It is possible that sex and strain differences contributed to the differential efficacy patterns between studies, and the same lab has indicated that the reinforcing efficacy of the training drug used can influence the self-administration pattern in substitution studies (Vandewater et al., 2015). Furthermore, the schedule of reinforcement differed between studies, potentially contributing to the differences in both potency and relative efficacy. Further studies controlling for the sex, strain, and training-drug differences are warranted for an exploration into factors contributing to the reinforcing efficacy of these compounds.
Butylone’s relatively limited and methylone’s ambiguous reinforcing effects likely arise from their SERT substrate or serotonin-releasing properties and differential DAT substrate efficacy. Compounds selective for SERT over DAT are dramatically less reinforcing relative to their more dopaminergic counterparts in rhesus monkeys (Wee et al., 2005; Wee & Woolverton, 2006), and the reinforcement-limiting nature of serotonin release is also apparent in MDMA self-administration as rats lacking SERT acquire MDMA self-administration more rapidly and exhibit a higher breakpoint under a progressive ratio schedule of reinforcement than wild-type rats (Oakly et al., 2014). Although methylone had the in vitro mechanism and discriminative stimulus effects most comparable to MDMA, methylone resulted in a substantially greater breakpoint relative to MDMA. Because methylone, like MDMA, is a fairly-robust DAT/SERT substrate, it is possible that the reduced efficacy of methylone at SERT contributed to its greater reinforcing efficacy relative to MDMA. Butylone had unique effects at both transporters by producing very little inward current at DAT and a robust current at SERT. Previous work has suggested that “hybrid” compounds that function as dopamine uptake inhibitors and SERT substrates have limited abuse liability as the impulse-independent serotonin release inhibits the reinforcing effects of the amplified, impulse-dependent synaptic dopamine (Blough et al., 2014). Although the current data indicate butylone acts as a DAT substrate, it is a very weak-efficacy DAT substrate, potentially making its DAT effects analogous to an uptake inhibitor by preventing dopamine uptake without substantial impulse-independent dopamine release, while still producing substantial effects at SERT. Pentylone, conversely, produces more dopaminergic discriminative stimulus effects and is a fairly weak DAT substrate and a modestly-efficacious SERT substrate. Pentylone acts as a transporter blocker and is more selective for DAT over SERT than the other cathinones tested in the present study; thus its potentially limited serotonergic relative to dopaminergic efficacy may underlie its robust self-administration.
5. Conclusions
At this juncture, we can conclude that the drugs tested in the current study are likely to engender different use patterns, with methylone and butylone being used episodically like MDMA, whereas pentylone would more likely lead to compulsive use, like cocaine or other stimulant-type drugs. These data also provide insight into structure-activity relations of synthetic cathinones and implicate the alpha-carbon side chain in their relative dopaminergic and serotonergic efficacy. These results are particularly concerning giving the inadvertent use of these compounds in “Molly” formulations, given that “Molly” users typically believe they are consuming pure MDMA (Palamar et al., 2016b). Formulations containing butylone, and possibly methylone, are less likely to lead to uncontrolled use, although their potential toxicities are not to be dismissed; however, those containing pentylone may cause compulsive use of “Molly” or “Ecstasy,” either in the form of excessive re-dosing in acute settings, or more regular episodic use, potentially serving as an avenue for experimenting with more traditional and highly-addictive psychostimulants.
MDMA, methylone, butylone, and pentylone are serotonin transporter substrates
Each drug produces methamphetamine-like discriminative stimulus effects
Methylone and butylone produce largely serotonergic discriminative stimulus effects
Pentylone produces predominately dopaminergic discriminative stimulus effects
Pentylone is self-administered to greater degree than butylone or MDMA
Acknowledgments
Funding: This work was supported by the National Institute on Drug Abuse [contract N01DA-13-8908, to MG], the National Institute on Aging [training grant T32AG020494, to SD]; the Texas Alzheimer’s Research and Care Consortium Investigator Grant Program (TARCC354528, to RH), and William and Ella Owens Medical Research Foundation (RN20024 to RH).
The authors would like to acknowledge Cynthia Taylor for excellent technical assistance in performing the surgeries for the self-administration experiments.
Footnotes
Abbreviations: β-keto-N-methylbenzodioxolylbutanamine (Butylone); dopamine transporter (DAT); drug-appropriate responding (DAR); 2,5-dimethoxy-4-methylamphetamine (DOM); 3,4-methylenedioxy-N-methamphetamine (MDMA); 3,4-methylenedioxy-N-methylcathinone (Methylone); β-Keto-N-methylbenzodioxolylpentanamine (Pentylone); 7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol (SCH23390); serotonin transporter (SERT); standard error of the mean (SEM)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Partilla JS, Baumann MH, Decker AM, Page KM, Rothman RN. Hybrid dopamine uptake blocker-serotonin releaser ligands: A new twist on transporter-focused therapeutics. ACS Med Chem Lett. 2014;5:623–627. doi: 10.1021/ml500113s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath slats components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013a;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 2013b;227:494–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97:20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecstasydata.org. [accessed on March 16, 2017];Test Results. 2017 http://www.Ecstasydata.org/results.php.
- Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243:55–60. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky AJ. Structure-activity relationships of substituted cathinones, with transporter binding, uptake and release. J Pharmacol Exp Ther. 2017;360:33–47. doi: 10.1124/jpet.116.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT. Reinforcing effects of binary mictures of common bath salt constituents: studies with 3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylendioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.141. https://doi.org/10.1038/npp.2017.141. [DOI] [PMC free article] [PubMed]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology. 2015;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav Pharmacol. 2016;27:497–505. doi: 10.1097/FBP.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AK, Baker LE. A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (+/−)-MDMA in rats. Exp Clin Psychopharmacol. 2000;8:415–423. doi: 10.1037//1064-1297.8.3.415. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE. Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a three-choice discrimination. Pharmacol Biochem Behav. 2003;74:987–995. doi: 10.1016/s0091-3057(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Grecco GG, Kisor DF, Magura JS, Sprague JE. Impact of common clandestine structural modifications on synthetic cathinone “bath salt” pharmacokinetics. Toxicol Appl Pharmacol. 2017;328:18–24. doi: 10.1016/j.taap.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Harper DN, Langen AL, Schenk S. A 3-lever discrimination procedure reveals differences in the subjective effects of low and high doses of MDMA. Pharmacol Biochem Behav. 2014;116:9–15. doi: 10.1016/j.pbb.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.09.002. https://doi.org/10.1016/j.neuropharm.2017.09.002. [DOI] [PMC free article] [PubMed]
- National Research Council. Guide for the care and use of laboratory animals. 8. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Oakly AC, Brox BW, Schenk S, Ellenbroek BA. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry. 2014;19:534–535. doi: 10.1038/mp.2013.75. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Acosta P, Ompad DC, Cleland CM. Self-reported ecstasy/MDMA/“Molly” use in a sample of nightclub and dance festival attendees in New York City. Subst Use Misuse. 2016a;52:82–91. doi: 10.1080/10826084.2016.1219373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Vincenti M, Cleland CM. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/”Molly” users. Drug Alcool Depend. 2016b;161:200–205. doi: 10.1016/j.drugalcdep.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ. There’s something about Molly: The underresearched yet popular powder form of ecstasy in the United States. Subst Abus. 2017;38:15–17. doi: 10.1080/08897077.2016.1267070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentney AR. An exploration of the history and controversies surrounding MDMA and MDA. J Psychoactive Drugs. 2001;33:213–221. doi: 10.1080/02791072.2001.10400568. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Menchaca AA, Solis E, Jr, Cameron K, De Felice LJ. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br J Pharmacol. 2012;165:2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleemi S, Pennybaker SJ, Wooldridge M, Johnson MW. Who is “Molly”? MDMA adulterants by product name and the impact of harm-reduction services at raves. J Psychopharmacol. 2017;31:1056–1060. doi: 10.1177/0269881117715596. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Discriminative profile of MDMA. Pharmacol Biochem Behav. 1986;24:1533–1537. doi: 10.1016/0091-3057(86)90480-6. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) Phamacol Biochem Behav. 1988;31:817–824. doi: 10.1016/0091-3057(88)90390-5. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233:1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) Global Synthetic Drugs Assessment: Amphetamine-Type Stimulants and New Psychoactive Substances. United Nations; New York, New York: 2014. [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 2015;99:538–545. doi: 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazirian M, Jerry JM, James J, Dale RM. Bath salts in the emergency department: a survey of emergency clinicians’ experience with bath salts-intoxicated patients. J Addict Med. 2015;9:94–98. doi: 10.1097/ADM.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Warrick BJ, Wilson J, Hedge M, Freeman S, Leonard K, Aaron C. Lethal serotonin syndrome after methylone and butylone ingestion. J Med Toxicol. 2012;8:65–68. doi: 10.1007/s13181-011-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The Reinforcing and Rewarding Effects of Methylone, a Synthetic Cathinone Commonly Found in “Bath Salts”. J Addict Res Ther Suppl. 2012;9:002. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JI, Harper DN, Schenk S. Generalization of serotonin and dopamine ligands to the discriminative stimulus effects of different doses of +/−3,4-methylenedioxymethamphetamine. Behav Pharmacol. 2017;28:245–254. doi: 10.1097/FBP.0000000000000282. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolvertoon WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–43. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]