Abstract
To reduce the burden of chronic illness, prevention and management interventions need to be not only efficacious, but also be adopted and implemented with fidelity and to reach those who are at greatest risk. Yet, many research-tested interventions are slow to translate into practice and reach those at greatest risk. This paper describes how The University of North Carolina at Chapel Hill School of Nursing's NINR-funded institutional pre- and postdoctoral research-training program is addressing the imperative to speed knowledge translation. The training emphasizes the following six methods to speed translation across the research cycle: stakeholder engagement, patient-centered outcomes, sequential multiple randomized trial (SMART) designs, pragmatic trials, mixed methods approaches, and dissemination and implementation science strategies.
Graphical abstract
Introduction
Chronic illness is the leading cause of disability and death and accounts for most health care expenditures in the United States (US). Unhealthy behaviors such as insufficient physical activity, poor nutritional intake, and substance use, and poorly controlled modifiable risk factors are major contributors to the symptoms, functional impairments, and loss of life attributable to chronic illness (CDC, 2016). Preventing and managing chronic illness often requires complex interventions that are theory-based (Bartholomew & Mullen, 2011) and address interactions among factors at patient, family, clinician, health care system, and environmental levels (Lam, Spitz, Schully, & Khoury, 2013).
Since 1996, the National Institute of Nursing Research (NINR)-funded institutional research training program entitled “Interventions for Preventing and Managing Chronic Illness” (T32NR007091) has addressed the need for complex theory-based interventions by training nurse scientists to develop and test complex theory– based interventions to prevent or manage chronic illness particularly among populations vulnerable to greater burden due to higher risk for illness onset and poorer outcomes. The training program has focused on generating the scientific basis for new interventions or modification of existing interventions, using theory to develop interventions, and testing theory-based interventions with patients and families aiming at maximizing efficacy. Yet, to reduce the burden of chronic illness, interventions must not only be optimally efficacious, they also must be adopted and implemented with fidelity in practice with those who are at greatest risk (Onken, Carroll, Shoham, Cuthbert & Riddle, 2014).
This paper describes how The University of North Carolina at Chapel Hill's NINR-funded institutional pre- and postdoctoral research-training program (https://grants.nih.gov/grants/guide/pa-files/PA-16-152.html) addresses the imperative to speed the translation of theory-based nursing interventions into practice as a means to maximize health outcomes for patients, families, communities and populations affected by chronic illness.
Theory-Based Interventions to Prevent and Manage Chronic Illness
Theory provides a framework for identifying the structural factors, cognitions, and behaviors associated with a specific health-related problem and its antecedents, for specifying linkages in the causal model that can be targeted to produce beneficial changes, for selecting intervention components and delivery modalities, for determining which measurements to take and when, and for establishing procedures for evaluating the intervention. Theory also guides examination of factors such as sex, age, culture and other personal, clinical or biological factors that characterize subgroups with distinct classes of responses (moderators) to interventions, and for identifying essential mechanisms (intermediary variables or mediators) of change (Bartholomew & Mullen, 2011; Lipsey, 1993; Sidani & Braden, 2011). In turn, understanding of moderators and mediators informs the development of interventions that are both efficacious and implementable. For example, information about moderators informs the future tailoring or targeting of the intervention to those for whom the intervention does and does not work, and identifying features of providers, settings, and the wider context that can influence implementation. Information about mediators provides guidance about which particular intervention components are linked to essential mechanisms of change and how the intervention might be scaled up to be more efficacious or scaled down to be feasible for different populations or practice contexts without compromising efficacy (Kraemer, Wilson & Fairburn, 2002).
Research-Tested Theory-Based Interventions are Under Utilized
The National Institutes of Health (NIH) and other funding agencies have invested heavily in developing and testing theory-based interventions to prevent and manage chronic illness, so that patients, clinicians and others can now readily access a growing menu of research-tested interventions. However, time from intervention development and testing to implementation in practice or policy has been estimated to average 17 years (Balas, 2000; Westfall, Mold & Fagnan, 2007) and many research-tested interventions remain underused or misused. Thus, potential benefits to patients, caregivers and society are forfeited and research funds and other scarce resources are wasted.
Multiple factors can account for the underuse or misuse of research-tested interventions. Historically, scientists' priorities have driven the intervention development and testing process, with less attention to the priorities of stakeholders, including patients, caregivers, communities, clinicians, health care systems, payers, and policy makers. This inattention has led to interventions that can be irrelevant to potential adopters, and often too complex and too costly to be feasible in practice (Tunis, Stryer & Clancey, 2003). As a result, those implementing interventions deviate from the components or dose required to achieve the intended outcomes (Onken et al., 2014).
Moreover, training for nurse scientists has prioritized learning about internal validity and randomized controlled trial (RCT) designs at the expense of considering external validity and trial designs that might contribute to an intervention's potential to reach the intended population and be implemented with fidelity over time (Glasgow, 2008; Kessler & Glasgow, 2011). A central challenge to translating research-tested interventions into practice, therefore, is identifying ways to engage the full array of stakeholders including clinicians who understand practice constraints across the translational process, and to balance internal and external validity during intervention development and testing (Alfano, 2014; Meslin, 2013; Peek et al., 2014). In other words, to speed translation from research to practice, nurse scientists must reshape the development and testing of theory-based nursing interventions with implementation in mind (or “application in mind” as stated by Klesges et al., 2005).
Even when interventions are developed with implementation in mind, patients, caregivers and clinicians can be slow to implement them. As a result, scientists are also developing and testing a range of theory-based strategies to more effectively disseminate and implement research-tested intervention materials, for example, intervention manuals and work sheets (Leeman, Jilcott-Pitts & Myers, 2014). The NIH defines dissemination as “targeted distribution of information and intervention materials to a specific public health or clinical practice audience”, and implementation as “use of strategies to adopt and integrate evidence-based health interventions into clinical and community settings” (https://grants.nih.gov/grants/guide/pa-files/PAR-16-238.html). Thus, dissemination strategies inform the distribution and marketing of efficacious interventions with the goal of increasing specific audiences' awareness, capacity to use, and receptivity to the intervention (Leeman, Sommers, Leung & Ammerman, 2011), while implementation strategies aim to institutionalize interventions and optimize their reach, fidelity, and sustainability (Proctor, Powell & McMillen, 2013).
In summary, to speed the translation of research-tested theory-based interventions to practice, more nurse scientists must have expertise in developing and testing interventions that are relevant, optimally efficacious and feasible to implement, including expertise in dissemination and implementation strategies. For this 5-year funding period (07/01/2016-06/30/2021), “Interventions for Preventing and Managing Chronic Illness” has added a new focus on translational research. The research training includes didactic instruction and mentored experiences in six methods that can catalyze translation to address the time gap between research and practice.
Framework
The pre- and postdoctoral research training is framed by the translational research paradigm depicted in Figure 1 (adapted from Glasgow et al., 2012; Khoury, Gwinn & Ioannidis, 2010; Peek, Glasgow, Stange, Klesges, Purcell & Kessler, 2014).
Figure 1. Conceptual Framework.
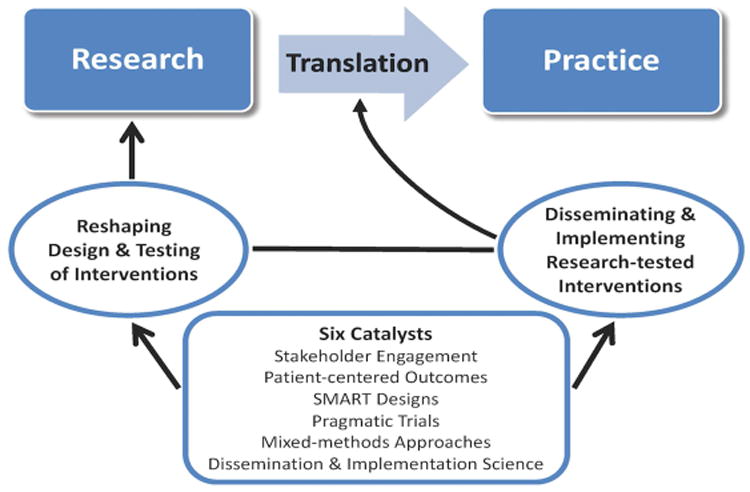
This paradigm suggests that translation can be accelerated by (a) reshaping the design and testing of interventions with implementation in mind, and (b) using rigorous scientific approaches to disseminate and implement findings in practice. The research training program highlights six methods (i.e., catalysts) that can speed translation across the research cycle: stakeholder engagement, patient-centered outcomes, sequential multiple randomized trial (SMART) designs, pragmatic trials, mixed methods approaches, and dissemination and implementation science strategies. Fundamental to this paradigm is the integration of intervention development and testing efforts with implementation efforts rather than viewing them as distinct areas of research (Onken et al., 2014).
The Six Catalysts
In recent years, scientists have increasingly recognized that closing the time gap between research and practice necessitates the use of methods that consider implementation as early as the intervention development stage. That is, rather than ultimately trying to fit research-tested interventions into the “round holes” of patients' lives, clinical practice and the health care system, scientists should methodically develop and test interventions better suited to these “round holes” (Onken et al., 2014). Relevant methods for doing this include engaging stakeholders at multiple levels in meaningful ways (Newhouse, Barksdale & Miller, 2015); targeting patient-centered outcomes (PCORI, 2013); employing frameworks for intervention optimization and pragmatic trial designs to produce evidence more readily applicable to practice (Ammerman, Smith & Calancie, 2014; Mullins, 2014); using mixed-methods to generate evidence related to how an intervention works, for whom and under what circumstances (Leeman & Sandelowski, 2012; Sidani, 2015; Song, Sandelowski & Happ, 2010); and capitalizing on theory-based dissemination and implementation strategies.
Stakeholder engagement
Stakeholders are those with vested interests in improving the health of individuals and populations; they comprise patients, families, clinicians, community leaders, healthcare administrators, policy makers, and scientists among others (Barksdale, Newhouse & Miller, 2014). Nurse scientists have a longstanding history of engaging patients and their families in self-management and treatment decision-making (Barksdale et al., 2014). Engaging stakeholders, including those with low health literacy levels, across the translation process is essential to increasing the feasibility, effectiveness, relevance and usability of research-tested interventions, and to promoting broad access and comprehension (Gaglio, 2016; NASEM, 2016). Participatory action research is one method to engage with stakeholders in clinical settings and the community thereby ensuring that research focuses on health outcomes that are high priority to them, and that interventions and implementation efforts are feasible and acceptable to those who are expected to enact them in their daily lives or in practice (Frank et al., 2015). Stakeholder partners (e.g., professional organizations) also play critical roles in disseminating and implementing interventions across interpersonal and professional networks (Dearing & Kreuter, 2010; Rogers, 2003).
Patient-centered outcomes
For research-tested, theory-based interventions to be relevant and implemented widely in practice, they must address health outcomes that are important to patients and their families. In other words, they must be patient-centered (PCORI, 2013). We highlight this catalyst in the research training given nursing's long tradition of research focused on patient-centered and patient- or parent-reported outcomes (PROs) such as symptoms (e.g., cognition ability, fatigue, negative mood, pain, sleep disturbances), physical functioning and the ability to perform valued social roles such as attending school, working for pay and parenting. Intentional use of common data elements (i.e., data elements that are operationally defined and assessed the same way across multiple studies [Redeker, et al., 2015]) to assess PROs can improve data quality and also facilitate synthesis of findings across studies that examine the efficacy of the same intervention or compare the effectiveness of two or more clinically relevant interventions (Reeve, Mitchell, Dueck, et al., 2014). Measuring PROs with rigorously validated adult or pediatric domain-specific item sets such as those that comprise the Patient-Reported Outcome Measurement System (PROMIS)® and other NIH-supported resources (https://www.nlm.nih.gov/cde/) allows nurse scientists to use common data elements to evaluate and compare the effects of theory-based nursing interventions on symptoms, function and health-related behavior across studies, the life cycle or illness trajectory, populations and settings, from the patient perspective thus advancing nursing science and accelerating translation of research-tested interventions that target patient-centered outcomes (Grady, 2015; Moore, et al., 2016; Redeker, et al., 2015).
Intervention optimization and sequential multiple randomized trials (SMART)
In recent years, intervention scientists have sought to optimize interventions targeting behavioral changes to improve health outcomes using the frameworks of adaptive treatments and multiphase optimization strategy (Collins, Murphy & Bierman, 2004; Collins, Murphy & Strecher, 2007; Collins, Nahum-Shani & Amirall, 2014). Such an interest coincides with recognition that the traditional approach to development and testing often does not take into account varying needs among individuals and over time resulting in interventions that are fixed in dose and/or components (“all-or-none” approach). The RCT designs, the “gold standard” for evaluating whether an intervention is efficacious (Shadish, Cook & Campbell, 2002), are based on this fixed treatment framework, and provide information limited to whether the intervention as a whole “works” with the assumption that everyone is given all components of the intervention at the same dose level. Even when interventions are to be highly individualized based on the participant's needs, decisions to alter dosage or components have been art rather than science that can be systematically tested.
To address these gaps, Murphy and colleagues (2007) proposed sequential multiple randomized trial (SMART) designs in which participants are randomized more than once when a decision to alter the treatment strategy in intervention type, components, and dose is needed based on the participant's response to the previous intervention. SMART designs characteristically include two or more interventions with known efficacy, variables to inform decisions to alter the treatment strategy (tailoring variables), and explicit if-then decision rules that are stated a priori and specify the when and what (alternative conditions) for the next stage of treatment (Almirall, Nahum-Shani, Sherwood & Murphy, 2014). These sequential randomizations generate evidence to inform decisions about how to modify interventions to maximize patient health outcomes, mimicking clinical practice (Song et al., 2016). Given the imperative to speed translation and limitations in the information to be gleaned from conventional RCTs, added training in SMART designs will provide nurse scientists with the background they need to conduct studies that generate evidence more relevant to clinical practice.
Pragmatic trials
Pragmatic trials, also referred to as practical clinical trials (Tunis, Stryer & Clancy, 2003) are conducted to generate evidence about outcomes of interest to various stakeholder groups (e.g., relative benefits, risks, costs) and at multiple levels (e.g., patient, provider, practice setting, society) when efficacious interventions are delivered within constraints of everyday practice and the daily lives of participants who reflect the diversity of real world patient populations (Ford & Norrie, 2016; Gaglio, Phillips, Heurtin-Roberts, Sanchez & Glasgow, 2014; Schwartz & Leilouch, 1967). Virtually no trials are purely explantory or pragmatic; rather they reside along the explanatory-pragmatic continuum (Loudon, Treweek, Sullivan, Donna, Thorpe & Zwarenstein, 2015). Characteristically, pragmatic trials enroll diverse samples representative of the broad array of patients seen in practice with the problem of interest from multiple heterogeneous settings, and have few inclusion and exclusion criteria (Glasgow, Magid, Beck, Ritzwoller & Estabrook, 2005). The interventions are clinically relevant and typically relatively simple, low-cost and easy to implement in practice given time and other resource constraints (Thorpe, 2009), and the comparison condition is a clinically relevant real-world alternative (such as usual care), not a placebo.
Pragmatic trials are built around routine care operations as much as possible with flexible study protocol. Because randomization can be unworkable for making decisions in practice, non-RCT designs that afford some level of experimental control are used, including interrupted time series designs in which each practice setting acts as it own control and delayed treatment designs such as a clustered-randomized trial in which each practice setting eventually receives the intervention but the control settings do so after some time interlude has elapsed (Glasgow et al., 2005). In fact, the cluster- randomized design is the most common pragmatic trial design since the intervention being tested is implemented by providers as part of their routine care and thus risk of experimental contamination should be minimized.
Alternatively, pragmatic trials can use non-random means to allocate patients within a setting to one of the interventions being compared, for example, following pre-specified rules based on explicit patient characteristics or in accordance with provider professional judgments and/or assessments of patient preferences. To address research questions about the influence of provider characteristics on study outcomes, balanced numbers of providers representing the various types practicing in the settings are educated about the rationale for the alternative interventions and involved in their delivery (Sidani, 2015)
Mixed-methods
Interventions in the realm of chronic illness tend to address complex behaviors and co-occurring symptoms and thus involve multiple components (Song, Sandelowski, & Happ, 2010), making it difficult to evaluate these interventions and investigate mechanisms of change using single-method approaches (Craig, Dieppe, Macintyre, Michie, Nazareth & Petticrew, 2008). Alternatively, integrating qualitative and bio-behavioral methods with standardized PRO measures and other common data elements (e.g., socio-demographic variables, clinical data) within a study can increase the breadth of understanding of intervention moderators, mechanistic processes and linkages between these processes and health outcomes (IOM & NRC, 2011; Tashakkori & Teddlie, 2010).
Mixed-methods approaches are also increasingly valued for their contributions to the development of instruments (Knafl, et al., 2007), generic item sets (Reeve, et al. 2017) and condition-specific modules (Follansbee-Junger, Mann, Guilfoyle, Morita, Varni & Modi, 2016); for optimizing an intervention's feasibility, safety, acceptability, and efficacy (Song, Sandelowski, & Happ, 2010) and for translating evidence into carefully designed stakeholder-driven formats that stakeholders can readily understand and use to aid their decision-making about intervention adoption and implementation (Arcia, Suero-Tejeda, Bales, Merrill, Yoon, Woollen & Bakken, 2016; Kneipp, Leeman, McCall, Lich, Bobashev & Schwartz, 2015).
Mixing complementary approaches can also help identify which components of an efficacious intervention may be critical to its effectiveness across settings, the contextual factors that require the intervention to be adapted to fit that setting, the patient- or family-level factors that require a complex intervention to be tailored to maximize its impact (Song, Sandelowski, & Happ, 2010), and the interventionist characteristics or behaviors that can influence intervention delivery and treatment outcomes (Santacroce et al., 2004). Mixed-methods also are increasingly recognized as critical for understanding the process, context, and outcomes of implementing research in practice (Palinkas et al., 2011a; Palinkas et al., 2011b), particularly with diverse populations who have low health literacy levels (NAM, 2016). Training in best practices for mixed-methods research (Creswell, et al. for the OBSSR, 2011) will aid nurse scientists in logically integrating complementary methods throughout the intervention development and testing process.
Dissemination and implementation science
Dissemination and implementation is an emerging field of research. Because only a limited number of universities offer training in this field (Meissner et al., 2013), the NIH Office of Behavioral and Social Sciences Research (OBSSR) and others collaborated to create the Training Institute for Dissemination and Implementation Research in Health ([TIDIRH] NIH OSSBR, 2017). The TIDIRH planning team and others identified specific areas for training, which include dissemination and implementation-related theory; study designs and measures; approaches to implementing change across multiple levels; understanding contextual factors that influence implementation; and balancing the adaptation of evidence-based interventions to new contexts while maintaining fidelity to components and underlying principles that were responsible for the effectiveness of those interventions (Glasgow et al., 2012; Klesges et al., 2005; Meissner et al., 2013). Training in dissemination and implementation science will aid also future nurse scientists in producing optimally efficacious interventions that can be implemented in practice with the intended population, thus completing the intervention development and testing process and speeding translation.
Integrating the Six Catalysts into Ongoing Research Training
To promote trainees' understanding of the complete intervention development and testing process, we have integrated the six catalysts into required coursework, and bi-weekly seminars series, and experiential research training. We revised the two courses that we have historically required of all T32 pre- and postdoctoral trainees. The first course, “From Theory to Intervention” has been renamed “From Theory to Intervention and Implementation” and includes a new course objectives related to implementation science theory and new content about stakeholder engagement across the translation process. The second course “Designing Theory-Based Intervention Studies” was revised to include content about trial designs to accelerate translation (e.g., SMART, pragmatic trials), the use of mixed method approaches in intervention testing and implementation research, and the responsible conduct of research issues that can arise with each of the translation catalysts. All trainees attend a school-wide, biweekly Research Seminar series that is mandatory for T32 trainees. These seminars are planned with input from members of the T32 Advisory Board and include a focus on the T32's six catalysts. Each year, we invite an external consultant to present one of the Research Seminars and also to meet individually with the T32 trainees and faculty to advance their research training and ongoing programs of research.
The research training also includes an experiential component. For 8 hours each week, pre-doctoral trainees must work closely with their T32 mentors on the mentor's research. Post-doctoral trainees may also work closely with their mentors to conduct secondary analyses of their mentor's data and/or translate the mentor's intervention research to another population. For both groups of trainees, the mentored experiences are expected to relate to the six catalysts. For example, pre-doctoral trainees have worked with their mentors to engage community stakeholders in designing either a lifestyle change intervention that fits the local culture and existing resources of a predominantly African American rural community, or appealing recruitment materials for an evidenced-based chronic illness self-management intervention that targets working age adults. Post-doctoral trainees have used mixed-methods to translate their mentor's theory- and evidenced-based interventions for use with related yet distinct populations. Other trainees have played roles in designing and piloting patient-centered data collection platforms for their mentor's research that consider the personal (e.g., developmental stage, health literacy level, familiarity with digital technology) and clinical characteristics (e.g., potential for fatigue, pain) of the target population. Trainees also have opportunities to engage in research experiences that relate to using implementation science and other theories to develop and implement interventions. Nurse-led adaptive treatment and pragmatic trials are new to our institution thus trainees seek experiences with faculty in other schools and departments (e.g., health behavior, nutrition, psychology) who are T32 participating faculty and using these designs in their intervention research. Individual mentors collaborate with the program directors to identify such faculty and make the necessary connections for their mentees. Through these research experiences, our trainees gain both hands-on experiences in applying the catalysts and opportunities to present their work in national and international forums.
Evaluating Training Outcomes
We will evaluate the success of the program and its trainees based on benchmarks that apply to all PhD program students, supplemented by indicators specific to the six translation catalysts. Indicators applicable to all PhD students include (a) progress in implementing the activities and goals stated the individual development plan (bi-annually); (b) peer reviewed presentations and publications (bi-annually); (c) grants submitted and those awarded funding (bi-annually); (d) successful completion of the qualifying examination (upon finishing the five core courses typically taken during the first year in the PhD program); (e) oral defense of the dissertation proposal (typically early in the third year in the PhD program); and (f) oral defense of the dissertation (at program completion). All T32 trainees must also demonstrate proficiency in at least two of the six translation catalysts in their presentations, publications, research proposals and completed research. The T32 Directors assess trainee proficiency through their works in progress presentations and peer reviews at T32 integrative seminars, their scholarly products (peer-reviewed presentations, publications, applications for funding), and their dissertation (pre-doctoral trainees only).
Summary
In this paper, we described an institutional pre- and postdoctoral research training program supported by NINR that addresses the imperative to speed the translation of theory-based interventions for preventing and managing chronic illness. While maintaining the emphasis on theory-based interventions, we have integrated six catalysts into our ongoing research training to expedite the dynamic process of intervention development, testing, dissemination, and implementation. These catalysts include stakeholder engagement, patient-centered outcomes, intervention optimization and sequential multiple randomized trials, pragmatic trials, mixed-methods, and dissemination and implementation science. With a variety of training activities, including didactic instruction, seminars and mentored research experiences, our research training focused on theory-based interventions and the six catalysts will generate future nurse scientists to speed the translation of theory-based nursing interventions into practice as a means to maximize health outcomes for patients, families, communities and populations affected by chronic illness.
Highlights.
Research-tested interventions are slow to translate into practice.
To accelerate research translation, nurse scientists need training in new methods.
We emphasize stakeholder engagement, PROs, new trial designs, and mixed methods
Implementation science also is key to accelerating translation of research-tested interventions into practice.
Acknowledgments
The institutional research training program described in this article is funded by the National Institutes of Health/National Institute of Nursing Research (T32NR007091).
We acknowledge Merle H. Mishel, PhD, RN, FAAN for her numerous contributions to nursing science including the origination and long time leadership of this research training program in interventions to prevent and manage chronic illness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfano CM, Smith T, de Moor JS, Glasgow RE, Khoury MJ, Hawkins NA, et al. Rowland JH. An action plan for translating cancer survivorship research into care. Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Translational Behavioral Medicine. 2014;4:260–74. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammerman A, Smith TW, Calancie L. Practice-based evidence in public health: Improving reach, relevance, and results. Annual Review of Public Health. 2014;35:47–63. doi: 10.1146/annurev-publhealth-032013-182458. [DOI] [PubMed] [Google Scholar]
- Arcia A, Sueron-Tejada N, Bales M, Merrill J, Yoon S, Woollen J, Bakken S. Sometimes more is more: iterative participatory design of infographics for engagement of community members with varying levels of health literacy. Journal of the American Informatics Association. 2016;23:174–183. doi: 10.1093/jamia/ocv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas E. Yearbook of Medical Informatics. Stuttgart, Germany: Schattauer; 2000. Managing clinical knowledge to health care improvement. [PubMed] [Google Scholar]
- Barksdale DJ, Newhouse R, Miller JA. The patient-centered outcomes research institute (PCORI): Information for academic nursing. Nursing Outlook. 2014;62:192–200. doi: 10.1016/j.outlook.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Bartholomew LK, Dolan P. Five roles for using theory and evidence in the design and testing of behavior change interventions. Journal of Public Health Dentistry. 2010;71:S20–33. doi: 10.1111/j.1752-7325.2011.00223.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Prevention & Control. Chronic disease overview. 2016 Accessed online at: https://www.cdc.gov/chronicdisease/overview/
- Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive prevention interventions. Prevention Science. 2004;5:185. doi: 10.1023/B:PREV.0000037641.26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Strecher V. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): New methods for potent ehealth interventions. American Journal of Prevention Medicine. 2007;32:S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colllins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial. Clinical Trials. 2014;11:426–434. doi: 10.1177/1740774514536795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: The new medical research council guidance. 2008;337 doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JW, Klassen AC, Plano Clark VL, Smither KC. Best practices for mixed methods research in the health sciences. National Institutes of Health: Office of Behavioral and Social Sciences Research; 2011. Retrieved from: https://obssr.od.nih.gov/training/mixed-methods-research/ [Google Scholar]
- Dearing JW, Kreuter MW. Designing for diffusion: How can we increase uptake of cancer communication innovations? Patient Education and Counseling. 81:S100–S110. doi: 10.1016/j.pec.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follanson-Junger K, Mann K, Guilfloyle S, Morita D, Varni J, Modi A. Development of the PedsQL™ Elilepsy Module: Focus grops and cognitive interviews. Epilepsy & Behavior. 2016;62:115–120. doi: 10.1016/j.yebeh.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Ford I, Norrie J. Pragmatic trials. New England Journal of Medicine. 2016;375:454–63. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- Frank L, Forsythe L, Ellis L, Schrandt S, Sheridan S, Gerson J, et al. Daugherty S. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Quality of Life Research. 2015:1–9. doi: 10.1007/s11136-014-0893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio B. Health literacy- an important element in patient-centered outcomes research. Journal of Health Communications. 2016;21:1–3. doi: 10.1080/10810730.2016.1184359. [DOI] [PubMed] [Google Scholar]
- Gaglio B, Phillips S, Heurtin-Roberts S, Sanchez N, et al. Glasgow R. How pragmatic is it? Lessons learned using PRECIS and RE-AIM for determing pragmatic characteristics of research. Implementation Science. 2014;9:96. doi: 10.1186/s13012-014-0096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE. What types of evidence are most needed to advance behavioral medicine? Annals of Behavioral Medicine. 2008;35:19–25. doi: 10.1007/s12160-0079008-5. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Magid D, Beck A, Ritzwoller D, Estabrooks P. Practical trials for translating research into practice. Design and measurement recommendations. Medical Care. 2005;43:551–57. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of Health approaches to dissemination and implementation science: Current and future directions. American Journal of Public Health. 2012;102:1274–81. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady P. Director's message: Research tools for patient-reported outcomes. 2014 Retrieved from http://www.ninr.nih.gov/aboutninr/directors-message.
- Institute of Medicine & National Research Council. Toward an integrated science of research on families: Workshop reports Committte on the Science of Research on Families. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
- Kessler R, Glasgow RE. A proposal to speed translation of healthcare research into practice: Dramatic change is needed. American Journal of Preventive Medicine. 2011;40:637–644. doi: 10.1016/j.amepre.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: From scientific discovery to population health impact. American Journal of Epidemiology. 2010;172:517–524. doi: 10.1093/aje/kwq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: Designing and planning health behavior change interventions to enhance dissemination. Annals of Behavioral Medicine. 2005;29 Suppl:66–75. doi: 10.1207/s15324796abm2902s_10. [DOI] [PubMed] [Google Scholar]
- Knafl K, Deatrick J, Gallo A, Holcombe G, Bakitas M, FDixon J, Grey M. The analysis and interpretation of cognitive interviews for instrument development. Research in Nursing & Health. 2007;30:224–234. doi: 10.1002/nur.20195. [DOI] [PubMed] [Google Scholar]
- Kneipp SM, Leeman J, McCall P, Hassmiller-Lich K, Bobasheve G, Schwartz TA, Gimore R, Gil B. Synthesizing marketing, community engagement, and systems science approacher for advancing translational research. ANS: Advances in Nursing Science. 2015;38:227–240. doi: 10.1097/ANS.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H, Wilson G, Fiarburn C. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2001;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Lam TK, Spitz M, Schully SD, Khoury MJ. “Drivers” of translational cancer epidemiology in the 21st century: Needs and opportunities. Cancer Epidemiology Biomarkers and Prevention. 2013;22:181–188. doi: 10.1158/1055-9965.epi-12-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman J, Jilcott-Pitts S, Myers A. Speeding the dissemination and implementation of evidence-based intervantions for cancer control and prevention. North Carolina Medicine. 2014;75:261–4. doi: 10.18043/ncm.75.4.261. [DOI] [PubMed] [Google Scholar]
- Leeman J, Sandelowski M. Practice-based evidence and qualitative inquiry. Journal of Nursing Scholarship. 2012;44(2):171–179. doi: 10.1111/j.1547-5069.2012.01449.x. [DOI] [PubMed] [Google Scholar]
- Leeman J, Sommers J, Leung MM, Ammerman A. Disseminating evidence from research and practice: A model for selecting evidence to guide obesity prevention. Journal of Public Health Management and Practice. 2011;17(2):133–140. doi: 10.1097/PHH.0b013e3181e39eaai. [DOI] [PubMed] [Google Scholar]
- Lipsey MW. Theory as method: Small theories of treatments. New Directions for Program Evaluation. 1993;1993:5–38. doi: 10.1002/ev.1637. [DOI] [Google Scholar]
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- Meissner HI, Glasgow RE, Vinson CA, Chambers D, Brownson RC, Green LW, et al. Mittman B. The U.S. training institute for dissemination and implementation research in health. Implementation Science. 2013;8:12. doi: 10.1186/1748-5908-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslin E, Blasimme A, Cambon-Thomsen A. Mapping the translational science policy ‘valley of death’. Clinical & Translational Medicine. 2013;2:14. doi: 10.1186/2001-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Schiffman R, Waldrop-Valverde D, Redeker N, McCloskey D, Kim M, et al. Grady P. Recommendations of common data elements to advance the science of self-management of chronic conditions. Journal of Nursing Scholarship. 2016;48:437–447. doi: 10.1111/jnu.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Lynch KG, Osline D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug & Alchohol Dependence. 2007;88(Suppl 2):S24–S30. doi: 10.1016/j.drugalcdep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine. Relevance of health literacy to precision medicine: Proceedings of a workshop. Washington, D.C.: The National Academies Press; 2016. [DOI] [PubMed] [Google Scholar]
- National Institute of Health Office of Behavioral & Social Science. Training Institute in Dissemination and Implementation Science. 2017 Retrieved from: https://obssr.od.nih.gov/training/training-institutes/training-institute-on-dissemination-and-implementation-research-tidirh/
- Newhouse R, Barksdale DJ, Miller JA. The Patient-Centered Outcomes Research Institute: Research done differently. Nursing Research. 2015;64:72–7. doi: 10.1097/NNR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shorham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: Unifying the discipline to improve public health. Clinical Psychological Science. 2014;2:22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinkas LA, Horwitz SM, Chamlerlain P, Hurlburt MS, Landsverk J. Mixed method designs in implementation research. Administraion & Policy in Mental Health. 2011a;38:44–53. doi: 10.1007/s10488-010-0314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinkas LA, Horwitz SM, Chamlerlain P, Hurlburt MS, Landsverk J. Mixed-methods deisgns in mental health services research: A review. Psychiatric Services. 2011b;62:255–263. doi: 10.1176/appi.ps.62.3.255. [DOI] [PubMed] [Google Scholar]
- Patient-Centered Outcomes Research Institute. 2017 Accessed online at: http://www.healthmeasures.net/explore-measurement-systems/promis.
- Peek C, Glasgow R, Stange K, Klesges L, Purcell E, Kessler R. The 5 R's: An emerging bold standard for conducting relevant research in a changing world. Annals of Family Medicine. 2014;12:447–455. doi: 10.1370/afm.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EK, Powell BJ, McMillen JC. Implementation strategies: Recommendations for specifying and reporting. Implementation Science. 2013;8:139. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker N, Anderson R, Bakken S, Corwin E, Docherty S, Dorsey S, Heitkemper M, McCloskey D, Moore S, Pullen C, Rapkin B, Schiffman R, Waldrop-Valverde D, Grady P. Advancing symptom science through the use of common data elements. Journal of Nursing Scholarship. 2015;47:379–388. doi: 10.1111/jnu.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve BB, McFatrich M, Pinheiro L, Freyer D, Basch E, Baker J, et al. Hinds P. Cognitive interiew-based validation of the patient-reported outcomes version of the Common Terminology Critieria for Adverse Events (PRO-CTCAE) in adolescents with cancer. Journal of Pain & Symptom Management. 2017 doi: 10.1016/j.jpainsymman.2016.11.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve BB, Mitchell SA, Dueck AC, Basch E, Cella D, et al. Bruner DW. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EM. Diffusion of innovations. New York, NY: Free Press; 2003. [Google Scholar]
- Santacroce SJ, Macarelli LM, Grey M. Intervention fidelity. Nursing Research. 2004;53:63–66. doi: 10.1097/00006199-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Shadish W, Cook T, Campbell D. Experimental and quasi-experimental designs for generalized causal inference. New York, NY: Houghton Mifflin Company; 2002. [Google Scholar]
- Sidani S. Health intervention research: Understanding research design and methods. Thousand Oaks, CA: Sage; 2015. [Google Scholar]
- Sidani S, Braden C. Design, evaluation and translation of nursing intervention studies. Philadelpia, PA: Wiley and Sons; 2011. [Google Scholar]
- Song MK, DeVito Dabbs A, Ward S. A SMART design to optimize treatment strategies for patient and family caregiver outcomes. Nursing Outlook. 2016;64:299–305. doi: 10.1016/j.outlook.2016.04.008. http://dx.doi.org/10.1016/j.outlook.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Song MK, Sandelowski M, Happ MB. Current practices and emerging trends in conducting mixed-methods intervention studies. In: Tashakkori A, Tashakkori C, editors. Mixed-methods in social and behavioural research. 2nd. Thousand Oaks, CA: Sage; 2010. pp. 727–747. [Google Scholar]
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutic trials. Journal of Chronic Diseases. 1967;20:637–648. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- Taskakkori A, Teddlie C. Handbook of mixed-methods in social and behavioral research. Thousand Oaks, CA: Sage; 2010. [Google Scholar]
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. Chalkidou K. A pragmatic-explanatory continuum indicator summary (PRECIS): A tool to help trial designers. Journal of Clinical Epidemiology. 2009;62:464–475. doi: 10.1016/j.clinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Tunis S, Stryer D, Clancey C. Practical clinical trials. Increasing the value of clinical research for decision making in clinical and health policy. Journal of the American Medical Association. 2003;290:1624–32. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Westfall JM, Mold J, Fagnan L. Practice-based research - “Blue Highways” on the NIH roadmap. Journal of the American Medical Association. 2007;297:403–6. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]


