Abstract
Objective
Black Americans (BAs) are at an elevated risk for morbidity and mortality in comparison to White Americans (WAs). Racial stressors are a common occurrence in American culture and is theorized to contribute to these disparities. When race-focused, stereotype threat (ST) is considered to be a factor that is detrimental to health in BAs; however few studies have directly investigated the impact of a ST manipulation on physiological function. Furthermore, it is proposed that racial stressors such as ST may have prolonged effects when more likely to perseverate (e.g. rumination) over the stressor and thus, those with greater trait perseveration may be more affected by ST. We sought to explore the impact of ST and trait perseveration on changes in vagus nerve activity – an indication of adaptive psychological and physiological well-being – as indexed by vagally mediated heart rate variability (vmHRV).
Design
Forty-three (24 females, mean age of 20, standard deviation of 3 years) apparently healthy BA individuals were randomly assigned to one of three experimental conditions in which they received either implicit (subtle), explicit (blatant), or no ST priming (control condition), prior to completing a cognitive task. Resting vmHRV was assessed both at baseline (pre-task) and recovery (post-task).
Results
BAs in the explicit ST condition exhibited the greatest decrease in vmHRV in comparison to the control group from pre-to post-task. BAs with moderate to high levels of trait perseveration showed the greatest decrease in vmHRV from pre-to post-task in comparison to those with lower levels of trait perseveration and BAs in the control group.
Conclusion
These data suggest that racial ST, especially when explicit and coupled with trait perseveration, can decrease vagal activity, as indexed by decreased vmHRV, which when experienced frequently can have significant consequences for health and longevity in BAs.
Keywords: Stereotype threat, heart rate variability, health disparities, perseverative cognition, race
1. Introduction
Racial stressors are a significant problem in the American culture – they uniquely and negatively affect individuals of color, particularly Black Americans (BAs),1 in comparison to White Americans (WAs; e.g. Pascoe and Smart Richman 2009; Steele and Aronson 1995). Converging evidence has linked such stressors with deleterious psychological and physiological outcomes, including cardiovascular disease, in BAs (see Brondolo et al. 2009; Schmader, Johns, and Forbes 2008; Williams and Mohammed 2009, for reviews). Therefore, racial stressors are thought to be important factors underlying health disparities found between BAs and WAs (e.g. Williams and Mohammed 2009). Specifically, BAs are at a greater risk for morbidity and mortality of the leading causes of death in America, including cardiovascular disease (American Heart Association 2016).
Stereotype threat (ST) occurs when a cue(s) in the environment brings to mind, either consciously or unconsciously, a negative group-based stereotype for which the individual belongs to; ST is defined as being at risk of confirming such a negative stereotype (Aronson et al. 2013; Burgess et al. 2010; Steele and Aronson 1995). When these stereotypes are race-based, ST is considered a racial stressor. It is important to note that racial ST is fundamentally different from other racial stressors, including racial discrimination. Racial discrimination involves negative behavior towards an individual based on their race, while racial ST is the threat of confirming a negative cognitive bias based on race. It is important to note here that the ST cue not need to come from another individual, but more broadly can be manifested via any environmental stimulus that may bring about a negative racial stereotype to the mind of the stigmatized individual (Smith 2004). It is the potential of confirming a negative racial stereotype that can have negative outcomes, such as decrements in working memory, in individuals of color, including BAs, and other negatively stigmatized groups (for review see Schmader and Johns 2003).
1.2. Stereotype threat and neural activity: potential consequences for working memory
In its original inception, ST was a stressor thought to contribute to the achievement gap found between BAs and WAs. Steele and Aronson (1995) showed that under ST conditions, BAs performed worse on a task (intelligence test) compared to all WAs and BAs in the control condition. Years later, converging evidence remains in support of this stance, having demonstrated that any ST (e.g. racial, gender) can decrease working memory capacity, as indexed by poorer performance on working memory and other cognitive tasks, in the respective stigmatized group compared to those under no threat and respective non-stigmatized groups (for review see Schmader and Johns 2003; Schmader, Johns, and Forbes 2008). One primary neurological mechanism by which ST is thought to disrupt working memory is the impact it can have on executive brain region activity, particularly the prefrontal cortex (PFC; responsible for the overall cognitive control and the recruiting of cognitive resources; Derks, Inzlicht, and Kang 2008; Schmader, Johns, and Forbes 2008). In this regard, two studies showed decreased activity in the PFC under ST conditions in women (Krendl et al. 2008; Wraga et al. 2006). Taken together, ST can decrease the neural resources needed to complete a cognitive task, and it is theorized that these brain areas may be otherwise occupied in the regulation of the stress/threat of potentially confirming a negative stereotype (Derks, Inzlicht, and Kang 2008; Schmader, Johns, and Forbes 2008). However, these few aforementioned studies have focused on women as the stigmatized group and thus, there is no direct evidence to support such a similar neurological mechanism in the context of race. Similar executive brain regions that are disrupted when experiencing ST (Derks, Inzlicht, and Kang 2008; Krendl et al. 2008; Wraga et al. 2006), have also been implicated in the regulation of the ANS (Thayer et al. 2012).
1.3. Stereotype threat and the autonomic nervous system: potential consequences for health
The autonomic nervous system (ANS) is a major homeostatic system, dually innervating many organs of the body (including the heart) and is of the most prominent factors linking somatic health to psychological processes (Thayer and Sternberg 2006; Weber et al. 2010). Greater executive brain activity, particularly in the medial PFC, is associated with greater activation of the parasympathetic nervous system (PNS; rest or digest) branch of the ANS. The vagus nerve is the primary nerve of the PNS and greater activity represents that of a major descending inhibitory pathway responsible for adaptively regulating inflammatory, immune, endocrine, and cardiovascular functions (Jarczok et al. 2014; Thayer and Lane 2009; Thayer and Sternberg 2006; Thayer, Yamamoto, and Brosschot 2010; Weber et al. 2010). In contrast, increased activation in brain areas that are associated with threat and stress, such as the amygdala, are related to activity increases in the sympathetic nervous system (SNS; fight or flight) branch of the ANS (Thayer et al. 2012). In a resting (e.g. sitting) state, the balanced and healthy individual displays greater PNS, relative to SNS, activity. ANS imbalance is characterized as a hypoactive PNS and hyperactive SNS, and is indicative of poorer physiological regulation due to lesser inhibitory control from the vagus (i.e. lower PNS activity) at rest (Thayer and Lane 2000; Thayer and Sternberg 2006). Thus, lower vagal tone and subsequent ANS imbalance in a resting state is associated with a number of disease states, including cardiovascular and inflammatory disease, and all-cause mortality (Thayer et al. 2012; Thayer, Yamamoto, and Brosschot 2010; Thayer and Sternberg 2006). Vagally-mediated heart rate variability (vmHRV) is a non-invasive method used to both index vagus nerve activity and predict ANS imbalance, and as such, has been shown to be predictive of mortality from cardiovascular and other diseases (Thayer et al. 2012; Thayer and Sternberg 2006; Thayer, Yamamoto, and Brosschot 2010). Therefore resting vmHRV can proxy both overall health and the degree to which executive brain regions are active and thus can readily self-regulate (e.g. cognitive control) in a given context (Thayer et al. 2012; Thayer and Lane 2000).
Given the importance of the vagus nerve in maintaining healthy bodily processes, understanding how ST influences vagal activity may provide insights into how racial stressors gets ‘under the skin’ (Hatzenbuehler, Nolen-Hoeksema, and Dovidio 2009) and affects physiological function in BAs. Previously only one study (Croizet et al. 2004) addressed the influence of ST on vmHRV; the study found vmHRV to be lower in stereotyped individuals in comparison to non-stereotyped individuals while performing a task under ST conditions but not under non-ST conditions. Indeed, vmHRV has been linked with cognitive control, such that those with greater vmHRV are often better at cognitive control (Thayer, Yamamoto, and Brosschot 2010; Williams, Thayer, and Koenig 2016) via greater PFC brain activity (Thayer et al. 2012). Thus, lower vmHRV during the task was construed as reflecting a decrease in working memory resources (Croizet et al. 2004) – a finding consistent with the hypothesis that ST decreases working memory capacity (Schmader, Johns, and Forbes 2008) and that vmHRV can index PFC activity (Thayer et al. 2012). It is important to note that stereotypes in this experiment were experimentally generated, and thus were not of a preexisting stereotype based on a demographic (e.g. race, gender). Moreover, this study did not examine vmHRV following the ST manipulation, as it would be important to know if ST remains subjectively active following the task, subsequently influencing resting-recovery (or prolonged) vmHRV activity. One study (Blascovich et al. 2001) showed that experimentally manipulated ST leads to increased blood pressure in BAs 20 min following the manipulation, demonstrating prolonged SNS activity; however, prolonged decrements in PNS (vagal) activity are better predictive of disease and death (Thayer and Sternberg 2006; Thayer, Yamamoto, and Brosschot 2010). In sum, while there is converging evidence illustrating the negative impact of ethnic discrimination and racism on both physiological and health outcomes (see Pascoe and Smart Richman 2009 for review) in BAs, little work has been conducted to explore potential physiological and health consequences of ST. Vagal activity following a stressor, or resting-recovery vmHRV, is thought to represent immediate physiological consequences of a stressor, that is, the degree to which the stressor is remaining subjectively active, which can have a detrimental impact one health and longevity (Brosschot, Gerin, and Thayer 2006; Brosschot, Verkuil, and Thayer 2010).
1.4. Resting-Recovery vagal tone and the perseverative cognition hypothesis
One model of health that assists in explaining the link between stress related changes in physiology and disease is the Perseverative Cognition Hypothesis. This hypothesis accounts for situations in which an individual may experience prolonged psychophysiological arousal both in anticipation to and following the presence of a stressor (Brosschot, Gerin, and Thayer 2006, 2010). Perseverative cognition represents a highly vigilant state, which may produce moderate but chronic levels of activation in the cardiovascular, hypothalamic pituitary adrenal, and immune systems via decreases in vagus nerve activity (Brosschot, Gerin, and Thayer 2006, 2010).
Perseverative cognition, or simply perseveration, can be defined as excessive rumination and/or worry over past or present (real or imagined) stressors (Brosschot, Gerin, and Thayer 2006). Worry can be defined as the repetitive negative thinking of possible future outcomes or events, whereas rumination can be defined as the repetitive thinking of past outcomes or events (Nolen-Hoeksema, Wisco, and Lyubomirsky 2008). Previous studies showed that when a stressor is no longer present, decreased PFC and increased amygdala activity following the experience of a stressor is characteristic of perseveration. This pattern of neural activity can lead to prolonged psychophysiological responses, for example, decreased resting-recovery vmHRV (Brosschot, Gerin, and Thayer 2006, 2010; Verkuil et al. 2010). This cycle of prolonged arousal and delayed recovery can permanently alter organ system functioning via the vagus, as indexed by vmHRV, effectively creating a pathway between stressor(s) and disease (Brosschot, Gerin, and Thayer 2006; Thayer, Yamamoto, and Brosschot 2010; Thayer and Sternberg 2010). Overall, the Perseverative Cognition Hypothesis posits that individuals experience prolonged periods of stress-related arousal through continued or repeated cognitive representations of the relevant stressor(s), and that subsequent vmHRV activity (recovery-resting vmHRV) could be used as an index to capture such a prolonged impact (Brosschot, Gerin, and Thayer 2006, 2010).
Thus, it is necessary to understand if ST can be a racial stressor that activates perseveration processes in BAs, where these individuals may experience not only the initial activation of the stereotype that can lead to both lower vmHRV reactivity (e.g. Croizet et al. 2004) and cognitive deficits (see Schmader, Johns, and Forbes 2008, for review), but also a ‘running dialogue’ as the threat may remain subjectively present and prolong vmHRV recovery. This would be particularly problematic in settings beyond the laboratory where individuals often endure multiple activations of ST. This idea has been applied to other racial stress factors. One recent study found resting-recovery vmHRV to be decreased following a racism manipulation (Neblett and Roberts 2013), while another report showed experiences of discrimination led to decreased resting vmHRV and increased resting mean HR a day following the negative racial discriminatory event, independent of trait perseveration (Hoggard et al. 2015). Indeed, such decreased vmHRV may contribute to aforementioned negative health outcomes seen in BAs, however research has not yet directly examined how ST may impact resting-recovery vmHRV in BAs. Such a study could also support for the idea that ST disrupts executive brain regions, and extend this idea to both demographic-based (in this case race-based) ST and prolonged activity (beyond the experience of the stressor itself). Moreover, if ST indeed leads to perseveration – as determined by decreased resting-recovery vmHRV – it is plausible that following the experience of ST, those with greater trait perseveration, or a general tendency to engage in perseveration, would show the greatest decrease in vmHRV compared to BAs with lower trait perseveration.
1.5. Present study
The present study attempts to illustrate the extent to which ST can impact prolonged vagal activity, as indexed by recovery-resting vmHRV, in BAs. More precisely, we investigated the extent to which BAs who experience ST will exhibit greater decreases in vagal activity in comparison to the BA control group.
In line with existing research, we hypothesized BAs to perform worse on a cognitive task following the ST manipulation in comparison to BAs in a control condition. We also hypothesized that BAs who experienced the ST manipulation would show a greater decrease in vmHRV from pre-task (baseline) to post-task (recovery) in comparison to BAs in the control group. We sought to explore the differential impact of both subtle/implicit and blatant/explicit ST on both cognitive performance and resting-recovery vmHRV. Considering the potential perseveration that ST may elicit, we also expected that BAs with a greater trait perseveration – in this instance rumination – would be most impacted by the ST conditions (i.e. exhibiting the greatest decrease in vmHRV).
2. Material and methods
2.1. Participants
Forty-three (24 females, mean age of 20, standard deviation of 3 years) BA individuals participated in the present study. Subjects were recruited via the Research Experience Program (REP) pool at The Ohio State University, allowing students to participate in research for partial class credit in an introductory level psychology course. Funding from The Ohio State University College of Social and Behavioral Sciences and College of Arts and Sciences also allowed us to recruit and compensate participants outside of the REP pool resulting in a diverse sample across the university (i.e. students from various majors and cohorts). The institutional review board approved the study, and all participants signed written informed consent.
2.2. Procedure
Participants were seated in a soundproof experimental room that was equipped with a camera and a microphone. Participants were given a detailed explanation of the procedure without indicating the underlying hypothesis or aim of the study. Upon completion of the consent form, height and weight were collected to assess body mass index. Electrocardiogram (ECG) leads were then attached to the subjects and while in a separate control room, the experimenter led the subjects to the initial phases of the experiment. Participants first completed a 5-minute baseline-resting period, in which they sat in a resting position with the television displaying a blank, grey screen, and were instructed not to move or fall asleep (spontaneous breathing). Immediately following this period, participants were to complete either a(n) (i) implicit ST, (ii) explicit ST, or (iii) control condition (randomly assigned). All participants then completed the cognitive task, immediately followed by a 5-minute resting-recovery period (same conditions as baseline), and ending the experiment with a set of self-report questionnaires.
2.3. Stereotype threat manipulations
In the implicit ST manipulation, participants viewed a screen that read, ‘The next phase of the experiment can serve as a measure of intelligence’; the following screen asked the participant to indicate their ethnicity. The purpose of this manipulation was to provide a subtle prime that would unconsciously elicit any negative stereotypes that may be associated with intelligence and the individual’s racial (BA) group.2 In the explicit ST manipulation, participants viewed the same two screens viewed by those in the implicit ST manipulation; however, the third screen read, ‘The following cognitive task has been shown to produce ethnic differences in the past. The average performance of majority participants was better than the performance of ethnic minority participants’ following the first two screens. Here we intended to provide a blatant prime that would consciously elicit any negative stereotypes associated with the individual’s racial group and intelligence (For discussion on our conceptual differences between ‘race’ and ‘ethnicity’, please see Footnote 1 in the Introduction). In the control condition, participants went directly from the baseline period to the cognitive task instructional screen.
2.4. Modified flanker task
Next, participants completed a modified flanker task, (adopted from Baars and Gage 2010), in which they were responsible for responding to the positioning (left or right) of a target (dot) in the face of a distracting cue (flanker-arrow). The task was administered via E-Prime 1.0 software (Psychology Software Tools, Inc., Sharpsburg, PA, USA).
Participants were first instructed to place their left index finger on the ‘Z’ key and their right index finger on the ‘M’ key. Participants began the trial by fixating on a cross in the middle of the screen for 1000 ms. Then, a flaker-arrow appeared for a brief period (250 ms) positioned directly to the left or right of the fixation cross. The direction for which the arrow pointed was consistent with the arrow placement (e.g. a cue arrow pointing left will always be placed to the left of the fixation cross). If the arrow pointed in the same direction of the target (left or right), the trial was considered congruent. If the arrow pointed in the opposite direction of the target, the trial was considered incongruent. Participants completed 120 trials, with 24 incongruent (20%) and the remaining 96 trials (80%) were congruent – a ratio of trials previously proposed for tasks attempting to test attention abilities (Baars and Gage 2010; Posner 1980; Posner and Petersen 1990). There was an even split of trials in which the target occurred on the left or right side (60 trials each side). Participants were instructed to respond to the positioning of the dot as quickly and as accurately as they could, ‘ignoring all other information’. Each trial was terminated immediately upon the participants’ selection, and the next trial began (inter-trial-interval of 1000 ms). In this task, the arrow is considered a distracting flanker that is difficult to ignore (Baars and Gage 2010). Individuals must work to appropriately direct attention in order to respond to the correct spatial positioning target, especially on incongruent trials (Baars and Gage 2010; Posner 1980). Thus, incongruent trial performance was of particular interest, as ST is most likely to disrupt processing on these trial types. Accuracy rates (ACC; proportion of the trials correct), and reaction time (RT; reaction time of responding on correct trials only) were recorded for incongruent trials. Upon the completion of this task, participants completed a 5-minute resting-recovery phase similar to baseline.
2.5. Perseverative cognition: ruminative responses scale
Upon completion of the task, participants completed a set of questionnaires, including the 22-item Ruminative Responses Scale (RRS; Treynor, Gonzalez, and Nolen-Hoeksema 2003). Participants answered on a scale from 1 (almost never) to 4 (almost always), (sample item: How often do you think ‘What am I doing to deserve this?’), with higher numbers representing greater trait rumination. The RRS contains three subscales, including brooding rumination (wallowing and sulking), dampening rumination (sadness and despair), and reflective rumination (problem solving and analyzing). In the present analysis, only total RRS scores were used as an index of perseveration.
2.6. Vagally mediated heart rate variability
Cardiac activity data was recorded continuously via a 3-lead electrocardiogram (ECG) at a sampling rate of 1000 Hz using a Mindware™ 2000D Impedance Cardiograph package. Electrodes were placed (1) below the right clavicle, (2) on the left side of the abdomen (below the heart), and (3) on the right side of the abdomen. VmHRV assessed during (i) a 5-minute resting-baseline period and (ii) a 5-minute post-task recovery period were used for analysis. Differences between the two segments were calculated as change scores. The variability between successive R-spikes (or variability of the inter-beat-intervals) was obtained from ECG recordings to calculate vmHRV. Participants’ successive inter-beat-intervals, in milliseconds, were extracted using HRV 3.0.25 Analysis software (Mindware Technologies, LTD), written in a text file, and analyzed using Kubios HRV analysis package 2.0 (Tarvainen et al. 2014), allowing for the calculation of time- and frequency-domain indices of vmHRV. Artifacts within the R-to-R series were visually detected, and we applied an artifact correction level that would differentiate and remove artifacts (differing abnormal inter-beat-intervals from the mean inter-beat-intervals) using a piecewise cubic spline interpolation method. The root mean square of successive differences (RMSSD), measured in milliseconds, was calculated and is considered to be a stable and valid time-domain measure of vmHRV (Thayer, Yamamoto, and Brosschot 2010). Autoregressive estimates were also calculated, yielding high frequency (HF) power HRV (HF-HRV, 0.15–0.4 Hz), which is also considered both a stable and valid index of vmHRV (Thayer, Yamamoto, and Brosschot 2010). RMSSD and HF values were natural log transformed (ln) to fit the assumptions of linear analyses. In the present study lnRMSSD correlated highly with lnHF-HRV (r = .927, p < .001) –thus, we only report lnHF-HRV results as the measure of vmHRV. Results were identical when using lnRMSSD (results not shown).
2.7. Statistical methods
All statistical tests were performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY) and StatsSoft Statistica 6.0 (StatSoft, Inc., Tulsa, OK). All tests were two-tailed level and evaluated with an alpha of .05.
Both gender (e.g. male = 1, female = 2) and experimental condition (control group as ‘1’, implicit ST group as ‘2’, and explicit ST group as ‘3’) were given dummy codes. Demographic covariates that have been shown to influence vmHRV were also added in all vmHRV analyses, including body mass index (Koenig et al. 2014), age (Choi et al. 2006), and gender (Koenig and Thayer 2016).
To test the effects of ST on vmHRV from pre- (baseline) to post- (recovery) task, we used a between-within factor analysis of variances (ANOVAs). The within-subject factor (TIME) includes vmHRV at two phases of the experiment (baseline and post task recovery). CONDITION (implicit, explicit, and no ST) was used as the between subject factor and aforementioned covariates were controlled included. Task performance was analyzed using multiple 1-way ANOVAs, where the between factor included CONDITION, and dependent variables included the aforementioned indices of SE task performance. Preplanned contrasts were used to evaluate hypothesized comparisons and pattern differences (from pre-task baseline to post-task recovery) in vmHRV between groups (Rosnow and Rosenthal 1995). We computed r coefficients to indicate the strength of the association between the experimental manipulation and changes vmHRV. The 95% confidence interval (in square brackets) associated with corresponding mean difference (MD) is also provided to indicate precision.
In order to test if trait perseveration (total RRS scores) moderated the relationship between the ST manipulations and vmHRV changes in BAs, the SPSS macro PROCESS was used (Hayes 2012). We used ‘Model 1’ to test how rumination impacted changes in vmHRV in BAs who experienced ST. We first created three different contrast variables: the variable ‘Contrast 1’ included dummy codes for individuals in the control and implicit ST (but not the explicit ST) groups; ‘Contrast 2’ included codes for control and explicit ST (but not implicit ST) groups; and ‘Contrast 3’ included dummy codes for implicit ST and explicit ST (but not the control) groups. Any contrast(s) found to be significant in the ANOVA preplanned contrast analyses (see above) were used as the independent variable (IV); trait perseveration (total RRS scores) as the moderating (M) variable; and change scores from baseline- to recovery-resting vmHRV as the dependent variable (DV; with negative scores representing a greater decrease in vagal activity). If the 2-way interaction is significant, it suggests that the relationship between the IV and DV changes at different levels of M (for review, see Hayes 2012; see Figure 1 for conceptual diagram). The nature of the interaction was determined using PROCESS’ conditional effects, that is, how the IV-DV relationship changes at different levels of M. Higher and lower values for the predictor variables are derived using +/− 1SD from the mean (respectively), allowing the program to yield predicted values of the DV at varying levels of the predictor variables (lower, moderate, and higher levels of the predictor variable). Statistics reported include unstandardized beta (B) coefficients, standard errors (SE; in brackets), partial correlation coefficients (for interactions), and confidence intervals.
Figure 1.
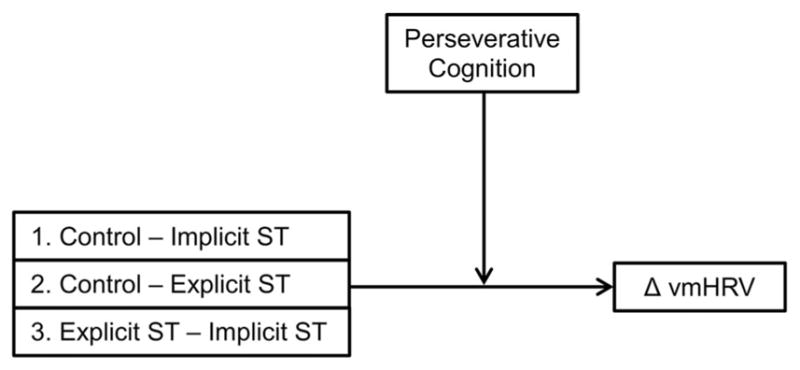
Conceptual diagram of proposed moderation model. This figure provides a conceptual illustration of the proposed (and tested) model. The three contrasts serves as the independent variable(IV), trait perseverative cognition served as the moderator variable (M), and vmHRV change scores from pre-to post-task served as the dependent variable (DV).
3. Results
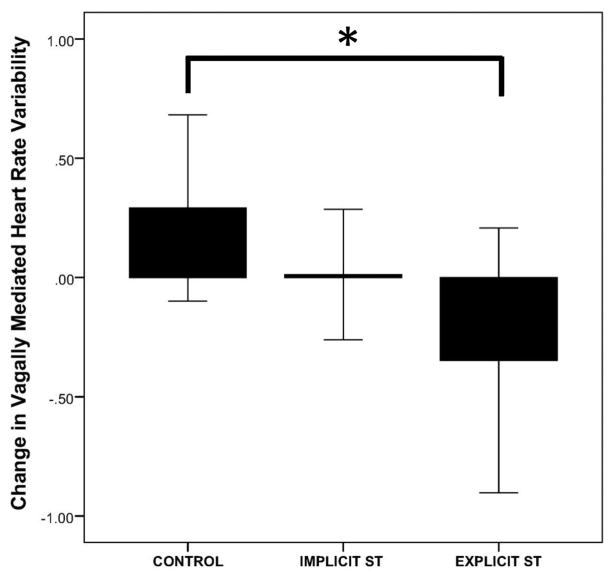
Table 1 shows means (standard deviation in brackets) of all variables split by experimental condition. ANOVA results showed a null main effect of TIME (F(1,37) = .98, r = .161, p = .328), a significant main effect of CONDITION (F(2,37) = 5.43, r = .476, p = .008), and a trending interaction between the two (F(2,37) = 2.70, r = .357, p = .080). Preplanned contrasts showed that BAs in the ST manipulation (both implicit and explicit) had a significant decrease in vmHRV from pre- to post-task compared to the control group (F(1,37) = 4.44, r = .327, p = .042). When considering both types of ST, BA individuals in the explicit ST showed a significant decrease in vmHRV (F(1, 37) = 5.37, MD =−.63, [−1.18, −.080], r = .356, p = .026), whereas BAs in the implicit ST condition did not show a significant decrease in vmHRV (F(1, 37) = 1.51, MD = −.31, [−.81, .20], r = .198, p = .227) from pre- to post-task compared to the control group. No significant differences were found between BAs in the implicit and explicit conditions (F(1, 37) = 1.44, MD = .27, [−.88, .23], r = .194, p = .237). Relative vmHRV change scores from baseline to post-task recovery and their 95% confidence intervals are illustrated in Figure 2.
Table 1.
Means and standard deviations of all variables by split by experimental condition.
| Control | Implicit ST | Explicit ST | Total | |
|---|---|---|---|---|
| n | 16 | 16 | 11 | 43 |
| Age, years | 20.8 (3.5) | 19.9 (1.9) | 19.7 (1.5) | 20.2 (2.5) |
| BMI, kg/m2 | 25.0 (4.4) | 26.6 (6.6) | 24.5 (3.9) | 25.5 (5.2) |
| HR, bpm | 73.6 (7.7) | 79.2 (10.4) | 72.8 (6.6) | 75.3 (8.8) |
| Trait Perseveration | 43.0 (9.9) | 42.9 (13.2) | 41.7 (11.8) | 42.7 (11.4) |
| Baseline vmHRV | 7.02 (0.59) | 6.59 (0.86) | 6.70 (0.91) | 6.78 (0.79) |
| Post-task vmHRV | 7.31 (0.77) | 6.59 (0.86) | 6.36 (0.83) | |
| vmHRV | 0.29 (.73) | 0.01 (.54) | −0.35 (.83) | |
| ICG-ACC (%) | 96.4 (6.3) | 98.1 (2.6) | 96.6 (4.4) | |
| ICG-RT (ms) | 459 (82) | 445.44 (67.62) | 468.35 (65.96) |
Note: This table shows mean (standard deviation in brackets) values on all variables of interest measures by experimental condition and full sample statistics (for baseline variables). Age was calculated in years, heart rate (HR) in beats per minute, Body mass index (BMI) was calculated in kg/m2, and vmHRV (lnHF-HRV, see methods) in ms2. Negative vmHRV change scores indicate a decrease in vmHRV. Higher Ruminative Responses Scale scores reflect greater trait perseveration. Incongruent accuracy (ICG-ACC) was measured as the percentage (%) of correct responses on incongruent trials. Incongruent reaction time (ICG-RT) was measured in milliseconds.
Figure 2.
VmHRV Change scores by condition. This figure represents change scores in vmHRV from baseline to post-task recovery split by condition.
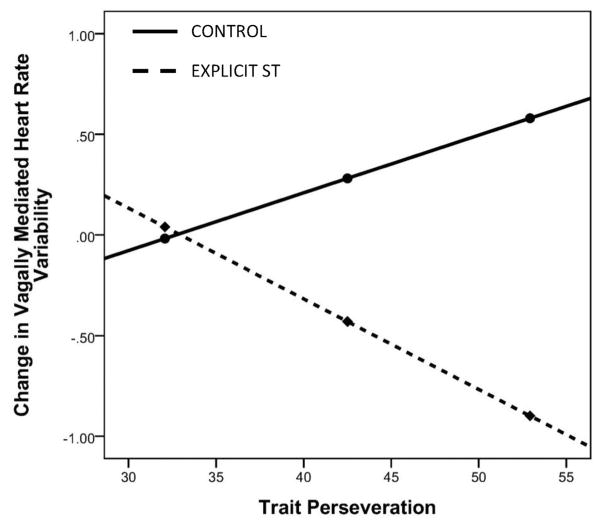
Thus using PROCESS, ‘Contrast 2’ (control coded as 1 and explicit ST coded as 2) was used to explore how perseveration possibly moderated this change in vmHRV. ‘Contrast 2’ was used as the IV, trait perseveration as the M, and change score values from baseline to recovery as the DV. Results showed a strong interaction between variables to predict change in vmHRV (B = −.037 (.015), [−.068, −.006], rpartial = −.497, p = .022). Conditional effects showed that those in the explicit ST group who also yielded moderate (B = −.356 (.16), [−.684, −.026], p = .036) and higher (B = −.33 (.22), [−1.21, −.274], p = .004) RRS scores showed decreases in vmHRV from baseline to recovery, whereas BAs who scored lower on the RRS did not show similar decreases compared to the control group (B = −.03 (.22), [−.43, −.48], p = .895; Figure 3). On task performance (both congruent and incongruent trials), we found no significant differences in BAs between conditions (each p > .521).
Figure 3.
Moderating effect of trait perseveration on changes in vmHRV. This figure represents the moderating effect of trait perseveration on the link between the ST manipulation and changes in vmHRV, specifically between the control and explict ST condition (‘ Contrast 2’, see Methods). Results showed that those with moderate and higher RRS scores showed the greatest decrease in vmHRV from baseline to post-task recovery in comparison to BAs lower in RRS scores and in the control group.
4. Discussion
It is theorized that racial stressors such as ST can have a profound and negative impact on health in BAs. However, little work has investigated the impact of ST on physiological function in negatively stigmatized individuals such as BAs, and no study had previously demonstrated how trait perseveration may moderate this association. To our knowledge, the present study is the first to investigate the impact of ST on changes in vagal activity –vital in maintaining good health – from baseline to recovery and how trait perseverative tendencies may moderate such effects in BAs.
Our results support the general impact of ST on physiological function, showing that BAs under ST conditions can experience decreased baseline- to recovery-resting vmHRV; this pattern of results were particularly evident in the explicit ST group compared to the control group. Within the explicit ST group, our data suggest that those with moderate to high levels of trait perseveration (i.e. rumination), were most impacted by the blatant ST manipulation. As previously mentioned, racial ST is fundamentally different from racial discrimination. Racial discrimination involves negative behavior towards an individual based on their race, while racial ST is the threat of confirming a negative cognitive bias based on race. Thus, our findings are novel in the sense that the conscious threat of confirming negative biases based on race can lead to prolonged decreases in vagal activity, which is necessary for the adaptive regulation of several vital bodily systems (Thayer and Sternberg 2006; Thayer, Yamamoto, and Brosschot 2010), in BAs. In sum, racial ST – particularly when blatant – can prolong decreased vmHRV, which can have downstream consequences for health and thereby supports the ideology that ST and other racial stressors are important factors underlying health disparities found between BAs and WAs.
4.1. Implications
As prolonged decreased vmHRV in a resting state following a stressor is often evident of perseveration (Brosschot, Gerin, and Thayer 2006, 2010), it is plausible that BAs in the explicit ST condition perseverated (whether conscious or unconscious) over ST. These results are consistent with the aforementioned studies that have examined how race related stressors and perseverative processes may influence physiology (Hoggard et al. 2015; Neblett and Roberts 2013). Thus, our results extend these previous reports by showing that ST, particularly when explicit, can be a racial stress that also leads to decreases in resting-recovery vagal activity. We also showed that BAs who typically perseverate may experience an even greater decrease in resting-recovery vmHRV following an (explicit) ST manipulation compared to those lower in trait rumination. This suggests that trait rumination can elevate the degree to which blatant ST prolongs vmHRV recovery. It is important to note that we do not suggest that having lower perseverative tendencies will compensate for the possible negative outcomes related to race-related stressors (e.g. ST) in BAs; this idea has been supported previously (e.g. Hoggard et al. 2015) and in the current data. Specifically, all tests of the ST manipulation impacting vmHRV remains significant when including rumination as a covariate. Additionally, trait rumination does not significantly mediate the association between experimental conditions and changes in vmHRV. Taken together, we suggest that having lower perseverative tendencies is will not solve the larger issue of the prevalence of racial stressors in America, but instead, lower perseverative tendencies in BAs is of particular importance, as the negative effects of race-related stressors can be exaggerated in those who tend to perseverate. In line with this idea, a recent meta-analysis found BAs to have greater resting vmHRV in comparison to WAs (Hill et al. 2015). However, BAs also tend to have greater resting peripheral resistanc in comparison to WAs – a pattern of results termed the ‘cardiovascular conundrum’ (Hill et al. 2015). It is proposed that greater vmHRV in BAs served as a compensatory mechanism. That is, with race-related stressors, such as ethnic discrimination and stereotype threat, BAs need greater emotion regulation capabilities to inhibit their expressions of anger in response to such unfair treatment. A recent report supported these claims, showing ethnic discrimination to mediate the association of darker skin color in AAs and greater resting vmHRV (Kemp et al. 2016). However, another report showed that within AAs, lower vmHRV was associated with greater experience of discrimination (Williams et al. in press). Taken together, although BAs show higher resting vmHRV in comparison to WAs, lower resting vmHRV within BAs may still represent lesser emotion regulation capabilities as previously theorized in all individuals (Thayer and Lane 2000). The current study extends this idea to emotion regulation strategies, such that maladaptive coping mechanisms such as rumination may be particularly harmful for BAs’ (and other individuals of color) unique and negative day-to-day encounter with ST and other racial stressors. From another perspective, Burgess et al. (2010) suggested that, in addition to maladaptive health behaviors, there are multiple internal mechanisms driving the relationship between ST and health, particularly in medical settings. Physiological arousal is considered one of the seven mechanisms behind ST and health set forth by Burgess et al. (2010). We propose that decreased vmHRV following racial stressors such as ST represents the psychophysiological mechanism linking such stressors unfavorable health outcomes, emphasizing the vagus nerve as an important pathway of the stress response in BAs who experience ST.
Given the volume of previous research proposing that ST decreases working memory, we expected to see significant differences between conditions on our cognitive task, which has been previously shown to activate brain areas involved with working memory (Baars and Gage 2010). However, there were no strong differences in accuracy or reaction time between BAs in any condition. Previous studies have examined the effects of ST on cognitive performance by using intelligence tasks such as math tests, or fluid intelligence tasks such as the Raven’s Progressive Matrices, where working memory is crucial in the completion of these tasks (Blascovich et al. 2001; Croizet et al. 2004; Schmader, Johns, and Forbes 2008; Schmader and Johns 2003; Shapiro and Neuberg 2007), and possibly more so in comparison to our modified Flanker Task. This point is important, as the lack of impact ST had on performance could be seen as a limitation in verifying that ST actually occurred. Here, it is important to note that given the definition of ST, performance decrements are an outcome, and not requirement, of the experience of ST. Our data showed participants as a whole to have little difficulty with the task (all accuracy rates > .95). Therefore, we propose that the task may not have been difficult enough to reveal performance deficits given the importance of task difficulty in examining ST effects on performance (see Keller 2007, for emperical evidence; for review see, Nguyen and Ryan 2008). In fact, our observed effects – that is decreased vmHRV in BAs following explicit ST – might underestimate the prolonged impact of ST, as it may have been stronger had the task been more difficult. Importantly, the present results suggest that the decreased vmHRV associated with the explicit ST condition was likely not due to poor performance on the task, and instead likely due to the threat/stress associated with ST. Additionally, while vmHRV during the task is beyond the scope of this report and thus data are not shown, it is important to note there were no significant results found using vmHRV during the task as the Croizet et al. (2004) report, suggesting that these prolonged reductions in vmHRV cannot be attributed to actually confirming the stereotype. Instead, the mere threat of confirming the stereotype (under explicit conditions) lowered prolonged resting (and not reactive) vmHRV in BAs – a pattern of activity that can be detrimental for health. Finally, here it is also important to highlight the positive association between resting vmHRV and brain areas involved in working memory (e.g, PFC; see Thayer et al. 2012, for meta-analysis). Therefore, while traditional ST effects did not show on our task parameters, by theoretical and empirical extension, we provide psychophysiological evidence that ST occurred in our experiment; decreased vmHRV represents lesser vagal tone and PNS activity, which reflect lesser activity in brain areas involved in working memory theoretical (see Thayer et al. 2009, 2012 for reviews), suggesting disruption of these areas (e.g. Croizet et al. 2004) at least under explicit ST conditions.
Overall, a growing body of research has linked decreased vagal tone to cardiovascular and other diseases (Brosschot, Gerin, and Thayer 2006; Brosschot, Van Dijk, and Thayer 2007; Brosschot, Verkuil, and Thayer 2010; Elliot et al. 2011; Thayer et al. 2012, 2010; Thayer and Lane 2000, 2009; Thayer and Sternberg 2010). While the current investigation shows that experimentally manipulated ST can have acute-prolonged negative effects on vagal activity as indexed by vmHRV, overtime, the repeated exposure to ST may be detrimental to the aforementioned physiological systems and subsequent health. Thus, loss in vagal control could be an underlying mechanism linking ST with health and longevity. However, longitudinal research beyond the laboratory setting is needed to address this claim directly.
4.2. Limitations and future directions
As previously mentioned, some may consider the lack of performance decrements following the experience of ST in this investigation as a limitation. Considering this viewpoint, future investigations should replicate our procedure using a more difficult paradigm (e.g. math testing) compared to the current used modified Flanker Task. As a result, researchers could examine if the results would be different/stronger in the event that participants show decreases in cognitive performance in response to ST. Additionally, given the impact ST can have on other stigmatized groups, such as women, this effect should be examined in alternative target groups. Our present ST manipulation did not focus on gender as the primary target stigma-group and thus, we could not analyze this in our data. Finally, ST is a phenomenon that can occur in any individual who identifies with a negatively stigmatized group. Thus, future research should examine how ST impacts vmHRV in other negatively stereotyped groups.
4.3. Conclusions
It is well-documented that racial stressors can have a detrimental impact on health in BAs (see Brondolo et al. 2009; Schmader, Johns, and Forbes 2008; and Williams and Mohammed 2009, for reviews). The present investigation is among the few (e.g. Hoggard et al. 2015; Hill et al. 2015; Williams et al. in press) to propose vmHRV as an underlying physiological mechanism linking racial stressors – in this instance ST – with health outcomes in BAs. We propose that decreased vagal activity due to the experience of ST and other racial stressors’ is an important mechanism underlying the health disparities in our society. Additionally, our results show that higher trait perseveration can lead to greater decreases of vagal activity following the experience of ST and thus for BAs (and possible other negatively stigmatized groups), lesser trait perseveration may be of particular importance given the unique and negative impact of day-to-day racial stressors such as ST.
Acknowledgments
Funding
This research was supported by funding from The Ohio State University College of Social and Behavioral Sciences to the first author (D.P.W.).
Footnotes
It is important to note that ethnicity (e.g. African American) and race (e.g. Black American) represent very different constructs as it relates to identities, status, and inequality. Nevertheless, when referring to Black and White Americans throughout the manuscript, we are referring to those of African and European descent, respectively. As it relates to the impact of race-related stressors, we acknowledge that it can potentially impact many other individuals of color and minority groups, and not just African Americans. However, as it relates to health disparities, these have been shown to be particularly prevalent in African American individuals and as such, Black Americans are considered African Americans in the present report for the purposes of providing a clear hypothesized link between race-related stressors aimed at Black Americans, and health outcomes in African Americans.
Implicit ST does not have to elicit stereotypes unconsciously, per se. The important components of implicit ST are both the ambiguity and subtleness of the threat (Mendoza-Denton et al. 2009).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- American Heart Association. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Aronson J, Burgess D, Phelan SM, Juarez L. Unhealthy Interactions: The Role of Stereotype Threat in Health Disparities. American Journal of Public Health. 2013;103(1):50–56. doi: 10.2105/AJPH.2012.300828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ, Gage NM. Consciousness and Attention. In: Baars BJ, Gage NM, editors. Cognition, Brain, and Consciousness. 2. London, UK: Academic Press; 2010. pp. 238–303. [DOI] [Google Scholar]
- Blascovich J, Spencer SJ, Quinn D, Steele C. African Americans and High Blood Pressure: The Role of Stereotype Threat. Psychological Science. 2001;12(3):225–229. doi: 10.1111/1467-9280.00340. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Ver Halen NB, Pencille M, Beatty D, Contrada RJ. Coping with Racism: A Selective Review of the Literature and a Theoretical and Methodological Critique. Journal of Behavioral Medicine. 2009;32(1):64–88. doi: 10.1007/s10865-008-9193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The Perseverative Cognition Hypothesis: A Review of Worry, Prolonged Stress-related Physiological Activation, and Health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Van Dijk E, Thayer JF. Daily Worry is Related to Low Heart Rate Variability During Waking and the Subsequent Nocturnal Sleep Period. International Journal of Psychophysiology. 2007;63(1):39–47. doi: 10.1016/j.ijpsycho.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Verkuil B, Thayer JF. Conscious and Unconscious Perseverative Cognition: Is a Large Part of Prolonged Physiological Activity Due to Unconscious Stress? Journal of Psychosomatic Research. 2010;69(4):407–416. doi: 10.1016/j.jpsychores.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, Warren J, Phelan S, Dovidio J, van Ryn M. Stereotype Threat and Health Disparities: What Medical Educators and Future Physicians Need to Know. Journal of General Internal Medicine. 2010;25(Suppl 2):169–177. doi: 10.1007/s11606-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, Dimsdale JE. Age and Ethnicity Differences in Short-term Heart-rate Variability. Psychosomatic Medicine. 2006;68(3):421–426. doi: 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- Croizet JC, Després G, Gauzins ME, Huguet P, Leyens JP, Méot A. Stereotype Threat Undermines Intellectual Performance by Triggering a Disruptive Mental Load. Personality & Social Psychology Bulletin. 2004;30(6):721–731. doi: 10.1177/0146167204263961. [DOI] [PubMed] [Google Scholar]
- Derks B, Inzlicht M, Kang S. The Neuroscience of Stigma and Stereotype Threat. Group Processes & Intergroup Relations. 2008;11(2):163–181. doi: 10.1177/1368430207088036. [DOI] [Google Scholar]
- Elliot AJ, Payen V, Brisswalter J, Cury F, Thayer JF. A Subtle Threat Cue, Heart Rate Variability, and Cognitive Performance. Psychophysiology. 2011;48(10):1340–1345. doi: 10.1111/j.1469-8986.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How Does Stigma ‘Get Under the Skin’? The Mediating Role of Emotion Regulation. Psychological Science. 2009;20(10):1282–1289. doi: 10.1111/j.1467-9280.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling 2012 [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers JJ, Kapuku G, Wang X, Snieder H, Thayer JF. Ethnic Differences in Resting Heart Rate Variability. Psychosomatic Medicine. 2015;77(28):16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard LS, Hill LK, Gray DL, Sellers RM. Capturing the Cardiac Effects of Racial Discrimination: Do the Effects ‘Keep Going’? International Journal of Psychophysiology. 2015;97(2):163–170. doi: 10.1016/j.ijpsycho.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard LS, Hill LK, Gray DL, Sellers RM. Capturing the Cardiac Effects of Racial Discrimination: Do the Effects ‘Keep Going’? International Journal of Psychophysiology. 2015;97(2):163–170. doi: 10.1016/j.ijpsycho.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. Lower Heart Rate Variability Predicts Increased Level of C-Reactive Protein 4 Years Later in Healthy, Nonsmoking Adults. Journal of Internal Medicine. 2014;276:667–671. doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- Keller J. Stereotype Threat in Classroom Settings: The Interactive Effect of Domain Identification, Task Difficulty and Stereotype Threat on Female Students’ Maths Performance. British Journal of Educational Psychology. 2007;77(2):323–338. doi: 10.1348/000709906X113662. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Koenig J, Thayer JF, Bittencourt MS, Pereira AC, Santos IS, Benseñor IM. Race and Resting-State Heart Rate Variability in Brazilian Civil Servants and the Mediating Effects of Discrimination: An ELSA-Brasil Cohort Study. Psychosomatic Medicine. 2016;78(8):950–958. doi: 10.1097/PSY.0000000000000359. [DOI] [PubMed] [Google Scholar]
- Koenig J, Jarczok MN, Warth M, Ellis RJ, Bach C, Hillecke TK, Thayer JF. Body Mass index is Related to Autonomic Nervous System Activity as Measured by Heart Rate Variability--a Replication Using Short Term Measurements. The Journal of Nutrition, Health & Aging. 2014;18(3):300–302. doi: 10.1007/s12603-014-0022-6. [DOI] [PubMed] [Google Scholar]
- Koenig J, Thayer JF. Sex Differences in Healthy Human Heart Rate Variability: A Meta-Analysis. Neuroscience & Biobehavioral Reviews. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Richeson JA, Kelley WM, Heatherton TF. The Negative Consequences of Threat: An fMRI Investigation of the Neural Mechanisms Underlying Women’s Underperformance in Math. Psychological Science. 2008;19(2):168–175. doi: 10.1111/j.1467-9280.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Mendoza-Denton R, Shaw-Taylor L, Chen S, Chang E. Ironic Effects of Explicit Gender Prejudice on Women’s Test Performance. Journal of Experimental Social Psychology. 2009;45(1):275–278. doi: 10.1016/j.jesp.2008.08.017. [DOI] [Google Scholar]
- Neblett EW, Roberts SO. Racial identity and Autonomic Responses to Racial Discrimination. Psychophysiology. 2013;50(10):943–953. doi: 10.1111/psyp.12087. [DOI] [PubMed] [Google Scholar]
- Nguyen HD, Ryan AM. Does Stereotype Threat Affect Test Performance of Minorities and WomenA Meta-Analysis of Experimental Evidence. Journal of Applied Psychology. 2008;93(6):1314–1334. doi: 10.1037/a0012702. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Pascoe EA, Smart Richman L. Perceived Discrimination and Health: A Meta-Analytic Review. Psychological Bulletin. 2009;135(4):531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of Attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The Attention System of the Human Brain. Annual Review of Neuroscience. 1990;13(1):25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. ‘SOME THINGS YOU LEARN AREN’T SO’. Cohen’s Paradox, Asch’s Paradigm, and the Interpretation of Interaction. Psychological Science. 1995;6(1):3–9. doi: 10.1111/j.1467-9280.1995.tb00297.x. [DOI] [Google Scholar]
- Schmader T, Johns M. Converging Evidence That Stereotype Threat Reduces Working Memory Capacity. Journal of Personality and Social Psychology. 2003;85(3):440–452. doi: 10.1037/0022-3514.85.3.440. [DOI] [PubMed] [Google Scholar]
- Schmader T, Johns M, Forbes C. An Integrated Process Model of Stereotype Threat Effects on Performance. Psychological Review. 2008;115(2):336–356. doi: 10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JR, Neuberg SL. From Stereotype Threat to Stereotype Threats: Implications of a Multi-Threat Framework for Causes, Moderators, Mediators, Consequences, and Interventions. Personality and Social Psychology Review. 2007;11(2):107–130. doi: 10.1177/1088868306294790. [DOI] [PubMed] [Google Scholar]
- Smith JL. Understanding the Process of Stereotype Threat: A Review of Mediational Variables and new Performance Goal Directions. Educational Psychology Review. 2004;16(3):177–206. [Google Scholar]
- Steele CM, Aronson J. Stereotype Threat and the Intellectual Test Performance of African Americans. Journal of Personality and Social Psychology. 1995;69(5):797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–Heart Rate Variability Analysis Software. Computer Methods And Programs in Biomedicine. 2014;113(1):210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. http://www.ncbi.nlm.nih.gov/pubmed/11163422. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the Heart-Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neuroscience and Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond Heart Rate Variability: Vagal Regulation of Allostatic Systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural Aspects of Immunomodulation: Focus on the Vagus Nerve. Brain, Behavior, and Immunity. 2010;24(8):1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural Aspects of Immunomodulation: Focus on the Vagus Nerve. Brain, Behavior, and Immunity. 2010;24(8):1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The Relationship of Autonomic Imbalance, Heart Rate Variability and Cardiovascular Disease Risk Factors. International Journal of Cardiology. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Verkuil B, Brosschot J, Gebhardt W, Thayer J. When Worries Make You Sick: A Review of Perseverative Cognition, the Default Stress Response and Somatic Health. Journal of Experimental Psychopathology. 2010;1(1):87–118. doi: 10.5127/jep.009110. [DOI] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC. Low Vagal Tone is Associated with Impaired Post Stress Recovery of Cardiovascular, Endocrine, and Immune Markers. European Journal of Applied Physiology. 2010;109(2):201–211. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed S. Discrimination and Racial Disparities in Health: Evidence and Needed Research. Journal of Behavioral Medicine. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Pandya KP, Hill LK, Kemp AK, Way BM, Koenig J, Thayer JF. Rumination Moderates the Association Between Resting High Frequency Heart Ratr Variability and Percieved Ethnic Discriminatin. Journal of Psychophysiology in press. [Google Scholar]
- Williams DP, Thayer JF, Koenig J. Resting Cardiac Vagal Tone Predicts Intraindividual Reaction Time Variability During an Attention Task in a Sample of Young and Healthy Adults. Psychophysiology. 2016;53(12):1843–1851. doi: 10.1111/psyp.12739. [DOI] [PubMed] [Google Scholar]
- Wraga M, Duncan L, Jacobs EC, Helt M, Church J. Stereotype Susceptibility Narrows the Gender Gap in Imagined Self-Rotation Performance. Psychonomic Bulletin & Review. 2006;13(5):813–819. doi: 10.3758/bf03194002. [DOI] [PubMed] [Google Scholar]