Abstract
Objective
White adipose tissue (WAT) fibrosis – the buildup of extracellular matrix (ECM) proteins, primarily collagen – is now a recognized hallmark of tissue dysfunction and is increased with obesity and lipodystrophy. While growth hormone (GH) is known to increase collagen in several tissues, no previous research has addressed its effect on ECM in WAT. Thus, the purpose of this study is to determine if GH influences WAT fibrosis.
Design
This study examined WAT from four distinct strains of GH-altered mice (bGH and GHA transgenic mice as well as two tissue specific GH receptor gene disrupted lines, fat growth hormone receptor knockout or FaGHRKO and liver growth hormone receptor knockout or LiGHRKO mice). Collagen content and adipocyte size were studied in all cohorts and compared to littermate controls. In addition, mRNA expression of fibrosis-associated genes was assessed in one cohort (6 month old male bovine GH transgenic and WT mice) and cultured 3T3-L1 adipocytes treated with GH.
Results
Collagen stained area was increased in WAT from bGH mice, was depot-dependent, and increased with age. Furthermore, increased collagen content was associated with decreased adipocyte size in all depots but more dramatic changes in the subcutaneous fat pad. Notably, the increase in collagen was not associated with an increase in collagen gene expression or other genes known to promote fibrosis in WAT, but collagen gene expression was increased with acute GH administration in 3T3-LI cells. In contrast, evaluation of 6 month old GH antagonist (GHA) male mice showed significantly decreased collagen in the subcutaneous depot. Lastly, to assess if GH induced collagen deposition directly or indirectly (via IGF-1), fat (Fa) and liver (Li) specific GHRKO mice were evaluated. Decreased fibrosis in FaGHRKO and increased fibrosis in LiGHRKO mice suggest GH is primarily responsible for the alterations in collagen.
Conclusions
Our results show that GH action is positively associated with an increase in WAT collagen content as well as a decrease in adipocyte size, particularly in the subcutaneous depot. This effect appears to be due to GH and not IGF-1 and reveals a novel means by which GH regulates WAT accumulation.
Keywords: growth hormone, bGH mice, GHA mice, FaGHRKO mice, LiGHRKO mice, adipose tissue, fibrosis
1. Introduction
White adipose tissue (WAT) fibrosis is a characteristic feature of obesity and lipodystrophy and associated with inflammation, insulin resistance, and decreased adipocyte size [19]. In addition, it is believed to negatively impact metabolism by limiting the ability of adipocytes to expand [33, 51, 53] and by contributing to other WAT dysfunctions such as hypoxia and immune cell infiltration[28, 43, 48]. Indeed, mice lacking collagen VI, a collagen preferentially expressed in WAT, display dramatic WAT expansion accompanied by significant improvements in glucose and lipid metabolism as well as reduced WAT immune cell infiltration and inflammation [33]. Though WAT fibrosis is currently associated with the obese state, it is possible that it is a general feature of unhealthy WAT and, thus, a consequence of other disease states as well.
Growth hormone (GH) altered mice provide a unique perspective on the relationship of WAT and health due to the profound impact that GH has on WAT mass and distribution as well as its negative effect on lifespan and healthspan [6, 8, 42, 49]. That is, mice with global excess in GH action are lean but suffer drastic metabolic and lifespan consequences, whereas mice with a reduction in GH action are obese but lack the metabolic dysfunction associated with obese states [7]. These contradictory and counterintuitive phenotypes – unhealthy leanness and healthy obesity – allow us to examine WAT fibrosis independent of the normal adiposity/health relationship and determine if WAT fibrosis is a hallmark of unhealthy WAT irrespective of obesity status.
We focused mainly on bovine GH transgenic (bGH) mice, which have life-long, chronic, high serum levels of GH, insulin-like growth factor 1 (IGF-1), and insulin resulting in a giant, lean phenotype. Important to consider is that their pattern of GH secretion is non-pulsatile, and thus pathologic. Despite their leanness, bGH mice are unhealthy and suffer from insulin resistance [23, 34, 45], increased cancer incidence [3], and shortened lifespans [9]. Similar complications are seen in human patients with acromegaly [2, 5, 21, 41]. Notably, high levels of GH have been shown to promote fibrosis in numerous tissues such as muscle, bone, heart and kidney [12, 20, 27, 38, 54, 56]. However, no results have been reported on the effect of GH on the ECM in WAT. Thus, the primary objective of this study was to examine the effect of chronic excess GH exposure on collagen accumulation in WAT using bGH mice. For comparison, we also assessed a mouse line with decreased GH stimulus, the GH receptor antagonist (GHA) mouse, to determine if the inhibition of GH action decreased collagen content. Lastly, to determine if GH induced increased collagen deposition directly or indirectly (via IGF-1), we studied WAT samples from adipose tissue-specific growth hormone receptor (GHR) knockout animals (FaGHRKO) [36] and liver-specific GHR knockout animals (LiGHRKO) [37], the latter of which have elevated circulating GH but reduced endocrine IGF-1.
2. Materials and methods
2.1 Animals
All mice were housed in the facility at the Edison Biotechnology Institute where they were kept on a 14-hour light/10-hour dark cycle and had ad libitum access to water and normal chow unless otherwise noted. Development and breeding of bGH and GHA transgenic mice, both on a C57BL/6J background, have been described previously [9, 13–15]. Generation of adipose tissue-specific (FaGHRKO) and liver-specific (LiGHRKO) GHR mice, both in the C57BL/6 background, have also been described previously [36, 37]. All animal procedures were approved by the Ohio University Institutional Animal Care and Use Committee.
Four separate cohorts of mice were utilized in this study and will be referred to as cohort 1 through 4 for clarity. Cohort 1 consisted of two male and female bGH and wild type (WT) control mice from three different age groups (26 weeks, 42 weeks, and 64 weeks). Cohort 2 was comprised of 6-month old male bGH mice and WT littermate control mice. Males were solely used in this cohort due to availability of samples. Since bGH mice have very little WAT, it was not possible to perform all of the experiments for this group on the same mice. Thus, separate mice from this cohort were necessary to complete the immunohistochemistry (n=8), hydroxyproline (n=10), qPCR expression (n=8) and RNA-seq (n=3) analyses. Cohort 3 consisted of 6-month old male GHA mice and WT littermate controls (n=7). Cohort 4 included a subset of the adipose tissue samples (n=8) from FaGHRKO and LiGHRKO male mice along with floxed littermate controls collected and described previously [36, 37].
2.2 Body Composition
Body composition and body weight were measured one day prior to dissection (cohorts 2–4). Body weight was measured using a Mettler Toledo PL 202-S balance and body composition was measured using the Minispec mq Benchtop Nuclear Magnetic Resonance analyzer (Bruker Instruments, Minispec ND2506) as previously described [10].
2.3 Tissue Weights
Mice were fasted for 12 hours prior to being sacrificed by cervical dislocation. For all cohorts, four distinct WAT depots [inguinal subcutaneous (sc), perigonadal (peri), mesenteric (mes), and retroperitoneal (retro)] were collected and weighed. WAT used for histological measures was fixed in a 10% formalin solution, then rinsed and stored in a 70% ethanol solution. For gene expression (RNA) analysis, the harvested WAT depot was flash frozen in liquid nitrogen and stored at −80 °C until further processing.
2.4 Immunohistochemistry
Formalin-fixed WAT samples from cohorts 1–4 were sent to AML Labs (Baltimore, MD) for paraffin embedding, sectioning and staining with picrosirius red, a general collagen stain. Slides were analyzed using a Nikon Eclipse E600 microscope. Images for both collagen staining and cell sizing were obtained using a Spot RT digital camera at 200× magnification. For collagen staining quantification, pictures of 20 non-overlapping fields were taken per WAT depot per mouse and then analyzed using ImageJ software [46]. Collagen staining [30] and cell size [36] were quantified as described previously.
2.5 Cell Culture
Adipogenic differentiation was induced by treating confluent 3T3-L1 preadipocyte cells with 1 µM dexamethasone, 0.5 µM isobutylmethylxanthine, 100 nM insulin, and 1 µM rosiglitazone in growth media (DMEM- high glucose with 10% FBS). After 2 days, the medium was replaced with growth medium containing 100 nM insulin and 1 µM rosiglitazone 2 more days, then for 2–3 days with growth medium alone to allow for differentiation. Mature 3T3-L1 adipocytes were starved for four hours in DMEM-high glucose alone and treated with or without 500 ng/mL of recombinant bGH (ProSpec) for 24 hours.
2.6 Quantitative PCR and RNAseq
Total RNA was isolated using a QIAzol™ Lysis Reagent (Qiagen). 1 µg of total RNA was reverse transcribed in 20 µl using the High Capacity cDNA Reverse Transcription (RT) Kit (Applied Biosystems). A portion (5 µl) of diluted (1/10) RT reaction was amplified with specific primers (300 nM each) in a 20 µl PCR reaction using an iQ™ SYBR® Green Supermix (BioRad). Analysis of gene expression was carried out in a BioRad MyiQ™ sequence detector with initial denaturation at 95°C for 3 min, followed by 40 PCR cycles, each cycle consisting of 95°C for 10 s and 58°C for 1 min. SYBR green fluorescence emissions were monitored after each cycle. For each gene, mRNA expression was calculated relative to acidic ribosomal protein 36B4 (36B4) expression. Amplification of specific transcripts was confirmed by the melting-curve profiles (cooling the sample to 68°C and heating slowly to 95°C with measurement of fluorescence) at the end of each PCR. Primer sequences used included: Col1a1 forward 5’-GGGTCTAGACATGTTCAGCTTT-3’; Col1a1 reverse 5’-ACCCTTAGGCCATTGTGTATG-3’; Col3a1 forward 5’-CCCTTCTTCATCCCACTCTTATT-3’; Col3a1 reverse 5’-GATCCTGAGTCACAGACACATATT -3’; Col4a1 forward 5’-TGGCTTCTGCTGCTCTTC-3’; Col4a1 reverse 5’-ACGCCATGACAGTCACATT-3’; Col5a1 forward 5’-GCCCTGCTGCTGTCTTC-3’; Col5a1 reverse 5’-GCACAGAAACCTGTGGT-3’; Col6a1 forward 5’-GGCGACCCTGGGTATGA-3’; Col6a1 reverse 5’-TACCCGACTGGTCCAAGAT-3’.
For RNA-Seq studies, mRNA from frozen WAT samples (cohort 2) was isolated and sequenced at the Ohio University Genomics Facility using the Ion Torrent Personal Genome Machine as previously described [4].
2.7 Hydroxyproline Determination
Measurement of hydroxyproline was performed as previously reported [55] with minor modifications. Briefly, 100mg of frozen WAT was heated in 6N HCl at 110°C for 24 hrs. Samples were centrifuged and diluted; the infranatant was incubated for 20 min in Chloramine-T solution (1.4% chloramine-T in water). Next, 3.15M perchloric acid was added. Following 5 min incubation at room temperature, p-DMAB (20% w/v p–dimethylaminobenzaldehyde in 2-methoxyethanol) was added, and samples were incubated at 60°C for 20 minutes. After cooling, the absorbance was read at 550 nm, and the concentration was determined from the standard curve using hydroxyl-L-proline (Sigma Aldrich).
2.8 Statistics
Statistics were analyzed using Statistical Package for the Social Sciences (SPSS version 17.0, 2008; IBM, Armonk, NY). Data are shown as mean ± SEM. Comparisons of two means was done by unpaired student’s t test. Genotype and depot differences for body weight, composition, tissue weight, staining, cell size, hydroxyproline, and gene expression were subjected to two-way ANOVA. Within and between-group comparisons were analyzed using a one-way analysis of variance followed by contrast tests. Corrected degrees of freedom were used if the groups failed Levene’s test for homogeneity of variance (p < 0.05). RNA-seq analysis was conducted using the mm10 mouse database as a reference genome, the on-line tool Galaxy, and the Cufflinks program version 2.1.0 as previously described [4]. Differences in gene expression were considered to be statistically significant at p < 0.05.
3. Results
3.1 Preliminary longitudinal analysis of fibrosis in bGH mice
Samples were collected from four depots – inguinal subcutaneous (sc), perigondal (peri), retroperitoneal (retro), and mesenteric (mes) – of male and female bGH mice and WT controls at three different ages (cohort 1). Picrosirius red staining revealed increased collagen content with differences becoming greater with increasing age (Supplementary Fig. 1A), in all WAT depots when compared to WT controls (Supplementary Fig. 1B) and in both male and female bGH mice compared to WT mice (Supplementary Fig. 1C). Importantly, staining was the most increased in the sc depot and the least altered in the peri depot; thus, for some of the subsequent experiments, these two depots were selected to represent the two extremes in collagen staining. Although there appears to be a few differences between sexes, a clear trend was not readily discernable. That is, fibrosis appeared to be increased in males in the sc depot (Supplementary Fig. 1C), while females seemed to have greater collagen content in the peri depot. Moreover, increasing fibrosis appeared to correlate with decreasing adipocyte size. Neither staining nor adipocyte size were quantified in this cohort due to the small sample size. Subsequent studies focused on the 6 month time point since this was a time at which fibrosis was readily detectable, and because samples from numerous other mouse lines were available for comparison.
3.2 Body weight, depot weight of 6 months old bGH mice
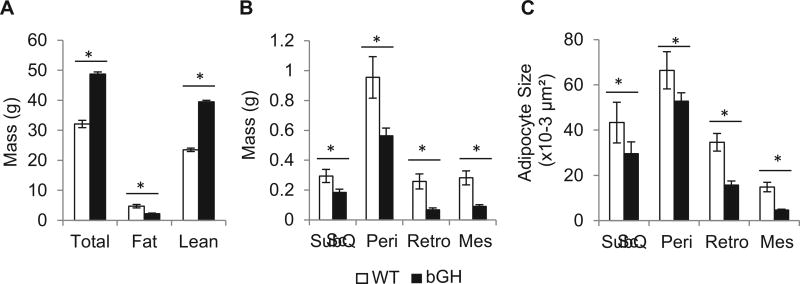
Next, a more in-depth investigation of WAT fibrosis in bGH mice was performed in 6 month old males (cohort 2). As reported previously, we confirmed that bGH mice had significantly greater body weights (p<0.001) and lean mass (p<0.001), but significantly less fat mass (p<0.001) than their WT controls (Fig. 1A) [9]. All measured WAT depot weights were significantly decreased in the bGH mice (sc: p<0.05; peri: p< 0.05; retro: p <0.01; mes: p <0.01; Fig. 1B). Importantly, adipocyte size was significantly decreased in bGH mice in all depots compared to WT (sc: p < 0.01; peri: p<0.001; retro: p<0.001; mes: p<0.001, Fig. 1C).
Fig. 1.
Comparison of A, body composition, B, individual adipose depot weight, and C, cell size of WT and bGH mice at 6 months of age (n=8 for both groups). In panel B, bGH tissue weights were significantly lower when analyzed by univariate analysis (F1,56 = 26.3, p = 3.7 × 10−6). In panel C, a two-way ANOVA found significant main for genotype (F1,302 = 130.7, p = 2.2 × 10−25), depot (F3,302 = 79.2, p = 7.9 × 10−38), and the interaction of genotype and depot (F3,302 = 12.0, p = 2.0 × 10−7) on adipocyte size. All data are expressed as mean ± SEM of eight mice per group. Sc, subcutaneous; Peri, perigonadal; Retro, retroperitoneal; Mes, mesenteric. *, P < 0.05 versus WT using one way ANOVA with Student’s t test (panel A) or contrasts tests (B, C).
3.3 Analysis of fibrosis at 6 months of age in bGH mice
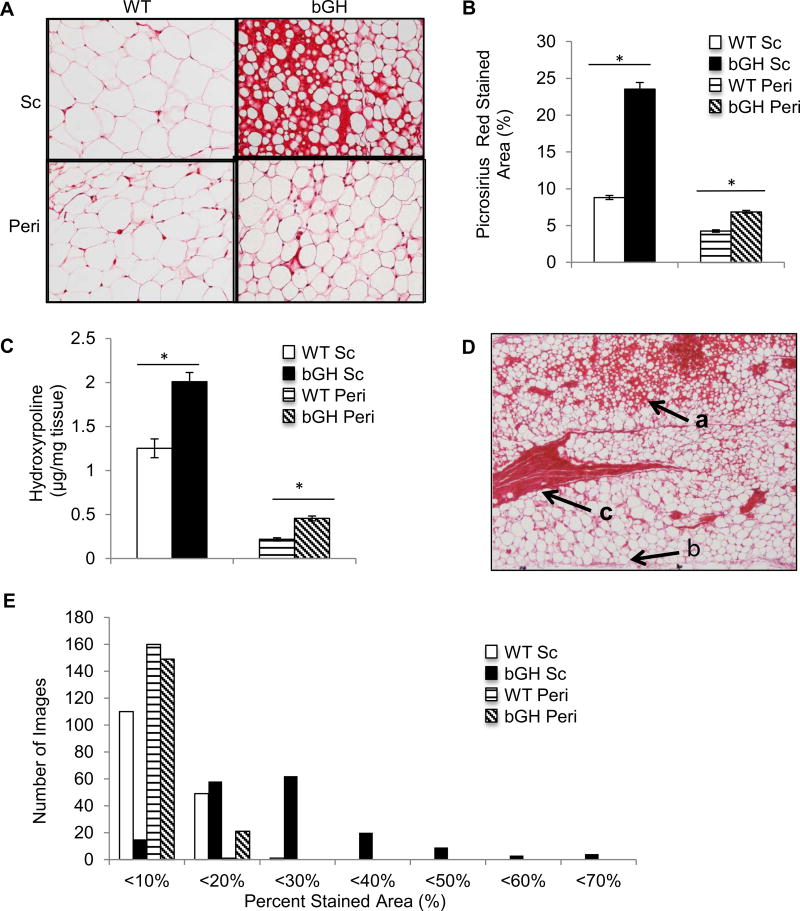
Picrosirius red staining indicated an apparent increase in the collagen content of bGH WAT in both the sc and peri depots compared to WT controls (cohort 2) (fig. 2A). Quantification of stained area confirmed that bGH mice had a significantly higher percentage of stained area in both the sc (p<0.01) and peri (p<0.05) depots compared to WT mice (Fig. 2B). Moreover, the sc depot had significantly greater collagen staining than the peri depot independent of genotype (p<0.01). As an additional measure of collagen content in WAT, the concentration of hydroxyproline was determined and found to closely mirror the results of the staining quantification. That is, the hydroxyproline concentration was significantly higher in bGH WAT compared to WT in both the sc (p<0.001) and peri depots (p<0.001, Fig. 2C), and the sc depot had a significantly higher hydroxyproline concentration than the peri depot (p<0.001). To ensure our results were a reflection of increased collagen and not an artifact of smaller adipocyte size, hydroxyproline was also calculated relative to depot weight. Normalized hydroxyproline concentration (µg/mg hydroxyproline*mg weight of depot) was also significantly increased in the sc depot (0.48 for bGH vs. 0.33 for WT, p<0.001) and in the peri depot (0.25 for bGH vs. 0.15 for WT, p>0.001). As is apparent in Fig. 2D, the distribution of collagen within a given bGH WAT depot, particularly the sc depot, was not homogeneous. bGH WAT contained areas of high fibrosis and small adipocytes (Fig. 2D, arrow indicated as a), as well as areas with less fibrosis and larger adipocytes (Fig. 2D, arrow indicated as b). In addition, bGH WAT contains abundant collagenous streaks, similar to what is seen in obese WAT [28](Fig. 2D, arrow indicated as c). This heterogeneity in staining is quantified in Fig. 2E, which shows the distribution of picrosirius red stained area in the various WAT depots. The WT sc and peri depots as well as the bGH peri depot show minimal variation in the percent stained area with no images having greater than 20% staining. In contrast, in the bGH sc depot, the amount of staining varies greatly from < 10% to < 70% picrosirius red stained area (Fig. 2E).
Fig. 2.
Analysis of collagen deposition in WAT of 6 month old male bGH and WT mice (n=8 for both groups). A, Representative images of sc and peri WAT from WT and bGH mice stained with picrosirius red (200× magnification). B, Quantification of picrosirius red stained area vs. total area in sc and peri WAT sections. Two way ANOVA analysis indicated significant main effects of genotype (F1,28 = 22.8, p =5.1 × 10−5), depot (F1,28 = 36.3, p = 1.7 × 10−6), and the interaction of genotype and depot (F1,28 = 11.5, p = 0.002). C, Hydroxyproline concentration was measured in sc and peri a WAT from bGH and WT mice. Two way ANOVA found significant main effects of genotype (F1,36=34.9, p=9.2×10−7), depot (F1,36=272.2, p=2.3×10−18), and the interaction of genotype and depot (F1,36=13.0, p=9.2×10−4). D, A representative picrosirius red stained WAT section showing an example of the bGH mouse sc depot at 4× magnification. The arrow labeled a indicates an area of high collagen deposition and small adipocytes. Arrow c indicates a characteristic collagenous streak, and arrow b indicates an area with relatively less collagen and larger adipocytes size. E, Shows the number of images in each 10% picrosirius red stained percentage range. *, P < 0.05 versus WT in the same depot using one way ANOVA with contrasts tests.
3.4 Analysis of collagen and fibrosis-related gene expression in bGH mice
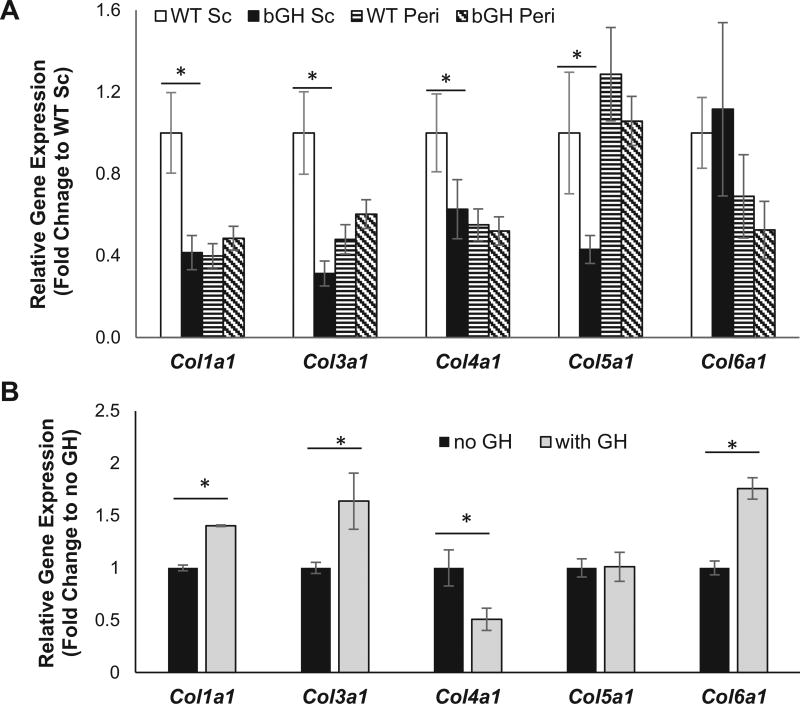
As a further assessment of fibrosis in bGH mice at 6 mo of age, qPCR was performed to determine mRNA transcript levels of collagen subunit genes in bGH versus control WAT (Fig. 3A). Curiously, none of the collagen genes evaluated were significantly upregulated in the bGH sc depot compared to the WT sc depot. In fact, we found significantly lower expression of Col1a1 (p<0.01), Col3a1 (p<0.01) Col4a1 (p<0.05) and Col5a1 (p<0.05) in the bGH sc depot compared to controls. No significant differences in mRNA expression were found in the peri fat pad.
Fig. 3.
Analysis of collagen gene expression. A, Collagen gene expression of 6 month old WT and bGH mice. Two way ANOVA found significant main effects of genotype for Col1a1 (F1, 39= 7.2, p=0.01), Col3a1 (F1,35=8.1, p=0.007), and Col5a1 (F1,35=4.6, p=0.04). There was also a significant main effect of depot for Col1a1 (F1,35=8.0, p=0.008), Col4a1 (F1,35=5.1, p=0.3) and Col5a1 (F1,35=4.4, p=0.4) as well as a significant interaction of genotype and depot for Col1a1 (F1,35=12.0, p=0.001) and Col3a1 (F1,35=15.4, p=0.0003). All data are expressed as mean ± SEM of ten mice per group. *, P < 0.05 versus WT of the same depot using one way ANOVA with contrasts tests. B, Collagen gene expression in 3T3-L1 cells with and without 24 treatment with exogenous bGH. An unpaired student’s t-test, revealed a significant difference in Col1a1 (p=0.002), Col3a1 (p=0.008), Col4a1 (p=0.01) and Col6a1 (p=0.001) expression. *, P < 0.05.
As expression of collagen genes provided little insight into the cause of the WAT fibrosis in bGH mice, we turned to a more global approach to evaluate differentially expressed, fibrosis-related genes by using a RNA-seq dataset previously generated [4]. We examined genes for collagen proteins, ECM remodeling enzymes, as well as other genes that have been associated with WAT fibrosis. A full list of genes analyzed and statistical comparisons can be viewed in the supplementary data (Supplementary Tables 1 & 2). Regarding genes coding for collagen subunits, no genes were significantly upregulated in the bGH sc depot compared to the WT sc depot, while Col4a2, Col5a1, Col5a3, Col6a1, Col15a1, and Col18a1 were all significantly downregulated in the bGH sc depot. In the perigonadal depot, Col1a1, Col4a2, Col5a2, and Col6a2 were significantly upregulated in bGH WAT, while no collagen subunits were significantly downregulated. Next, the expression of ECM remodeling enzymes, including matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), were examined. When comparing genotypes, only Timp-4 was altered with significantly lower expression in the bGH sc depot compared to littermate controls but with no difference between perigonadal depots.
As the tissue expression data for chronic exposure to GH excess could not explain the increased collagen deposition, we turned to an in vitro system in which we evaluated the effect of acute administration of GH to 3T3-L1 cells. After a 24 hour bGH treatment of differentiated cells, expression of Col1a1, Col3a1 and Col6a1 were significantly increased (Fig. 3B) while no change or a decreased in expression was observed for Col5a1 and Col4a1, respectively
3.5 Body composition, WAT distribution, Adipocyte Size, and WAT collagen content in GHA mice
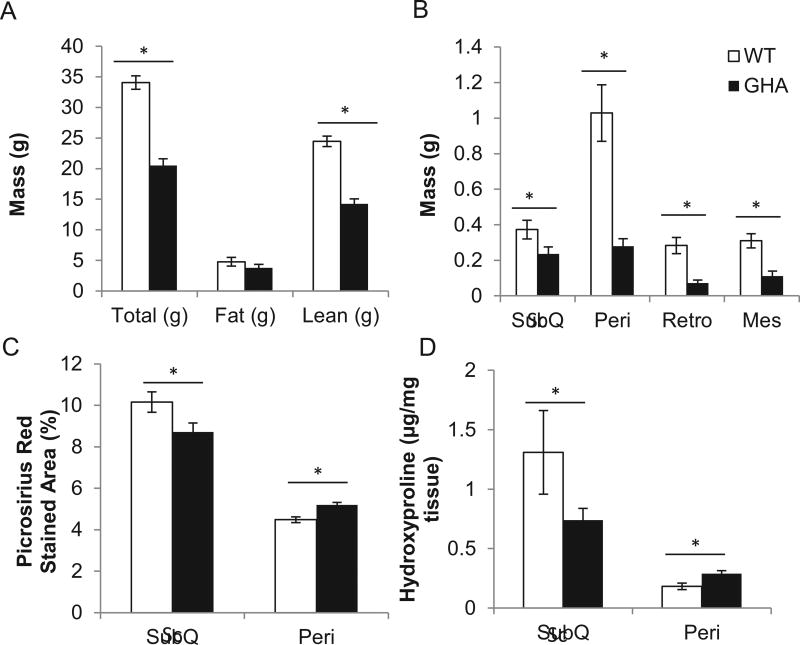
Next, we examined WAT collagen content in 6 month old male GH receptor antagonist (GHA) transgenic mice, which have a reduction in GH induced signaling. As expected, these mice were found to have decreased total body weight (p<0.001) and decreased lean mass (p<0.001) but normal absolute fat mass (Fig 4A). When normalized to body weight, GHA mice were relatively obese (18.2% fat mass) compared to littermate controls (13.9% fat mass) (data not shown) similar to previously documented GHA cohorts [11]. Regarding WAT distribution, GHA mice showed reductions in the absolute mass of all depots (sc, p<0.05; peri, p<0.001; retro, p<0.01; and mes, p<0.01; Fig 4B), but the mass of the sc depot was the only depot significantly enlarged when normalized to total body weight (p<0.01). A difference in mean adipocyte size was found for the sc depot (19.2×10−3 µm2 for WT vs. 29.5×10−3 µm2 for GHA, p<0.001) and trended but did not reach statistical significance for the peri depot (16.2×10−3 µm2 for WT vs. 18.7×10−3 µm2 for GHA, p<0.001). To assess WAT collagen content, picrosirius red staining and hydroxyproline measurements were performed. Comparison between genotypes revealed a significant decrease in both picrosirius red staining (p<0.01) as well as hydroxyproline (p<0.05) in the GHA sc depot (Fig. 4C&D). However, the results were less clear in the peri depot where there was a significant increase in staining (p<0.001) but with no difference in hydroxyproline content. When comparing depots, the sc depot had greater collagen content than the peri depot for both picrosirius red staining (p<0.01) and hydroxyproline measurements (p<0.001; Fig. 4C & D).
Fig. 4.
A, Body weight, fat, and lean mass of GHA and WT mice. B, Comparison of WAT depot weights between GHA and WT mice. Two-way ANOVA found significant main effects of genotype (F(1,62)=94.1, P=4.8×10−14), depot (F(3,62)=36.2, P=1.2×10−13), and the interaction of genotype and depot (F(3,62)=17.5, P=2.4×10−8). C, Comparison of picrosirius red stained area from WAT histology sections. Two-way ANOVA revealed a significant effect of genotype (F(1,504)=17.4, P=3.7×10−5) and depot (F(1,504)=181.5, P=1.5×10−35) but not the interaction of genotype and depot (F(1,504)=.1, P=.7). D, Comparison of hydroxyproline concentration in WAT tissue from GHA and WT mice. Two-way ANOVA found a significant effect of depot (F(1,32)=14.6, p=.001), but not genotype or the interaction of genotype and depot on hydroxyproline concentration. *, p<.05 using one way ANOVA with contrasts tests.
3.6 WAT collagen content in FaGHRKO and LiGHRKO mice
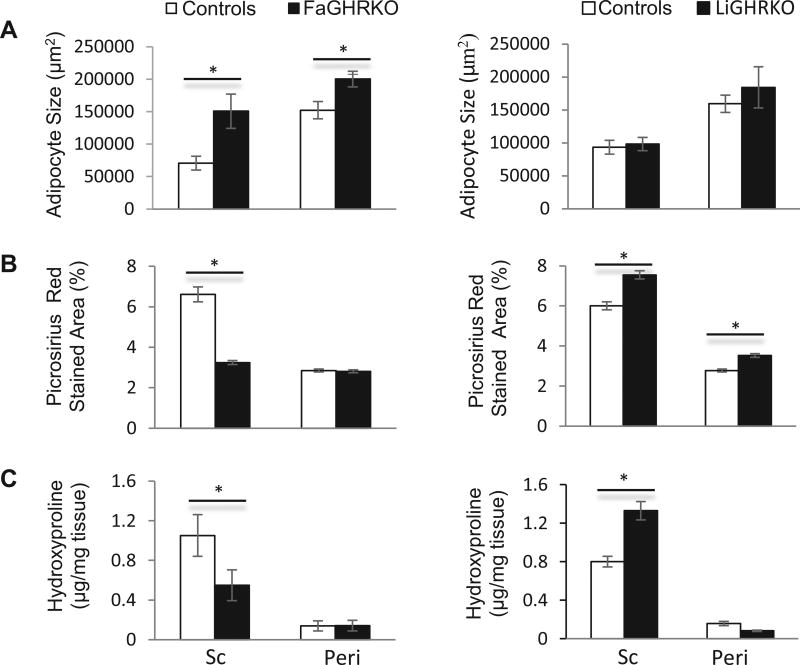
Finally, in order to evaluate if GH alters collagen content directly or indirectly via IGF-1, we examined two additional mouse lines with tissue specific disruption of GHR in either adipose tissue or liver (Fig. 5). As reported previously, male FaGHRKO mice are obese with slightly elevated IGF-1 levels along with normal GH levels and normal glucose homeostasis whereas the male LiGHRKO mice are leaner with increased GH but dramatically decreased endocrine IGF-1 and exhibit impairment in glucose homeostasis [36, 37]. Thus, these mice provided an in vivo model in which IGF-1 and GH levels are disconnected. Interestingly, FaGHRKO mice had larger adipocyte sizes in both the sc (p<0.05) and peri depots (p<0.05) (Fig 5A, left panel), but only the sc depot had significantly decreased picrosirius red staining (p<0.001) (Fig. 5B, left panel) and hydroxyproline content (p<0.05) (Fig. 5C, left panel). For the LiGHRKO mice, there was no difference in adipocyte size (Fig 5A, right panel) yet the sc depot had significantly increased quantification of the picrosirius red staining (p<0.001) and hydroxyproline content (p<,0.001) (Fig 5B & C, right panel). The results with the peri depot were less consistent with significantly increased picrosirius red staining (p<0.001) and a trend but nonsignificant difference in hydroxyproline content (p=0.06).
Fig. 5.
Comparison of A, average adipocyte size; B, picrosirius red stained area; and C, hydroxyproline content in FaGHRKO mice (left panels) and LiGHRKO mice (right panels) compared to floxed littermate controls at 6 months of age (n=8 for all groups). In panel A and using a two-way ANOVA, FaGHRKO mice had a significant main effect of genotype (F1, 28=14.4, p=0.001) and depot (F1,28 =15.1, p=0.001) on adipocyte size. In panel B, a two-way ANOVA found significant main effects for genotype (FaGHRKO: F1,476=76.4, p=4.1×10−17; LiGHRKO: F1,446 =55.0, p=6.1×10−13), depot (LiGHRKO: F1,476=115.3, p=3.1×10−24; LiGHRKO: F1, 446=550.4, p 7.1×10−80) and the interaction of genotype and depot (FaGHRKO: F1,476=72.5, p=2.2×10−16; LiGHRKO: F1, 446=6.6, p=0.01) for picrosirius red stained area for both FaGHRKO and LiGHRKO samples compared to floxed controls. In panel C, a two-way ANOVA found significant main effects for genotype (FaGHRKO: F1,28=3.5, p=0.05; LiGHRKO: F1,24=6.2, p=0.02) and depot (FaGHRKO: F1,28=16.5, p=3.6×10−4; LiGHRKO: F1, 446 =93.1, p 9.3×10−10) but only a significant the interaction of genotype and depot in LiGHRKO samples (F1,24=12.9, p=0.001) compared to floxed controls. All data are expressed as mean ± SEM of eight mice per group. *, P < 0.05 using one way ANOVA with contrasts tests.
4. Discussion
The purpose of this study was to analyze the influence of chronic, life-long alterations to the GH/IGF-1 axis on the collagen content of WAT. Our major findings reveal a positive relationship between GH action and collagen content in WAT of mice as well as an inverse relationship between GH and adipocyte size. In addition, mRNA expression of collagen and fibrosis-related genes were decreased in 6 month old male bGH mice, a trend that was opposite of the amount of collagen deposition. However, in vitro studies showed that acute GH stimulation results in an increased collagen expression, suggesting that GH may not be the cause of decreased collagen mRNA expression but rather a secondary consequence of chronic GH exposure. Our investigation of tissue-specific GHRKO mice suggests that collagen content may be more dependent on GH than on IGF-1 (discussed below). The results reveal a distinct effect on collagen content and adipocyte size at the tissue level that may represent a novel mechanism by which GH alters fat mass either directly or indirectly via lipolysis and, as a result, influences metabolic health.
Analyses of picrosirius red staining and hydroxyproline concentration in WAT reveal that GH has a striking, positive effect on collagen content. This study is the first evidence that GH increases the collagen content of WAT, though previous studies have shown that GH increases collagen content in other tissues such as liver, kidney, heart, bone and muscle [12, 20, 27, 38, 54]. Our preliminary study on bGH mice suggests that collagen content increases with age and may have sex differences; however, larger studies are needed to confirm these findings. Importantly, both our preliminary study and further investigation of 6 month old bGH males reveal that GH had the greatest influence on collagen content in the sc depot. This reinforces conclusions from previous research showing that the sc depot is the most affected by GH action at least in mice [10]. Analysis of GHA mice confirmed that decreased GH signaling had the opposite result: decreased WAT collagen content. Notably, this difference was primarily apparent in the sc depot, as collagen staining and hydroxyproline were both only significantly different between genotypes in this depot. Importantly, clinical studies suggest a more significant effect on the visceral adipose depots in obese, GH-deficient states with GH treatment [22, 31] and in acromegaly in which a visceral adiposity index has been suggested as an indicator of the severity of disease state [17, 18]. In contrast, our results in mice suggest a more dramatic impact on sc depots. It is unknown whether this represents a species difference or whether the reduction in WAT mass in humans is correlated with depot alterations in fibrosis. Regardless, these data highlight the importance of investigating multiple WAT depots in future studies and are consistent with GH action being directly correlated with collagen content in WAT.
One of the primary actions of GH is the stimulation of IGF-1 production. Predominantly secreted by the liver, it is also a potent growth factor and stimulator of somatic growth [35]. However, GH and IGF-1 have different actions in WAT as IGF-1, like its namesake insulin, prevents lipolysis, promotes lipogenesis, and is insulin sensitizing [32]. In order to differentiate the effects of GH and IGF-1 signaling on WAT collagen, we studied two tissue-specific KO mouse lines: FaGHRKO and LiGHRKO mice. FaGHRKO mice lack GH signaling only in WAT; thus, the GH/IGF-1 axis is relatively normal with the exception of a modest elevation in serum IGF-1 in males; conversely, LiGHRKO mice lack GH signaling in the liver and thus have significantly decreased serum IGF-1 but elevated circulating GH [36, 37]. Therefore, these tissue-specific GHR gene disrupted mouse lines allow us to begin to separate the effects of GH and IGF-1 signaling on collagen in WAT. Our results reveal that FaGHRKO mice have lower collagen content than WT, similar to the GHA mice results. In contrast, LiGHRKO mice, with elevated circulating GH and decreased endocrine IGF-1, have increased collagen content, similar to bGH mice. Thus, our results show that collagen content has a positive relationship with GH action in WAT, and does not seem to be dependent on or altered by changes in endocrine IGF-1.
WAT health appears to be inversely related to ECM deposition. This might be expected as you would need a highly flexible ECM for a tissue that has to adapt to changing nutrient conditions. Indeed, an increased and rigid ECM, as seen in both obese and lipodystrophic states, has been shown to impede WAT growth and promote local and systemic pathologies, including chronic inflammation, insulin resistance and cell death [1, 19, 33, 52]. The central role of a flexible WAT ECM in metabolic health is illustrated by knocking out collagen VI, which weakens the ECM and results in larger adipocytes but also improved metabolic outcomes, including decreased WAT inflammation and improved insulin sensitivity [33]. Likewise, a dramatic phenotype is seen in the absence of MTP1-MMP (MMP14) in which a rigid ECM develops in the sc depots due to abnormal accumulation of collagens. These MTP1-MMP null mice have a developmental fibrotic response that dramatically blunts adipose expansion and promotes complete lipodystrophy [16]. Results from our study show a strong positive correlation between GH and WAT fibrosis. GH has long been known to impair WAT health and decrease adipocyte size/WAT mass, which has been largely attributed to GH’s stimulation of lipolysis and inhibition of lipogenesis [24, 25]. Results from the current study suggest that increased fibrosis, whether directly caused by GH excess or a secondary effect of increased lipolysis due to GH action, occurs in bGH WAT. As the ability to reverse fibrosis is thought to be limited, the resultant, rigid WAT ECM in bGH mice would ultimately alter overall metabolic fitness of the fat pad and the health of the organism. To that end, bGH mice have been repeatedly shown to have greater insulin resistance and WAT inflammation [9, 30, 39]. Conversely, inhibition of GH signaling, as seen in GHA mice, appears to weaken the ECM and allows adipocytes to expand, promoting obesity but a without the metabolic dysfunction commonly associated with obese states. Thus, GH’s influence on WAT fibrosis may be a novel means by which GH alters or controls WAT mass and health.
The ECM of WAT is comprised of many of the same collagens found in other tissues. Specifically, collagens I, III, IV, V, and VI have been identified as ECM components involved in WAT fibrosis [19, 40, 47]. Collagen VI is of particular importance as it is preferentially expressed in WAT with little expression present in other tissues [33, 40]. Collagen gene expression is known to be altered in other tissues such as muscle and bone in response to GH [20] leading us to hypothesize that the bGH mice, with their increased collagen deposition, would have increased collagen mRNA expression in WAT. However, our results with WAT from mice were unexpected and not consistent between depots. For example, in the sc depot, many collagen genes were downregulated in bGH mice when compared to controls as well as when compared to the bGH peri depot. Thus, gene expression and fibrosis showed opposite trends with the bGH sc depot having the greatest collagen deposition but the lowest gene expression. Overall, the existing RNAseq data agreed with the qPCR data and also showed that other fibrosis-associated genes showed opposite trends of what we would expect.
Several possible explanations exist for an increased ECM deposition in bGH mice without a concomitant increase in expression of fibrosis-associated genes. First, GH may affect post-translational modification of collagen or other relevant proteins rather than mRNA expression. In support of this, post-translational modifications of signaling intermediates in the TGF-β and PDGFRα signaling pathways as well as a generation of a C-terminal cleavage product of COL6α3 called endotrophin, have been implicated in the WAT fibrosis associated with obesity [26, 29, 44, 50]. Second, whole tissue may mask gene expression changes in a subset of cells within WAT. For example, in obese human WAT, the expression of collagens 1α1, 3α1, and 6α1 are significantly greater in the stromal vascular fraction (SVF) cells when compared to their expression in mature adipocytes or whole tissue [19]. GH is known to alter the SVF cell population. Specifically, bGH mice have an altered WAT immune cell populations [4], with an overall increase in immune cell numbers as well as a substantial shift toward an M2 macrophage phenotype, particularly in the sc depot [4]. Interestingly, M2 macrophages, also known as alternatively activated macrophages, have been associated with increased ECM remodeling and decreased insulin sensitivity in subcutaneous WAT from obese human subjects [48]. Thus, a disproportionate number of these cells would be expected to influence mRNA expression data from whole tissue. Finally, chronic exposure to excess GH, as occurs in bGH mice, may result in a largely dysfunctional and inactive tissue by 6 months of age. Importantly, our data from 3T3-L1 cells treated with pharmacological doses of GH reveal at least a transient increase in expression of Col1a1, Col3a1, and Col6a1, which may dissipate with prolonged exposure. Regardless, expression of the obvious collagen and fibrosis-associated genes do not appear to provide insight into how GH increases fibrosis and further studies are needed to elucidate the mechanism responsible for the increase in fibrosis observed in WAT of these mice.
Notably, this study included different experimental mouse models that have important clinical correlations. bGH mice experience chronic GH excess similar to patients with acromegaly; GHA mice have reduced GH action through a mechanism similar to the drug Pegvisomant, which is used to decrease GH action in patients with acromegaly. In light of our findings and previous research showing increased fibrosis in other tissues, we believe that fibrosis should be an important factor to consider in patient management. Specifically, we believe that drugs for the treatment of acromegaly should be evaluated for their antifibrotic properties. There are a number of available therapeutic strategies to reduce fibrosis albeit they have mainly been studied in the context of pulmonary, liver, and kidney fibrotic disorders. Perhaps antifibrotic drugs in combination therapy with pharmaceutical agents that reduce the activity of the GH/IGF-1 axis would further attenuate the metabolic disturbances that accompany acromegaly. Lastly, we believe that the potential for fibrosis and subsequent alterations to normal tissue function represent an added caution to be considered when utilizing GH supplementation.
In summary, GH-altered mice provide a unique perspective in that we are able to study hallmarks of obese WAT in lean unhealthy mice and obese healthy mice. This study has revealed genotypic and depot differences in collagen content and adipocyte size, strengthening the argument that unhealthy WAT has similar characteristics in both obese and lean states. Thus, associated diseases of obesity may be attributed to the health of the tissue, rather than the amount of tissue. In addition, this study reveals that chronic high levels of GH increase fibrosis in WAT and decrease adipocyte size. This appears to be due primarily to the effects of GH and not to IGF-1. In obese WAT, fibrosis has been associated with limiting the storage capacity of adipocytes thus inhibiting their function, leading us to question whether this is also true in GH influenced WAT. GH is known to decrease fat mass by increasing lipolysis and decreasing lipogenesis, and it is important to acknowledge that our data do not discern whether the alterations in fibrosis are due to GH’s lipolytic effect or to GH directly. Perhaps future studies that employ other lipolytic factors, such as beta and renergic receptor agonists, could be evaluated for their impact on WAT fibrosis; this would determine whether fibrosis is a common phenomenon with other potent lipoloytic molecules or unique to the lipolytic action of GH. Regardless, if the increased collagen/fibrosis seen in our study does, in fact, limits the storage capacity of adipocytes, then the increased collagen content in bGH mice could be a contributing factor to the decreased fat mass caused by GH. Therefore, our results suggest that restriction of adipocyte size via enhanced collagen deposition may be a novel mechanism by which GH may reduce fat mass beyond lipogenesis or lipolysis.
Supplementary Material
HIGHLIGHTS.
GH action is positively associated with collagen content in adipose tissue (AT).
Effect of GH on AT is depot-dependent with the subcutaneous fat pad most impacted.
Fibrosis-related RNA expression is not related to AT collagen levels in bGH mice.
WAT fibrosis appears to be a direct consequence of GH action and not IGF-1.
Acute GH stimulation in vitro increases collagen mRNA expression.
Acknowledgments
We would like to acknowledge Stephen Bell and Delaney Geitgey for careful review of the manuscript.
Funding
This work was supported by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll (J.J.K.), National Institutes of Health (AG031736 to J.J.K., D.E.B., E.O.L.; DK112237 to K.Y.L; AG051869 to D.E.B., E.O.L); the Diabetes Institute at Ohio University; the Research and Scholarly Advancement Fellowship at Ohio University (L.A.H); and the AMVETS (J.J.K., C.W.).
Abbreviations
- GH
growth hormone
- GHR
growth hormone receptor
- GHRKO
growth hormone receptor knockout
- FaGHRKO
fat growth hormone receptor knockout
- LiGHRKO
liver growth hormone receptor knockout
- IGF-1
insulin-like growth factor 1
- WAT
white adipose tissue
- ECM
extracellular matrix
- bGH
bovine growth hormone
- GHA
growth hormone receptor antagonist
- AT
adipose tissue
- Sc
subcutaneous
- Mes
mesenteric
- Peri
perigonadal
- ret
Retro
- MMPs
matrix metalloproteinases
- TIMPs
tissue inhibitors of inhibitors of MMPs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K, Winlove CP. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab. 2013;305:E1427–1435. doi: 10.1152/ajpendo.00111.2013. [DOI] [PubMed] [Google Scholar]
- 2.Ayuk J, Sheppard MC. Does acromegaly enhance mortality? Rev Endocr Metab Disord. 2008;9:33–39. doi: 10.1007/s11154-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 3.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 4.Benencia F, Harshman S, Duran-Ortiz S, Lubbers ER, List EO, Householder L, Al-Naeeli M, Liang X, Welch L, Kopchick JJ, Berryman DE. Male Bovine GH Transgenic Mice Have Decreased Adiposity With an Adipose Depot-Specific Increase in Immune Cell Populations. Endocrinology. 2015;156:1794–1803. doi: 10.1210/en.2014-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengtsson BA, Brummer RJ, Eden S, Bosaeus I, Lindstedt G. Body composition in acromegaly: the effect of treatment. Clin. Endocrinol. (Oxf) 1989;31:481–490. doi: 10.1111/j.1365-2265.1989.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 6.Berryman D, List E, Sackmann-Sala L, Lubbers E, Munn R, Kopchick J. Growth hormone and adipose tissue: Beyond the adipocyte. Growth Horm. IGF Res. 2011;21:113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: Lessons from animal models. Growth Horm. IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berryman DE, Glad CA, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9:346–356. doi: 10.1038/nrendo.2013.64. [DOI] [PubMed] [Google Scholar]
- 9.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O'Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life-span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:131–141. doi: 10.1093/gerona/glt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogazzi F, Lombardi M, Strata E, Aquaro G, Di Bello V, Cosci C, Sardella C, Talini E, Martino E. High prevalence of cardiac hypertophy without detectable signs of fibrosis in patients with untreated active acromegaly: an in vivo study using magnetic resonance imaging. Clin. Endocrinol. (Oxf) 2008;68:361–368. doi: 10.1111/j.1365-2265.2007.03047.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen WY, White ME, Wagner TE, Kopchick JJ. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology. 1991;129:1402–1408. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- 14.Chen WY, Wight DC, Chen NY, Coleman TA, Wagner TE, Kopchick JJ. Mutations in the third alpha-helix of bovine growth hormone dramatically affect its intracellular distribution in vitro and growth enhancement in transgenic mice. The Journal of biological chemistry. 1991;266:2252–2258. [PubMed] [Google Scholar]
- 15.Chen WY, Wight DC, Mehta BV, Wagner TE, Kopchick JJ. Glycine 119 of bovine growth hormone is critical for growth-promoting activity. Mol. Endocrinol. 1991;5:1845–1852. doi: 10.1210/mend-5-12-1845. [DOI] [PubMed] [Google Scholar]
- 16.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 17.Ciresi A, Amato MC, Pizzolanti G, Giordano Galluzzo C. Visceral adiposity index is associated with insulin sensitivity and adipocytokine levels in newly diagnosed acromegalic patients. The Journal of clinical endocrinology and metabolism. 2012;97:2907–2915. doi: 10.1210/jc.2012-1518. [DOI] [PubMed] [Google Scholar]
- 18.Ciresi A, Radellini S, Guarnotta V, Giordano C. The visceral adiposity index is associated with insulin sensitivity and IGF-I levels in adults with growth hormone deficiency. Endocrine. 2016 doi: 10.1007/s12020-016-1076-5. [DOI] [PubMed] [Google Scholar]
- 19.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clement K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A, Kjaer M. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. The Journal of physiology. 2010;588:341–351. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foss MC, Saad MJ, Paccola GM, Paula FJ, Piccinato CE, Moreira AC. Peripheral glucose metabolism in acromegaly. J. Clin. Endocrinol. Metab. 1991;72:1048–1053. doi: 10.1210/jcem-72-5-1048. [DOI] [PubMed] [Google Scholar]
- 22.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J. Clin. Endocrinol. Metab. 2005;90:1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 23.Frick F, Bohlooly YM, Linden D, Olsson B, Tornell J, Eden S, Oscarsson J. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab. 2001;281:E1230–1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- 24.Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinol. Metab. Clin. North Am. 2012;41:283–295. v–vi. doi: 10.1016/j.ecl.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Goodman HM, Gorin E, Schwartz Y, Tai LR, Chipkin SR, Honeyman TW, Frick GP, Yamaguchi H. Cellular effects of growth hormone on adipocytes. The Chinese journal of physiology. 1991;34:27–44. [PubMed] [Google Scholar]
- 26.Grandl G, Muller S, Moest H, Moser C, Wollscheid B, Wolfrum C. Depot specific differences in the adipogenic potential of precursors are mediated by collagenous extracellular matrix and Flotillin 2 dependent signaling. Mol Metab. 2016;5:937–947. doi: 10.1016/j.molmet.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granot I, Halevy O, Hurwitz S, Pines M. Growth hormone and insulin-like growth factor I regulate collagen gene expression and extracellular collagen in cultures of avian skin fibroblasts. Mol. Cell. Endocrinol. 1991;80:1–9. doi: 10.1016/0303-7207(91)90137-h. [DOI] [PubMed] [Google Scholar]
- 28.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29:1106–1119. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jara A, Benner CM, Sim D, Liu X, List EO, Householder LA, Berryman DE, Kopchick JJ. Elevated systolic blood pressure in male GH transgenic mice is age dependent. Endocrinology. 2014;155:975–986. doi: 10.1210/en.2013-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J. Clin. Endocrinol. Metab. 1997;82:727–734. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol. Metab. Clin. North Am. 2007;36:75–87. doi: 10.1016/j.ecl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annu. Rev. Nutr. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- 35.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 36.List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol. 2013;27:524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, Dall R, Boroujerdi MA, Bassett EE, Healy ML, Pentecost C, Wallace JD, Powrie J, Jorgensen JO, Sacca L. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. The Journal of clinical endocrinology and metabolism. 2000;85:1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- 39.Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J. Endocrinol. 2013;216:363–374. doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melmed S. Acromegaly pathogenesis and treatment. The Journal of clinical investigation. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 43.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. The Journal of clinical endocrinology and metabolism. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pessin JE, Kwon H. How does high-fat diet induce adipose tissue fibrosis? J. Investig. Med. 2012;60:1147–1150. doi: 10.231/JIM.0b013e318271fdb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124:40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- 46.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. The Journal of clinical endocrinology and metabolism. 2011;96:E1990–1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 2014;6:575–586. doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, Hamaguchi M, Nishimura S, Manabe I, Matsuda T, Kimura K, Inoue H, Inagaki Y, Aoe S, Yamasaki S, Ogawa Y. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 53.Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui P, Grober J, Stich V, Rossmeislova L, Zakaroff-Girard A, Bouloumie A, Viguerie N, Moro C, Tavernier G, Langin D. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7:1116–1129. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 54.Wilson VJ, Rattray M, Thomas CR, Moreland BH, Schulster D. Growth hormone increases IGF-I, collagen I and collagen III gene expression in dwarf rat skeletal muscle. Mol. Cell. Endocrinol. 1995;115:187–197. doi: 10.1016/0303-7207(95)03690-3. [DOI] [PubMed] [Google Scholar]
- 55.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 56.Yokota F, Arima H, Hirano M, Uchikawa T, Inden Y, Nagatani T, Oiso Y. Normalisation of plasma growth hormone levels improved cardiac dysfunction due to acromegalic cardiomyopathy with severe fibrosis. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.12.2009.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.