Abstract
Background
Ketamine induces rapid and robust antidepressant effects, and many patients also describe dissociation, which is associated with antidepressant response. This follow-up study investigated whether antidepressant efficacy is uniquely related to dissociative symptom clusters.
Methods
Treatment-resistant patients with major depressive disorder (MDD) or bipolar disorder (BD) (n=126) drawn from three studies received a single subanesthetic (0.5mg/kg) ketamine infusion. Dissociative effects were measured using the Clinician-Administered Dissociative States Scale (CADSS). Antidepressant response was measured using the 17-item Hamilton Depression Rating Scale (HAM-D). A confirmatory factor analysis established the validity of CADSS subscales (derealization, depersonalization, amnesia), and a general linear model with repeated measures was fitted to test whether subscale scores were associated with antidepressant response.
Results
Factor validity was supported, with a root mean square error of approximation of .06, a comparative fit index of .97, and a Tucker-Lewis index of .96. Across all studies and timepoints, the depersonalization subscale was positively related to HAM-D percent change. A significant effect of derealization on HAM-D percent change was observed at one timepoint (Day7) in one study. The amnesia subscale was unrelated to HAM-D percent change.
Limitations
Possible inadequate blinding; combined MDD/BD datasets might have underrepresented ketamine’s antidepressant efficacy; the possibility of Type I errors in secondary analyses.
Conclusions
From a psychometric perspective, researchers may elect to administer only the CADSS depersonalization subscale, given that it was most closely related to antidepressant response. From a neurobiological perspective, mechanistic similarities may exist between ketamine-induced depersonalization and antidepressant response, although off-target effects cannot be excluded.
Keywords: dissociation, ketamine, depression
INTRODUCTION
The glutamatergic modulator ketamine is a U.S. Food and Drug Administration (FDA)-approved anesthetic. Distinct from traditional anesthetics, ketamine has been called a “dissociative anesthetic” due to the occurrence of dissociative symptoms (Sleigh et al., 2014). Dissociation can be loosely defined as a detachment from reality and manifests across a spectrum of severity. Pathological dissociation can include depersonalization (detachment from self), derealization (detachment from surroundings), amnesia and, in more severe, persistent manifestations, fugue states and dissociative identity disorder. At typical clinical doses, ketamine-induced dissociation is mild to moderate in severity and transient in duration, with patients returning to their premorbid mental state within hours of administration. Ketamine is thought to derive its anesthetic, psychotomimetic, and dissociative properties from noncompetitive, high-affinity antagonism at the N-methyl-D-aspartate (NMDA) receptor (MacDonald and Nowak, 1990).
A single, subanesthetic-dose ketamine infusion has repeatedly been demonstrated to reduce depressive symptoms in a matter of hours (Berman et al., 2000; Diazgranados et al., 2010; Ibrahim et al., 2012b; Murrough et al., 2013; Valentine et al., 2011; Zarate et al., 2006). NMDA receptor antagonism has been proposed as an initiating molecular event in ketamine’s antidepressant effects (Maeng and Zarate, 2007), although recent preclinical evidence supports non-NMDA receptor-mediated antidepressant properties of the ketamine metabolite (2R,6R)-hydroxynorketamine (Zanos et al., 2016); as predicted in animal models, the latter is not associated with clinically problematic side effects such as psychomotor agitation, prepulse inhibition, and addictive-like behaviors (Green and Johnson, 1990; Zanos et al., 2016). While other NMDA receptor antagonists with lower and/or more specific affinities have demonstrated antidepressant efficacy without psychotomimetic or dissociative side effects (Ibrahim et al., 2012a; Zarate et al., 2013), these other NMDA receptor antagonists generally have much less robust and sustained antidepressant effects than ketamine; it is presently unclear whether this represents qualitative and/or quantitative differences at the receptor (Aan Het Rot et al., 2012; Zanos et al., 2016).
The relationship between ketamine’s psychoactive side effects and antidepressant response has been explored in several studies. A study of 10 subjects with treatment-resistant major depressive disorder (MDD) found no association between maximum change in dissociative symptoms (as measured by the Clinician-Administered Dissociative States Scale (CADSS)) and change in depressive symptom scores (as measured by the Hamilton Depression Rating Scale (HAM-D)) in response to a single, subanesthetic-dose ketamine infusion (Valentine et al., 2011). Another study of 27 depressed hospitalized patients found a correlation between antidepressant response (as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS)) and psychotomimetic symptoms (as measured by the Brief Psychiatric Rating Scale (BPRS)) seven days post-ketamine infusion (Sos et al., 2013). Finally, a previous study from our laboratory reported that increased dissociative symptoms—but not psychotomimetic, hypomanic, or sympathomimetic symptoms—correlated with antidepressant response to ketamine at 230 minutes and at seven days post-ketamine infusion in 108 treatment-resistant hospitalized inpatients with either MDD or bipolar disorder (BD) (Luckenbaugh et al., 2014).
These conflicting findings prompted the present investigation into the relationship between ketamine-induced dissociative symptoms and antidepressant efficacy. This follow-up study uses a larger sample (n=126, inclusive of the previous sample (Luckenbaugh et al., 2014)) and explores empirically validated subdimensions of dissociation for the first time. We hypothesized that increased dissociative symptoms would predict improvement in depressive symptom scores after ketamine infusion, but that subdimensions of dissociation, as derived by Bremner and colleagues (Bremner et al., 1998), would uniquely predict antidepressant response.
METHODS
Participants and procedures
Data from 126 treatment-resistant depressed patients were analyzed (18–65 years old; 84 MDD, 42 BD). Patient data were obtained from one of three studies, colloquially identified as Ketamine-Bipolar (Ket-BD, n=39) (Diazgranados et al., 2010; Zarate et al., 2012), Ketamine-Riluzole (Ket-Riluzole, n=52) (Ibrahim et al., 2012b), or Ketamine-MOA (Ket-MOA, n=35) (Nugent et al., in press) (Clinical Trials Identifier: NCT0088699; NIH Protocol 04-M-0222, substudies 2, 3, and 4, respectively). All subjects were studied as inpatients at the National Institute of Mental Health’s Clinical Research Center in Bethesda, MD, USA and provided written informed consent as approved by the NIH Combined Neuroscience Institutional Review Board.
At screening, all patients were currently experiencing a major depressive episode without psychotic features that had lasted at least two weeks, diagnosed according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First et al., 2002); this depressive episode was judged to be of at least moderate severity (as defined by >20 on the 10-item MADRS or >18 on the 17-item HAM-D). Subanesthetic-dose (0.5mg/kg) ketamine was administered over 40 minutes in either double-blind, placebo (saline)-controlled (Ket-MOA and Ket-BD) or open-label (Ket-Riluzole) designs. Treatment resistance was defined via a modified version of the Antidepressant Treatment History Form (ATHF), and all subjects had failed to respond to at least one adequate antidepressant trial during the current or during a past major depressive episode. Patients were medication-free for at least two weeks prior to the first ketamine or placebo infusion (five weeks for fluoxetine), except for BD patients who were maintained on therapeutic levels of lithium (0.6–1.2 mEq/L) or valproate (50–125 μg/mL).
Instruments
Depressive and dissociative symptoms were assessed using the 17-item HAM-D (Hamilton, 1960) and the 19-item CADSS (Bremner et al., 1998), respectively. Each item on the CADSS is scored on a five-option Likert scale ranging from 0 (not at all) to 4 (extremely). Three non-empirically derived subscales for the CADSS have been proposed: amnesia (two items), depersonalization (five items), and derealization (12 items) (Bremner et al., 1998). CADSS scores were calculated as change between baseline (60 minutes prior to infusion) and 40 minutes post-infusion, when maximal dissociative effects were observed.
Statistical Analysis
To establish the factor validity of the CADSS subscales described by Bremner and colleagues (Bremner et al., 1998), a confirmatory factor analysis for ordered categorical indicators was performed. Model fit was evaluated using standard interpretation of fit indices, including the root mean square error of approximation, comparative fit index, and Tucker-Lewis index (Hu and Bentler, 1999).
To confirm our previous finding that ketamine’s dissociative effects predict improvement in depressive symptoms, and to evaluate whether subdimensions of dissociation (CADSS subscale scores) were uniquely related to antidepressant response, we fitted a general linear model with maximum likelihood estimation and repeated measures with a compound symmetry covariance structure (selected based on relative fit indices) to HAM-D percent change scores from 230 minutes, one day, and seven days post-infusion. Fixed effects of time, study, CADSS score, and their interactions were included in this model. Significant interactions were probed by estimating and comparing the simple slopes for each study by time point.
Statistical significance was evaluated at p ≤ .05, two-tailed. Analyses were performed in Mplus Version 7 and SAS Version 9.4.
RESULTS
Characterization of Sample
Participant demographics are shown in Table 1. Several demographic features differed significantly across studies; partially to account for this fact, study was entered as a covariate in all analyses.
Table 1.
Demographic information
| Ket-MOA (n=35) | Ket-BD (n=39) | Ket-Riluzole (n=52) | Omnibus Test | Post-hoc comparisons, unadjusted p | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M (SD) | M (SD) | M (SD) | MOA vs. BD | MOA vs. Ril | BD vs. Ril | ||
| Age, years | 36.2 (10.3) | 45.7 (10.6) | 47.9 (13.0) | F(2,122)=11.3, p<.001 | 0.001 | <.001 | 0.37 |
| BMI, kg/m2 | 27.2 (6.8) | 29.8 (5.8) | 29.8 (6.4) | F(2,122)=2.2, p=.12 | |||
| Age of depression onset, years | 15.3 (6.5) | 17.5 (6.9) | 19.9 (11.8) | F(2,120)=2.6, p=.08 | |||
| Length of illness, years | 20.6 (12.0) | 28.2 (10.5) | 28.1 (13.8) | F(2,120)=4.6, p=.01 | 0.01 | 0.007 | 0.95 |
| Length current major depressive episode, mos. | 43.1 (67.6) | 18.6 (21.0) | 86.8 (13.8) | F(2,118)=6.5, p=.002 | 0.25 | 0.03 | 0.001 |
| Clinical ratings at baseline | |||||||
| HAM-D (17-item) | 22.0 (4.3) | 21.2 (4.1) | 21.0 (4.2) | F(2,123)=0.7, p=.49 | |||
| BDI | 30.5 (8.0) | 28.5 (8.2) | 27.1 (8.5) | F(2,123)=1.8, p=.18 | |||
| MADRS | 34.2 (4.4) | 33.0 (4.4) | 33.1 (4.7) | F(2,123)=0.8, p=.43 | |||
| CADSS ratings at 40 minutes | |||||||
| Derealization | 12.7 (9.3) | 13.5 (9.7) | 14.0 (10.6) | F(2,123)=0.2, p=.84 | |||
| Depersonalization | 6.3 (5.9) | 7.1 (6.0) | 7.3 (5.9) | F(2,123)=0.3, p=.72 | |||
| Amnesia | 2.9 (2.4) | 2.5 (2.0) | 2.8 (2.7) | F(2,123)=0.3, p=.76 | |||
| Total Score | 25.1 (18.6) | 25.7 (18.1) | 27.1 (20.5) | F(2,123)=0.1, p=.88 | |||
| Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Prior Psychiatric Hospitalizations | 1 (0 – 2) | 2.5 (1 – 5.25) | 1 (0 – 2) | χ2(2,117)=12.8, p<.001 | <.001 | 0.53 | 0.003 |
| Total Lifetime Antidepressant Trials | 9.5 (7.8 – 13.5) | 7 (4 – 10) | 7 (4 – 10) | χ2(1,96)=7.2, p=.03 | 0.007 | 0.02 | 0.61 |
| n (%) | n (%) | n (%) | |||||
| Sex, female | 23 (65.7) | 23 (59.0) | 19 (36.5) | χ2(2,124)=8.4, p=.02 | 0.56 | 0.008 | 0.03 |
| Education, college graduate | 18 (51.4) | 15 (38.5) | 36 (69.2) | χ2(2,122)=8.0, p=.02 | 0.25 | 0.13 | 0.005 |
| Lifetime Diagnosis | |||||||
| Alcohol Use Disorder | 4 (11.4) | 21 (53.8) | 10 (19.2) | χ2(2,113)=16.7, p<.001 | 0.001 | 0.65 | <.001 |
| Substance Use Disorder (non-nicotine) | 7 (20.0) | 18 (46.2) | 21 (40.4) | χ2(2,117)=3.9, p=.15 | |||
| Lifetime History of | |||||||
| Abuse | |||||||
| Physical | 2 (5.7) | 6 (15.4) | 14 (26.9) | χ2(2,107)=3.6, p=.16 | |||
| Sexual | 6 (17.1) | 11 (28.2) | 10 (19.2) | χ2(2,107)=1.8, p=.41 | |||
| Suicide Attempt | 14 (40.0) | 18 (46.2) | 20 (38.5) | χ2(2,122)=1.0, p=.61 | |||
| Family History of | |||||||
| Alcohol Use Disorder, 1st Degree Relative | 14 (40.0) | 14 (53.9) | 19 (36.5) | χ2(2,122)=0.1, p=.95 | |||
| Alcohol Use Disorder, 2nd Degree Relative | 6 (17.1) | 20 (51.3) | 16 (30.8) | χ2(2,114)=10.6, p=.005 | 0.001 | 0.12 | 0.05 |
| Mood Disorder | 26 (74.3) | 35 (89.7) | 44 (84.6) | χ2(2,122)=3.4, p=.18 | |||
| Suicide Attempt | 9 (25.7) | 10 (25.6) | 17 (32.7) | χ2(2,111)=0.7, p=.72 | |||
Note: Some data missing (see df in omnibus test). Omnibus tests were ANOVA for continuous variables, Kruskal-Wallis test for ordinal variables, and chi-squared test for categorical variables. Some patients enrolled in each study were excluded from analysis if they did not receive ketamine or if they were missing CADSS ratings at baseline and 40 minutes post-ketamine (Ket-MOA, n=4; Ket-BD, n=2).
Abbreviations: BDI: Beck Depression Inventory; BMI: Body Mass Index; BD: bipolar disorder; CADSS: Clinical Administered Dissociative States Scale; HAM-D: Hamilton Depression Rating Scale; IQR: Inter-Quartile Range; Ket: Ketamine; M: Mean; MADRS: Montgomery-Åsberg Depression Rating Scale; MOA: Mechanism of Action; Ril: Riluzole; SD: Standard Deviation
Factor Validity of the CADSS Subscales
Factor validity of the CADSS subscales was evaluated using the 40-minute data (n=126). The three-factor model was a good fit to the data, with a Room Mean Square Error of Approximation (RMSEA) of .06 (95% CI: .042 – .077), a Comparative Fit Index (CFI) of .97, and a Tucker-Lewis Index of .96. Full results of the confirmatory factor analysis are shown in Supplementary Tables S1–S3.
Ketamine-Induced Dissociation and Antidepressant Response
The results of the four mixed models—CADSS Total Score as well as the Derealization, Depersonalization, and Amnesia subscales—are presented in Table 2. When predicting percent change in HAM-D score, a significant three-way interaction was observed between CADSS total score, study, and time (F(4,204)=2.70, p=.03); specifically, greater CADSS total score predicted greater percent change in HAM-D score at Day 7 within the Ket-MOA study (B=−0.62, SE=0.30, t(204)=−2.09, p=.04). This differed significantly from the slope observed for the Ket-BD study (B=0.20, SE=0.29, t(204)=0.71, p=.48; comparison, t(204)=−1.99, p=.048). The slope for the Ket-Riluzole study was non-significant, and did not differ significantly from that seen in the Ket-MOA study. Across all studies, the relationship between CADSS total score and change in HAM-D score was negative but non-significant at 230 minutes (B=−0.25, SE=0.14, t(204)=−1.70, p=.09) and Day 1 (B=−0.10, SE=0.14, t(204)=−0.72, p=.47).
Table 2.
CADSS Total/Subscale Scores and HAM-D Percent Change Linear Mixed Models
| Model Effect | Numerator df | Denominator df | F | p |
|---|---|---|---|---|
| CADSS Total Score | ||||
| Time | 2 | 204 | 4.03 | .02 |
| Study | 2 | 118 | 1.24 | .29 |
| CADSS | 1 | 118 | 1.92 | .17 |
| Time*Study | 4 | 204 | 0.76 | .55 |
| CADSS*Time | 2 | 204 | 0.53 | .59 |
| CADSS*Study | 2 | 118 | 0.43 | .65 |
| CADSS*Time*Study | 4 | 204 | 2.70 | .03 |
| CADSS Derealization | ||||
| Time | 2 | 204 | 3.37 | .04 |
| Study | 2 | 118 | 2.22 | .11 |
| CADSS | 1 | 118 | 2.66 | .11 |
| Time*Study | 4 | 204 | 0.86 | .49 |
| CADSS*Time | 2 | 204 | 0.42 | .66 |
| CADSS*Study | 2 | 118 | 1.31 | .27 |
| CADSS*Time*Study | 4 | 204 | 2.73 | .03 |
| CADSS Depersonalization | ||||
| Time | 2 | 204 | 9.27 | .0001 |
| Study | 2 | 118 | 0.90 | .41 |
| CADSS | 1 | 118 | 4.28 | .04 |
| Time*Study | 4 | 204 | 0.60 | .66 |
| CADSS*Time | 2 | 204 | 1.23 | .29 |
| CADSS*Study | 2 | 118 | 0.26 | .77 |
| CADSS*Time*Study | 4 | 204 | 0.97 | .42 |
| CADSS Amnesia | ||||
| Time | 2 | 204 | 4.06 | .02 |
| Study | 2 | 118 | 0.90 | .41 |
| CADSS | 1 | 118 | 0.02 | .90 |
| Time*Study | 4 | 204 | 0.64 | .63 |
| CADSS*Time | 2 | 204 | 1.19 | .31 |
| CADSS*Study | 2 | 118 | 0.13 | .88 |
| CADSS*Time*Study | 4 | 204 | 1.04 | .39 |
Note: Time is a three-level fixed variable (230 minutes, Day 1, Day 7). Study is a three-level fixed variable (Ket-MOA, Ket-BD, and Ket-Riluzole). Statistically significant (p ≤.05) effects are bolded.
Abbreviations: BD: bipolar disorder; CADSS: Clinician Administered Dissociative States Scale; df: Degrees of Freedom; HAM-D: Hamilton Depression Rating Scale; Ket: Ketamine; MOA: Mechanism of Action
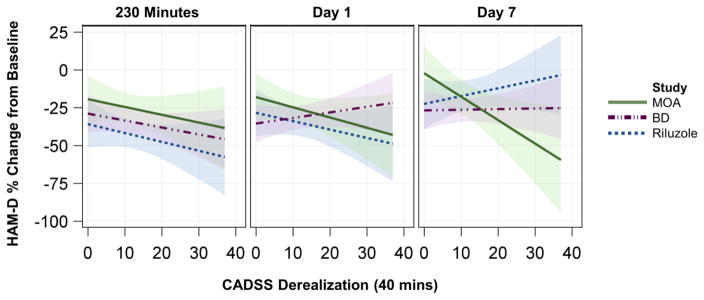
A very similar result was observed for the Derealization subscale, with a significant three-way interaction noted for Derealization, study, and time (F(4,204)=2.73, p=.03); specifically, increased ketamine-induced Derealization predicted greater antidepressant response. As with total score, this was explained by a significant effect of Derealization on percent change in HAM-D score at Day 7 in the Ket-MOA study only (B=−1.55, SE=0.65; t(204)=−2.37, p=.019) (Figure 1). The slope for the Ket-MOA study at Day 7 differed significantly from that of both the Ket-BD (B=0.51, SE=0.52, t(204)=0.99, p=0.33; comparison: t(204)=−2.47, p=0.01) and Ket-Riluzole (B=0.04, SE=0.39, t(204)=0.11, p=0.92; comparison: t(204)=−2.09, p=0.038) studies. Across all studies, the relationship between the Derealization subscale and percent change in HAM-D score was negative but non-significant at both 230 minutes (B=−0.52, SE=0.28, t(204)=−1.89, p=.06) and Day 1 (B=−0.29, SE=0.27, t(204)=−1.04, p=.30).
Figure 1. CADSS Derealization Subscale and Antidepressant Response to Ketamine.
A statistically significant three-way interaction was observed between the CADSS Derealization subscale score, study, and time (F(4,208)=2.7, p=.03. Slope was significant only for the Ket-MOA study at Day 7 (B=−1.55, SE=0.65; t(204)=−2.37, p=.019). Predicted values from the linear mixed model are plotted; shaded areas represent the 95% confidence limits. Abbreviations: BD: bipolar disorder; CADSS, Clinician Administered Dissociative States Scale; HAM-D: Hamilton Depression Rating Scale; MOA: Mechanism of Action; B: unstandardized regression coefficient; SE: Standard Error
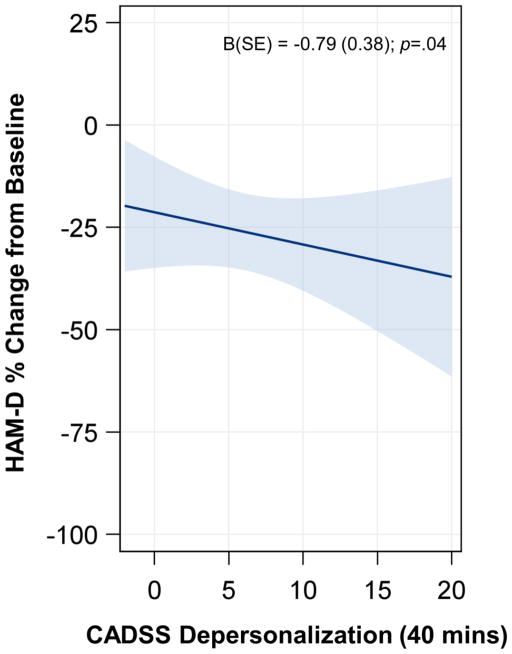
The Depersonalization subscale was also negatively related to percent change in HAM-D score (B=−0.79, SE=0.38, t(118)=−2.07, p=.04), but this effect did not vary by time or study. In other words, across all studies and timepoints, greater ketamine-induced Depersonalization predicted greater antidepressant response (Figure 2). The amnesia subscale was not related to percent change in HAM-D score; all effects were non-significant.
Figure 2. CADSS Depersonalization Subscale and Antidepressant Response to Ketamine.
A main effect of CADSS Depersonalization subscale score was observed (t(118)=−2.07, p=.04; interactions with study and time were non-significant). Fit line was computed for visualization at Day 1 for KET-MOA. Shaded areas represent the 95% confidence limits. Abbreviations: CADSS, Clinician Administered Dissociative States Scale; HAM-D: Hamilton Depression Rating Scale; B: unstandardized regression coefficient; SE: Standard Error
DISCUSSION
The present study extends previous work from our laboratory investigating ketamine-induced dissociation and antidepressant response (Luckenbaugh et al., 2014). In the initial report, intra-infusion dissociation positively correlated with antidepressant response to ketamine at two post-infusion timepoints: 230 minutes and Day 7; however, the previous analysis did not address whether specific dimensions of dissociation were uniquely related to antidepressant response. Using a larger sample and a statistical model allowing the inference of causality, the present study observed that increased Derealization and Depersonalization were modest predictors of antidepressant response to subanesthetic dose ketamine infusion.
The association between ketamine-induced Derealization and antidepressant response was observed only at Day 7 and only in the Ket-MOA study, with a significant difference observed between the Ket-MOA and Ket-BD studies. Given the magnitude of the p-values, it is probable that these differences represent a Type I (false positive) error. However, some methodological and demographic differences may be pertinent. First, Ket-BD subjects were maintained on therapeutic doses of the mood stabilizers lithium or valproate, while the Ket-MOA subjects were unmedicated for at least two weeks prior to ketamine administration. Second, Ket-BD subjects also tended to have a longer duration and more severe course of illness (specifically, they had more psychiatric hospitalizations) than patients in the Ket-MOA study, as well as a shorter duration of antidepressant response. In addition, a recent meta-analysis noted diagnostic differences in baseline dissociation, with BD patients being the least likely to experience dissociation compared to other major neuropsychiatric disorders, including MDD (Lyssenko et al., 2017). It should be noted, however, that we observed no significant diagnostic (MDD vs. BD) differences in dissociation in the current analysis (Supplementary Table S4), suggesting that diagnosis alone cannot explain the observed differences across studies.
In contrast to Derealization, Depersonalization was associated with greater antidepressant response across all time points and studies; the consistency suggests that this result is less likely to be due to a Type I (false positive) error. It is possible that the underlying neurobiological constructs measured by the Depersonalization subscale of the CADSS may relate to ketamine’s antidepressant mechanism(s) of action, though it is necessary to acknowledge that potential off-target effects may exist. We hypothesize a potential causal link between Depersonalization and antidepressant response, initiated by glutamate receptor modulation—indeed, ketamine has repeatedly been found to induce glutamate release (DeLorenzo et al., 2015; Lorrain et al., 2003; Moghaddam et al., 1997; Stone et al., 2012), and its dissociative properties may result from either excess or insufficient glutamatergic tone (Aan Het Rot et al., 2012). This modulation, in turn, would result in circuit-level responses, including changes in default mode network connectivity (Abdallah et al., 2017; Bonhomme et al., 2016; Nugent et al., 2016).
This study is the first reported validation of the CADSS subscales proposed by Bremner and colleagues (Bremner et al., 1998), which were initially developed for use in dissociative disorders, including post-traumatic stress disorder, as well as in healthy volunteers. Although a recent study questioned the instrument’s ability to fully capture ketamine’s psychoactive properties (van Schalkwyk et al., 2017), we found that both the validity of the CADSS subscales and their ability to meaningfully capture distinct aspects of ketamine-induced dissociation were supported. In fact, coupled with the evidence of psychometric validity, the differential relationship observed with antidepressant response suggests that ketamine researchers may wish to focus primarily on the Depersonalization subscale of the CADSS.
This study has several important limitations. First, there is the potential for inadequate blinding, as, due to its psychoactive and sympathomimetic effects even at low doses, many patients can distinguish ketamine from an inert (saline) placebo. Similarly, increased levels of dissociation could potentially unblind clinical/research staff, who may think or behave differently around subjects exhibiting high levels of dissociative symptoms (e.g., giving them greater individual attention and monitoring during the post-infusion period than subjects who received ketamine without a significant side effect burden). In this regard, both subject and staff unblinding may overestimate antidepressant response to ketamine in placebo-controlled studies, especially in subjects who exhibit more dissociative side effects. Next, our combined MDD and BD datasets, while maximizing available power, may underrepresent ketamine’s antidepressant efficacy, as MDD patients display greater ketamine-induced antidepressant improvement than patients with bipolar depression (McGirr et al., 2015). Finally, as mentioned above, Type I (false positive) errors may have been possible in our secondary (exploratory) analyses. Future studies are required to a priori confirm these observations.
In conclusion, our data support the factor validity of the CADSS dissociative subscales in an actively depressed population with MDD and BD. If time constrained, clinicians administering ketamine for depression may opt to focus solely on the Depersonalization subscale of the CADSS as a predictor of subsequent antidepressant response. It should also be noted that while off-target effects cannot be excluded and confounding factors exist, a mechanistic overlap that warrants future neurobiological investigation may nevertheless exist between ketamine-induced depersonalization and antidepressant response.
Supplementary Material
HIGHLIGHTS.
Intra-infusion dissociation is associated with antidepressant response to ketamine.
Antidepressant response may be uniquely related to dissociative symptom clusters.
Depersonalization was globally associated with antidepressant response.
Derealization was discriminately associated with antidepressant response.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Role of Funding Source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857; NIH Protocol 04-M-0222), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These agencies had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
MJN: conceptualized the study; designed the study; drafted the manuscript; revised the manuscript; edited the manuscript for critical intellectual content; helped interpret the statistical analysis; provided research supervision; approved the final version of the article.
BJS: conceptualized the study; designed the study; drafted the manuscript; revised the manuscript; conducted the statistical analysis; approved the final version of the article.
BAJ: conceptualized the study; designed the study; drafted the manuscript; revised the manuscript; conducted the statistical analysis; approved the final version of the article.
CF: revised the manuscript; edited the manuscript for critical intellectual content; assisted in statistical design, analysis, and interpretation; conducted and helped interpret the statistical analysis; approved the final version of the article.
DAL: revised the manuscript; edited the manuscript for critical intellectual content; assisted in statistical design, analysis, and interpretation; conducted and helped interpret the statistical analysis; approved the final version of the article.
NEB: conceptualized the study; designed the study; revised the manuscript; approved the final version of the article.
LTP: conceptualized the study; designed the study; provided research supervision; approved the final version of the article.
EDB: revised the manuscript; edited the manuscript for critical intellectual content; provided research supervision; approved the final version of the article.
CAZ: conceptualized the study; revised the manuscript; edited the manuscript for critical intellectual content; helped interpret the statistical analysis; provided research supervision; approved the final version of the article.
Declaration of Interest
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857; NIH Protocol 04-M-0222), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. Dr. Zarate is listed as a coinventor on a patent for the use of ketamine and its metabolites in major depression and suicidal ideation. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aan Het Rot M, Zarate CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2017;42:1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno MA, Jaquet O, Bahri MA, Plenevaux A, Boly M, Boveroux P, Soddu A, Brichant JF, Maquet P, Laureys S. Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology. 2016;125:873–888. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, Yang J, Wen R, Mann JJ, Krystal JH. In vivo ketamine-induced changes in [11 C] ABP688 binding to metabotropic glutamate receptor subtype 5. Biol Psychiatry. 2015;77:266–275. doi: 10.1016/j.biopsych.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Biometrics Research. New York, NY: New York State Psychiatric Institute; 2002. Structured clinical intervies for DSM-IV-TR axis I disorders, research version (SCID-I-RV) [Google Scholar]
- Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, review and implications. Ann Emerg Med. 1990;19:1033–1046. doi: 10.1016/s0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche NE, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012a;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, DiazGranados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, Potter WZ, Zarate CA., Jr A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012b;32:551. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain D, Baccei C, Bristow L, Anderson J, Varney M. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko L, Schmahl C, Bockhacker L, Vonderlin R, Bohus M, Kleindienst N. Dissociation in Psychiatric Disorders: A Meta-Analysis of Studies Using the Dissociative Experiences Scale. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17010025. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Nowak LM. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol Sci. 1990;11:167–172. doi: 10.1016/0165-6147(90)90070-O. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CAJ. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim M, Bond D, Fleck M, Yatham L, Lam R. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Matthew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. doi: 10.1038/s41380-018-0028-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Robinson SE, Coppola R, Zarate CA. Preliminary differences in resting state MEG functional connectivity pre-and post-ketamine in major depressive disorder. Psychiatry Res. 2016;254:56–66. doi: 10.1016/j.pscychresns.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J, Harvey M, Voss L, Denny B. Ketamine–More mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4:76–81. [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34:287–293. [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1 H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schalkwyk GI, Wilkinson ST, Davidson L, Silverman WK, Sanacora G. Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: analysis of the Clinician Administered Dissociative State Scale. J Affect Disord. 2017;227:11–16. doi: 10.1016/j.jad.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.