Abstract
This study used mice to evaluate whether coupling expression of corticotropin-releasing hormone (CRH) and angiotensin converting enzyme 2 (ACE2) creates central interactions that blunt endocrine and behavioral responses to psychogenic stress. Central administration of diminazene aceturate, an ACE2 activator, had no effect on restraint-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis; however, mice that ubiquitously overexpress ACE2 had reduced plasma corticosterone (CORT) and pituitary expression of POMC mRNA. The Cre-LoxP system was used to restrict ACE2 overexpression to CRH synthesizing cells and probe whether HPA axis suppression was the result of central ACE2 and CRH interactions. Within the paraventricular nucleus of the hypothalamus (PVN), mice with ACE2 overexpression directed to CRH had a ≈ 2.5 fold increase in ACE2 mRNA, which co-localized with CRH mRNA. Relative to controls, mice overexpressing ACE2 in CRH cells had a decreased CORT response to restraint as well as decreased CRH mRNA in the PVN and CEA and POMC mRNA in the pituitary. Administration of ACTH similarly increased plasma CORT, indicating that the blunted HPA axis activation that accompanies ACE2 overexpression in CRH cells is centrally mediated. Anxiety-like behavior was assessed to determine whether the decreased HPA axis activation was predictive of anxiolysis. Mice with ACE2 overexpression directed to CRH cells displayed decreased anxiety-like behavior in the elevated plus maze and open field when compared to that of controls. Collectively, these results suggest that exogenous ACE2 suppresses CRH synthesis, which alters the central processing of psychogenic stress, thereby blunting HPA axis activation and attenuating anxiety-like behavior.
Keywords: corticosterone, CRH, HPA, PVN, stress, anxiety
1. Introduction
Anxiety disorders are comorbid with hypertension (Sandstrom et al., 2016) and common pathophysiology may contribute to each disease. Augmentation of the renin-angiotensin-system (RAS) increases the synthesis of the angiotensin II (Ang-II) and its activation of the angiotensin type- 1a receptor (AT1aR) is established to promote hypertension; however, emerging evidence suggests that the RAS also contributes to the onset of anxiety disorders. Psychogenic stress increases Ang-II and interventions that inhibit AT1aR(s) attenuate anxiety-like behavior and hypothalamic-pituitary-adrenal (HPA) axis activity (Saavedra et al., 2005). Angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) alleviate hypertension. Interestingly, patients with posttraumatic stress disorder (PTSD) taking ACEi or ARBs have fewer traumatic stress symptoms relative to PTSD patients taking other types of blood pressure lowering medications (Khoury et al., 2012). Dysregulation of the HPA axis frequently occurs in patients with anxiety disorders (Abelson et al., 2007; Brand et al., 2011; Vreeburg et al., 2010) and chronic administration of an ARB corrects this dysregulation and improves affect (Pavlatou et al., 2008). These results implicate the RAS in the etiology of affective disorders and suggest it may serve as a viable target for therapeutic interventions.
The relatively recent discovery of angiotensin converting enzyme 2 (ACE2) revealed a ‘protective limb’ of the RAS that opposes many of the deleterious consequences of AT1aR activation (Xu et al., 2011). ACE2 metabolizes Ang-II into angiotensin 1-7 (Ang1-7) which promotes cardio-protection by activating the Mas receptor (MasR) (Xia and Lazartigues, 2010). Levels of ACE2 and Ang1-7 are elevated in patients taking ACEi and ARBs (Furuhashi et al., 2015; Luque et al., 1996), suggesting that blocking the synthesis of Ang-II or its actions at the AT1aR augments ACE2 activity (Ferreira et al., 2010). We recently discovered that increasing ACE2 activity in the brain is potently anxiolytic in mice (Wang et al., 2016a) but whether increasing ACE2 activity alters activation of the HPA axis, another indicator of stress responsiveness, is unknown.
Activation of the HPA axis is initiated by neurons in the paraventricular nucleus of the hypothalamus (PVN) that secrete corticotropin-releasing-hormone (CRH) into the median eminence to stimulate the release of adrenocorticotropic hormone (ACTH), which drives adrenal glucocorticoid (CORT) secretion. Anxiety disorders are associated with HPA axis dysfunction (Abelson et al., 2007; Brand et al., 2011; Vreeburg et al., 2010) and we discovered that the majority of CRH neurons in the PVN express AT1aR(s) and their deletion down-regulates CRH mRNA (de Kloet et al., 2013; de Kloet et al., 2017; Wang et al., 2016b). Neuropsychiatric illnesses are associated with impaired CRH signaling in the brain (Banki et al., 1987; Nemeroff et al., 1988; Nemeroff et al., 1984) and coupling CRH transcription and ACE2 overexpression may inhibit the stimulation of AT1aR expressed on CRH neurons and down-regulate its production. Increasing ACE2 activity also has the beneficial effect of elevating levels of Ang1-7, which promotes anxiolysis by activating MasR(s) (Kangussu et al., 2017; Moura Santos et al., 2017; Wang et al., 2016a). Probing CRH and ACE2 interactions within the CNS may reveal novel strategies for reducing stress responsiveness by dampening HPA axis activation and attenuating anxiety.
This study used mice to evaluate whether central CRH and ACE2 interactions dampen endocrine and behavioral responses to psychogenic stress. We administered mice diminazene aceturate (DIZE) or engineered mice that ubiquitously overexpress ACE2 in order to evaluate whether pharmacological up-regulation of endogenous ACE2 or genetic overexpression of exogenous ACE2 alters HPA axis activity. Next, we used the Cre-LoxP system to direct ACE2 overexpression to CRH transcription to probe whether altered HPA axis activation was the result of a CRH and ACE2 interaction. Anxiety-like behavior was assessed to determine whether any differences in HPA axis activation were associated with altered behavioral responses to psychogenic stressors. The results suggest that directing ACE2 overexpression to CRH transcription creates central interactions that inhibit stress-induced HPA axis activation and attenuate anxiety-like behavior.
2. Materials and Methods
2.1. Animals
All mice were male and 8–12 weeks-old at the initiation of the study. Mice were given ad libitum access to pelleted rodent chow and water and were individually-housed on a 12h/12h light-dark cycle. The light phase started at 0700 h and the dark phase started at 1900 h. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
C57BL/6 mice
We used C57BL/6 mice obtained from the Harlan Laboratories to evaluate the effects of centrally administered DIZE, an ACE2 activator (De Maria et al., 2016; Qi et al., 2013a), on stress-induced activation of the HPA axis.
ACE2 KI mice
We have previously described the generation of ACE2 KI mice and their wild-type littermate controls (WT) (Wang et al., 2016a). Briefly, ACE2 KI mice have the expression of ACE2 driven by ROSA26. Both ACE2 KI mice and WT are maintained on a 129/B6 mixed background. We have previously validated that ACE2 KI mice have significantly increased ACE2 mRNA expression and ACE2 activity in central and peripheral tissues (Qi et al., 2016; Wang et al., 2016a).
CRH ACE2 KI mice
To overexpress ACE2 specifically in CRH cells, we first generated mice homozygous for floxed STOP ACE2 (ACE2 gene was preceded by a stop codon that was flanked by two loxP sites) after the ROSA26 promoter (floxed STOP ACE2 mice). These floxed STOP ACE2 mice were bred to mice heterozygous for Cre recombinase driven by the gene encoding for CRH (CRH-Cre mice, Jackson Laboratory Stock # 012704) to generate mice carrying both floxed STOP ACE2 and CRH-Cre (CRH ACE2 KI mice) as well as littermate controls carrying only the floxed STOP ACE2 gene (CON mice).
CRH-Cre mice
To evaluate whether Cre recombinase expression, in and of itself, affects HPA axis activity, we bred heterozygous CRH-Cre mice to C57BL/6J mice (Jackson Laboratory) to generate CRH-Cre mice and littermate wild-type controls (littermate WT CON).
2.2. Intracerebroventricular (ICV) infusion of DIZE
To determine whether activating endogenous ACE2 in the brain influences HPA axis activity, we chronically administered normal saline or DIZE (low dose group: 0.11 μg/h, medium dose group: 1.1 μg/h, high dose group: 11 μg/h) into the lateral ventricles of C57BL/6 mice for 2 weeks. The icv infusions were conducted using micro-osmotic pumps (Model 1004, flow rate: 0.11 mL/h, ALZET, Cupertino, CA, USA). Prior studies determined that central delivery of DIZE, at the medium and high doses used here, significantly decreases anxiety-like behavior (Wang et al., 2016a). The procedures for preparation and implantation of micro-osmotic pumps have been described previously (Wang et al., 2016a). Two weeks after implantation of micro-osmotic pumps, the plasma corticosterone (CORT) response to restraint stress was assessed.
2.3. Assessment of plasma CORT
We examined plasma CORT concentrations before, during and after a 30 min restraint challenge in DIZE-infused mice, ACE2 KI mice, CRH ACE2 KI mice, and their respective controls. Between 0800 h–0900 h, we restrained mice in clear plastic ventilated tubes and collected tail vein blood samples (40 μl) within 3 min to measure morning basal levels of CORT. Thirty-minutes after the onset of restraint, we collected another set of blood samples, and mice were released and returned to home cages. Additional blood samples were collected at 60 and 120 min after the onset of restraint. Blood samples were centrifuged at 3500 rpm for 15 min at 4° C to isolate plasma. Plasma samples were stored at −80° C.
To assess afternoon basal CORT levels in CRH ACE2 KI mice and CON mice, blood samples were taken during the light phase between 1800 h–1900 h and plasma was isolated and stored at −80° C.
Plasma concentration of CORT was measured using an I125 radioimmunoassay kit (MP Biomedicals, Orangeburg, NY) as previously described (Krause et al., 2011; Krause et al., 2008).
2.4. Assessment of pro-opiomelanocortin (POMC) expression in the pituitary gland
We used semiquantitative real-time polymerase chain reaction (PCR) to measure mRNA expression of POMC in the pituitary. Pituitary glands were extracted immediately after decapitation of mice that were pre-anesthetized with isoflurane. We have previously described the procedures for real-time PCR (de Kloet et al., 2013; Wang et al., 2016a). Briefly, RNA was extracted from pituitary glands using RNeasy columns (Qiagen Sciences, Germantown, MD, USA), cDNA was synthesized using iScript (Bio-Rad, Hercules, CA, USA), and real-time PCR was conducted using TaqMan probes and TaqMan Gene Expression Master Mix in a 7900HT Fast Real-time PCR system (Thermofisher Scientific, Waltham, MA, USA). Specific TaqMan probes used in the present study were POMC (Mm00435874; Thermofisher Scientific, Waltham, MA, USA) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, house-keeping gene, Mm99999915). After obtaining the Ct values, we calculated the relative expression of POMC using the 2ΔΔCt method.
2.5. RNAscope in situ hybridization
We performed RNAscope in situ hybridization in brain nuclei that synthesize CRH, the PVN and central nucleus of the amygdala (CEA), and another that does not, the globus pallidus (GP), to confirm that coupling ACE2 overexpression to the CRH gene significantly increases ACE2 mRNA within CRH cells but does not affect its expression in brain nuclei devoid of CRH (Cummings et al., 1983). Reagents used for RNAscope in situ hybridization were purchased from Advanced Cell Diagnostics (Newark, CA, USA) unless otherwise specified. We have previously described the procedures for tissue preparation and RNAscope in situ hybridization (de Kloet et al., 2016; Smith et al., 2014; Wang et al., 2016a). Briefly, mice were euthanized with an overdose of sodium pentobarbital and transcardially perfused with 4% RNase free paraformaldehyde (PFA). Brains were quickly extracted, submerged in RNase free 4% PFA for 4 h, and then transferred into RNase free 30% sucrose at 4° C. Subsequent to cryoprotection, brains were coronally sectioned at 20 μm using a Leica CM3050 S cryostat. Brain sections were rinsed twice in RNase free PBS and mounted onto Superfrost Plus Gold slides. Mounted brain sections were air-dried for 20 min at room temperature and then stored at −80° C. On the day of conducting RNAscope in situ hybridization, brain sections were air-dried for 30 min at room temperature, incubated in protease IV (Catalog No. 322340) for 20 min, and then hybridized with specific probes following procedures described in the RNAscope® Multiplex Fluorescent Kit User Manual PART 2 (Advanced Cell Diagnostics, Newark, CA, USA). The specific probes used in this study were: (1) DapB, negative control, (2) Ubc, positive control, (3) CRH (Catalog No. 316091), (4) ACE2 (Catalog No. 417081-C2). CRH and ACE2 mRNAs were co-labeled on the same brain section with CRH mRNA labeled with Atto-550 and ACE2 mRNA labeled with Atto-647.
2.6. Image capturing and analysis
Fluorescent images for RNAscope in situ hybridization were captured using an AxioImager M.2 fluorescent Apotome microscope (Carl Zeiss Microscopy, Thornwood, NY, USA). When capturing the images, we first determined the optimal exposure time for each channel based on the sections hybridized with Ubc (positive control) and then confirmed that no visible fluorescence was detected in sections hybridized with DapB (negative control) under this exposure time. Then we captured all images under 20 × magnification using the same exposure time obtained from the positive control. To analyze the density of mRNA signals, we used ImageJ. Specifically, we converted the images to 8-bit greyscale and then measured the density of dots representing mRNA signals. We first measured the negative control images to determine nonspecific background and then measured the images representing CRH mRNA or ACE2 mRNA. Subsequently, we subtracted the nonspecific background from the signal generated by CRH mRNA or ACE2 mRNA to obtain the density of mRNA transcript for each gene within a region of interest.
2.7. Plasma ACE2 activity assay
Plasma ACE2 activity was assayed as previously described (Qi et al., 2016) to evaluate whether directing ACE2 overexpression to the CRH gene affects systemic indices of ACE2 activity. Briefly, tail blood samples (40 μl) were collected with EDTA (ethylenediaminetetraacetic acid) from CRH ACE2 KI and CON mice and centrifuged at 3500 rpm for 15 min at 4° C to isolate plasma. Plasma was diluted at 1:10 ratio in buffer that consisted of 1 M NaCl, 75 mM Tris–HCl, and 0.5 mM ZnCl2 (pH 7.4) and 25 nM ACE2 enzyme was used as a positive control. Human recombinant ACE2 (catalog 933-ZN-010) were obtained from R&D Systems along with fluorogenic ACE2 substrate (fluorogenic peptide VI (FPS VI), Mca-YVADAPK(Dnp)OH, catalog ID ES007). All reactions were performed in a total volume of 100 μl using a fluorescence plate reader (Synergy HT, Biotek, USA) at an excitation wavelength of 320 nm and emission wavelength of 405 nm as described previously. All samples were read at 37 °C for 4 h immediately after the addition of fluorogenic substrate.
2.8 ACTH stimulation Test
To evaluate whether overexpressing ACE2 in CRH cells affects adrenal responsiveness to ACTH, we injected mice with dexamethasone (4 μg/kg, sc). This dose of dexamethasone suppresses the release of endogenous CORT in mice (de Kloet et al., 2015). Two hours after the injection of dexamethasone, tail vein blood samples were collected, and subsequently, mice were delivered a low dose of ACTH (0.01 mg/kg, sc). This dose of ACTH was previously determined to stimulate CORT release in mice that is ≈ 50% of the maximal response (de Kloet et al., 2015). Fifteen minutes after the injection of ACTH, another set of tail vein blood samples was collected and plasma CORT was measured as described above.
2.8. Assessment of anxiety-like behavior
Anxiety-like behavior was assessed using the elevated plus maze (EPM) between 0800 h–1200 h. The EPM consists of two opposing open (31 cm × 6 cm) and two opposing closed arms (31 cm × 6 cm) that were elevated 41 cm off the floor. To start the test, a mouse was placed in the center of the EPM and allowed to explore the maze for 5 min. Each testing session was recorded by a ceiling-mounted camera connected to a PC running TopScan software (CleverSys, Reston, VA). The TopScan software automatically analyzed the movement of mice in the EPM. We manually summarized time spent in the open arms, the number of open arm entries, and the total distance travelled.
Anxiety-like behavior was also assessed using the open field apparatus. The open field apparatus consists of a walled (height: 20.3 cm) square-shaped arena (27.3 cm × 27.3 cm). Two regions were defined in the arena: the center, which accounts for 25% of the total area and the periphery, which accounts for the remaining 75% of the total area. The open field test was conducted between 0800-1200 h. To start the test, a mouse was placed in the center and allowed to explore the open field for 5 min. Each testing session was video recorded and the movements of mice were automatically analyzed by the same PC system used for the EPM test. We then manually summarized the time spent in the center, the number of center entries, and the total distance travelled.
2.9. Experimental Design and Statistical Analysis
All experiments adopted between-subjects designs, in which ACE2 KI mice and CRH ACE2 KI mice were compared to their respective littermate controls. Experiments were conducted using age and temporally matched cohorts of mice. Plasma CORT subsequent to restraint was assessed using two cohorts of mice: 1st cohort: WT (n=7) and ACE2 KI (n=4); 2nd cohort: WT (n=10) and ACE2 KI (n=10). CRH and ACE2 mRNA expression were assessed using two cohorts of mice: 1st cohort: CON (n=3) and CRH ACE2 KI (n=3); 2nd cohort: CON (n=8) and CRH ACE2 KI (n=6). Basal plasma CORT sampled in the afternoon was assessed using two cohorts of mice: 1st cohort: CON (n=12) and CRH ACE2 KI (n=13); 2nd cohort: CON (n=13) and CRH ACE2 KI (n=14). EPM testing was conducted using two cohorts of mice: 1st cohort: CON (n=3) and CRH ACE2 KI (n=4); 2nd cohort: CON (n=9) and CRH ACE2 KI (n=12). All other experiments were conducted using one cohort of mice.
All data were analyzed and graphed using Prism 5 (GraphPad Software, La Jolla, CA, USA) and presented as mean ± SEM. All CORT results were analyzed using a two-way ANOVA. All other results were analyzed using a two-tailed student t-test with the exception of the results from the open field test, which were analyzed using a one-tailed student t-test due to a directional hypothesis based on the findings from the EPM. Exact P values were reported when available from Prism 5. P values less than 0.0001 and P values for the Bonferroni post-hoc analysis were only available as ranges and are reported accordingly.
3. Results
3.1. Exogenous ACE2 is sufficient for stress-evoked HPA axis dampening
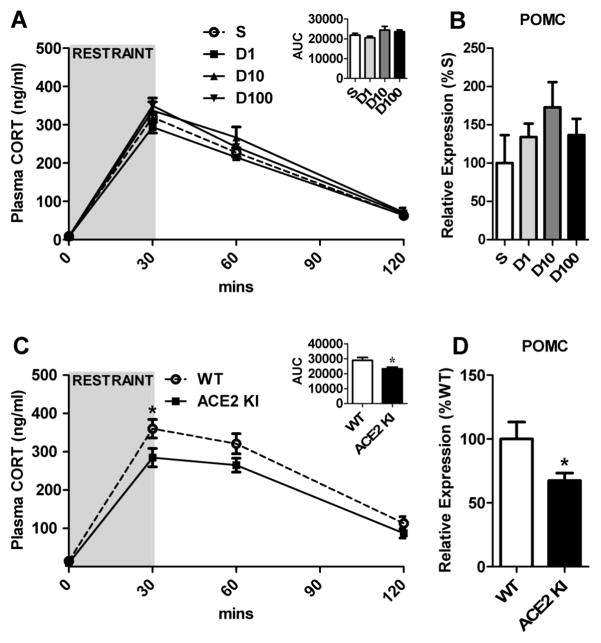
Mice were infused (icv) with normal saline (n=9) or DIZE (low dose, n=10; medium dose, n=12; high dose, n = 9) for 2 weeks to evaluate the effects of ACE2 activation on the plasma CORT response to psychogenic stress. As expected, restraint significantly (F(3, 108) = 562, P < 0.0001) elevated plasma CORT relative to basal levels at 30 and 60 min (Figure 1A). However, central delivery of DIZE did not affect basal or restraint-induced elevations in plasma CORT or pituitary expression of POMC mRNA (Figure 1B). Taken together, these results suggest that administration of DIZE into the brain is insufficient to suppress HPA axis activity.
Figure 1. The effects of chronic central administration of DIZE or ubiquitous ACE2 overexpression on the HPA axis.
(A) Plasma CORT levels in response to 30 min restraint and (B) pituitary expression of POMC in mice receiving icv infusion (0.11 μl/h) of normal saline (S) or DIZE (D1, 0.11 μg/h; D10, 1.1 μg/h; D100, 11 μg/h). AUC: Area under the curve indicating the integrated response. (C) Plasma CORT levels in response to 30 min restraint and (D) pituitary expression of POMC in WT and ACE2 KI mice. Bars represent mean and SEM. * = P < 0.05.
To determine whether ubiquitous overexpression of ACE2 attenuates the HPA axis response to psychogenic stress, ACE2 KI mice (n=14) and their WT littermate controls (n=17) were subjected to a 30 min restraint challenge. As expected, there was an effect of time (F(3, 87) = 159, P < 0.0001) with restraint significantly elevating plasma CORT at 30 and 60 min relative to baseline levels (Figure 1C). There was also an effect of genotype (F(1, 29) = 5.97, P = 0.021) with ACE2 KI mice having significantly lower plasma CORT relative to WT littermate controls. Post-hoc analysis found that both groups of mice had similar baseline levels of plasma CORT; however, 30 min after the onset of restraint the plasma CORT of ACE2 KI mice was significantly lower than that of WT littermate controls (Bonferroni post-hoc analysis, t(116) = 2.86, P < 0.05). This blunted stress-induced HPA axis activation was also evident in the integrated response (AUC; t(29) = 2.34, P = 0.026; Figure 1C inset). Additionally, ACE2 KI mice (n=6) had significantly (t(10) = 2.23, P = 0.0498, Figure 1D) decreased pituitary expression of POMC mRNA compared to WT littermate controls (n=6). Collectively, these results suggest that delivery of exogenous ACE2 is necessary to observe dampened HPA axis activation.
3.2. Coupling ACE2 overexpression to CRH transcription increases ACE2 mRNA expression in the PVN and CEA but has no effect on plasma ACE2 activity
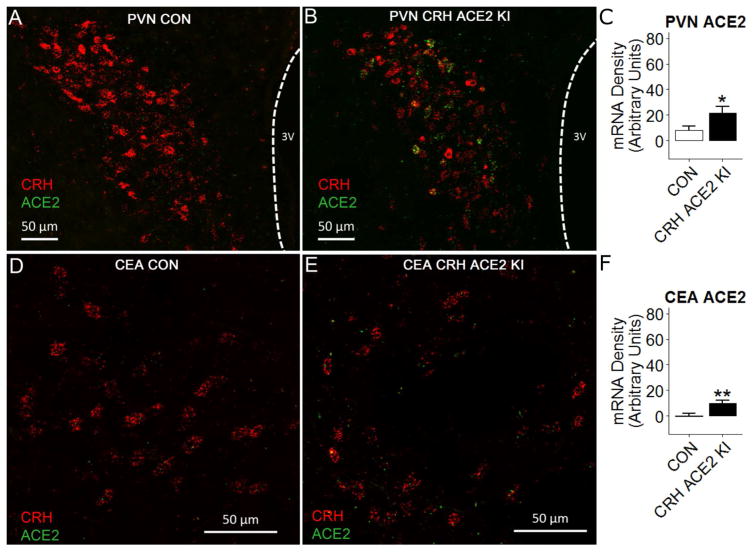
To confirm that CRH ACE2 KI mice indeed overexpress ACE2 in CRH cells, we conducted RNAscope in situ hybridization for CRH mRNA and ACE2 mRNA in the PVN and CEA. Control mice had modest levels of ACE2 mRNA in the PVN and this expression rarely co-localized with CRH mRNA (Figure 2A). In contrast, the ACE2 mRNA observed in CRH ACE2 KI mice frequently co-localized with CRH mRNA in the PVN and the CEA (Figure 2B and 2E). Quantitative analysis found that relative to CON mice, CRH ACE2 KI mice had significantly increased expression of ACE2 mRNA in CRH-producing regions of the PVN (CON; n =10, CRH ACE2 KI; n = 9, t(17) = −2.39, P = 0.029, Figure 2C) and the CEA (CON; n =11, CRH ACE2 KI; n = 9, t(18) = −3.13, P = 0.0058, Figure 2F). Importantly, no differences in ACE2 mRNA (P = 0.85; CON; n = 8, CRH ACE2 KI; n= 7) were observed in the GP, indicating that ACE2 overexpression was specifically directed to CRH synthesis. Plasma ACE2 activity was also similar (P = 0.77) between CON mice (n = 7; 27.5 ± 2.99 RFU\min\μl) and CRH ACE2 KI mice (n =8; 24.5 ± 2.49 RFU\min\μl), suggesting that directing ACE2 overexpression to the CRH does not affect ACE2 activity in the periphery. Taken together, these results confirm that ACE2 overexpression was directed to CRH transcription, resulting in a significant increase in ACE2 mRNA in the PVN and CEA.
Figure 2. Expression of ACE2 mRNA in CRH-producing regions in CON and CRH ACE2 KI mice.
(A and B) mRNA expression of ACE2 (punctate green dots) and CRH (punctate red dots) in the paraventricular nucleus of hypothalamus (PVN) of (A) a CON mouse and (B) a CRH ACE2 KI mouse. (C) Integrated density of ACE2 mRNA signal within the PVN. (D and E) mRNA expression of ACE2 (punctate green dots) and CRH (punctate red dots) in the central nucleus of amygdala (CEA) of (D) a CON mouse and (E) a CRH ACE2 KI mouse. (F) Integrated density of ACE2 mRNA signal within the CEA. Bars represent mean and SEM. * = P < 0.05, * = P < 0.01.
3.3. Overexpressing ACE2 in CRH cells attenuates stress-induced HPA axis activation
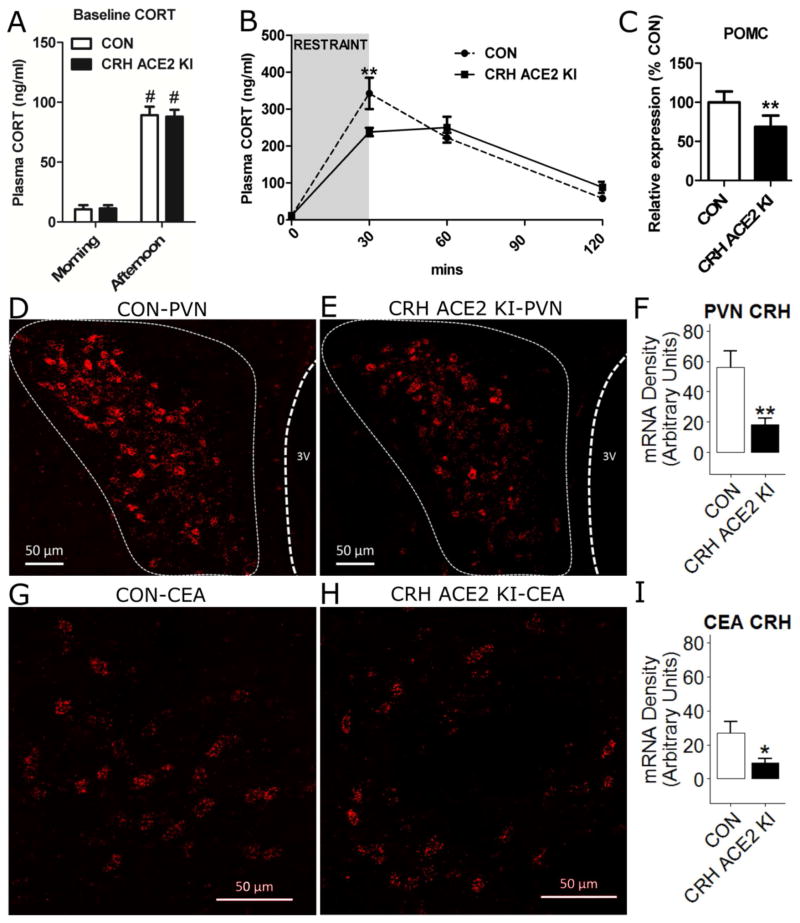
To study whether overexpressing ACE2 in CRH cells affected the diurnal rhythm of the HPA axis, we measured non-stressed levels of plasma CORT in the morning (CON mice; n = 8; CRH ACE2 KI mice; n = 10) and in the afternoon (CON mice; n = 25, CRH ACE2 KI mice; n = 27). As expected, there was an effect of time (F(1, 66) = 97.1, P < 0.0001) with plasma CORT in the afternoon being significantly higher than that in the morning (Bonferroni post-hoc analysis; CON mice, morning vs. CON afternoon, t(66) = 6.75, P < 0.0001; CRH ACE2 KI mice, morning vs. afternoon, t(66) = 7.22, P < 0.0001; Figure 3A). However, these differences were not affected by genotype (F(1, 66) = 0.0009, P = 0.976) indicating that overexpression of ACE2 in CRH cells does not disrupt the diurnal rhythm of CORT secretion.
Figure 3. Overexpressing ACE2 in CRH cells attenuates HPA axis activation during restraint stress.
(A) Basal levels of plasma CORT in the morning and in the afternoon of CON and CRH ACE2 KI mice. (B) Plasma CORT levels in response to 30 min restraint and (C) pituitary expression of POMC in CON and CRH ACE2 KI mice. (D and E) Expression of CRH mRNA (punctate red dots) in the PVN of (D) a CON mouse and (E) a CRH ACE2 KI mouse. (F) Integrated density of CRH mRNA signal within the PVN. (G and H) Expression of CRH mRNA (punctate red dots) in the CEA of (G) a CON mouse and (H) a CRH ACE2 KI mouse. (I) Integrated density of CRH mRNA signal within the CEA. Bars represent mean and SEM. * = P < 0.05, ** = P < 0.01, # = P < 0.0001 vs. Morning.
To investigate how overexpressing ACE2 in CRH cells affects plasma CORT subsequent to psychogenic stress, CON mice (n = 8) and CRH ACE2 KI mice (n = 10) were subjected to a 30 min restraint challenge. Again, there was an effect of time (F(3, 60) = 89.9, P < 0.0001) with restraint significantly elevating plasma CORT above baseline at 30 and 60 min (Figure 3B). The increased plasma CORT at 30 min was significantly attenuated in CRH ACE2 KI mice relative to CON mice (Bonferroni post-hoc analysis, t(60) = 3.73, P < 0.01; Figure 3B). These results demonstrate that overexpressing ACE2 in CRH cells attenuates activation of the HPA axis in response to acute psychogenic stress. To explore whether the reduced plasma CORT was centrally-mediated, mRNA expression of POMC in the pituitary gland and CRH in the PVN was measured using real-time PCR and RNAscope in situ hybridization, respectively. CRH ACE2 KI mice (n = 6) had significantly (t(5) = 4.68, P = 0.0054) decreased expression of POMC mRNA in the pituitary gland compared to CON mice (n = 6) (Figure 3C), suggesting that overexpressing ACE2 in CRH cells may inhibit the production of ACTH. In addition, we found that CRH ACE2 KI mice had significantly less CRH mRNA transcripts in the PVN (CON; n =11, CRH ACE2 KI; n = 9, t(18) = 2.97, P = 0.0081; Figure 3D–F) and CEA (CON; n =10, CRH ACE2 KI; n = 8, t(16) = 2.19, P = 0.043; Figure 3G–I) when compared to that of controls. These results indicate that overexpressing ACE2 in CRH cells suppresses stress-induced HPA axis activation and down-regulates the synthesis of CRH in the PVN and CEA.
3.4. Directing Cre recombinase to the CRH gene does not affect HPA axis activity
Basal and stressed levels of plasma CORT were measured in CRH-Cre mice and their wild-type littermate controls to address the possibility that directing Cre recombinase expression to the CRH gene, in and of itself, affects HPA axis activity. CRH-Cre (n=11) mice and littermate WT CON mice (n=7) had similar basal plasma CORT (CRH-Cre mice, 6.34 ± 0.685 ng/ml vs. littermate WT CON mice, 6.16 ± 1.25 ng/ml) and similar stress-induced plasma CORT (30 min after restraint, CRH-Cre mice, 249 ± 26.0 ng/ml vs. littermate WT CON mice, 208 ± 28.6 ng/ml; 60 min after restraint, CRH-Cre mice, 273 ± 24.2 ng/ml vs. littermate WT CON mice, 259 ± 29.6 ng/ml; 120 min after restraint, CRH-Cre mice, 130 ± 16.9 ng/ml vs. littermate WT CON mice, 114 ± 19.8 ng/ml). These results suggest that the blunted HPA axis activation that was observed in the CRH ACE2 KI mice cannot be attributed to the presence of Cre recombinase, but rather, is the result of increased ACE2 production.
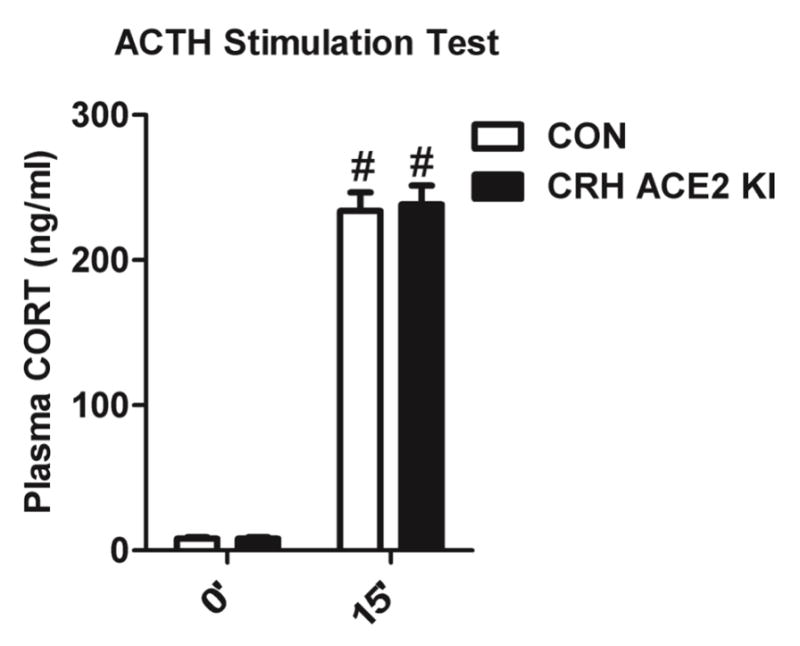
3.5. Overexpressing ACE2 in CRH cells does not affect adrenal responsiveness to ACTH
We subjected CRH ACE2 KI mice and their respective control mice to an ACTH stimulation test to evaluate whether the decreased plasma CORT observed in CRH ACE2 KI mice subsequent to stress was the result of altered adrenal responsiveness to ACTH. Administration of dexamethasone was associated with similarly low levels of endogenous CORT (8.03 ng/ml in CON; 8.06 ng/ml in CRH ACE2 KI mice; Figure 4, 0′). Delivery of exogenous ACTH elevated plasma CORT (F(1, 46) = 615, P < 0.0001; Figure 4); however, this response did not differ as a function of genotype (F(1, 46) = 0.06, P = 0.802; Figure 4, 15′). Collectively, these results suggest that overexpressing ACE2 in CRH cells does not affect adrenal responsiveness to ACTH.
Figure 4. Overexpressing ACE2 in CRH cells does not affect adrenal responsiveness to ACTH.
Plasma CORT levels of CON and CRH ACE2 KI mice 2 h after subcutaneous injection of 4 μg/kg dexamethasone (0′) and 15 min after subcutaneous injection of 0.01 mg/kg ACTH (15′). Bars represent mean and SEM. # = P < 0.0001 vs. 0′.
3.6. Overexpressing ACE2 in CRH cells attenuates anxiety-like behavior
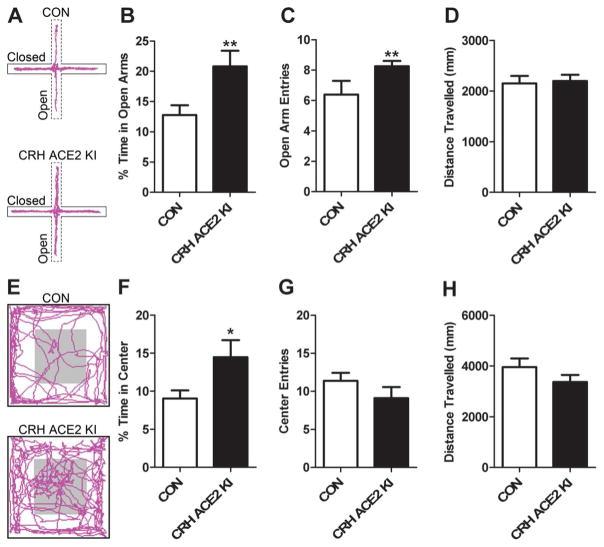
To investigate whether the attenuated stress-induced activation of the HPA axis observed in CRH ACE2 KI mice was predictive of altered behavioral responses to psychogenic stress, we assessed anxiety-like behavior in the EPM. Relative to CON mice (n = 12), CRH ACE2 KI (n = 16) mice spent more time in the open arms (t(26) = 2.95, P = 0.007; Figure 5B), entered more frequently to the open arms (t(26) = 3.10, P = 0.005; Figure 5C), but travelled similar distances throughout the test (t(26) = 0.709, P = 0.485, Figure 5D). To further validate the anxiolytic behavior observed in the EPM, we tested mice in the open field arena and found that compared to CON mice (n = 8), CRH ACE2 KI mice (n = 11) spent more time in the center of the open field arena (t(17) = 1.94, P = 0.03; Figure 5F) but travelled similar distances throughout the test (t(17) = 1.36, P = 0.10, Figure 5H). Together, these results suggest that overexpressing ACE2 in CRH cells attenuates anxiety-like behavior without altering locomotor activity.
Figure 5. Overexpressing ACE2 in CRH cells attenuates anxiety-like behavior.
(A) Representative locomotor traces of (top) a CON mouse and (bottom) a CRH ACE2 KI mouse in the elevated plus maze (EPM). (B) Average percentage of time the mice spent in the open arms of the EPM. (C) Average number of times mice entered the open arms of the EPM. (D) Average total distance mice travelled in the EPM. (E) Representative locomotor traces of (top) a CON mouse and (bottom) a CRH ACE2 KI mouse in the open field arena. (F) Average percentage of time mice spent in the center of the open field arena. (G) Average number of times mice entered the center of the open field arena. (H) Average total distance mice travelled in the open field arena. Bars represent mean and SEM. * = P < 0.05, ** = P < 0.01.
4. Discussion
This series of experiments evaluated the impact of increasing the activity and/or expression of ACE2 on indices of stress responding. Targeting endogenous ACE2 activity in the brain with central delivery of DIZE had no effect on the HPA axis; however, mice with ubiquitous overexpression of ACE2 had blunted stress-induced activation of the HPA axis. We coupled CRH transcription and ACE2 overexpression to evaluate whether doing so recapitulates the HPA dampening observed with ubiquitous ACE2 overexpression. Indeed, relative to littermate controls harboring only the STOP floxed ACE2 gene, CRH ACE2 KI mice had an attenuated HPA response to restraint stress. Importantly, basal and stress-evoked plasma CORT levels were similar amongst CRH-Cre mice and their wild-type littermates indicating that the presence of Cre recombinase, in and of itself, did not contribute to HPA axis suppression. Additionally, directing ACE2 overexpression to CRH synthesizing cells had no effect on the diurnal rhythm of the HPA axis or adrenal responsiveness to ACTH but did reduce CRH mRNA in the PVN and CEA as well as POMC mRNA in the pituitary. To determine whether these central alterations were predictive of decreased anxiety-like behavior, mice were tested in the EPM and open field. As predicted, CRH ACE2 KI mice displayed decreased anxiety-like behavior in the EPM and open field relative to controls. Collectively, these results suggest that delivery of exogenous ACE2 suppresses CRH synthesis in the PVN and CEA, which alters the central processing of psychogenic stress, thereby blunting HPA axis activation and attenuating anxiety-like behavior.
The present study found that pharmacological up-regulation of endogenous ACE2 with chronic central administration of the ACE2 activator, DIZE (De Maria et al., 2016; Qi et al., 2013b), had no effect on basal or stress-induced HPA axis activity. In contrast, ACE2 KI mice had decreased plasma CORT subsequent to a 30 min restraint challenge relative to their wild-type littermate counter-parts. The conflicting effects that DIZE and ACE2 overexpression have on the HPA axis may be due to different molecular mechanisms of action. In this regard, DIZE increases the activity of ACE2 by binding to the hinge region of the enzyme, which facilitates its ability to convert Ang-II into Ang1-7 (Kulemina and Ostrov, 2011; Shenoy et al., 2013). Consequently, the efficacy by which DIZE elicits cleavage of Ang-II is dependent on the availability of endogenous ACE2; however, we found that control mice had relatively low ACE2 mRNA expression in the PVN (see Figure 2A). Given that neurons in the PVN initiate activation of the HPA axis, it is possible that DIZE had no effect on plasma CORT because insufficient amounts of endogenous ACE2 were available. Collectively, these results suggest that exogenous ACE2 is sufficient to observe blunted HPA axis activation subsequent to psychogenic stress exposure and the implication is that recombinant ACE2 may be well suited to attenuate HPA axis hyperactivity.
We generated CRH ACE2 KI mice to determine whether selectively targeting ACE2 overexpression to CRH transcription recapitulates the HPA axis dampening observed with ubiquitous overexpression. Relative to controls, CRH ACE2 KI mice had increased levels of ACE2 mRNA in the PVN and CEA. The basal plasma CORT of control and CRH ACE2 KI mice were similar in the morning and afternoon, indicating that ACE2 overexpression did not alter the diurnal rhythm of the HPA axis. When CRH ACE2 KI mice were challenged with restraint an attenuated HPA axis response was revealed. Specifically, plasma CORT assessed 30 min after the onset of restraint was significantly lower in CRH ACE2 KI mice when compared to that of controls. However, CRH ACE2 KI mice were compared to littermates harboring only the STOP flox ACE2 gene, which presents the possibility that the presence of Cre recombinase, in and of itself, suppresses stress-induced HPA axis activation. Consequently, stress-induced elevations in plasma CORT were assessed in CRH-Cre mice and their wild-type littermate counterparts. In this cohort of mice, the maximum plasma CORT response to restraint tended to be lower compared to that of mice carrying the floxed STOP ACE2 gene (WT; 259 ± 29.6 ng/ml vs. CRH-Cre, 273 ± 24.2 ng/ml vs. CON; 342 ± 42.6 ng/ml see Figure 3). Genetic influences, litter effects and/or methodological dissimilarities between these groups may have contributed to this apparent difference. Nonetheless, CRH-Cre mice and their wild-type littermate controls had similar plasma CORT before, during and after restraint stress, suggesting that the HPA dampening observed in CRH ACE2 KI mice cannot be attributed to the presence of Cre recombinase, but rather, occurs downstream of ACE2 overexpression.
In this regard, CRH ACE2 KI mice had lower levels of CRH and POMC mRNA in the PVN and pituitary, respectively. POMC is the precursor for the glucocortoid secretagogue, ACTH, and previous in vitro and in vivo studies determined that levels of CRH in the PVN regulate POMC synthesis and the release of ACTH from the pituitary (for review see (Aguilera, 1994; Antoni, 1993)). Taking these results into account, we hypothesized that the blunted HPA axis activation that occurs in CRH ACE2 KI mice was centrally mediated. Consistent with this hypothesis, directing ACE2 overexpression to CRH cells had no effect on plasma ACE2 activity and delivery of ACTH to control and CRH ACE2 KI mice treated with dexamethasone similarly increased plasma CORT, indicating that the blunted HPA axis activation is likely not attributed to systemic changes in ACE2 activity or altered adrenal responsivity to ACTH. Taken together, these results suggest the dampened HPA axis activation that occurs in CRH ACE2 KI mice is centrally-mediated and manifests from decreased CRH production in the PVN.
The precise molecular mechanism by which ACE2 down-regulates the expression of CRH mRNA and dampens HPA axis activation is unknown. Consistent with prior research (Aguilera et al., 1995; Oldfield et al., 2001), a recent study from our laboratory found that nearly every CRH producing neuron in the PVN also expresses AT1aR(s) and selective optogenetic excitation of such neurons robustly activates the HPA axis (de Kloet et al., 2017). Therefore, it is possible that ACE2 overexpression suppresses HPA activation by converting Ang-II into Ang1-7, leading to reduced activation of AT1aR(s) in the PVN and decreased CRH transcription. Consistent with this notion, systemic administration of an ARB that crosses the blood-brain-barrier significantly reduces CRH mRNA in the PVN as well as indices of HPA axis activity subsequent to isolation stress (Armando et al., 2001; Armando et al., 2007). To determine whether antagonism of AT1aR(s) in the PVN mediate HPA axis suppression, we engineered mice with AT1aR(s) selectively deleted from the PVN (de Kloet et al., 2013; Wang et al., 2016b). Intriguingly, selective deletion of AT1aR(s) from the PVN significantly decreased hypothalamic CRH mRNA (de Kloet et al., 2013; Wang et al., 2016b) but had no effect on basal or stress-evoked levels of plasma CORT (Wang et al., 2016b). These results suggest that stimulation of AT1aR(s) in the PVN likely influences CRH transcription but selective inhibition of these receptors is insufficient to reduce HPA axis activation.
In humans, pharmacological inhibition of Ang-II production or AT1aR(s) increases levels of ACE2 and Ang1-7 (Furuhashi et al., 2015; Luque et al., 1996) and there is growing appreciation that up-regulation of the ‘protective limb’ of the RAS contributes to the beneficial effects of ACEi and ARBs. As mentioned, ACE2 metabolizes Ang-II into Ang1-7, which serves as a ligand for the MasR, and accumulating pre-clinical evidence suggests that MasR(s) residing in the CNS are potent mediators of mood and affect. Knockout of the MasR is anxiogenic in mice (Walther et al., 1998) and genetic overexpression of Ang1-7 in rats decreases stress-evoked cardiac reactivity and anxiety-like behavior (Kangussu et al., 2017; Moura Santos et al., 2017). Because CRH and HPA axis dysregulation are heavily implicated in the etiology of anxiety disorders (Abelson et al., 2007; Banki et al., 1987; Brand et al., 2011; Nemeroff et al., 1988; Nemeroff et al., 1984; Vreeburg et al., 2010), we predicted that the decreased CRH mRNA and dampened HPA axis activity observed in CRH ACE2 KI mice would be accompanied by decreased anxiety-like behavior. As predicted, CRH ACE2 KI mice spent more time in the open arms and center of the EPM and open field, respectively. These results are consistent with our previous study demonstrating that increasing brain ACE2 activity potently elicits anxiolysis by activating central MasR(s) (Wang et al., 2016a). The present study found that DIZE had no effect on HPA axis activation; however, this previous study found that central administration of DIZE decreased anxiety-like behavior and that increasing brain ACE2 activity was associated with enhanced GABAergic synaptic transmission in the basolateral amygdala (Wang et al., 2016a). Our interpretation of these results is that low endogenous levels of ACE2 in the PVN may prohibit effects of DIZE on the HPA axis but the anxiolysis that follows central delivery of DIZE is likely the result of augmented GABA release in the amygdala. Collectively, these suggest that increasing the production of ACE2 in the brain may constrain the endocrine, cardiovascular and behavioral nodes of the stress response and that centrally residing MasR(s) may mediate these effects.
In summary, the present study revealed that coupling ACE2 overexpression to CRH transcription attenuates acute stress-induced activation of the HPA axis and anxiety-like behavior. Ubiquitously overexpressing ACE2 increased ACE2 mRNA > 1000-fold in the hypothalamus (Wang et al., 2016a), but selectively overexpressing ACE2 in CRH neurons modestly increased ACE2 expression by ≈ 2.5 fold in the PVN. Despite these differences in the magnitude of ACE2 overexpression both approaches promote anxiolysis (Wang et al., 2016a) and attenuate stress-evoked HPA axis activation, suggesting that targeting ACE2 overexpression to CRH is sufficient to dampen physiological and behavioral responses to psychogenic stress. Consequently, ACE2 may be a promising target to treat diseases featuring CRH dysregulation, like anxiety and HPA axis over-activation.
Highlights.
Exogenous ACE2 is necessary for dampening stress-evoked HPA axis activation.
Overexpressing ACE2 in CRH cells attenuates stress-induced HPA axis activation.
Overexpressing ACE2 in CRH cells reduces anxiety-like behavior.
Acknowledgments
This work was supported by the National Institutes of Health [HL122494 (EGK), HL139868 (EGK), HL125805 (ADK), and HL033610 (MKR)].
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology. 1995;61:437–444. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terron JA, Falcon-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation Stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–99. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- Brand S, Wilhelm FH, Kossowsky J, Holsboer-Trachsler E, Schneider S. Children suffering from separation anxiety disorder (SAD) show increased HPA axis activity compared to healthy controls. J Psychiatr Res. 2011;45:452–459. doi: 10.1016/j.jpsychires.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Krause EG, Solomon MB, Flak JN, Scott KA, Kim DH, Myers B, Ulrich-Lai YM, Woods SC, Seeley RJ, Herman JP. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology. 2015;56:110–119. doi: 10.1016/j.psyneuen.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33:4825–4833. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016;221:891–912. doi: 10.1007/s00429-014-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, Nguyen D, Cahill KM, Sumners C, Stern JE, Krause EG. A Unique “Angiotensin-Sensitive” Neuronal Population Coordinates Neuroendocrine, Cardiovascular, and Behavioral Responses to Stress. J Neurosci. 2017;37:3478–3490. doi: 10.1523/JNEUROSCI.3674-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria ML, Araujo LD, Fraga-Silva RA, Pereira LA, Ribeiro HJ, Menezes GB, Shenoy V, Raizada MK, Ferreira AJ. Anti-hypertensive Effects of Diminazene Aceturate: An Angiotensin- Converting Enzyme 2 Activator in Rats. Protein Pept Lett. 2016;23:9–16. doi: 10.2174/0929866522666151013130550. [DOI] [PubMed] [Google Scholar]
- Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- Kangussu LM, Almeida-Santos AF, Moreira FA, Fontes MAP, Santos RAS, Aguiar DC, Campagnole-Santos MJ. Reduced anxiety-like behavior in transgenic rats with chronically overproduction of angiotensin-(1-7): Role of the Mas receptor. Behav Brain Res. 2017;331:193–198. doi: 10.1016/j.bbr.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16:878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. Journal of Hypertension. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- Moura Santos D, Ribeiro Marins F, Limborco-Filho M, de Oliveira ML, Hamamoto D, Xavier CH, Moreira FA, Santos RA, Campagnole-Santos MJ, Peliky Fontes MA. Chronic overexpression of angiotensin-(1-7) in rats reduces cardiac reactivity to acute stress and dampens anxious behavior. Stress. 2017;20:189–196. doi: 10.1080/10253890.2017.1296949. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol. 2001;13:139–146. doi: 10.1046/j.1365-2826.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papassotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP. Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic-pituitary-adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: preliminary results. Stress. 2008;11:62–72. doi: 10.1080/10253890701476621. [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, Song C, Pepine CJ, Katovich MJ, Raizada MK. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013a;62:746–752. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YF, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, Song CJ, Pepine CJ, Katovich MJ, Raizada MK. Diminazene Aceturate Enhances Angiotensin-Converting Enzyme 2 Activity and Attenuates Ischemia-Induced Cardiac Pathophysiology. Hypertension. 2013b;62:746. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YF, Zhang J, Wang L, Shenoy V, Krause E, Oh SP, Pepine CJ, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J Mol Med (Berl) 2016;94:37–49. doi: 10.1007/s00109-015-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept. 2005;128:227–238. doi: 10.1016/j.regpep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sandstrom YK, Ljunggren G, Wandell P, Wahlstrom L, Carlsson AC. Psychiatric comorbidities in patients with hypertension--a study of registered diagnoses 2009–2013 in the total population in Stockholm County, Sweden. Journal of Hypertension. 2016;34:414–420. doi: 10.1097/HJH.0000000000000824. discussion 420. [DOI] [PubMed] [Google Scholar]
- Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ, Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiology & Behavior. 2014;136:91–96. doi: 10.1016/j.physbeh.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Hoogendijk WJ, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72:340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem. 1998;273:11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, Raizada MK, Krause EG. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016a;105:114–123. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hiller H, Smith JA, de Kloet AD, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol Genomics. 2016b;48:667–676. doi: 10.1152/physiolgenomics.00029.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]