Abstract
Although the mechanisms underlying the anti-seizure effects of the high-fat ketogenic diet (KD) remain unclear, a long-standing question has been whether ketone bodies (i.e., beta-hydroxybutyrate [BHB], acetoacetate [ACA] and acetone), either alone or in combination, contribute mechanistically. The traditional belief has been that while ketone bodies reflect enhanced fatty acid oxidation and a general shift toward intermediary metabolism, they are not likely to be the key mediators of the KD’s clinical effects, as blood levels of BHB do not correlate consistently with improved seizure control. Against this unresolved backdrop, new data support ketone bodies as having anti- seizure actions. Specifically, BHB has been shown to interact with multiple novel molecular targets such as histone deacetylases, hydroxycarboxylic acid receptors on immune cells, and the NRLP3 inflammasome. Clearly, as a diet-based therapy is expected to render a broad array of biochemical, molecular, and cellular changes, no single mechanism can explain how the ketogenic diet works. Specific metabolic substrates or enzymes are only a few of many important factors influenced by the KD that can collectively influence brain hyperexcitability and hypersynchrony. This review summarizes recent novel experimental findings supporting the anti-seizure and neuroprotective properties of ketone bodies.
Keywords: epilepsy, ketogenic diet, ketone bodies, beta-hydroxybutyrate, acetoacetate, neuroprotection
Introduction
The ketogenic diet (KD) is a high-fat, low-carbohydrate and adequate-protein formulation that has been used for nearly a century to treat medically intractable epilepsy. Although the mechanisms underlying the KD’s clinical effects remain unclear (Rho & Stafstrom, 2012; Rogawski et al, 2016), it remains controversial whether any or all of the major ketone bodies (e.g., beta-hydroxybutyrate [BHB], acetoacetate [ACA] and acetone) produced by the liver are directly responsible for the KD’s anti-seizure profile. One reason for this persistent uncertainty is the clinical observation that blood ketone (i.e., BHB) levels do not correlate well with seizure control (Kossoff et al, 2009; Kossoff & Rho, 2009; but see Gilbert et al., 2000; van Delft et al., 2010), although local ketone levels at the neuronal or synaptic level may be a more accurate reflection of ketone effects on excitability (Stafstrom, 2004). Further, another diet that has been used successfully to treat patients with medically intractable epilepsy, the low glycemic index treatment (LGIT), does not induce systemic ketosis (Muzykewicz et al, 2009). While definitive evidence in this regard is not yet forthcoming, recent clinical data indicate that ketone bodies (specifically, BHB) may yet be relevant to an anti-seizure effect (Buchhalter et al, 2017). In contrast, experimental data are more compelling, with recent studies highlighting pleiotropic actions of BHB and novel molecular targets (Puchalska & Crawford, 2017). While some of these mechanistic observations have yet to be firmly and causally linked to an attenuation of seizure activity, the scientific rationale for both ketone-induced anti-seizure and neuroprotective effects has grown substantially over the past few years (Gano et al, 2014; Puchalska & Crawford, 2017). This review summarizes the evidence for ketone bodies as important contributors to ketogenic diet effects in the clinical setting.
Evidence for the Anti-seizure Activity of Ketone Bodies in In Vivo Seizure Models
Since the introduction of the KD and the hypothesis that ketone bodies are responsible for its therapeutic effects, there has been a relative paucity of in vivo studies demonstrating the therapeutic efficacy of ketones against seizures. However, since the turn of the 21st century, evidence has been quickly accumulating, supporting the notion that ketone bodies can indeed contribute to seizure control. Initial screening for anti-seizure activity usually begins with determination of dose-dependent protection of an acutely administered compound against an induced seizure in normal (i.e., non-epileptic) animals. In the case of ketone bodies, the first documented testing began with Keith (1933, 1935) who found that acetone and acetoacetate, but not β-hydroxybutyrate, protected rabbits against seizures induced by thujone, a constituent of wormwood oil and a known antagonist of γ-aminobutyric acid, type A (GABAA) receptors (Höld et al, 2000).
Nearly seventy years passed before interest in ketone bodies was revived. Acetone and acetoacetate, but not β-hydroxybutyrate, protected against sound-induced seizures in the Frings audiogenic seizure-susceptible mouse model (Rho et al. 2002). Shortly thereafter, acetone was found to increase the seizure threshold of rats in multiple models of seizure induction, including the maximal electroshock test which models tonic-clonic seizures, the pentylenetetrazol test which models typical absence seizures, the amygdala kindling test which models complex partial (more recently termed focal with impaired awareness) seizures with bilateral spread, and the AY-9944 test which models chronic atypical absence seizures (Likhodii et al., 2002, 2003). Collectively, these data suggest that acetone has a broad-spectrum anti-seizure profile similar to the clinical experience with the KD. Effects of acetone in the pentylenetetrazol test and electroshock test have been confirmed, and extended to protection against tonic seizures in the 4-aminopyridilne (4-AP) test and reduced seizure severity during lithium-pilocarpine status epilepticus (Gasior et al., 2007; Inoue et al., 2009; Hasebe et al. 2010). Similar to acetone, acetoacetate reduced seizures induced by intrahippocampal 4-AP infusion in rats (Juge et al., 2010). Furthermore, acetoacetate and its analog 2-phenylbutyrate decreased hippocampal seizure activity in the intrahippocampal kainate model of chronic epilepsy (Kadowaki et al., 2017).
Recently, ketone esters have been investigated as a potential “pro-drug” capable of sustained elevation of ketone bodies (D’Agostino et al., 2013). The R,S-1,3-butanediol acetoacetate diester (BD-AcAc2) resulted in elevated blood acetone, acetoacetate and β-hydroxybutyrate levels in rats and increased the latency to hyperbaric oxygen-induced seizures. In contrast, 1,3-butandiol raised only blood β-hydroxybutyrate levels and failed to affect seizure latencies. Single or repeated dosing of BD-AcAc2 has been further demonstrated to increase the threshold of pentylenetetrazole-induced seizures in rats (Viggiano et al., 2015, 2016) and audiogenic- and kainate-induced seizures in a mouse model of Angelman syndrome (Ciarlone et al., 2017).
Based on the aforementioned studies, it would appear that β-hydroxybutyrate does not contribute to the anti-seizure efficacy of the ketogenic diet. However, three recent studies indicate that β-hydroxybutyrate may yet play a role. In an effort to determine if the higher endogenous β-hydroxybutyrate levels in suckling neonates confers seizure protection, Minlebaev and Khazipov (2011) performed a series of depth electrode experiments on postnatal day 5–9 non-anesthetized rat pups. Inhibiting ketogenesis had no effect on seizures provoked by a single flurothyl exposure. However, seizures during a second exposure were exacerbated. This effect was reversed with administration of exogenous β-hydroxybutyrate, suggesting a reduction of hyperexcitability and a direct role of β-hydroxybutyrate in raising the seizure threshold in neonates (Minlebaev and Khazipov, 2011). The second study investigated the effects of β-hydroxybutyrate in the betamethasone-NMDA model of infantile spasms (Yum et al., 2015). A single administration of β-hydroxybutyrate failed to affect the spasms, but repeated injections over three days increased the latency and decreased the number of spasms. Furthermore, this anti-seizure effect was enhanced with repeated bouts of NMDA-triggered spasms (Yum et al., 2015). The third study involved chronic infusion of β-hydroxybutyrate via osmotic minipumps over a two-week period in a genetic model of epilepsy and reported that β-hydroxybutyrate reduced spontaneous recurrent seizures similar to a ketogenic diet (Kim et al., 2015). Collectively, these studies suggest that the anti-seizure effects of β-hydroxybutyrate in vivo may have been missed in previous experiments due to limited, single dosing and/or the use of acute seizure models rather than models replicating aspects of spontaneous recurrent seizures.
In pediatric and adult patients or animals clinically used KDs generally raise plasma levels of ketone bodies to between 1 and 10 mM (Gilbert et al., 2000; van Delft et al., 2010; Simeone et al., 2016, 2017). Importantly, all of the in vivo studies described above used doses that would raise plasma ketones to within this range (e.g., an intraperitoneal injection of 10 mmol/kg β-hydroxybutyrate raises β-hydroxybutyrate to 5–7 mM, injection of 8 mmol/kg acetone raises acetone to 4–6 mM and intragastric administration of the pro-drug BD-AcAc2 raises acetone to 1 mM, β-hydroxybutyrate to 4 mM and acetoacetate to 4 mM; Eiger et al., 1980; Likhodii et al., 2002, 2003; D’Agostino et al., 2013; Yum et al., 2015). Moreover, those studies performing dose response experiments calculated anti-seizure ED50’s that would result in this mM range.
Before delving into the in vitro evidence of ketone-mediated inhibition of network excitability and mechanistic studies, it is important to briefly address how plasma ketone levels are related to brain ketone levels as this informs the physiological relevance of in vitro experiments. Ketone bodies enter the brain via monocarboxylate transporters which are expressed more highly in suckling than mature animals (Nehlig, 2004), although dietary ketosis may increase expression in adults (Leino et al., 2001). Acetoacetate and β-hydroxybutyrate exist in a stoichiometric relationship (Owen et al., 1967). The ratio of acetoacetate to β-hydroxybutyrate is approximately 1:3 in plasma of fasting or KD-treated humans and 1:2 in cerebral spinal fluid (CSF) of KD-treated humans and brain of KD-fed rats (Balasse et aI., 1978; De Vivo et al., 1978; Nordii & De Vivo, 1997). Magnetic resonance spectroscopy (MRS) studies in KD-treated epileptic children and fasted adults have demonstrated that dietary ketosis dramatically increases brain β-hydroxybutyrate, and that brain and plasma concentrations are linearly related with brain β-hydroxybutyrate being approximately 26% of plasma concentrations (Shen et al., 1998; Pan et al., 2000). This is in rough agreement with the relationship found between brain and plasma of KD-fed mice (18%; Samala et al., 2011). However, microdialysis in the hippocampus of KD-fed mice indicate that β-hydroxybutyrate in extracellur fluid is 5% of the plasma concentration. In contrast, acetone has been found to have a 1:1 relationship between blood and CSF (Likhodii et al., 2002, 2003). Collectively, these findings suggest that the relevant physiological concentrations for β-hydroxybutyrate, acetoacetate and acetone range from 0.05–2.5 mM, 0.025–1.25 mM and 1–10 mM, respectively. Therefore, in the following sections concerning in vitro studies regarding ketone body effects on cellular excitability and potential mechanisms, we make a point to state whether concentrations used fall within physiological relevance or, even better, whether the study involved full concentration responses spanning physiological levels. Studies using concentrations outside the physiological range are also presented, but should be interpreted with caution.
Evidence of Inhibitory Properties of Ketone Bodies In Vitro
In general, ketone body effects on neuronal and network excitability have been investigated using acutely prepared hippocampal slices from normal mice or rats. Thus far, the results have been inconsistent.Juge et al. (2010) reported that a high concentration of acetoacetate (10 mM) reduced mEPSC frequency and amplitude in CA1 neurons using whole-cell patch clamp techniques. However, using extracellular recording conditions, multiple groups have found that physiologically relevant concentrations of acetoacetate (1–3 mM) and β-hydroxybutyrate (1–3 mM) exert no effects on evoked field potentials, population spikes or long-term potentiation (Thio et al., 2000; Kimura et al., 2012; Kim et al., 2015; Youseff, 2015). Similarly, seizure-like events (SLEs) induced by application of pentylenetetrazole or low-magnesium were not reduced when exposed to high concentrations of either acetoacetate (10 mM) or β-hydroxybutyrate (10 mM) (Chang et al., 2016). In contrast, pretreatment with acetoacetate and β-hydroxybutyrate (co-application of 1 mM each) prevented synaptic dysfunction due to mitochondrial electron transport chain inhibitors or exogenously applied reactive oxygen species (ROS) (Maalouf & Rho, 2008; Kimura et al., 2012; Kim et al., 2010, 2015). Further, 0.5–10 mM β-hydroxybutyrate restored synaptic function during glucose deprivation, but only in slices from rats postnatal day 30 or younger; however, β-hydroxybutyrate provided protection against glucose deprivation-mediated morphological damage at every age (Izumi et al., 1998).
The root cause(s) of these in vitro inconsistencies may reflect challenges observed in vivo, e.g. duration of exposure to ketone bodies may be too short. In an attempt to circumvent this limitation of acute slices, two studies used organotypic hippocampal slice cultures. Samoilova et al., (2010) applied 10 mM β-hydroxybutyrate to slice cultures for 3 days and provoked SLEs under five different conditions: 4-aminopyridine (4-AP), low-magnesium, bicuculline, high-frequency stimulation and oxygen-glucose deprivation (OGD). β-hydroxybutyrate did not affect the properties of SLEs under any of these provocative manipulations except the metabolic insult of OGD. Specifically, upon re-perfusion after OGD, β-hydroxybutyrate prevented the development of SLEs that are ordinarily observed in >80% of slice cultures (Samoilova et al., 2010). Using a slightly different paradigm,Kim et al. (2015) generated hippocampal slice cultures from epileptic Kcna1-null mice that developed spontaneous SLEs. Incubation with a cocktail of 1 mM acetoacetate and 5 mM β-hydroxybutyrate for two-weeks reduced the frequency, duration and intensity of the SLEs (Kim et al., 2015). It is worth noting that acutely isolated mitochondria from Kcna1-null hippocampi are dysfunctional and likely contribute to the synaptic and network hyperexcitability underlying seizure genesis in this mouse model (Simeone et al., 2014a; Kim et al., 2015).
Overall, these studies with specific ketone bodies mirror in vitro experiments with acute hippocampal slices from mice and rats fed a KD. Specifically, a KD did not affect synaptic function in normal rats or prevent low-magnesium provoked hyperexcitability (Stafstrom et al., 1999; Bough et al., 2006), but it did prevent synaptic depression by glucose-deprivation (Bough et al., 2006). Furthermore, unprovoked network and synaptic hyperexcitability of slices from KD treated epileptic mice and rats were significantly dampened (Stafstrom et al., 1999; Simeone et al., 2014b).
Even though there are experimental design and technical differences among the cited studies, taken together, it is reasonable to conclude that ketone bodies have minimal or no effect on normal synaptic and network excitability, but do reduce the consequences of diverse metabolic insults. Moreover, ketone bodies may be unable to overcome hyperexcitability due to widespread, near-complete modulation of receptor and ion channel systems (i.e., non-selective inhibition of GABAA receptors with bicuculline or pentylenetetrazole, non-selective block of potassium channels with 4-AP, and disinhibition of NMDA receptors with low magnesium). However, it should be noted that in these experiments, concentrations of modulators that elicit maximum effects were used. Perhaps performing concentration-response experiments with these pro-convulsant modulators may reveal meaningful ketone body effects.
Based on the above, it is tempting to speculate why the KD is ineffective in some compliant patients. Perhaps a metabolic derangement plays a minimal role in the patient’s specific epilepsy syndrome. Alternatively, genetic or epigenetic factors may significantly alter receptor or channel function. At this time, these hypotheses await experimental and clinical verification.
Molecular Targets
The in vivo and in vitro studies discussed thus far provide solid evidence that ketone bodies do indeed exert anti-seizure effects. Although the primary determinants of neuronal excitability have traditionally focused on the major excitatory and inhibitory neurotransmitter systems in the brain (i.e., glutamate and γ-aminobutyric acid or GABA, respectively), recently there has been growing recognition of additional factors that influence neuronal excitability directly. These include mitochondrial health (their ability to produce ATP, buffer calcium and generate ROS), antioxidant systems, inflammatory mediators, and histone deacetylation status (which modifies transcription of genes). Here, we review the key studies supporting ketone body modulation of these diverse mechanisms.
Mitochondria
Several elegant studies have demonstrated that mitochondria regulate synaptic transmission via three mechanisms: (1) production of ATP, (2) generation of ROS, and (3) sequestration of cytosolic calcium (Lee et al., 2007; Lee et al., 2012; Harris et al., 2012). Thus, any perturbation of mitochondrial health will send ripples of dysregulation across synaptic, neuronal and network activities. Moreover, prolonged excitotoxicity and concomitantly rising intracellular calcium levels can initiate the opening of the mitochondrial membrane permeability transition pore (mPTP), through which pro-apoptotic factors are released into the cytosol and which invariably lead to cell death. Stabilizing mitochondria could be a mechanism by which ketone bodies reduce seizure activity and cellular demise.
Krebs cycle
At the heart of a long-standing hypothesis concerning the role of ketone bodies in KD action is that they are a more efficient fuel source than glucose. β-hydroxybutyrate converts to acetoacetate via the enzyme β-hydroxybutyrate dehydrogenase and acetoacetate skips glycolysis, directly entering the Krebs cycle at the level of acetyl-CoA. Using nuclear magnetic resonance (NMR) techniques in rat hippocampal slices, Valente-Silva et al. (2015) reported that given equal amounts of substrate, ketone bodies (5 mM) out-compete glucose for neuronal acetyl-CoA by inhibiting glycolytic flux upstream of pyruvate kinase. A similar finding was made in brain homogenates from rats fed a KD using isotopomer mass spectrometry analysis (Zhang et al., 2015). In the heart, ketone body metabolism maximizes the redox difference between the mitochondrial NAD+/NADH couple of Complex I and the co-enzyme Q/QH2 couple of Complex II, resulting in an increased proton gradient, enhanced potential for ATP synthesis and increases in the free energy of ATP hydrolysis. Collectively, these changes indicate that more energy is stored in ATP and released when ATP becomes ADP (Sato et al., 1995; Veech et al., 2001). Ketone body metabolism also decreases mitochondrial free radical production through at least two mechanisms: (1) decreasing the reduced form of co-enzyme Q (which normally reacts with O2 to form the superoxide radical O −.) and (2) increasing the ratio of [NADPH]/[NADP+] (which promotes glutathione reductase to reduce glutathione and restore antioxidant capacity) (Veech et al., 2001). These actions serve to maintain Complex I-driven mitochondrial respiration and ATP synthesis under stressful conditions (Maalouf et al., 2007; Kim et al., 2010).
Membrane Permeability Transition Pore (mPTP)
mPTP is a massive channel (>1nS conductance) that is believed to regulate the homeostatic status of mitochondria (Nicholls and Budde, 2000). Prolonged opening of the mPTP is promoted by excessive concentrations of calcium and/or ROS. The resultant permeability transition results in collapse of the mitochondrial membrane potential, inhibition of ATP production via uncoupling of the electron transport system from ATP synthase, mitochondrial swelling, and the release of calcium, ROS and pro-apoptotic proteins such as cytochrome c into the cytosol, which together can trigger cell-death (Sullivan et al., 2005). A series of studies over the past decade have demonstrated that acetoacetate and β-hydroxybutyrate (individual or co-application, 0.1–3 mM) can inhibit mPTP opening indirectly via decreased ROS levels as well as facilitate enhanced mitochondrial respiration, NADH oxidation and ATP production (Maalouf et al., 2007, 2008; Kim et al., 2007, 2010, 2015). Ultimately these actions lead to decreased interaction of cyclophilin D (CypD), a key regulator of mPTP calcium sensitivity (Hurst et al., 2016), with mPTP, as ketone bodies were ineffective in mitochondria lacking the CypD subunit (Kim et al., 2015). Furthermore, the importance of mPTP and CypD for the KD’s anti-seizure effects were demonstrated when in vivo administration of atractyloside, a mPTP opener, blocked KD-induced reduction of spontaneous recurrent seizures in epileptic Kcna1-null mice, whereas NIM811, a selective CypD inhibitor, mimicked the KD with respect to attenuation of seizure activity (Kim et al., 2015).
BCL-2-associated Agonist of Cell Death (BAD)
The mitochondrial membrane potential depends on several factors, an important one being the balanced presence of anti-apoptotic Bcl-2 and Bcl-xl and pro-apoptotic Bad and Bax. Upon injury, Bad and Bax are dephosphorylated and translocate to mitochondria, bind Bcl-2 and Bcl-xl, and depolarize mitochondria. Significant depolarization of the mitochondrial membrane potential can lead to the release of pro-apoptotic factors such as cytochrome C, caspase 9 and caspase 3 (Youle and Strasser, 2008). The phosphorylation state of three serines, Ser112, Ser136 and Ser155, determine BAD function. Phosphorylation of Ser112 and Ser136 promotes binding of BAD to 14-3-3 proteins, sequestering BAD away from the mitochondrial membrane (Tan et al., 2000). The KD increased phosphorylation of BAD Ser136 and the interaction between BAD and 14-3-3, actions which may underlie its neuroprotective properties against kainic acid-induced status epilepticus (Noh et al., 2006). Similarly, in vivo administration of β-hydroxybutyrate increased 14-3-3 mRNA and phosphorylation of BAD Ser136 and decreased pulmonary apoptosis in a rat model of hemorrhagic shock (Jaskille et al., 2004).
Phosphorylation of Ser155 blocks binding of BAD to BCL-xl (Tan et al., 2000), but also induces glucokinase binding and increases the rate of glycolysis (Danial et al., 2003). Thus, phosphorylated BAD S155 shifts metabolism in favor of glucose. Gimenez-Cassina et al. (2012) hypothesized that dephosphorylated BAD S155 may confer preference for ketone body metabolism and raise seizure thresholds. Indeed, when glucose was the substrate, mitochondrial respiration rates were lower in neuronal and astrocyte primary cultures from BAD knockout mice and BAD S155A mutant mice compared to wild-type controls. The opposite occurred when β-hydroxybutyrate (5 mM) was the substrate, i.e., respiration rates were higher in neuronal and astrocyte cultures from BAD mutants relative to wild-type mice. Also, seizure severity during kainate- or pentylenetetrazole-induced status epilepticus was reduced in BAD knockout mice and BAD S155A mutant mice compared to wild-type controls. The authors inferred that metabolic alterations occurred in vivo with glucose and ketone metabolism, decreasing and increasing, respectively, in the BAD mutant mice, and were hence responsible for the increased seizure thresholds. However, this was not directly tested (Gimenez-Cassina et al., 2012). While intriguing, this study failed to demonstrate directly the relevance of BAD Ser155 to the anti-seizure or neuroprotective effects of a KD or β-hydroxybutyrate. Thus, how BAD relates specifically to β-hydroxybutyrate-evoked anti-seizure mechanisms remains unclear.
Neurotransmitter systems
In general, studies have failed to demonstrate a direct action of ketone bodies on GABA or glutamate receptors at physiologically relevant concentrations (Thio et al., 2000; Yang et al., 2007). Alternatively, ketone bodies may induce homeostatic effects on these neurotransmitter systems, but whether this occurs, and in which direction, are debated questions. Ketone bodies have been shown to increase GABA levels in rat synaptosomes prepared from the forebrains of rodents injected with β-hydroxybutyrate and in cultured astrocytes (Erecinska et al. 1996; Daikhin and Yudkoff, 1998; Suzuki et al., 2009). Supporting these pre-clinical data, patients on a KD showed increased GABA levels in the cerebrospinal fluid, consistent with what was reported using magnetic resonance spectroscopy (Wang et al. 2003; Dahlin et al. 2005). However, more often than not, in vivo and in vitro studies have reported that ketone bodies do not change total GABA or glutamate content, but rather the preferential source of amino acid carbons shifts from glucose to ketone bodies (Yudkoff et al., 2001; Lund et al., 2009, 2011; Valente-Silva et al., 2015; Zhang et al., 2015). Yet, a consistent finding in these studies is that ketone body metabolism decreases aspartate. Aspartate is a known inhibitor of glutamate decarboxylase, an enzyme involved in the generation of GABA from glutamate. Therefore, the GABA hypothesis remains viable, as a decrease in aspartate could theoretically promote the synthesis of GABA (McNally and Hartman, 2012).
Another method to regulate neurotransmission is to alter synaptic vesicle loading. Through a series of elegant experiments,Juge et al. (2010) demonstrated that acetoacetate inhibits SLC17 vesicular neurotransmitter transporters. In this family of transporters are vesicular glutamate transporters (VGLUTs) which are responsible for filling presynaptic vesicles with glutamate in a Cl−-dependent manner. Reconstituting purified VGLUTs in proteoliposomes, these investigators demonstrated that acetoacetate competitively inhibits the allosteric activation by Cl− of VGLUTs. Significant inhibition occurred in the low µM range, and near complete inhibition was achieved in the low mM range (concentrations that are physiologically relevant). Impressively, Juge and colleagues showed that acetoacetate reversibly inhibited glutamate release from both cultured rat neurons and mouse CA1 pyramidal neurons in hippocampal slices, observations that correlated with decreased miniature excitatory postsynaptic current EPSC amplitude and frequency. Importantly, acetoacetate failed to affect VGATs (vesicular GABA transporters) and miniature IPSCs. Finally, acetoacetate was able to suppress in vivo glutamate release and seizures in rat brains exposed to 4-AP (Juge et al., 2010). If there is any criticism of this intricate and challenging study, it is that only the 10 mM acetoacetate concentration was used in the cultures, slices and in vivo experiments, leaving open the question of what effects a ~50% inhibition by µM concentrations of acetoacetate or β-hydroxybutyrate would have on synaptic neurotransmission and seizure activity. Finally, acetoacetate was found to be as efficacious and potent an inhibitor of other SLC17 transporters, VNUT (vesicular nucleotide transporter) and VEAT (vesicular excitatory amino acid transporter). In the case of VNUT, which loads ATP into vesicles, this action seems paradoxical because it would lower synaptic inhibition by ATP and adenosine release. However, this loss of inhibition may be outweighed by the reduction of released glutamate.
ATP-Sensitive Potassium (KATP) channels
Potassium channels are a heterogeneous group of ion channels that largely function to hyperpolarize the cell membrane. Inwardly rectifying ATP-dependent potassium (KATP) channels are inhibited by ATP and open when ATP levels are low. Thus, they represent a direct link between membrane excitability and the metabolic state of the cell. Although there are clear data demonstrating that acetoacetate and β-hydroxybutyrate (0.5–1 mM) increase intracellular ATP levels (Kim et al, 2010), experiments in substantia nigra pars reticulata GABAergic neurons and hippocampal dentate granule cells revealed that KATP channels are activated indirectly in the presence of acetoacetate (2 mM) or β-hydroxybutyrate (2 mM), thereby decreasing neuronal excitability (Ma et al., 2007; Tanner et al., 2011). Opening of KATP channels resulted in decreased spontaneous firing rates, and was prevented by the KATP channel inhibitor, tolbutamide. Further, the ketone body effect was abrogated in Kir6.2 knockout mice (lacking the KATP channel alpha subunit).
Interestingly, the neuroprotective effects of ketone bodies (cocktail of 1 mM acetoacetate and 1 mM β-hydroxybutyrate) during oxidative stress were shown to involve both surface KATP channels and mitochondrial KATP channels (Kim et al., 2015a). Also, 1mM β-hydroxybutyrate-induced opening of KATP channels was involved in neurotransmission of cultured cerebellar neurons, as addition of glibenclamide, another KATP channel inhibitor, increased D-[2,3-3H]-aspartate release (Lund et al., 2015). Thus far, the most reasonable explanation for the collective and discrepant findings has been proposed by Kawamura et al. (2010). Conducting patch-clamp experiments on hippocampal CA3 pyramidal cells, they found that under conditions of low intracellular glucose and elevated ATP, the large pannexin-1 hemichannels open and release ATP. ATP is then broken down to adenosine by ectonucleotidases in the extracellular space. The increase in extracellular adenosine was shown to activate G-protein coupled A1 receptors which were then linked to the opening of KATP channels via second messenger signaling (Kawamura et al., 2010). Thus, β-hydroxybutyrate-induced increased ATP production has been proposed as a form of metabolic autocrine regulation that ultimately reduces neuronal and network excitability.
Histone (Lysine) Deacetylases and Gene Regulation
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from lysine residues on histones and other proteins such as transcription factors and other enzymes. Deacetylation of histones loosens the tight wrapping of DNA, thus enabling gene transcription, whereas deacetylation of transcription factors or enzymes can increase or decrease their activity.Shimazu et al. (2013) found that β-hydroxybutyrate inhibits HDAC1, HDAC3 and HDAC4 with IC50s of 5.3, 2.4 and 5.4 mM, respectively – suggesting the physiological relevance of this mechanistic action. Furthermore, they showed that β-hydroxybutyrate-induced HDAC inhibition resulted in up-regulation of genes in the FOXO3A transcription factor network, including the anti-oxidants catalase, mitochondrial superoxide dismutase (SOD2) and metallothionein 2, collectively leading to reduced oxidative stress in kidney cells (Shimazu et al., 2013). Supporting this downstream effect on anti-oxidants, co-application of acetoacetate (1 mM) and β-hydroxybutyrate (1 mM) were earlier found to increase catalase activity in hippocampal slices during oxidative stress (Kim et al., 2010). HDAC3 is also part of the corepressor complex that inhibits peroxisome proliferator-activated receptor (PPAR) γ, a transcription factor that also regulates many of the same antioxidant genes. Therefore, an HDAC inhibitor would be expected to increase the transcriptional activity of PPARγ (Ye et al., 2013). In cultured HT22 cells, 5 mM acetoacetate increased PPARγ expression and in in vivo experiments, genetic or pharmacologic loss of PPARγ attenuated the anti-seizure effects of the KD (Jeong et al., 2011; Simeone et al., 2017).
An alternative and complementary action could be that ketone bodies increase the expression and activity of the NAD+-dependent histone deacetylase sirtuin 3 (SIRT3). In a mouse model of ischemic stroke, administration of ketone bodies reduced the infarct size and oxidative stress and increased protein expression of mitochondrial SIRT3, FOXO3A and SOD2 (Yin et al., 2015). In primary neuronal cultures, ketone bodies (cocktail of 0.4 mM β-hydroxybutyrate and 0.45 mM acetoacetate) prevented cell death from rotenone-induced mitochondrial dysfunction and increased mitochondrial content of SIRT3, FOXO3A and Mn-SOD. Importantly, ketone body neuroprotection was lost when SIRT3 shRNA, and FOXO3A and SOD2 expression were inhibited (Yin et al., 2015).
Anti-Inflammatory Effects
Experimental and clinical research within the last two decades has provided convincing evidence of the importance of inflammatory mediators and related molecules in ictogenesis, epileptogenesis, and the exacerbation of seizure severity. Importantly, seizures induce inflammation and increase levels of cytokines and chemokines such as interleukin-1β (IL-1β) (Vezzani et al., 2005; 2011). These factors are secreted from both activated glia and neurons in many experimental models of acute and chronic seizures and increase neuronal and network hyperexcitability. Inflammatory mediators also increase the permeability of the blood-brain barrier allowing the infiltration of monocytes and macrophages into the brain, which further enhances the inflammatory process (Marchi et al., 2011; 2014). Recently, β-hydroxybutyrate has been found to modulate two inflammatory mechanisms.
HCA2
Hydroxy-carboxylic acid receptor 2 (HCA2, GPR109A) is a Gi protein-coupled receptor that is activated by β-hydroxybutyrate.Rahman et al. (2014) sought to determine whether HCA2 contributes to the neuroprotective effects of the KD and β-hydroxybutyrate. They induced ischemic strokes by occluding the distal middle cerebral artery. Mice fed a KD or administered β-hydroxybutyrate via subcutaneous minipumps achieved plasma concentrations of 1 mM and developed smaller infarcts than control mice. This protection by the KD and β-hydroxybutyrate was lost in HCA2−/− mice. Furthermore, using cell ablation techniques and chimeric mice, these investigators demonstrated that HCA2 is expressed in infiltrating bone marrow-derived monocytes and macrophages in the brain, and that HCA2 activation on these monocytes and macrophages is required for the neuroprotective effect (Rahman et al., 2014).
NRLP3
The innate immune sensor NOD-like receptor protein 3 (NLRP3) inflammasome is a multi-protein complex that controls the activation of caspase-1 and the release of the proinflammatory cytokines IL-1β and IL-18 in macrophages. In response to diverse damage-associated molecular patterns such as excess glucose ceramides and amyloids, the macrophage experiences a loss of cytoplasmic K+, promoting oligomerization of apoptosis-associated speck-like protein containing a caspase activation and recruitment domain or ASC, speck formation, and the assembly of the inflammasome (Youm et al., 2015). In an elegant study, Youm and colleagues found that β-hydroxybutyrate (1–10 mM), but not acetoacetate (10 mM), inhibits NLRP3 inflammasome assembly by preventing K+ efflux. Although the exact mechanism(s) of β-hydroxybutyrate inhibition remain unclear, this effect on NRLP3 assembly was not dependent on chirality or starvation-regulated mechanisms like AMP-activated protein kinase (AMPK), ROS, autophagy or glycolytic inhibition. β-hydroxybutyrate inhibition was also independent of uncoupling protein-2, SIRT2 and HCA2 (Youm et al., 2015). Interestingly, another study suggested that in vivo and in vitro inhibition of the inflammasome by 1 mM β-hydroxybutyrate occurs through suppression of endoplasmic reticulum-related oxidative stress (Bae et al., 2016). Additionally, β-hydroxybutyrate increased SOD2 and catalase by mediating FOXO3 through AMPK activation, implying that decreasing ROS may have prevented inflammasome activation. Nevertheless, β-hydroxybutyrate reduced NLRP3 inflammasome–mediated IL-1β and IL-18 production in human monocytes. Finally, in multiple mouse models of NLRP3-mediated diseases in which mutant NLRP3 is constitutively active, in vivo administration of β-hydroxybutyrate or a KD attenuated caspase-1 activation and IL-1β secretion (Youm et al., 2015).
Summary
Within only the past few years, there has been remarkable progress in our understanding of ketone bodies and their numerous biological effects (Puchalska & Crawford, 2017). Indeed, circling back to the original question of whether ketone bodies exert anti-seizure effects, accumulating evidence suggests that they play a pivotal role and are not just epiphenomena. While some of the novel biological effects observed and targets activated by ketone bodies such as β-hydroxybutyrate have not yet been shown in brain, it is reasonable to extrapolate these findings as fundamental mechanisms such as HDAC inhibition would not be expected to be restricted to only one tissue type. Clearly, ketone bodies can no longer be considered simply energy molecules or substrates for membrane biosynthesis during development (Morris, 2005; Puchalska & Crawford, 2017). Rather, it is increasingly becoming apparent that ketone bodies contribute to the anti-seizure efficacy of the KD. However, ultimate validation of their role in the clinical setting has yet to be firmly demonstrated.
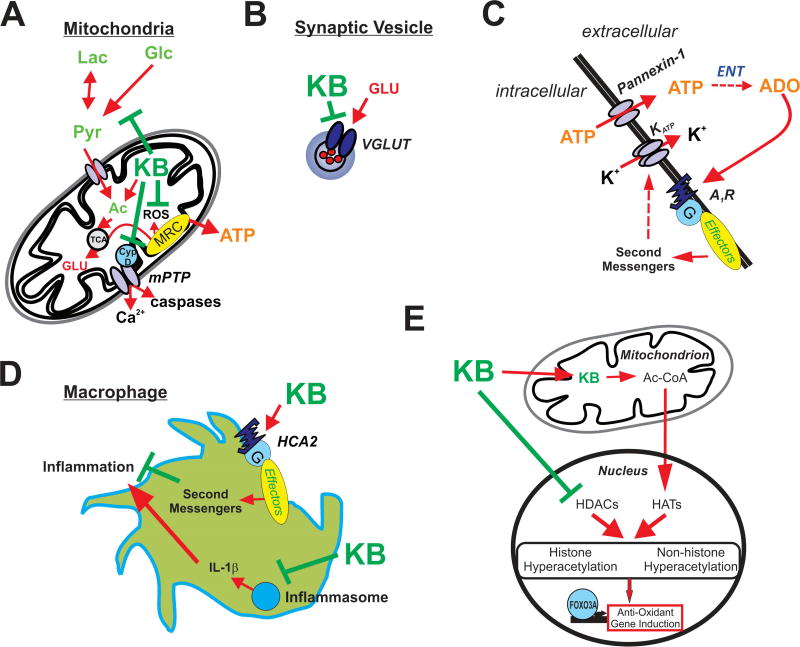
Figure 1.
Illustrations depicting potential mechanisms underlying ketone body-mediated attenuation of CNS hyperexcitability and neuroprotection. (A) Ketone bodies (KB) may enhance ATP production by providing acetyl-CoA (Ac) and inhibit production of reactive oxygen species (ROS) and the mitochondrial permeability transition (mPT) pore, thereby protecting the cell against oxidative injury and preventing excessive release of calcium. (B) KB may inhibit vesicular glutamate transporters (VGLUT), decreasing the amount of glutamate loaded in vesicles and reducing the size of glutamate quanta released during synaptic transmission. (C) KB-mediated increases in intracellular ATP and subsequent release through pannexin channels lead to adenosine (ADO) synthesis via ectonucleotidases (ENT) in the extracellular space. ADO in turn binds to inhibitory adenosine type 1 receptors (A1Rs) which are coupled to the indirect opening of KATP channels. (D) KB activate HCA2 receptors and inhibit the assembly of the NRLP3 inflammasome; thus, KB attenuate inflammatory mediators produced by infiltrating macrophages. (E) KB also promote histone and non-histone hyperacetylation by increasing acetyl-CoA, a substrate for histone acetyltransferases (HATs), and directly inhibiting histone deacetylases (HDACs) – with the end result of increasing endogenous anti-oxidants (among other actions).
Table 1.
Ketone Body efficacy in in vivo and in vitro models of seizures and hyperexcitability.
| In vivo | Acetone | AcAc | BHB | References |
|---|---|---|---|---|
| Induced Seizures | ||||
| Thujone | + | + | − | a, b |
| Audiogenic | + | + (BD-AcAc2, AS mice) | − | c, m |
| MES | + | N.D. | N.D. | d |
| PTZ | + | + (BD-AcAc2) | N.D. | d, e, g, k, l |
| Amygdala Kindling | + | N.D. | N.D. | d |
| AY-9944 | + | N.D. | N.D. | d |
| 4-AP | + | + | N.D. | e, h |
| Li-pilo SE | + | N.D. | N.D. | f |
| Hyperbaric Oxygen | N.D. | + (BD-AcAc2) | − (1,3-butandiol) | j |
| Kainate | N.D. | + (BD-AcAc2, AS mice) | N.D. | m |
| Repeated flurothyl | N.D. | N.D. | + (rat neonates) | n |
| Spontaneous Seizures | ||||
| Intrahippocamapal Kainate | N.D. | + | N.D. | i |
| betamethasone-NMDA | N.D. | N.D. | + | o |
| Kcna1-null mice | N.D. | N.D. | + | p |
|
| ||||
| In vitro | Acetone | AcAc | BHB | References |
|
| ||||
| Cellular and population excitability | ||||
| mEPSCs | N.D. | + | N.D. | h |
| Field Potentials | N.D. | − | − | q, p, s |
| Population Spikes | N.D. | − | − | q, s |
| LTP | N.D. | − | − | p, r, s |
| Mitochondrial mediated synaptic dysfunction | ||||
| ETC inhibitors | N.D. | + | + | p, r, v |
| ROS | N.D. | + | + | p, u, v |
| Induced Seizure-like events in slices | ||||
| 4-AP | N.D. | N.D. | − | w |
| Low Mg2+ | N.D. | N.D. | − | t, w |
| PTZ | N.D. | − | − | t |
| bicuculline | N.D. | N.D. | − | |
| High frequency stimulation | N.D. | N.D. | − | w |
| OGD | N.D. | N.D. | + | w |
| Kcna1-null mice | N.D. | + | + | p |
Abbreviations: AcAc, acetoacetate; BHB, β-hydroxybutyrate; MES, maximal electroshock; PTZ, pentylenetetrazole; 4-AP, 4-aminopyridine; Li-pilo, lithium pilocarpine; SE, status epilepticus; HFS, high-frequency stimulation; OGD, Oxygen-glucose deprivation; N.D., not determined; +, attenuation effect; −, inactive; BD-AcAc2, R,S-1,3-butanediol acetoacetate diester; AS, Angelman syndrome; mESPC, miniature excitatory post-synaptic current; LTP, long-term potentiation; ETC, electron transport chain; ROS, reactive oxygen species.
a. Keith, 1933
b. Keith, 1935
m. Ciarlone et al., 2017
Highlights.
Ketone bodies (such as beta-hydroxybutyrate, acetoacetate and acetone) are a hallmark feature of the ketogenic diet – a high-fat, low-carbohydrate and adequate protein intervention for medically intractable epilepsy.
In addition to their neuroprotective activity, ketone bodies have been shown in a growing number of scientific reports to counter neuronal hyperexcitability in vitro and to exert antiseizure effects in vivo.
Recent studies have revealed a broad array of biological actions of ketone bodies, beyond their classical role as energy substrates, including epigenetic, anti-oxidant, mitochondrial and antiinflammatory effects.
Acknowledgments
This work was supported by Citizens United for Research in Epilepsy Foundation (KAS), NIH NS072179 (KAS), NIH NS085389 (TAS), and the Canadian Institutes of Health Research (JMR). Please note, the second author has published under the names K Dorenbos, KA Fenoglio, KA Fenoglio-Simeone and KA Simeone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, Chung KW, Kim SM, Im, Chung HY. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–66454. doi: 10.18632/oncotarget.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse EO, Fery F. Ketone body production and disposal: effects of fasting. Diabetes and exercise. Diabetes Metab. Rev. 1978;5:247–270. doi: 10.1002/dmr.5610050304. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Buchhalter JR, D’Alfonso S, Connolly M, Fung E, Michoulas A, Sinasac D, Singer R, Smith J, Singh N, Rho JM. The relationship between D-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open. 2017 doi: 10.1002/epi4.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, Hardege JD, Chen PE, Walker MC, Williams RS. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139:431–43. doi: 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlone SL, Grieco JC, D'Agostino DP, Weeber EJ. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol. Dis. 2016;96:38–46. doi: 10.1016/j.nbd.2016.08.002. [DOI] [PubMed] [Google Scholar]
- D'Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R829–836. doi: 10.1152/ajpregu.00506.2012. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–6. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Daikhin Y, Yudkoff M. Ketone bodies and brain glutamate and GABA metabolism. Dev. Neurosci. 1998;20:358–364. doi: 10.1159/000017331. [DOI] [PubMed] [Google Scholar]
- Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–125. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Devivo DC, Leckie MP, Ferrendelli JS, McDougal DB. Chronic ketosis and cerebral metabolism. Ann. Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- Eiger SM, Kirsch JR, D'Alecy LG. Hypoxic tolerance enhanced by beta-hydroxybutyrate-glucagon in the mouse. Stroke. 1980;11:513–517. doi: 10.1161/01.str.11.5.513. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014;55:2211–28. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia. 2007;48:793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Pyzik PL, Freeman JM. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J. Child Neurol. 2000;15:787–90. doi: 10.1177/088307380001501203. [DOI] [PubMed] [Google Scholar]
- Giménez-Cassina A, Martínez-François JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, Tanner GR, Lutas A, Yellen G, Danial NN. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–730. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hasebe N, Abe K, Sugiyama E, Hosoi R, Inoue O. Anticonvulsant effects of methyl ethyl ketone and diethyl ketone in several types of mouse seizure models. Eur. J. Pharmacol. 2010;642:66–671. doi: 10.1016/j.ejphar.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Höld KM, Sirisoma NS, Ikeda T, Narahashi T, Casida JE. Alpha-thujone (the active component of absinthe): gamma-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc Natl Acad Sci U S A. 2000;97:3826–3831. doi: 10.1073/pnas.070042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst S, Hoek J, Sheu SS. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2016;49:27–47. doi: 10.1007/s10863-016-9672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue O, Sugiyama E, Hasebe N, Tsuchiya N, Hosoi R, Yamaguchi M, Abe K, Gee A. Methyl ethyl ketone blocks status epilepticus induced by lithium-pilocarpine in rats. Br. J. Pharmacol. 2009;158:872–878. doi: 10.1111/j.1476-5381.2009.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Ishii K, Katsuki H, Benz AM, Zorumski CF. beta-Hydroxybutyrate fuels synaptic function during development. Histological and physiological evidence in rat hippocampal slices. J. Clin. Invest. 1998;101:1121–1132. doi: 10.1172/JCI1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskille A, Alam HB, Rhee P, Hanes W, Kirkpatrick JR, Koustova E. D-lactate increases pulmonary apoptosis by restricting phosphorylation of bad and eNOS in a rat model of hemorrhagic shock. J. Trauma. 2004;57:262–269. doi: 10.1097/01.ta.0000133841.95455.73. [DOI] [PubMed] [Google Scholar]
- Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 2011;232:195–202. doi: 10.1016/j.expneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki A, Sada N, Juge N, Wakasa A, Moriyama Y, Inoue T. Neuronal inhibition and seizure suppression by acetoacetate and its analog, 2-phenylbutyrate. Epilepsia. 2017;58:845–857. doi: 10.1111/epi.13718. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith HM. Factors influencing experimentally produced convulsions. Arch. Neurol. Psychol. 1933;29:148–154. [Google Scholar]
- Keith H. Experimental convulsions induced by administration of thujone. Arch. Neurol. Psychiatry. 1935;34:1022–1040. [Google Scholar]
- Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, Rho JM. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007;101:1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- Kim DY, Abdelwahab MG, Lee SH, O'Neill D, Thompson RJ, Duff HJ, Sullivan PG, Rho JM. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS One. 2015a;10:e0119316. doi: 10.1371/journal.pone.0119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015b;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Vallejo J, Rho JM. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 2010;114:130–41. doi: 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Ma LY, Wu C, Turner D, Shen JX, Ellsworth K, Wakui M, Maalouf M, Wu J. Acute exposure to the mitochondrial complex I toxin rotenone impairs synaptic long-term potentiation in rat hippocampal slices. CNS. Neurosci. Ther. 2012;18:641–646. doi: 10.1111/j.1755-5949.2012.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Bergqvist C, Blackford R, Buchhalter JR, Caraballo RH, Cross HJ, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Liu C, Nation J, Nordli DR, Jr, Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EP The Charlie Foundation, Practice Committee of the Child Neurology Society. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee KH, Ho WK, Lee SH. Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J. Neurosci. 2007;27:13603–13613. doi: 10.1523/JNEUROSCI.3985-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim KR, Ryu SY, Son S, Hong HS, Mook-Jung I, Lee SH, Ho WK. Impaired short-term plasticity in mossy fiber synapses caused by mitochondrial dysfunction of dentate granule cells is the earliest synaptic deficit in a mouse model of alzheimer's disease. J. Neurosci. 2012;32:5953–5963. doi: 10.1523/JNEUROSCI.0465-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhodii SS, Burnham WM. Ketogenic diet: does acetone stop seizures? Med. Sci. Monit. 2002;8:HY19–24. [PubMed] [Google Scholar]
- Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC, 3rd, Burnham WM. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 2003;54:219–226. doi: 10.1002/ana.10634. [DOI] [PubMed] [Google Scholar]
- Lund TM, Risa O, Sonnewald U, Schousboe A, Waagepetersen HS. Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured neurons. J. Neurochem. 2009;110:80–91. doi: 10.1111/j.1471-4159.2009.06115.x. [DOI] [PubMed] [Google Scholar]
- Lund TM, Obel LF, Risa Ø, Sonnewald U. β-Hydroxybutyrate is the preferred substrate for GABA and glutamate synthesis while glucose is indispensable during depolarization in cultured GABAergic neurons. Neurochem. Int. 2011;59:309–318. doi: 10.1016/j.neuint.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Lund TM, Ploug KB, Iversen A, Jensen AA, Jansen-Olesen I. The metabolic impact of β-hydroxybutyrate on neurotransmission: Reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J. Neurochem. 2015;132:520–531. doi: 10.1111/jnc.12975. [DOI] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J. Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM. Oxidative impairment of hippocampal long-term potentiation involves activation of protein phosphatase 2A and is prevented by ketone bodies. J. Neurosci Res. 2008;86:3322–3330. doi: 10.1002/jnr.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Johnson AJ, Puvenna V, Johnson HL, Tierney W, Ghosh C, Cucullo L, Fabene PF, Janigro D. Epilepsia. 2011;52:1627–1634. doi: 10.1111/j.1528-1167.2011.03080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J. Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minlebaev M, Khazipov R. Antiepileptic effects of endogenous beta-hydroxybutyrate in suckling infant rats. Epilep. Res. 2011;95:100–109. doi: 10.1016/j.eplepsyres.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. J. Inherit. Metab. Dis. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–1126. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:265–275. doi: 10.1016/j.plefa.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nordli DR, Jr, de Vivo DC. The ketogenic diet revisited: back to the future. Epilepsia. 1997;38:743–749. doi: 10.1111/j.1528-1157.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J. Clin. Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J. Cereb. Blood Flow Metab. 2000;20:1502–1507. doi: 10.1097/00004647-200010000-00012. [DOI] [PubMed] [Google Scholar]
- Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, Pokorná B, Vollbrandt T, Stölting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- Rho JM, Anderson GD, Donevan SD, White HS. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43:358–361. doi: 10.1046/j.1528-1157.2002.47901.x. [DOI] [PubMed] [Google Scholar]
- Rho JM, Stafstrom CE. The ketogenic diet: What has science taught us? Epilepsy Res. 2012;100:210–217. doi: 10.1016/j.eplepsyres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W, Rho JM. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016;6(5) doi: 10.1101/cshperspect.a022780. pii, a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Klein J, Borges K. The ketogenic diet changes metabolite levels in hippocampal extracellular fluid. Neurochem. Int. 2011;58:5–8. doi: 10.1016/j.neuint.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Samoilova M, Weisspapir M, Abdelmalik P, Velumian AA, Carlen PL. Chronic in vitro ketosis is neuroprotective but not anti-convulsant. J. Neurochem. 2010;113:826–835. doi: 10.1111/j.1471-4159.2010.06645.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Shen J, Novotny EJ, Rothman DL. In vivo lactate and beta-hydroxybutyrate editing using a pure-phase refocusing pulse train. Magn Reson Med. 1998;40:783–788. doi: 10.1002/mrm.1910400520. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone KA, Matthews SA, Samson KK, Simeone TA. Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures. Exp. Neurol. 2014a;251:84–90. doi: 10.1016/j.expneurol.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Samson KK, Matthews SA, Simeone KA. In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv 1.1α knockout mice. Epilepsia. 2014b;55:e44–49. doi: 10.1111/epi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol. 2017;287:54–64. doi: 10.1016/j.expneurol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE. Dietary treatment of epilepsy: old and new options on the menu. Epilepsy Curr. 2004;4:215–222. doi: 10.1111/j.1535-7597.2004.46001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Wang C, Jensen FE. Electrophysiological observations in hippocampal slices from rats treated with the ketogenic diet. Dev. Neurosci. 1999;21:393–399. doi: 10.1159/000017389. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takahashi H, Fukuda M, Hino H, Kobayashi K, Tanaka J, Ishii E. Beta-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009;1268:17–23. doi: 10.1016/j.brainres.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Tan Y, Demeter MR, Ruan H, Comb ML. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J. Bio. Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. J. Neurosci. 2011;31:8689–8696. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–331. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- Valente-Silva P, Lemos C, Köfalvi A, Cunha RA, Jones JG. Ketone bodies effectively compete with glucose for neuronal acetyl-CoA generation in rat hippocampal slices. NMR Biomed. 2015;28:1111–1116. doi: 10.1002/nbm.3355. [DOI] [PubMed] [Google Scholar]
- van Delft R, Lambrechts D, Verschuure P, Hulsman J, Majoie M. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure. 2010;19:36–39. doi: 10.1016/j.seizure.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–247. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- Viggiano A, Pilla R, Arnold P, Monda M, D'Agostino D, Coppola G. Anticonvulsant properties of an oral ketone ester in a pentylenetetrazole-model of seizure. Brain Res. 2015;1618:50–54. doi: 10.1016/j.brainres.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Viggiano A, Pilla R, Arnold P, Monda M, D'Agostino D, Zeppa P, Coppola G. Different calorie restriction treatments have similar anti-seizure efficacy. Seizure. 2016;35:45–49. doi: 10.1016/j.seizure.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy-exploration of GABA levels in a ketogenic diet. Magn. Reson. Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI, 2nd, Sonner JM. Anesthetic properties of the ketone bodies beta-hydroxybutyric acid and acetone. Anesth. Analg. 2007;105:673–679. doi: 10.1213/01.ane.0000278127.68312.dc. [DOI] [PubMed] [Google Scholar]
- Ye J. Improving Insulin Sensitivity With HDAC Inhibitor. Diabetes. 2013;62:685–687. doi: 10.2337/db12-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Han P, Tang Z, Liu Q, Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J. Cereb. Blood Flow Metab. 2015;35:1783–1789. doi: 10.1038/jcbfm.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef FF. Ketone bodies attenuate excitotoxic cell injury in the rat hippocampal slice under conditions of reduced glucose availability. Neurol. Res. 2015;37:211–216. doi: 10.1179/1743132814Y.0000000430. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A. Brain amino acid metabolism and ketosis. J. Neurosci. Res. 2001;66:272–281. doi: 10.1002/jnr.1221. [DOI] [PubMed] [Google Scholar]
- Yum MS, Lee M, Woo DC, Kim DW, Ko TS, Velíšek L. β-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 2015;117:125–132. doi: 10.1016/j.eplepsyres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang S, Marin-Valencia I, Puchowicz MA. Decreased carbon shunting from glucose toward oxidative metabolism in diet-induced ketotic rat brain. J. Neurochem. 2015;132:301–312. doi: 10.1111/jnc.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]