Abstract
Lagging behind rapid changes to state laws, societal views, and medical practice is the scientific investigation of cannabis’s impact on the human brain. While several brain imaging studies have contributed important insight into neurobiological alterations linked with cannabis use, our understanding remains limited. Here, we sought to delineate those brain regions that consistently demonstrate functional alterations among cannabis users versus non-users across neuroimaging studies using the activation likelihood estimation meta-analysis framework. In ancillary analyses, we characterized task-related brain networks that co-activate with cannabis-affected regions using data archived in a large neuroimaging repository, and then determined which psychological processes may be disrupted via functional decoding techniques. When considering convergent alterations among users, decreased activation was observed in the anterior cingulate cortex, which co-activated with frontal, parietal, and limbic areas and was linked with cognitive control processes. Similarly, decreased activation was observed in the dorsolateral prefrontal cortex, which co-activated with frontal and occipital areas and linked with attention-related processes. Conversely, increased activation among users was observed in the striatum, which co-activated with frontal, parietal, and other limbic areas and linked with reward processing. These meta-analytic outcomes indicate that cannabis use is linked with differential, region-specific effects across the brain.
Keywords: Cannabis, marijuana, activation likelihood estimation (ALE) meta-analysis, functional magnetic resonance imaging (fMRI), anterior cingulate cortex, dorsolateral prefrontal cortex (DL-PFC), striatum, cognitive control, reward processing, pain
Introduction
Cannabis, the flowering plant genus that includes three species, C. sativa, C. indica, and C. ruderalis, contains psychotropic and non-psychotropic compounds, including delta-9-tetrahydracannabinol (THC) and cannabidiol (CBD). These compounds interact with endogenous cannabinoid receptors distributed throughout the central nervous system, with particularly high receptor densities found in the thalamus, cingulate cortex, and primary and supplementary motor areas (Freund et al., 2003; Howlett et al., 2002). In 2015, there were an estimated 22.2 million past-month cannabis users in the United States (US), a number expected to continue rising in the coming years (Substance Abuse and Mental Health Services Administration, 2016). As of September 2017, 29 states within the US and the District of Columbia have enacted legislation that permits cannabis use for the treatment of various medical conditions, with several states even permitting recreational use. Lagging behind these rapid changes to state laws, as well as societal views and medical practice, is insight into the cognitive, behavioral, and neurobiological consequences of cannabis use. Deeper understanding of cannabis’s impact on brain functioning is important for informed decision-making about its use.
Functional neuroimaging modalities – including functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) – provide a means to examine the effects of substance use and abuse on the human brain (Jia et al., 2011; Sutherland et al., 2015, 2016; Volkow et al., 2013). Regarding cannabis, some insights into region-specific effects are beginning to emerge. Specifically, previous studies suggest attenuated activity among users in brain regions supporting executive functions, such as cognitive control, error processing, and learning, yet enhanced activity in regions supporting reward processing. For example, Hester et al. (2009) examined the neural correlates of error processing among cannabis users employing a modified Go/No-Go response inhibition task. Those authors observed behavioral deficits in error awareness (but not error commission) among users versus non-users, and that more severe reductions in anterior cingulate cortex (ACC) activation were associated with greater error awareness deficiencies. Similarly, Jager et al. (2007) documented reduced activation in the dorsolateral prefrontal cortex (DL-PFC), among other regions, in abstinent cannabis users completing an associative memory paradigm, such that cannabis-related reduced activation within prefrontal regions was more pronounced during associative learning relative to retrieval task phases. In another example, Nestor et al. (2010) used a monetary incentive delay paradigm to examine reward anticipation and evaluation among cannabis users. Those researchers reported increased ventral striatum responding during reward anticipation among users versus non-users. Importantly, cannabis-use patterns (i.e. lifetime joints consumed) were positively correlated with increased striatal activation, providing evidence for heightened sensitivity to reward-predictive stimuli with increasing exposure. Moving forward, an important challenge facing the field is to synthesize findings from functional neuroimaging studies on cannabis use to establish aggregate patterns of neurobiological changes associated with non-acute exposure.
Towards this goal, several narrative reviews have sought to summarize the neural, cognitive, and behavioral consequences of cannabis use (Bhattacharyya et al., 2012a; Bhattacharyya and Sendt, 2012; Bossong et al., 2014; Kowal et al., 2013; Martin-Santos et al., 2010; Wrege et al., 2014). En masse, these reviews provide variant yet complimentary perspectives. Specifically, some reports have advocated that cannabis use has detrimental effects on cognitive processes, such as attention and decision-making (Bhattacharyya et al., 2012a; Martin-Santos et al., 2010; Wrege et al., 2014), which have been ascribed to decreased activation within frontal and prefrontal regions, including the ACC (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Hester et al., 2009) and DL-PFC (Bolla et al., 2005). Others have focused on salience processing and impulsiveness among cannabis users, highlighting enhanced activation within reward circuits (Bhattacharyya et al., 2012a; Wrege et al., 2014), including the striatum (Bhattacharyya et al., 2012b). Despite these high-level interpretations, results from specific studies have been inconsistent, with several reports suggesting discordant outcomes (Volkow et al., 2016). For example, Gruber et al. (2012) used a multi-source inference task to examine impulsive-behavior inhibition among cannabis users. Using a region-of-interest (ROI) approach, those authors identified increased ACC activation among users, despite comparable behavioral performance to non-using controls. In another example, Martz et al. (2016) demonstrated decreased striatal responsiveness among young adult users during reward anticipation relative to controls in a monetary incentive delay task. These variant, and at times contradicting outcomes may not be surprising when one considers the limited, yet growing corpus of functional neuroimaging studies that have examined the non-acute effects of cannabis (e.g. long-term recreational use) on brain function. Accordingly, unbiased procedures that assess statistical convergence across published neuroimaging studies are needed to determine which brain regions most consistently show cannabis-related alterations.
Neuroimaging meta-analytic tools present opportunities to quantitatively synthesize and more fully interpret published neuroimaging results. As such, we sought to determine which brain regions most consistently demonstrate functional alterations among cannabis users (across various neuroimaging tasks) by examining published findings within the activation likelihood estimation (ALE) framework (Eickhoff et al., 2009; Laird et al., 2005; Turkeltaub et al., 2002). To do so, we first identified studies reporting functional brain differences between cannabis users and non-users using fMRI or PET in the context of cognitive, social cognitive, affective, perceptual, and motor tasks. This holistic approach permitted the consideration of an expanded experiment assortment, enhancing statistical power, and revealing cannabis-related neurobiological effects irrespective of ontological task classifications (Sutherland et al., 2015). In a primary assessment, we identified convergent cannabis-related activity decreases (users < non-users) and increases (users > non-users) across functional neuroimaging studies. In a first ancillary assessment, we then determined the brain networks in which cannabis-affected regions were embedded using meta-analytic connectivity modeling (MACM). MACM delineates those brain areas that tend to co-activate with user-specified ‘seed’ regions across various task classifications, revealing potential network-level targets of drug-related effects. In a second ancillary assessment, we aimed to provide enhanced insight into brain–behavior relationships. Specifically, we performed a functional decoding assessment using data from a large repository of published neuroimaging coordinates and associated meta-data terms (BrainMap, http://www.brainmap.org) to characterize psychological processes potentially linked to functional alterations among cannabis users via quantitative forward- and reverse-inference analyses. Here, we use the term ‘functional decoding’ to represent the statistical approaches used to determine which psychological processes (e.g. working memory) are implicated given the observed meta-analysis results. This approach provides objective interpretations about the potential behavioral consequences of the convergent neurobiological alterations associated with cannabis use. We anticipated that users would demonstrate region-specific differential activation relative to non-users across brain regions. Moreover, we expected these neurobiological alterations to be associated with expansive task-based neural networks, and to correspond with distinct psychological constructs and processes. Clarifying cannabis’s effects on specific brain regions, delineating broader brain networks potentially impacted, and characterizing psychological processes that may be disrupted among users may provide a more complete and coherent understanding of the health-related impacts of cannabis.
Methods and materials
Literature search and selection
We conducted a comprehensive literature search to locate neuroimaging studies that reported brain activation differences between cannabis users and non-users. Primary searches were conducted using PubMed (www.ncbi.nlm.nih.gov/pubmed) and Web of Science (http://webofknowledge.com) with the search terms: MRI OR magnetic resonance imaging OR fMRI OR functional magnetic resonance imaging OR PET OR positron emission tomography OR neuroimaging AND cannabis OR marijuana. To expand our assemblage, we then reviewed the reference sections from the papers identified in the primary search as well as from several narrative reviews (Bhattacharyya et al., 2012a, 2012b; Bhattacharyya and Sendt, 2012; Bossong et al., 2014; Chang and Chronicle, 2007; Kowal et al., 2013; Martin-Santos et al., 2010; Wrege et al., 2014).
The current meta-analysis included studies that: (1) described between-group brain differences between cannabis users and non-users, (2) used fMRI and/or PET measures to derive these differences, (3) expressed differences as statistical parametric contrasts, encompassing the whole brain, with coordinates reported in standard stereotaxic space, and (4) utilized cognitive, social cognitive, affective, perceptual, and/or motor tasks. As a consequence, studies that assessed anatomical differences (e.g. voxel-based morphometry), functional resting-state differences, functional connectivity differences, and/or findings derived using ROI methods were excluded. Moreover, the current meta-analysis was limited to contrasts between cannabis users and non-users, omitting contrasts involving groups specifically selected for having mental health disorders and diseases. Published papers that examined one or more clinical groups (e.g. schizophrenia, bipolar disorder) were carefully reviewed using the inclusion/exclusion criteria. We note that although some studies reported results from several group-level comparisons (e.g. schizophrenia patients with cannabis-use histories > non-schizophrenia patients with cannabis-use histories > controls), coordinate extraction was restricted to just those comparisons between otherwise normal users and non-users (e.g. non-schizophrenia patients with cannabis-use histories > controls). In addition, pharmacologic assessments characterizing acute cannabis administration were not considered. This meta-analysis reflects papers published through December 2016.
Coordinates that expressed decreased activation were taken from contrasts that reported attenuated activation among cannabis users compared with non-users (cannabis users < non-users), while increased activation coordinates were taken from contrasts that reported enhanced activation among users compared with non-users (cannabis users > non-users). Considering decreased and increased activation across included studies is common among neuroimaging meta-analyses (Cortese et al., 2016; Dehghan et al., 2016; Etkin and Wager, 2007; Radua et al., 2012). This approach can produce detailed interpretations about neurobiological differences reported across sampled studies. Moreover, by incorporating coordinates across task classifications (Sutherland et al., 2015), we allowed our meta-analytic outcomes to represent more general aspects of task-based processing. That is, results reported here may represent cannabis-related functional brain changes that supersede task-specific and domain-specific demands, revealing neurobiological consequences that are not constrained by variable neuroimaging ontologies and methodologies.
Primary assessment: ALE meta-analysis
To establish which brain regions showed consistent cannabis-related changes across functional neuroimaging studies, we conducted two coordinate-based ALE meta-analyses using GingerALE (version 2.3.6; http://brainmap.org/ALE/), which implements an updated version of the ALE algorithm (Eickhoff et al., 2009, 2012). ALE meta-analysis is used to assess statistical convergence across results from reported neuroimaging studies (Turkeltaub et al., 2002), producing quantitative outcomes that are otherwise unobtainable using more traditional narrative review approaches (Laird et al., 2005). In ALE, activation coordinates (foci) are modeled as centers of three-dimensional Gaussian probability distributions to account for uncertain variance associated with functional neuroimaging experiments. The extent of the aforementioned distribution is weighted using the given experiment’s sample size. The GingerALE software computes one modeled brain activation map per experiment, and then aggregates these maps to compute spatial convergence. The resulting ALE map provides statistical measures, via the ALE statistic, for every voxel in the brain, characterizing the extent to which that voxel is implicated in a given dataset. First, we assembled coordinates of cannabis-related decreased and increased activation into a database along with associated experimental characteristics as described elsewhere (detailed description of BrainMap taxonomy and workflows provided in Laird et al., 2009a) (Figure 1). This included relevant meta-data, including: experiment sample size, mean subject age, sex distribution, time since last cannabis-use episode, mean lifetime cannabis-use episodes, and task classification or paradigm used. Coordinates that were reported in Montreal Neurological Institute (MNI) space were transformed to Talairach space (Lancaster et al., 2007). These coordinates were then used to compute two distinct ALE meta-analysis maps, revealing clusters of convergent cannabis-related decreased activation and increased activation (pcluster-corrected < 0.01; pvoxel-level < 0.001) (Eickhoff et al., 2016). When considering decreased activations, clusters >3600 mm3 survived cluster-level thresholding, while for increased activations, clusters >2960 mm3 survived thresholding. We note that one inherent limitation of ALE meta-analysis is that the approach cannot consider null results (i.e. no observed activation differences between users and non-users). Thus, included studies do not represent the complete corpus of available cannabis-related neuroimaging studies, but rather the corpus of available studies that reported activation differences between users and non-users. Resultant meta-analytic clusters were reported as coordinates in Talairach space. Extrema labels were determined via the Talairach Daemon application (Lancaster et al., 1997, 2000). To facilitate future research, ROIs created using resultant meta-analytic clusters have been made available via NeuroVault (http://neurovault.org/collections/2508).
Figure 1.
Meta-analytic data pipeline. Schematic illustration of the meta-analytic tools employed to identify convergent functional alterations among cannabis users and provide enhanced interpretation of such alterations. Step 1 (literature search): Published papers that reported functional brain alterations among cannabis users relative to non-users were identified. Step 2 (primary meta-analysis): Statistical convergence among reported coordinates was assessed using GingerALE to produce separate maps delineating regions showing decreased activations (users < non-users) and increased activations (users > non-users). Step 3 (connectivity modeling): Using the BrainMap database, MACM was implemented to locate archived neuroimaging experiments that reported co-activations with resultant meta-analytic clusters. Step 4 (functional decoding): Quantitative forward-and reverse-inference analysis techniques were applied to determine which commonly used functional neuroimaging paradigms were associated with cannabis-affected regions.
ALE: activation likelihood estimation; MACM: meta-analytic connectivity modeling.
Ancillary assessment: MACM
To delineate the large-scale brain networks in which cannabis-affected regions were embedded, we used MACM and computed whole-brain co-activation patterns for each resultant ALE meta-analysis cluster. MACM leverages the BrainMap database (www.brainmap.org), an online repository of human neuroimaging studies and associated meta-data, to determine which regions co-activate with a given user-specified seed region (Cauda et al., 2012; Clos et al., 2013; Eickhoff et al., 2011; Robinson et al., 2010, 2012). Results from published functional neuroimaging studies are archived in the database using a rigorous classification scheme and taxonomy that describes peak-activation coordinates in the context of experimental conditions used to derive them (for a complete listing of meta-data terms, please see http://www.brainmap.org/scribe/BrainMapLex.xls). Using Sleuth (version 2.4; www.brainmap.org/sleuth), we searched the database for studies that reported foci within the resulting meta-analysis clusters (i.e. seed regions). Sleuth is an online application that searches the BrainMap database for relevant studies, given user-specified search parameters. Inclusion was limited to databased statistical contrasts that reported activations (i.e. task > baseline) and normal mapping (i.e. non-patient populations), as described in (Robinson et al., 2010, 2012). At the time of analyses, the database contained 116,639 foci from 3026 papers, representing functional neuroimaging data from more than 60,000 subjects. Contrasts that reported coordinates within the seed regions were extracted from the database and assessed for statistical convergence using ALE meta-analysis to establish each cluster’s whole-brain co-activation pattern (pcluster-corrected < 0.01 pvoxel-level < 0.001). MACM maps were computed in Talairach space.
Ancillary assessment: Functional decoding
To quantify potential psychological, physiological, or behavioral processes associated with each meta-analysis cannabis-affected cluster, we used a data-driven forward- and reverse-inference analytic approach that capitalizes on the BrainMap database’s meticulous classification protocol (Cieslik et al., 2013; Laird et al., 2015; Nickl-Jockschat et al., 2015). Forward inference describes the likelihood that a specific volume (e.g. voxel, anatomically-defined region, network) will activate given the recruitment of mental processes (e.g. working memory, fear, finger tapping), while reverse inference describes the likelihood that various mental processes are being recruited given activation within some volume. Together, these techniques provide important information about brain–behavior relationships (Yarkoni et al., 2011). Here, we used intact primary meta-analysis cluster volumes to search the database, effectively circumventing error or bias associated with creating three-dimensional ROIs centered around local maxima. In addition, forward- and reverse-inference analyses were constrained to foci from databased studies that reported activations – as opposed to deactivations – from otherwise normal subjects. Having identified these studies, we examined the distribution of meta-data terms used to catalog them, including various task classifications (e.g. Stroop Task, Monetary Incentive Delay, Flashing Checkerboard). To establish statistical significance, we examined the relations between the representation of meta-data terms contributing to the (a) volume and (b) complete database (Poldrack, 2006; Yarkoni et al., 2011). That is, we used forward inference to examine the relations between the conditional probability of activation (P(Activation|Process)) and the baseline probability of activation (P(Activation)) being observed within each meta-analytic cluster (pFDR-corrected < 0.05). For forward inference, statistical significance was calculated using a binomial test. These measures (i.e. P(Activation), P(Process), and P(Activation|Process)) were then used to calculate reverse inference (P(Process|Activation)) using Bayes’ rule (pFDR-corrected < 0.05). For reverse inference, statistical significance was calculated using a Chi-square test. When considering functional decoding interpretations, we note that subject-level inferences about psychological processes that may be impacted among cannabis users (e.g. working memory) are made using modern machine learning techniques that leverage cross-validation to make out-of-sample generalizations (Bzdok and Yeo, 2017; Yarkoni and Westfall, 2017). These analytic approaches produce models with better predictive value, providing augmented understanding about the relationship between brain regions and associated psychological processes.
Results
Our systematic search yielded 35 peer-reviewed papers meeting inclusion criteria (Table 1; Figure 1). The corpus of published results included 88 task-based statistical contrasts, reporting 202 coordinates representing decreased activation among 472 cannabis users and 466 non-users, as well as 161 coordinates representing increased activation among 482 cannabis users and 434 non-users.
Table 1.
Published papers meeting inclusion criteria. Numbering (no.) corresponds with published papers (reference) meeting specific inclusion criteria. Subjects were cannabis users (n = 647, mean age = 24.0 years) and non-users (n = 625, mean age = 24.3). Extracted variables were cannabis-use patterns (cannabis use), other substance-use patterns (co-use), time since previous use episode (abstinence), and the task classification used (paradigm).
| No. | Reference | Cannabis users
|
Non-users
|
Additional details regarding cannabis users
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age | % M | n | Age | % M | Cannabis use | Co-use | Abstinent | Paradigm | ||
| 1 | Abdullaev et al. (2010) | 14 | 19.5 | 71% | 14 | 19.7 | 71% | 673.2 EPL* | Alcohol | 48 h | Attention |
| 2 | Acheson et al. (2015) | 14 | 17.6 | 76% | 14 | 17.3 | 76% | 6.7 EPW | Alcohol; tobacco | ~12 h | Reward |
| 3 | Behan et al. (2014) | 17 | 16.5 | 94% | 18 | 16.1 | 94% | 4168.1 EPL | Alcohol; tobacco | ~12 h | Go/No-Go |
| 4 | Block et al. (2002) | 18 | N/R | N/R | 13 | N/R | N/R | 7 EPW | Alcohol | 26 h | Working memory |
| 5 | Bolla et al. (2005) | 11 | 26 | 100% | 11 | 31 | 100% | 41 EPW | Alcohol; tobacco | 25 d | Iowa gambling |
| 6 | Carey et al. (2015) | 15 | 22.4 | 73% | 15 | 23.27 | 86% | 7341.4 EPL | Alcohol; tobacco | 101 h | Associate recall |
| 7 | Chang et al. (2006) | 24 | 28.77 | 63% | 19 | 30.57 | 58% | 2709 EPL | Alcohol | 4 h / 38 m | Tracking |
| 8 | Cousijn et al. (2012b) | 32 | 21.4 | 66% | 41 | 22.2 | 63% | 1611.2 EPL | Alcohol; tobacco | 1.6 d | Iowa gambling |
| 9 | Eldreth et al. (2004) | 11 | 25 | 100% | 11 | 29 | 100% | 34.7 EPW | Alcohol; tobacco | 25 d | Stroop |
| 10 | Enzi et al. (2015) | 15 | 26.33 | 100% | 15 | 27.13 | 100% | 13.27 EPW | Alcohol; tobacco | 1.1 d | Monetary incentive delay |
| 11 | Filbey et al. (2013) | 59 | 23.49 | 78% | 27 | 30.32 | 18% | 7467.9 EPL | Alcohol | 72 h | Monetary incentive delay |
| 12 | Gruber et al. (2009) | 15 | 25 | 93% | 15 | 26 | 93% | 25.6 EPW | Alcohol | 12 h | Faces |
| 13 | Heitzeg et al. (2015) | 20 | 19.84 | 60% | 20 | 20.51 | 70% | 618.12 EPL | N/R | 48 h | Emotion elicitation |
| 14 | Hester et al. (2009) | 16 | 24.6 | 94% | 16 | 25.2 | 94% | 11,628 EPL | Alcohol | 38 h | No/No-Go |
| 15 | Jager et al. (2007) | 20 | 24.5 | 65% | 20 | 23.6 | 65% | 1900 EPL | Alcohol; tobacco | 7 d | Encoding |
| 16 | Kanayama et al. (2004) | 12 | 37.9 | 83% | 10 | 27.8 | 60% | 19,200 EPL | N/R | 6-36 h | Perception/working memory |
| 17 | King et al. (2011) | 30 | 21.75 | 53% | 30 | 23.75 | 53% | 9.75 EPW | Alcohol | ~12 h | Finger tapping |
| 18 | Kober et al. (2014) | 20 | 26.65 | 100% | 20 | 29.2 | 100% | 2611.44 EPL* | Tobacco | N/R | Stroop |
| 19 | Lopez-Larson et al. (2012) | 24 | 18.2 | 92% | 24 | 18 | 71% | 1500.6 EPL | N/R | 24–48 h | Finger tapping |
| 20 | Nestor et al. (2008) | 14 | 24.4 | 86% | 14 | 24.1 | 86% | 7925 EPL | Alcohol | 80.8 h | Encoding |
| 21 | Nestor et al. (2010) | 14 | 22.1 | 86% | 14 | 23.1 | 79% | 7256 EPL | Alcohol; tobacco | 108 h | Monetary incentive delay |
| 22 | Padula et al. (2007) | 17 | 18.06 | 82% | 17 | 17.9 | 71% | 477.06 EPL | Alcohol | 28 d | N-back |
| 23 | Pillay et al. (2008) | 11 | 37.7 | 36% | 16 | 29.7 | 63% | 17,637 EPL | N/R | 28 d | Finger tapping |
| 24 | Pillay et al. (2004) | 9 | 37.3 | 89% | 16 | 29.4 | 63% | 16,711.5 EPL | N/R | 4-36 h | Finger tapping |
| 25 | Riba et al. (2015) | 16 | N/R | N/R | 16 | N/R | N/R | 42,000 EPL | Tobacco | N/R | Working memory |
| 26 | Roser et al. (2012) | 15 | 26.5 | 100% | 14 | 27.3 | 100% | 13.1 EPW | Tobacco | 25.1 h | Theory of mind |
| 27 | Schweinsburg et al. (2008) | 15 | 18.1 | 73% | 17 | 17.9 | 70% | 480 EPL | Alcohol; tobacco | 60.4 d | N-back |
| 28 | Smith et al. (2010) | 10 | 20 | 60% | 14 | 20 | 64% | 2697 EPL | Alcohol; Tobacco | N/R | N-back |
| 29 | Sneider et al. (2013) | 10 | 20.3 | 80% | 18 | 22.8 | 61% | 2268.4 JPL* | N/R | 12 h | Morris water maze |
| 30 | Tapert et al. (2007) | 16 | 18.1 | 75% | 17 | 17.9 | 71% | 475.6 JPL | Alcohol; tobacco | 28 d | Go/No-Go |
| 31 | Vaidya et al. (2012) | 46 | 24.32 | 61% | 34 | 24.72 | 53% | 589.92 JPL | Alcohol; tobacco | 24-29 h | Iowa gambling |
| 32 | Van Hell et al. (2010) | 14 | 24 | 93% | 13 | 24 | 85% | 3841 JPL | Alcohol; tobacco | 7 d | Monetary incentive delay |
| 33 | Wesley et al. (2011) | 16 | 26.4 | 56% | 16 | 26.6 | 38% | 29.4 D/M | Alcohol | 12 h | Iowa gambling |
| 34 | Wesley et al. (2016) | 17 | 25.1 | 53% | 16 | 27.1 | 31% | 8, 466.528 EPL* | Tobacco | ~12 h | Emotion elicitation |
| 35 | Yip et al. (2014) | 20 | 26.7 | 100% | 20 | 29.2 | 100% | N/R | Tobacco | 21 d | Monetary incentive delay |
d: days; D/M: days per month; EPL: reported episodes per lifetime; EPL*: estimated episodes per lifetime; EPW: episodes per week; h: hours; JPL: joints per lifetime; m: months; %M: percent of participant sample that was male; N/R: not reported.
Cannabis-related functional alterations
Regarding the neurobiological impact of cannabis use, our primary meta-analytic assessments revealed convergent decreased and increased activation among cannabis users versus nonusers across neuroimaging studies (Table 2; Figure 2). Specifically, clusters of convergent decreased activation were observed among cannabis users in the bilateral ACC (volume = 4920 mm3) and right DL-PFC (volume = 3744 mm3). In contrast, one cluster of convergent increased activation was observed among cannabis users in the right striatum (i.e. caudate, claustrum, putamen) extending into the insula (volume = 4200 mm3). We note that simultaneous inspection of decreased and increased activation clusters via conjunction analysis revealed no regions of spatial overlap among clusters, suggesting that our meta-analytic results speak to dissociable effects of cannabis use on the brain. Given considerable variance across included studies with respect to time since last cannabis-use episode (Table 1), we completed an exploratory assessment that involved parsing included studies into two groups: studies reporting short-term and long-term abstinence among users (see Supplemental Figure 1).
Table 2.
Convergent functional alterations associated with cannabis use. Numbering (left) corresponds to brain regions shown in Figure 2. Cluster 1, ACC, volume = 4920 mm3. Cluster 2, DL-PFC, volume = 3744 mm3. Cluster 3, Striatum, volume = 4200 mm3. Extrema labels were determined via the Talairach Daemon. Hemisphere column denotes in which cerebral hemisphere resultant meta-analytic clusters were observed. Coordinates (x, y, z) indicate location of local extrema in Talairach space.
| Cluster no. | Extrema label (within ± 5 mm) | BA | Hemisphere | x | y | z |
|---|---|---|---|---|---|---|
| Decreases (users < non-users) | ||||||
| 1 (ACC) | ||||||
| Cingulate gyrus | 24 | B | 4 | 20 | 28 | |
| Anterior cingulate gyrus | 32 | B | −4 | 34 | 22 | |
| Cingulate gyrus | 24 | B | −4 | 2 | 36 | |
| Medial frontal gyrus | 8 | B | 0 | 22 | 42 | |
| Medial frontal gyrus | 6 | B | 4 | 36 | 36 | |
| 2 (DL-PFC) | ||||||
| Middle frontal gyrus | 6 | R | 36 | −6 | 42 | |
| Precentral gyrus | 6 | R | 38 | −4 | 36 | |
| Precentral gyrus | 6 | R | 56 | −6 | 28 | |
| Middle frontal gyrus | 6 | R | 48 | 2 | 38 | |
| Increases (users > non-users) | ||||||
| 3 (striatum) | ||||||
| Caudate | NA | R | 12 | 6 | 8 | |
| Claustrum | NA | R | 32 | 12 | 4 | |
| Putamen | NA | R | 20 | 10 | −10 | |
| Insula | 13 | R | 34 | 22 | −2 | |
ACC: anterior cingulate cortex; B: bilateral; BA: Brodmann area; DL-PFC: dorsolateral prefrontal cortex; L: left; NA: not applicable; R: right.
Figure 2.

Convergent functional alterations associated with cannabis use. Across studies, cannabis use was associated with differential regional alterations. Specifically, convergent decreases in activity among users were observed in two clusters, one encompassing the cingulate gyrus, anterior cingulate gyrus, and medial frontal gyrus (Cluster 1 (ACC), blue), and a second encompassing the middle frontal gyrus and precentral gyrus (Cluster 2 (DL-PFC), blue). Conversely, convergent increases in activity among users were observed in a single cluster encompassing the caudate, claustrum, putamen, and extending into the insula (Cluster 3 (Striatum), red). ALE maps were computed in Talairach space and thresholded (pcluster-corrected < 0.01; pvoxel-level < 0.001). Labels and numbering correspond with those in Table 2.
ACC: anterior cingulate cortex; ALE: activation likelihood estimation; DL-PFC: dorsolateral prefrontal cortex.
MACM of affected brain regions
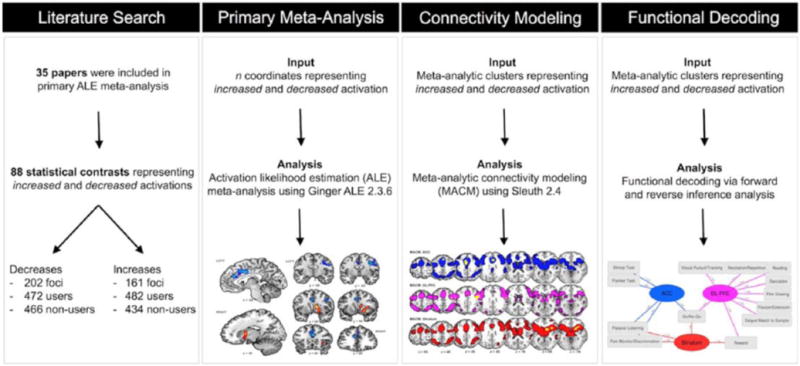
In a first ancillary assessment, we used the BrainMap database to delineate which brain regions tend to co-activate with the cannabis-affected meta-analytic clusters described above (Figure 3; Supplemental Table 1). MACM analyses revealed that Cluster 1 (ACC) was characterized by extensive whole-brain co-activation patterns, including associations with the insular cortex and caudate, medial frontal cortex, precuneus, fusiform gyrus, culmen, thalamus, and cingulate cortex (Figure 3, top row). In addition, Cluster 2 (DL-PFC) demonstrated considerable co-activation with the orbitofrontal cortex, parietal regions, fusiform gyrus, and occipital cortex (Figure 3, middle row). Finally, Cluster 3 (striatum) was associated with whole-brain co-activations that included the insular cortex, frontal cortex, superior parietal lobule, fusiform gyrus, and culmen (Figure 3, bottom row). These MACM outcomes demonstrate that cannabis-affected brain regions interact with broader brain networks across various neuroimaging tasks. Moreover, close inspection of each co-activation network suggested considerable spatial overlap within the medial frontal cortex, lateral frontal cortex, lateral parietal regions, insula, and limbic areas.
Figure 3.
MACM of impacted regions. MACM delineated other brain areas showing significant co-activation with resultant meta-analytic clusters impacted by cannabis (yellow) when considering all neuroimaging experiments archived in the BrainMap database. Modeled co-activation maps (pcluster-corrected < 0.01; pvoxel-level < 0.001) were visualized in Talairach space within the MRIcron environment. Cluster 1 (ACC, top row, blue) demonstrated co-activation with the insular cortex, caudate, medial frontal cortex, precuneus, fusiform gyrus, culmen, thalamus, and cingulate cortex. Cluster 2 (DL-PFC, middle row, purple) showed co-activation with the orbitofrontal cortex, fusiform gyrus, and occipital cortex. Cluster 3 (Striatum, bottom row, red) demonstrated co-activation with the insular cortex, frontal cortex, superior parietal lobule, fusiform gyrus, and culmen. See Supplemental Table 1 for coordinates.
ACC: anterior cingulate cortex; DL-PFC: dorsolateral prefrontal cortex; MACM: meta-analytic connectivity modeling.
Functional decoding of primary meta-analytic clusters
In a second ancillary assessment, we used quantitative forward- and reverse-inference analyses to characterize which psychological phenomena were linked to those regions showing cannabis-related functional alterations. Because we were interested in describing the likelihood that mental processes were being recruited given activation in specified brain regions, the results from the reverse-inference analysis are reported in Figure 4. However, those results from the forward-inference analysis can be found in Supplemental Table 2. Functional decoding revealed significant associations between Cluster 1 (ACC) and the BrainMap task classifications: Stroop Task, Flanker Task, Passive Listening, Pain Monitor/Discrimination and Go/No-Go (Figure 4, ACC). Cluster 2 (DL-PFC) showed significant associations with the task classifications: Delayed Match to Sample, Visual Pursuit/Tracking, Flexion/Extension, Recitation/Repetition, Film Viewing, Saccades, Reading, Reward, and Go/No-Go (Figure 4, DL-PFC). Finally, decoding results identified significant associations between Cluster 3 (striatum) and the task classifications: Pain Monitor/Discrimination, Passive Listening, Reward, and Go/No-Go (Figure 4, Striatum). We note that several tasks demonstrated associations with more than one cluster, including Pain Monitor/Discrimination and Passive Listening (ACC, striatum), Reward (DL-PFC, striatum), and Go/No-Go (ACC, DL-PFC, and striatum).
Figure 4.
Functional decoding of primary meta-analytic clusters. Reverse-inference analysis characterized psychological processes that were significantly associated with functionally impacted brain regions among cannabis users. Meta-data term associations were visualized using Cytoscape version 3.4.0 (Shannon et al., 2003). Lines between meta-data terms (squares, task classifications) and meta-analytic clusters (circles, impacted brain regions) indicate that the observed probability reached statistical significance. Probabilities next to each line (pFDR-corrected < 0.05) express the likelihood that a specific psychological process is engaged given activation in a specified brain region (P(Process|Activation)), where higher values indicated greater likelihoods.
ACC: anterior cingulate cortex; DL-PFC: dorsolateral prefrontal cortex.
Discussion
To clarify the neurobiological impact of cannabis use, we compiled results from 35 task-based functional neuroimaging papers, which compared cannabis users and non-users, within the ALE meta-analysis framework. In a primary meta-analytic assessment, we identified statistical convergence of reported decreased activation (users < non-users), revealing functional alterations in the ACC and DL-PFC, and of increased activation (users > non-users), showing convergent disruptions in the striatum. In two ancillary assessments, we used archived functional neuroimaging coordinates and associated meta-data to more thoroughly characterize these meta-analytic outcomes. Specifically, we observed co-activation between the ACC and frontal, parietal, and limbic system regions, with demonstrated associations to cognitive, inhibitory, and pain processing tasks. Additionally, the DL-PFC was characterized by co-activation patterns with frontal, parietal, and occipital regions, and was linked to tasks involving cognitive and visual processes. Finally, we observed extensive co-activation between the striatum and frontal, parietal, and limbic regions, and associations to tasks involving reward, inhibition, and pain.
Overall neurobiological impact of cannabis use
Aberrant ACC activation among cannabis users is perhaps not surprising, given the known architecture of the endocannabinoid system. Indeed, several studies have shown dense concentrations of endogenous cannabinoid (CB1) receptors within the ACC (Eggan and Lewis, 2007; Glass et al., 1997; Herkenham et al., 1990, 1991; Svizenska et al., 2008). Despite these concentrations, findings regarding structural alterations within the ACC among users are mixed (Cousijn et al., 2012a; Weiland et al., 2015). A critical region in task-dependent and task-independent, or resting-state, processes (Binder et al., 1999; Laird et al., 2009b; Mazoyer et al., 2001; Shulman et al., 1997), the ACC has been linked to error monitoring, conflict detection, and engaging the DL-PFC to resolve such conflicts (Botvinick et al., 2004; Carter et al., 1998; Carter and van Veen, 2007; Laird et al., 2005). Increased activation within the ACC, specifically following high-conflict challenges, is thought to promote enhanced DL-PFC activation to reduce conflict and error commissions on subsequent challenges (Kerns et al., 2004). In this way, the ACC, working in concert with the DL-PFC, is believed to modulate cognitive control processes following high-conflict situations including error detection. Furthermore, the DL-PFC is involved in sustained attention, important for maintaining sensory, motor, and cognitive information ‘online’ for subsequent encoding and retrieval (Miller and Cohen, 2001). Our primary meta-analytic results identified convergent cannabis-related decreased activation in the ACC and DL-PFC. One interpretation of these outcomes is that functional alterations within cognitive control nodes and their associated brain networks may underlie task-performance disruptions among cannabis users. Indeed, several studies have reported poorer performance among users relative to non-users when considering tasks which probe cognitive control and executive functions (e.g. Crane et al., 2013a, 2013b; Gruber and Yurgelun-Todd, 2005; Sagar et al., 2015; Thames et al., 2014). Results from our functional decoding assessments are consistent with such accounts, suggesting that the ACC and DL-PFC were associated with behavioral control paradigms, including Flanker and Stroop Tasks, and with paradigms involving learning and memory, including Delayed Match to Sample and Recitation/Repetition (Figure 4, DL-PFC, purple).
Mesocorticolimbic and nigrostriatal dopaminergic systems are implicated in various aspects of reward processing, including evaluating social judgments (Bzdok et al., 2011; Kampe et al., 2001), and substance use (Delgado, 2007; Haber and Knutson, 2010; Wise, 2009). Specifically, drug-related hyperactivation throughout dopaminergic circuitry, including the striatum, has been linked to long-term substance use across various drugs, including opioids (Chu et al., 2015), heroin (Li et al., 2012), alcohol (Gilman et al., 2012; van Holst et al., 2014), cocaine (Jia et al., 2011), amphetamines (O’Daly et al., 2014), nicotine (Addicott et al., 2012), and cannabis (Abdullaeva et al., 2010; Acheson et al., 2015; Cousijn et al., 2012a; Enzi et al., 2015; Hester et al., 2009; Nestor et al., 2010; van Hell et al., 2010). Our meta-analytic results are consistent with these reports, as we observed increased activation within the striatum among cannabis users. In addition, functional decoding revealed an association between the striatum and the task classification of Reward, suggesting that users may demonstrate cannabis-related enhanced activation in brain regions that subserve reward processing. These conclusions are supported by previous accounts that link the striatum to reward-processing features, including anticipation and appraisal (Delgado, 2007; Haber and Knutson, 2010; Wise, 2009). Indeed, increased reward-seeking behavior following cannabis exposure has been demonstrated in animal (for an extensive review, see Tanda and Goldberg, 2003) and human studies (Gilman et al., 2015; Lyvers et al., 2013). Moreover, a large-scale longitudinal assessment of adolescent risky decision-making found that increased substance use, including cannabis, correlated with attentional biases towards reward-predictive cues (van Hemel-Ruiter et al., 2013). However, across drugs of abuse, both decreased and increased striatal responding has been reported during reward anticipation. Regarding cannabis, such discrepant outcomes may reflect distinct methodological decisions made across studies. For example, one study (van Hell et al., 2010), which required cannabis-using participants to have a negative THC urine toxicology screen before scanning, reported comparable striatal activity during reward anticipation relative to non-users. In another study (Nestor et al., 2010), in which users were required to have a positive THC toxicology screen, cannabis-using participants showed increased striatal activity despite similar task demands as in the previous example. As such, it is plausible that these seemingly discordant results are indicative of residual intoxication/pharmacological effects among users with positive toxicology results (Balodis and Potenza, 2015). Nevertheless, the convergent increased striatal activation observed among users here, coupled with quantitatively demonstrated associations to reward-related tasks, provides a potential neurobiological explanation for previously characterized links between cannabis use and sensitization towards reward-predicting cues and associated outcomes.
The evolution from recreational substance use to dependence is believed to represent a transition from cortical-mediated to striatal-mediated behavioral control processes (Volkow et al., 2013). For example, functional neuroimaging studies have shown decreased activations in the ACC and PFC among cocaine users; deficits that persisted more than 3 months following detoxification (Volkow and Wang, 1992). Such alterations in cognitive control neurocircuitry have been linked to impulsive behaviors (Holmes et al., 2016). Taken together, decreased activation of the ACC and DL-PFC, paired with increased activation of the striatum, may represent a systems-level neurobiological mechanism through which problematic, and potentially addictive cannabis use patterns develop. From a related perspective, it is noteworthy that each of the three meta-analytic clusters observed were associated with the Go/No-Go task classification, a behavioral inhibition paradigm requiring participants to make/withhold motor responses. These results are consistent with contemporary views about the relations between the PFC, striatum, and one’s abilities to regulate problematic behaviors (Goldstein and Volkow, 2011). Here, the fact that distinct region-specific disruptions were linked with the same task classification may be indicative of a cannabis-related compound effect manifest across studies. In other words, a diminished capacity to inhibit problematic behaviors may be linked to concurrent reduction of prefrontal activity (ACC and DL-PFC) and elevation of striatal activity.
Implications from functional decoding
Given the absence of pain-related studies in this meta-analysis, it is noteworthy that two clusters, the ACC and striatum, were linked with the pain monitor/discrimination meta-data task classifications (Figure 4). Indeed, these structures have demonstrated involvement in distinct aspects of pain processing (Freund et al., 2009; Jahn et al., 2016; Lieberman and Eisenberger, 2015). From a pharmacotherapy perspective, cannabinoids represent a potential option for pain treatment and management. Data from more than 40 clinical trials using cannabinoids provide evidence for its antinociceptive effects in both chronic and neuropathic pain (Hill, 2015). A recent meta-analysis explored the beneficial and adverse effects of cannabinoids for medical use (Whiting et al., 2015). When considering data from 79 studies, representing 6462 participants, those researchers found a 37% pain reduction among medicinal cannabis users. Despite these and other findings, the antinociceptive properties of cannabis remain poorly understood at the neurobiological level. Results from one pharmacologic neuroimaging investigation involving cannabis administration suggest that a potential mechanism for THC-related analgesic effects may be through cingulate-limbic connectivity (Lee et al., 2013). Our meta-analytic outcomes and subsequent functional decoding results demonstrate the utility of using meta-analytic approaches to coalesce results from published neuroimaging studies involving substance use, revealing neuroimaging paradigms that may warrant additional investigation among cannabis-using populations (e.g. pain processing).
Limitations
When interpreting these results, we considered several methodological issues. First, the observed meta-analytic effects of cannabis should be considered preliminary, given the sample of studies included (N = 35). In addition, similar limitations barred the meta-analytic assessment of structural differences (e.g. voxel-based morphometry), acute cannabis effects (e.g. drug-administration studies), and null effects (i.e. studies reporting no differences between users and non-users). Second, as is the case with most reviews and meta-analytic reports, our results are constrained by the current state of the functional neuroimaging literature. Studies included here used tasks designed to probe specific psychological constructs, such as working memory, spatial memory, reward processing, and response inhibition. Importantly, the range of tasks used to identify use-related functional brain alterations may be constrained by a priori conceptualizations about cannabis’s impact on brain and behavior. That is, it is possible that the findings reported here reflect an overabundance of cognition- and reward-related investigations, and that the inclusion of more affective, perceptual, or motor investigations would refine the current results, revealing alternative brain regions that may be impacted among cannabis users. Third, the studies included in this meta-analysis were all cross-sectional, which limits causal inferences that can be made about the observed functional brain alterations and cannabis use. With large-scale longitudinal assessments currently underway, such as the Adolescent Brain Cognitive Development study, dissociable antecedents and consequences of cannabis use will become more evident. Fourth, we note that these findings represent cannabis-related decreased and increased activation that are task-general. The included studies represent the expanding corpus of functional neuroimaging studies on cannabis use that used fMRI or PET, and reported whole-brain findings. As new neuroimaging data are assembled, a more dissociative approach should become possible, permitting assessment of task-specific effects (e.g. cannabis users versus non-users during working-memory challenges). Fifth, the studies included here did not adequately consider sex-specific effects of cannabis use on brain function. Specifically, women were under-sampled among included studies, with 79% of users and 73% of non-users being men. Additionally, whole-brain contrasts comparing men and women are sparse, despite emerging indications of sex-specific effects. For example, one recent report showed that men, but not women, were responsive to the antinociceptive effects of cannabis, showing significant reductions in pain sensitivity (Cooper and Haney, 2016). Similar sex differences have also been reported regarding neurocognitive performance (Crane et al., 2013a, 2013b). Future studies should take into consideration potential sex differences when assessing the neurobiological effects of cannabis. Finally, cannabis-use assessment, including amount and frequency estimates, as well as duration since last cannabis-use episode, varied across the included studies. Despite considerable variance regarding these measures, with some studies reporting recreational use and others reporting more severe, problematic use patterns, convergent results were indeed detected. Furthermore, an exploratory assessment that involved parsing included studies using abstinence measures revealed distinct neurobiological changes associated with short-term versus long-term durations since last use episode (Supplemental Figure 2). Future investigations should take into consideration these critical factors to provide additional clarification regarding the impact of the amount, frequency, and currency of cannabis use on brain function.
Conclusions
The meta-analytic outcomes reported here suggest that cannabis use is linked with differential, region-specific effects on the brain, including decreased activation in the ACC and DL-PFC, and increased activation in the striatum. Ancillary analyses revealed that these cannabis-related functional alterations were embedded within expansive co-activation networks, and that several psychological processes may be impacted among users. Specifically, these functional alterations may manifest as alterations in cognitive control performance, enhanced reward seeking and responsiveness, and disrupted pain processing. This study highlights the utility of using meta-analytic tools to synthesize published neuroimaging results thereby elucidating neurobiological, cognitive, and behavioral processes associated with cannabis use. As policies and societal norms regarding cannabis undergo rapid changes, enhanced understanding of the impact of cannabis on the human brain is important for providing patients, healthcare providers, and policy makers with scientific information allowing for informed decision-making regarding cannabis use.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by awards from the National Institute on Drug Abuse, Florida International University, (U24-DA039832, R01-DA041353, R01-DA031176, R01-DA033156, K01-DA037819), and National Institute of Mental Health, Florida International University, (R56-MH097870, U54-MD012393: sub-project 5378).
Footnotes
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD
Julio A Yanes https://orcid.org/0000-0002-6620-4351
Meredith A Reid https://orcid.org/0000-0003-1946-0544
Matthew T Sutherland https://orcid.org/0000-0002-6091-4037
Supplemental material
Supplementary material is available for this article online.
References
- Abdullaev Y, Posner MI, Nunnally R, et al. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res. 2010;215:45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Acheson A, Ray KL, Hines CS, et al. Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J Addict. 2015;2015:783106. doi: 10.1155/2015/783106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addicott MA, Baranger DA, Kozink RV, et al. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: A preliminary study. Psychopharmacology. 2012;219:563–573. doi: 10.1007/s00213-011-2404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN. Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biol Psychiatry. 2015;77:434–444. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan B, Connolly CG, Datwani S, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, et al. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: A critical review of neuroimaging studies. Curr Pharm Des. 2012a;18:5045–5054. doi: 10.2174/138161212802884636. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, et al. Induction of psychosis by Δ-9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012b;68:27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Sendt KV. Neuroimaging evidence for cannabinoid modulation of cognition and affect in man. Front Behav Neurosci. 2012;6:1–4. doi: 10.3389/fnbeh.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, et al. Conceptual processing during the conscious resting state: A functional MRI study. J Cogn Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Jager G, Bhattacharyya S, et al. Acute and non-acute effects of cannabis on human memory function: A critical review of neuroimaging studies. Curr Pharm Des. 2014;20:2114–2125. doi: 10.2174/13816128113199990436. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, et al. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Struct Funct. 2011;215:209–223. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Yeo BT. Inference in the age of big data: Future perspectives on neuroscience. NeuroImage. 2017;155:549–564. doi: 10.1016/j.neuroimage.2017.04.061. [DOI] [PubMed] [Google Scholar]
- Carey SE, Nestor L, Jones J, et al. Impaired learning from errors in cannabis users: Dorsal anterior cingulate cortex and hippocampus hypoactivity. Drug Alcohol Depend. 2015;155:175–182. doi: 10.1016/j.drugalcdep.2015.07.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DME, et al. Meta-analytic clustering of the insular cortex: Characterizing the meta-analytic connectivity of the insula when involved in active tasks. NeuroImage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neuroscientist. 2007;13:422–432. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, et al. Marijuana use is associated with reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chu LF, Lin JC, Clemenson A, et al. Acute opioid withdrawal is associated with increased neural activity in reward-processing centers in healthy men: A functional magnetic resonance imaging study. Drug Alcohol Depend. 2015;153:314–322. doi: 10.1016/j.drugalcdep.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, et al. Is there “one” DL-PFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23:2677–2698. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, et al. Tackling the multifunctional nature of Broca’s region meta-analytically: Co-activation-based parcellation of area 44. NeuroImage. 2013;83:174–188. doi: 10.1016/j.neuroimage.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DZ, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend. 2016;167:112–120. doi: 10.1016/j.drugalcdep.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Castellanos FX, Eickhoff CR, et al. Functional decoding and meta-analytic connectivity modeling in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2016;80:896–904. doi: 10.1016/j.biopsych.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Shuster RM, Fusar-Poli P, et al. Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex difference. Neuropsuchol Rev. 2013a;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Shuster RM, Gonzalez Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. Neuropsychol Soc. 2013b;19:1009–1015. doi: 10.1017/S135561771300088X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, et al. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012a;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, et al. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict Biol. 2012b;18:1013–1023. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Dehghan M, Schmidt-Wilcke T, Pfleiderer B, et al. Coordinate-based (ALE) meta-analysis of brain activation in patients with fibro-myalgia. Hum Brain Mapp. 2016;37:1749–1758. doi: 10.1002/hbm.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann NY Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, et al. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, et al. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PM, et al. Implementation errors in the GingerALE software: Descriptions and recommendations. Hum Brain Mapp. 2016;38:7–11. doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CV1 receptor in the primate nerocortex: A regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, et al. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Enzi B, Lissek S, Edel MA, et al. Alterations of monetary reward and punishment processing in chronic cannabis users: An FMRI study. PLoS One. 2015;10:e0119150. doi: 10.1371/journal.pone.0119150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Myers US. Neural effects of positive and negative incentives during marijuana withdrawal. PLoS One. 2013;8:e61470. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Freund W, Klug R, Weber F, et al. Perception and suppression of thermally induced pain: A fMRI study. Somatosens Motor Res. 2009;26:1–10. doi: 10.1080/08990220902738243. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Calderon V, Curran MT. Young adult cannabis users report greater propensity for risk-taking only in non-monetary domains. Drug Alcohol Depend. 2015;147:26–31. doi: 10.1016/j.drugalcdep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, et al. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addict Biol. 2012;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RLM. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, et al. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. 2012;511:89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: An FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, et al. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cogn Neurosci. 2015;16:71–83. doi: 10.1016/j.dcn.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, et al. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA. 2015;313:2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, Roffman JL. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. Neuroscience. 2016;36:4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MML, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jahn A, Nee DE, Alexander WH, et al. Distinct regions within medial prefrontal cortex process pain and cognition. Neuroscience. 2016;36:12385–12392. doi: 10.1523/JNEUROSCI.2180-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia ZR, Worhunsky PD, Carroll KM, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, et al. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, et al. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- King GR, Ernst T, Deng W, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: Relationship to cortisol levels. Neuroscience. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, DeVito EE, DeLeone CM, et al. Cannabis abstinence during treatment and one-year follow-up: Relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal MA, Hazekamp A, Colzato LS, et al. Modulation of cognitive and emotional processing by cannabidiol: The role of the anterior cingulate cortex. Front Hum Neurosci. 2013;7:1–4. doi: 10.3389/fnhum.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, et al. ALE meta-analysis work-flows via the BrainMap database: Progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009a;3:1–11. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. Neuroscience. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, et al. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AL, Riedel MC, Sutherland MT, et al. Neural architecture underlying classification of face perception paradigms. NeuroImage. 2015;119:70–80. doi: 10.1016/j.neuroimage.2015.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Ploner M, Wiech K, et al. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain. 2013;154:124–134. doi: 10.1016/j.pain.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci. 2015;112:15250–15255. doi: 10.1073/pnas.1515083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang YR, Zhang Y, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: An event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Bogorodzki P, et al. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res. 2012;202:224–232. doi: 10.1016/j.pscychresns.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M, Jamieson R, Thorberg FA. Risky cannabis use is associated with alexithymia, frontal lobe dysfunction, and impulsivity in young adult cannabis users. J Psychoactive Drugs. 2013;45:394–403. doi: 10.1080/02791072.2013.844525. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, et al. Neuroimaging in cannabis use: A systematic review of the literature. Psychol Med. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, et al. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;73:838–844. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Roberts G, Garavan H, et al. Deficits in learning and memory: Parahippocampal hyperactivity and frontocortical hypoactivtiy in cannabis users. NeuroImage. 2008;40:1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Rottschy C, Thommes J, et al. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct Funct. 2015;220:2355–2371. doi: 10.1007/s00429-014-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Joyce D, Tracy DK, et al. Amphetamine sensitization alters reward processing in the human striatum and amygdala. PLoS One. 2014;9:e93955. doi: 10.1371/journal.pone.0093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interactions in abstinent adolescent marijuana user. Psychol Addict Behav. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: An fMRI study. Drug Alcohol Depend. 2004;76:261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G. Cannabis and motor function: fMRI changes following 28 days of discontinuation. Exp Clin Psychopharmacol. 2008;16:22–32. doi: 10.1037/1064-1297.16.1.22. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Riba J, Valle M, Sampedro F, et al. Telling true from false: Cannabis users show increased susceptibility to false memories. Mol Psychiatry. 2015;20:772–777. doi: 10.1038/mp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, et al. Meta-analytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser P, Lissek S, Tegenthoff M, et al. Alterations of theory of mind network activation in chronic cannabis users. Schizophr Res. 2012;139:19–26. doi: 10.1016/j.schres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Sagar KA, Dahlgren MK, Gonenc A, et al. The impact of initiation: Early onset of marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci. 2015;16:84–92. doi: 10.1016/j.dcn.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, et al. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith AM, Longo CA, Fried PA. Effects of marijuana on visuospatial working memory: An fMRI study in young adults. Psychopharmacology. 2010;210:429–438. doi: 10.1007/s00213-010-1841-8. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: Summary of National Findings. Rockville: Substance Abuse and Mental Health Services Administration; 2016. [Google Scholar]
- Sutherland MT, Ray KL, Riedel MC, et al. Neurobiological impact of nicotinic acetylcholine receptor agonists: An activation likelihood estimation meta-analysis of functional neuroimaging studies. Biol Psychiatry. 2015;78:711–720. doi: 10.1016/j.biopsych.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, et al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016;12:1–15. doi: 10.1186/s12993-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Gruber SA, Rogowska J, et al. A preliminary study of functional brain activation among marijuana users during a virtual water maze task. J Addict. 2013;2013:461029. doi: 10.1155/2013/461029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures: A short review. Pharmacol Biochem Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: Reward, dependence, and underlying neurochemical mechanisms—A review of recent preclinical data. Psychopharmacology. 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arbid N, Sayegh P. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict Behav. 2014;39:994–999. doi: 10.1016/j.addbeh.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Block RI, O’Leary DS, et al. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37:618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hell HH, Vink M, Ossewaarde L, et al. Chronic effects of cannabis use on the human brain reward system: An fMRI study. Eur Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Van Hemel-Ruiter ME, de Jong PJ, Oldehinkel AJ, et al. Reward-related attentional biases and adolescent substance use: The TRAILS study. Psychol Addict Behav. 2013;127:142–150. doi: 10.1037/a0028271. [DOI] [PubMed] [Google Scholar]
- Van Holst RJ, Clark L, Veltman DJ, et al. Enhanced striatal responses during expectancy coding in alcohol dependence. Drug Alcohol Depend. 2014;142:204–208. doi: 10.1016/j.drugalcdep.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry. 2016;73:292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, et al. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland B, Thayer RE, Depue BE, et al. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. Neuroscience. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lile JA, Hanlon CA, et al. Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology. 2016;233:1035–1044. doi: 10.1007/s00213-015-4180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal: Not just mesocorticolimbic—Dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrege J, Schmidt A, Walter A, et al. Effects of cannabis on impulsivity: A systematic review of neuroimaging findings. Curr Pharm Des. 2014;20:2126–2137. doi: 10.2174/13816128113199990428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, et al. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Westfall J. Choosing prediction over explanation in psychology: Lessons from machine learning. Perspect Psychol Sci. 2017;12:1100–1122. doi: 10.1177/1745691617693393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, DeVito EE, Kober H. Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: An exploratory study of relationships with abstinence during behavioral treatment. Drug Alcohol Depend. 2014;140:33–41. doi: 10.1016/j.drugalcdep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


